Introduction
Chemotherapy-induced thrombocytopenia is a
relatively common adverse drug reaction (ADR) in the oncological
setting; the effects of chemotherapy drugs such as oxaliplatin or
fluoropyrimidines on bone marrow functionality are well known
(1). Oxaliplatin is a
third-generation platin compound frequently used for colorectal
cancer; thrombocytopenia is a common adverse reaction during
treatment, occurring in up to 70% of patients (2,3).
Drug-induced thrombocytopenia is an ADR characterized by
accelerated platelet destruction or interference with regular bone
marrow function, resulting in impaired platelet growth (4). It is characterized by a severe and
rapid reduction of the platelet count, usually
<50×109/l, which may lead to clinically relevant
conditions such as purpura, spontaneous bleeding or even
haemorrhage, requiring blood transfusion in certain cases. Once
identified, the causative agent must be immediately suspended and
further re-exposure should be avoided, as it may lead to relapse of
the disorder (positive rechallenge) (5). The present case report described the
occurrence of acute thrombocytopenia, probably triggered by
panitumumab, a human anti-epidermal growth factor receptor
(anti-EGFR) monoclonal antibody (mAb), in an oncology patient.
Case report
The patient was a female aged 49 years who was
diagnosed with rectal adenocarcinoma in June 2018 at the Medical
Oncology Unit of E.O. Ospedali Galliera (Genoa, Italy). There, from
2018 to 2020, the patient was administered chemotherapy with
5-fluorouracil (5-FU), Oxaliplatin, L-leucovorin (FOLFOX4 regimen).
Afterwards, the patient was treated with Radiotherapy in
combination with Capecitabine up until 2021, when metastatic
progression was reported (pT3, N2b, M1). Therefore, at the end of
June 2021, the patient started therapy with FOLFOX6, a
chemotherapeutic regimen composed of 5-FU, oxaliplatin and folic
acid, in association with panitumumab, a human mAb (IgG2 subtype).
The intravenous (IV) dosage was as follows: Oxaliplatin 135 mg IV
infusion over 2 h, 5-FU 600 mg IV bolus injection + 4,000 mg IV 48
h infusion (corrected for body surface area), L-leucovorin 300 mg
IV infusion over 2 h and panitumumab 320 mg IV; panitumumab was the
first drug administered, followed by oxaliplatin with leucovorin,
followed by 5-FU. The therapy was well tolerated for 9 cycles, with
three more to complete before re-evaluation of the disease. No
life-threatening events of thrombocytopenia were reported nor
documented in the 4 years of treatment (from diagnosis to March
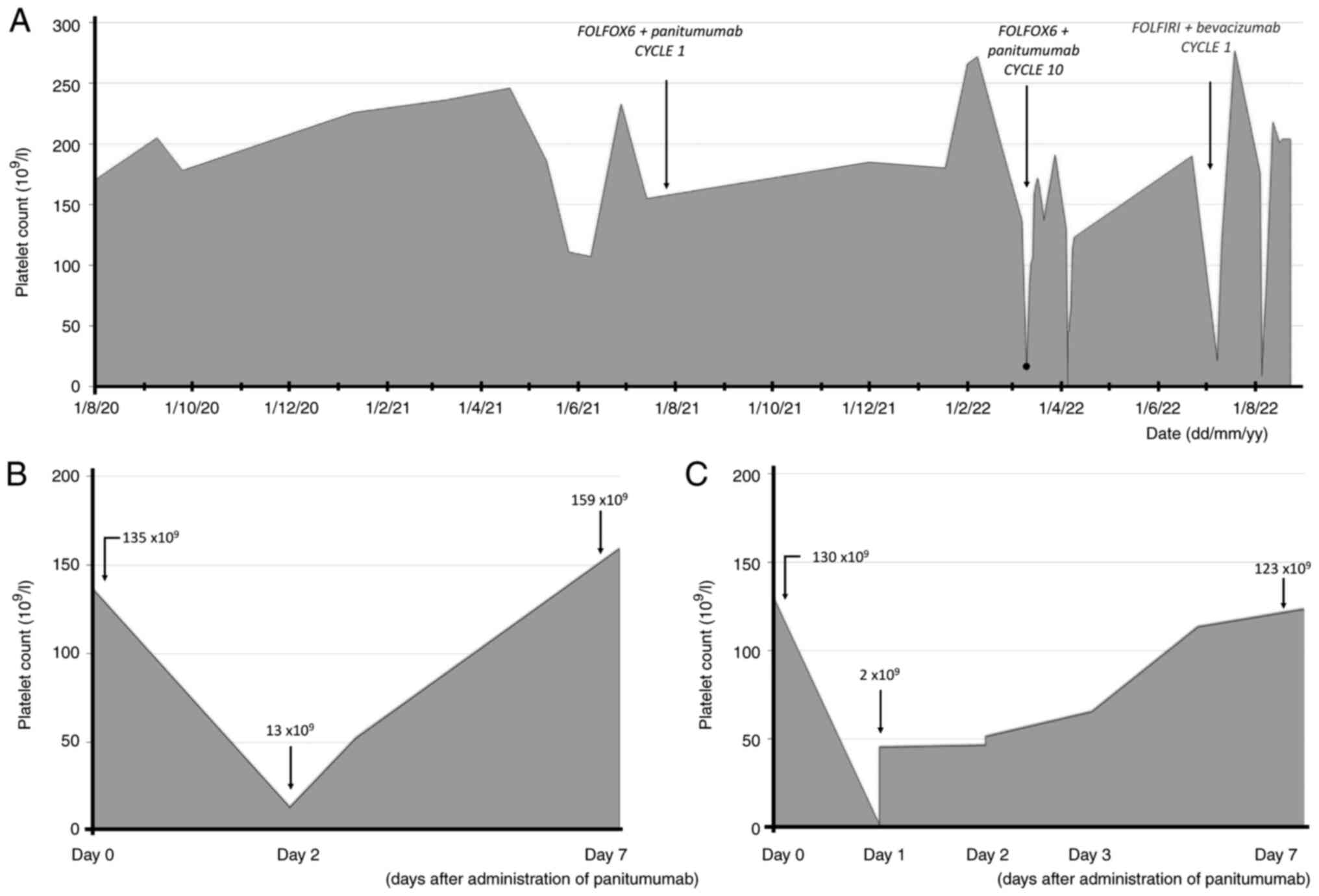
2022). In March 2022, the patient was administered her 10th cycle
of therapy; at 48 h after administration, the patient had developed
severe thrombocytopenia, as indicated by a low platelet count
(13×109/l; normal range, 169–359 ×109/l) and
purpura on both legs. The patient was rapidly treated with
transfusion of 1 bag of blood (450 ml) and was transferred to the
Internal Medicine Unit of E.O. Ospedali Galliera (Genoa, Italy),
where she was monitored. After 7 days, the patient's platelet count
increased to 158×109 (Fig.
1A), and the patient was then discharged. According to the most
recent Common Terminology Criteria for Adverse Events (AEs) v.5.0
(6), this event was classified as
grade 3 thrombocytopenia. The AE was regarded as being caused by
chemotherapy (CT), so at the next planned cycle of therapy (11th
cycle in April 2022), the CT dosage was overall reduced as follows:
Oxaliplatin 110 mg (20% reduction), 5-FU 500 mg IV bolus injection
(20% reduction) + 2,800 mg (30% reduction) IV infusion for 48 h,
L-leucovorin 300 mg (unchanged) and panitumumab 320 mg (unchanged).
Soon after the end of panitumumab IV infusion, the patient
developed acute back pain and shivering; therefore, subsequent CT
was suspended altogether. The next morning, the patient had
developed severe thrombocytopenia (platelet count
<2×109/l, double checked) and purpura, leading once
more to admission to the Internal Medicine Unit. The patient was
hospitalized and treated with transfusion of 1 bag of blood and
dexamethasone 6 mg IV infusion once per day. Blood tests indicated
increased d-dimer (1,293 ng/ml), while activated partial
thromboplastin time, prothrombin time, haemoglobin, white blood
cell count, neutrophil count and fibrinogen results were normal.
Immunology tests were conducted regarding anti-nucleus antibody
(ANA), anti-double-strand DNA antibodies, anti-Smith antibodies,
anti-ribonucleoprotein antibodies,
anti-Ro/Sjögren's-syndrome-related antigen A, anti-La/Sjögren's
syndrome-related antigen B, anti-platelet factor 4 antibodies, anti
Jo-1 antibodies, anti SCl-70 antibodies, IgM and IgG. These
autoimmunity test results were negative except for ANA (titration,
1:160) and increased levels of IgG (2,296 mg/dl). After 5 days of
therapy and observation, the patient's platelet count returned to
normal (Table I). No other AEs were
reported. The Clinical Pharmacology Unit of E.O. Ospedali Galliera
(Genoa, Italy) used Naranjo's algorithm to calculate the
probability of relationship between the reported adverse event and
the drug, resulting in a score of 6 (‘probable’) (7). The toxicity levels were deemed
clinically unacceptable by the oncologists; thus, treatment with
the FOLFOX6 regimen and panitumumab was interrupted and
re-evaluation of disease was anticipated. Positron emission
tomography taken in June 2022 documented progression of disease;
therefore, the oncologists decided to further treat the patient
with a different mAb both in terms of mechanism of action and in
IgG type. Thus, CT with the FOLFIRI regimen and bevacizumab, a
humanized anti-VEGF mAb (IgG1 subtype), was chosen for start in
July. The dosage of the new treatment option was as follows:
Irinotecan 280 mg IV infusion over 1 h, 5-FU 500 mg IV bolus
injection + 2,800 mg IV 48 h infusion, L-leucovorin 300 mg IV
infusion over 2 h and bevacizumab 270 mg IV; irinotecan and
leucovorin are administered first, followed by bevacizumab and
5-FU. However, 48 h after administration of the new treatment, the
patient experienced a similar event of thrombocytopenia (platelet
count, 21×109/l; Fig.
1A). Re-evaluation of the autoimmunity panel was then
conducted, but once again, the results were negative except for ANA
titration 1:160. After careful considerations, treatment with
monoclonal antibodies was suspended altogether. The next treatment
in August 2022 was conducted with only FOLFIRI. Once more, the
patient manifested with sings of acute thrombocytopenia (platelet
count, 12×109/l). Treatment with FOLFIRI was terminated
altogether. In September 2022, the patient started a new treatment
with trifluridine/tipiracil, 110/45 mg/day oral tablet. The patient
is currently undergoing this therapy without the occurrence of any
serious AEs.
 | Table I.Laboratory findings from day 0 to day
7 after administration of panitumumab (cycle 11). |
Table I.
Laboratory findings from day 0 to day
7 after administration of panitumumab (cycle 11).
| Parameter | Day 0 | Day 1 | Day 3 | Day 7 | Reference range |
|---|
| Hb, g/l | 14 | 13.2 | 12.9 | 13.3 | 12.5–15.5 |
| WBC,
×109/l | 5.99 | 9.32 | 10.52 | 6.36 | 4.19–9.35 |
| Neutrophil count,
×109/l | 3.52 | 7.72 | 9.06 | 4.06 | 1.81–6.74 |
| PLT,
×109/l | 130 | 2 | 55 | 123 | 169-359 |
| CRP, mg/dl | 0.06 | 0.08 | - | - | 0.00–0.05 |
| Fibrinogen,
mg/dl | 260 | 282 | - | - | 180-450 |
| d-dimer, ng/ml | 1,293 | - | - | - | <500 |
| IgG, mg/dl | - | - | 2,296 | - | 700-1600 |
| IgA, mg/dl | - | - | 104 | - | 70-400 |
| IgE, mg/dl | - | - | 100 | - | 40-230 |
| C3, mg/dl | - | - | 121.3 | - | 90.0–180.0 |
| ANA | - | - | 1:160 | - | Titration
<1:160 |
| ANCA | - | - | 1:5 | - | Titration
<1:10 |
| Sm-Ab | - |
| Negative | - | Not applicable |
| Anti-RNP | - |
| Negative | - | Not applicable |
| Anti-SS-A/Ro | - |
| Negative | - | Not applicable |
| Anti-SS-B/La | - |
| Negative | - | Not applicable |
| Anti-Scl-70 | - |
| Negative | - | Not applicable |
| Anti-Jo-1 | - |
| Negative | - | Not applicable |
| Anti-PF-4 | - |
| Negative | - | Not applicable |
Discussion
Panitumumab is a human monoclonal antibody
authorized for the treatment of metastatic colorectal cancer. This
drug is an IgG2 that binds to EGFR, inhibiting the growth of cells
expressing this receptor, such as epidermal and certain tumoral
cells. After the suspension of panitumumab and re-evaluation of
disease, which documented a progression, the oncologists decided to
treat the disease with another mAb with a different subtype and
mechanism of action; thus, treatment with bevacizumab was chosen.
Bevacizumab is a humanized mAb with antiangiogenic properties. It
is approved for the treatment of advanced colorectal cancer,
non-small cell lung cancer, breast cancer and renal cell cancer;
the drug is an IgG1 antibody that binds to VEGF, inhibiting
vascular growth. Drug-induced immune thrombocytopenia is a rare
ADR, with an incidence of 1 case per 100,000 patients (8); it is a particular case of ADR in which
certain medicines interact with platelets, accelerating their
destruction through immune-mediated interactions and reducing their
effective count. Among haematological AEs, the panitumumab
datasheet reports anemia (very common, ≥1/10) and leucopenia
(common, ≥1/100), but not thrombocytopenia (9). According to the European drug
vigilance database (EudraVigilance), from 2014 up to July 2022, a
total number of 138 cases of thrombocytopenia was reported;
however, it is unclear whether panitumumab was the causative agent
or just concurrent therapy (10).
Literature describing panitumumab-related thrombocytopenia is
scarce; however, it is worth noting that there is one other case
report describing a similar AE after panitumumab administration
(11). Bevacizumab is more
frequently the cause of immune-mediated thrombocytopenia, as proven
by the abundance of papers and reports that describe this type of
occurrence (12–15). The reason for the more frequent
immune-mediated AEs may be partially attributed to the nature of
bevacizumab, a humanized mAb, compared to panitumumab, which is a
fully human mAb.
In the case presented in the current study, it may
be assumed that platelets were targeted by a destructive mechanism
that was probably triggered by panitumumab. Several elements
strongly support this assumption: i) The temporal link between the
administration and the onset of clinical manifestations; ii) the
triggering of the event after re-administration of panitumumab
alone, before any other therapies (such as oxaliplatin or 5-FU);
iii) the absence of significant changes in immunological tests
except for ANA and IgG, which may be related to the presence of
panitumumab (a drug with a half-life of 7–10 days) in the patient's
serum; iv) the improvement of symptoms after dechallenge with the
suspected causative agent; v) the probability score of relationship
between the AE and the drug (score=6), using Naranjo's algorithm
(7). Albeit the mechanism of
thrombocytopenia was not completely understood, it may be assumed
that it is immune-related; the administration of bevacizumab, as a
second-choice therapy, probably triggered the same mechanism
induced by the first causative agent, a fully human mAb.
The rapid and severe clinical manifestation of
thrombocytopenia suggests that more attention should be directed to
the subsequent days after infusion of the mAbs; anticipating the
routine complete blood count tests to identify early signs of
severe thrombocytopenia would be a suggested course of action. In
conclusion, the evidence presented in this case report suggests
that thrombocytopenia may occur after administration of
panitumumab; however, the rarity of the clinical occurrence and
scarcity in the pre-existent literature makes it difficult to fully
describe the event and the correct diagnostic and therapeutic
approach. Physicians should be aware of this rare AE and, if
possible, should gather more data to better characterize the
pathophysiology and treatment of the occurrence.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the
current study are not publicly available for ethical reasons as per
the local guidelines but are available from the corresponding
author on reasonable request.
Authors' contributions
SPa, FM, LF, MC, GA, ADC and SPi provided the
necessary clinical data. GP and FM provided complementary
laboratory data. SPa, MC and FM wrote the manuscript. ADC and GA
also contributed to the writing of the report. MC and FM confirm
the authenticity of the data. All authors contributed to the review
of the manuscript and have read and approved the final version.
Ethics approval and consent to
participate
The research was conducted in accordance with the
ethical standards of the institutional and national research
committee and with the Helsinki declaration. All data were obtained
in a completely anonymized fashion to protect patient's
privacy.
Patient consent for publication
Written informed consent was obtained from the
patient at the time of admission to the Hospital to use the
clinical data for research purposes, following the privacy policy
of E.O. Ospedali Galliera (Genoa, Italy).
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Barreto JN, McCullough KB, Ice LL and
Smith JA: Antineoplastic agents and the associated myelosuppressive
effects: A review. J Pharm Pract. 27:440–446. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jardim DL, Rodrigues CA, Novis YAS, Rocha
VG and Hoff PM: Oxaliplatin-related thrombocytopenia. Ann Oncol.
23:1937–1942. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Erdem GU, Dogan M, Demirci NS and Zengin
N: Oxaliplatin-induced acute thrombocytopenia. J Cancer Res Ther.
12:509–514. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Danese E, Montagnana M, Favaloro EJ and
Lippi G: Drug-induced thrombocytopenia: Mechanisms and laboratory
diagnostics. Semin Thromb Hemost. 46:264–274. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marini I, Uzun G, Jamal K and Bakchoul T:
Treatment of drug-induced immune thrombocytopenias. Haematologica.
107:1264–1277. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
US Department of Health and Human
Services, . National institutes of health, national cancer
institute. Common terminology criteria for adverse events (CTCAE)
version 5.0. Published:. November 27–2007.
|
|
7
|
Naranjo CA, Busto U, Sellers EM, Sandor P,
Ruiz I, Roberts EA, Janecek E, Domecq C and Greenblatt DJ: A method
for estimating the probability of adverse drug reactions. Clin
Pharmacol Ther. 30:239–245. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vayne C, Guéry EA, Rollin J, Baglo T,
Petermann R and Gruel Y: Pathophysiology and diagnosis of
drug-induced immune thrombocytopenia. J Clin Med. 9:22122020.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
VECTIBIX® (panitumumab) SmPC.
Amgen Europe B.V.; Breda: 2022, https://www.ema.europa.eu/en/medicines/human/EPAR/vectibix
|
|
10
|
The European Medicines Agency, .
EudraVigilance-European database of suspected adverse drug reaction
reports. http://www.adrreports.eu/index.html
|
|
11
|
Kato K, Michishita Y, Oyama K, Hatano Y,
Nozawa T, Ishibashi M, Konda R and Sasaki A: A case of thrombotic
thrombocytopenic purpura in a patient undergoing FOLFOX6 plus
panitumumab therapy for unresectable recurrent rectal cancer with a
rapidly progressive course. Gan To Kagaku Ryoho. 43:133–136.
2016.(In Japanese). PubMed/NCBI
|
|
12
|
Dior M, Coriat R, Mir O, Brezault C,
Perkins G, Dhooge M, Goldwasser F and Chaussade S: A rare
hematological adverse event induced by bevacizumab: Severe
thrombocytopenia. Am J Med. 125:828–830. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kumar J, Bhargava M and Aggarwal S:
Bevacizumab-induced reversible thrombocytopenia in a patient with
adenocarcinoma of colon: Rare adverse effect of bevacizumab. Case
Rep Oncol Med. 2012:6954302012.PubMed/NCBI
|
|
14
|
Leal T and Robins HI: Bevacizumab induced
reversible thrombocytopenia in a patient with recurrent high-grade
glioma: A case report. Cancer Chemother Pharmacol. 65:399–401.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Meyer T, Robles-Carrillo L, Robson T,
Langer F, Desai H, Davila M, Amaya M, Francis JL and Amirkhosravi
A: Bevacizumab immune complexes activate platelets and induce
thrombosis in FCGR2A transgenic mice. J Thromb Haemost. 7:171–181.
2009. View Article : Google Scholar : PubMed/NCBI
|