Introduction
Epithelioid angiomyolipoma (EAML), which was first
described by Mai et al (1)
in 1996, is a rare variant of angiomyolipoma (AML). AMLs are the
most common mesenchymal neoplasms of the kidney with an incidence
of 0.1–0.3% in healthy adults (2).
AMLs are classified as neoplasms of the perivascular epithelioid
cell or perivascular epithelioid cell tumor ‘PEComa’, which also
includes lymphangioleiomyomatosis, and pulmonary and extrapulmonary
clear cell sugar tumors (3). Renal
EAMLs can occur sporadically or be associated with tuberous
sclerosis (TSC) (4). Approximately
one-third of EAMLs have been found to present with malignant
biological behavior (5). According
to a Japanese cohort study, the percentage of EAML cases among
non-classical AML cases, which included EAML and fat-poor AML, was
17.9% (6). Although most cases of
EAML follow a benign course, approximately one third of EAML cases
are characterized by aggressive biological behavior, such as local
recurrence after excision, enlarged lymph nodes, extension into the
venous system and distant metastases (3,4,7).
Unlike classical AML, which is a benign renal entity
composed of dysmorphic blood vessels, smooth muscle and adipose
tissue, EAML is characterized by minimal fat content and an
abundance of epithelioid cells (7).
Atypia within the epithelioid cells, the presence of mitotic
figures and necrosis are common and are associated with a more
aggressive course of disease (8).
Thus, EAML exhibits similar findings compared with renal cell
carcinoma (RCC) and fat-poor AML on standard CT scans, making
preoperative diagnosis challenging (9,10).
However, surgery is the treatment of choice and is curative in most
cases of EAMLs (3,6). The present report describes two cases
of renal EAML, which were treated successfully by laparoscopic
surgery. Although preoperative diagnosis was difficult to achieve,
EAML should be included in the differential diagnosis when a renal
lesion is found and correct histological diagnosis of this subtype
of renal AML is crucial. Erroneous diagnosis of simple renal AML
instead of EAML may lead to insufficient postoperative
management.
Case report
Case 1
A 34-year-old female patient complaining of acute
left flank pain was referred to The First Affiliated Hospital of
Shandong First Medical University and Shandong Provincial
Qianfoshan Hospital (Jinan, China) in November 2021. The patient
had no previous illnesses or family history of TSC, and no gross
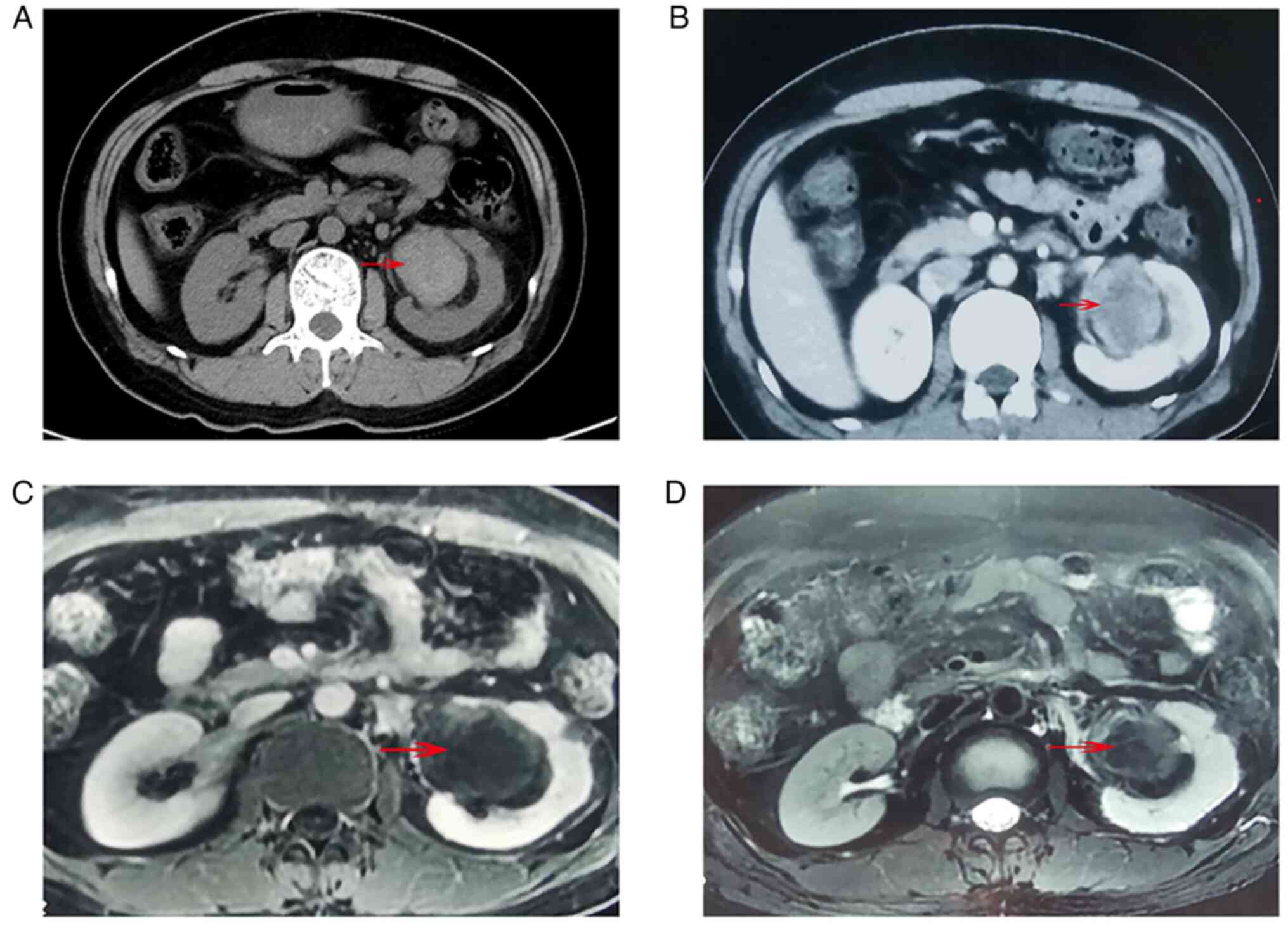
hematouria was recorded. CT scans revealed a large hyperdense left
renal mass arising from the renal sinus on non-contrast imaging
(Fig. 1A). After contrast medium
was administrated, the mass was enhanced and heterogeneous
(Fig. 1B). These findings were
similar to those of RCC in standard CT imaging (hypodense or
isodense mass in plain scans and hyperdense mass in
contrast-enhanced imaging) (9,10). The
tumor was hypointense in gadolinium enhanced T1-weighted MRI
(Fig. 1C), and also hypointense in
fat-saturated T2-weighted imaging (Fig.
1D).
In the differential diagnosis of the patient, three
types of disease were considered: RCC, renal pelvic carcinoma and
AML with acute hemorrhage. Due to its location in proximity to the
renal pedicle, biopsy of the tumor was not performed. Retrograde
ureteroscopy was conducted and no pelvic mass was found during the
procedure. After a negative metastatic workup, the patient
underwent a left radical nephrectomy using the retroperitoneal
laparoscopic technique.
Histologic examination revealed a 47×45-mm tumor
with hemorrhage, which could explain the onset of acute pain of the
patient, and the protocol for histopathological staining was as
follows: After appropriate tissue sampling, tumor blocks were fixed
in solution consisting of 10% formaldehyde in 0.01 M
phosphate-buffered saline (PBS) for 2 h at room temperature.
Subsequently, tissue blocks were loaded into the Tissue-Tek
VIP® 6 AI Tissue Processor (Sakura Finetek USA, Inc.)
and then embedded in paraffin. The paraffin blocks were cut into
sections with 5-µm thickness, dewaxed with xylene, and rehydrated
in a descending ethanol series (95, 90, 80 and 75%) and water.
Slices were immersed in Harris hematoxylin staining solution for 5
min at room temperature and then differentiated with 0.3% acid
alcohol, followed by incubation with 0.6% ammonia for 5 sec at room
temperature. Subsequently, samples were incubated with eosin
staining solution for 1–3 min at room temperature and then
dehydrated with ethanol and xylene at room temperature, and
finally, slides were mounted with neutral gum and observed under a
light microscope (LEICA DM2000; Leica Microsystems GmbH).
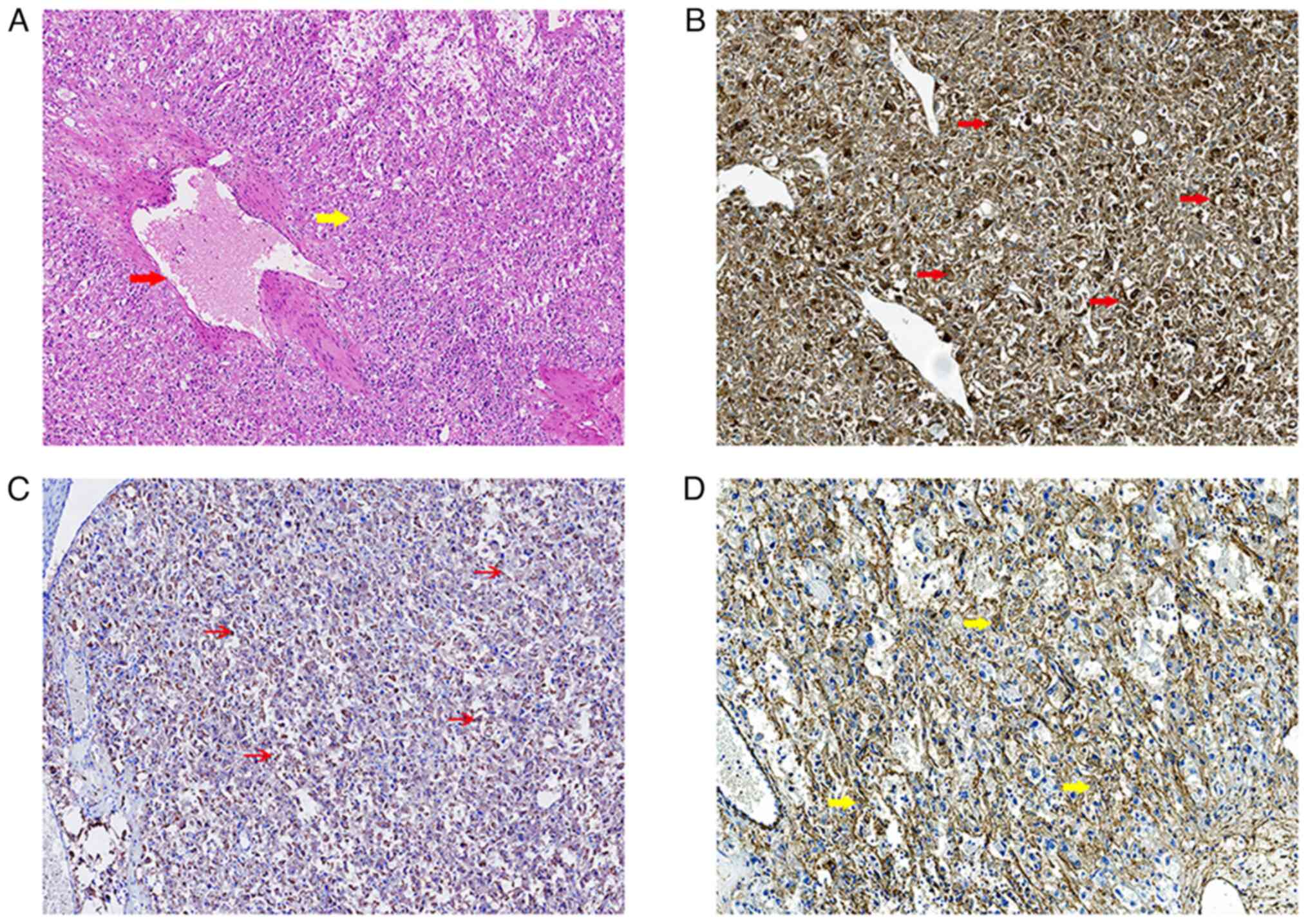
Histologically, spindle epithelioid cells, which were clustered
around blood vessels, could be observed in the tumor of the
patient. The tumor cells were mostly composed of eosinophilic
epithelioid cells. The cytoplasm of tumor cells was clear or
granular with vesicular chromatin and prominent nucleoli (Fig. 2A).
For immunohistochemistry (IHC), 5-µm-thick tumor
sections from a paraffin block were deparaffinized and rehydrated
as previously described (11). For
quenching of endogenous peroxidase activity, the slides were
incubated with 3% hydrogen peroxide solution in methanol for 30 min
at room temperature in the dark. Heat-induced epitope retrieval was
adopted; slides were rinsed three times with 0.01 M PBS, then
treated with antigen retrieval reagent (0.01 M citrate buffer
solution; pH 6.0) in a pressure cooker for 10 min. Slides were
washed three times with 0.01 M PBS (pH 7.4; 5 min/wash) at room
temperature, and were then blocked with 10% goat non-immune serum
(SP KIT-B3; Fuzhuo Maixin Biotech Co., Ltd.) for 30 min at room
temperature to prevent non-specific binding. Sections were rinsed
three times with 0.01 M PBS (pH 7.4; 5 min/wash), and were then
incubated with monoclonal primary anti-HMB45 (1:200; ab190913;
Abcam), anti-melan A (1:200; ab187369; Abcam) and anti-α-smooth
muscle actin (α-SMA; 1:500; ab247685; Abcam) antibodies at 4°C for
12 h. Primary antibodies were diluted with PBS. Slides were washed
three times with 0.01 M PBS (5 min/wash), then incubated with
Peroxidase-AffiniPure Goat Anti-Rabbit IgG (ready to use; cat. no.
KIT-9706; Fuzhou Maixin Biotech Co., Ltd,) for 30 min at 37°C.
Peroxidase-conjugated streptavidin (Invitrogen; Thermo Fisher
Scientific, Inc.) was applied, diaminobenzidine was used as the
chromogen and sections were counterstained with Mayer's hematoxylin
for 2 min at room temperature. Subsequently, slides were sealed
with Permount Mounting Medium and observed under a light microscope
(LEICA DM2000; Leica Microsystems GmbH). Images were captured with
Digital Pathology Scanner (PRECICE series 510; UNic medical Corp.).
The tumor cells were strongly positive for HMB-45 (Fig. 2B), weakly positive for melan A
(Fig. 2C) and diffusely positive
for α-SMA (Fig. 2D). The pathologic
diagnosis was most consistent with EAML, which was defined as an
epithelioid component ≥80% according to the 2016 World Health
Organization (WHO) Classification of Renal Neoplasms (12).
The patient recovered well and was discharged 8 days
after surgery, and no local recurrence or remote metastasis was
observed during 3-monthly follow-up with clinical evaluation and CT
scans of the chest, abdomen and pelvis. The follow-up would
continue for 2 years, then biannual follow-up up to 5 years must be
carried out, followed by annual follow-up in the future. At
present, the patient has undergone routine clinical evaluation and
CT scans of the chest, abdomen and pelvis at the outpatient clinic
(The First Affiliated Hospital of Shandong First Medical University
and Shandong Provincial Qianfoshan Hospital, Jinan, China), and no
recurrence or metastasis have been observed. Written informed
consent was obtained from the patient for publication of the
present report.
Case 2
A 20-year-old male patient presenting with right
flank pain for 7 h and hematouria for 2 h was admitted to The First
Affiliated Hospital of Shandong First Medical University and
Shandong Provincial Qianfoshan Hospital (Jinan, China) in October
2018. Past medical and family histories were unremarkable. CT scans
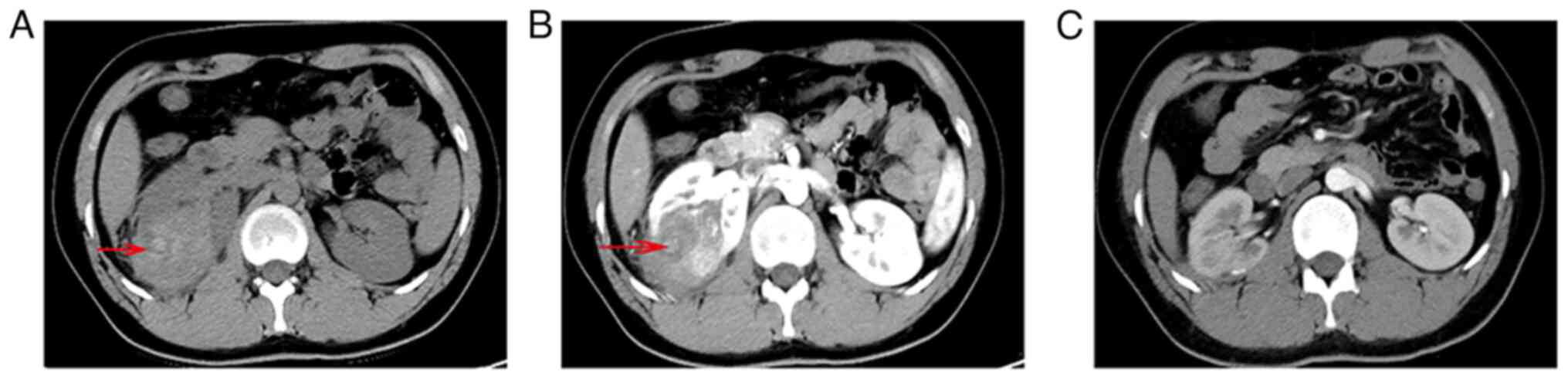
demonstrated a hyperdense mass of the right kidney, measuring 58×52
mm (Fig. 3A). After contrast medium
was administrated, the tumor was slightly enhanced and the renal
pelvis was invaded (Fig. 3B). AML
with hemorrhage was considered, which might lead to the flank pain
and hematouria of the patient. Since it was hard to distinguish AML
with hemorrhage from RCC, which might be treated by radical
nephrectomy, on CT imaging, a fine-needle biopsy was conducted
subsequently, but no tumor tissue was found, potentially due to
necrosis or hemorrhage of the mass (13). The patient underwent a partial right
nephrectomy using the retroperitoneal laparoscopic approach.
Similar to patient 1, gross examination also
revealed hemorrhage of the tumor, which led to presentation of
flank pain of the patient. The same protocol as described for
patient 1 was used for histopathological staining and IHC.
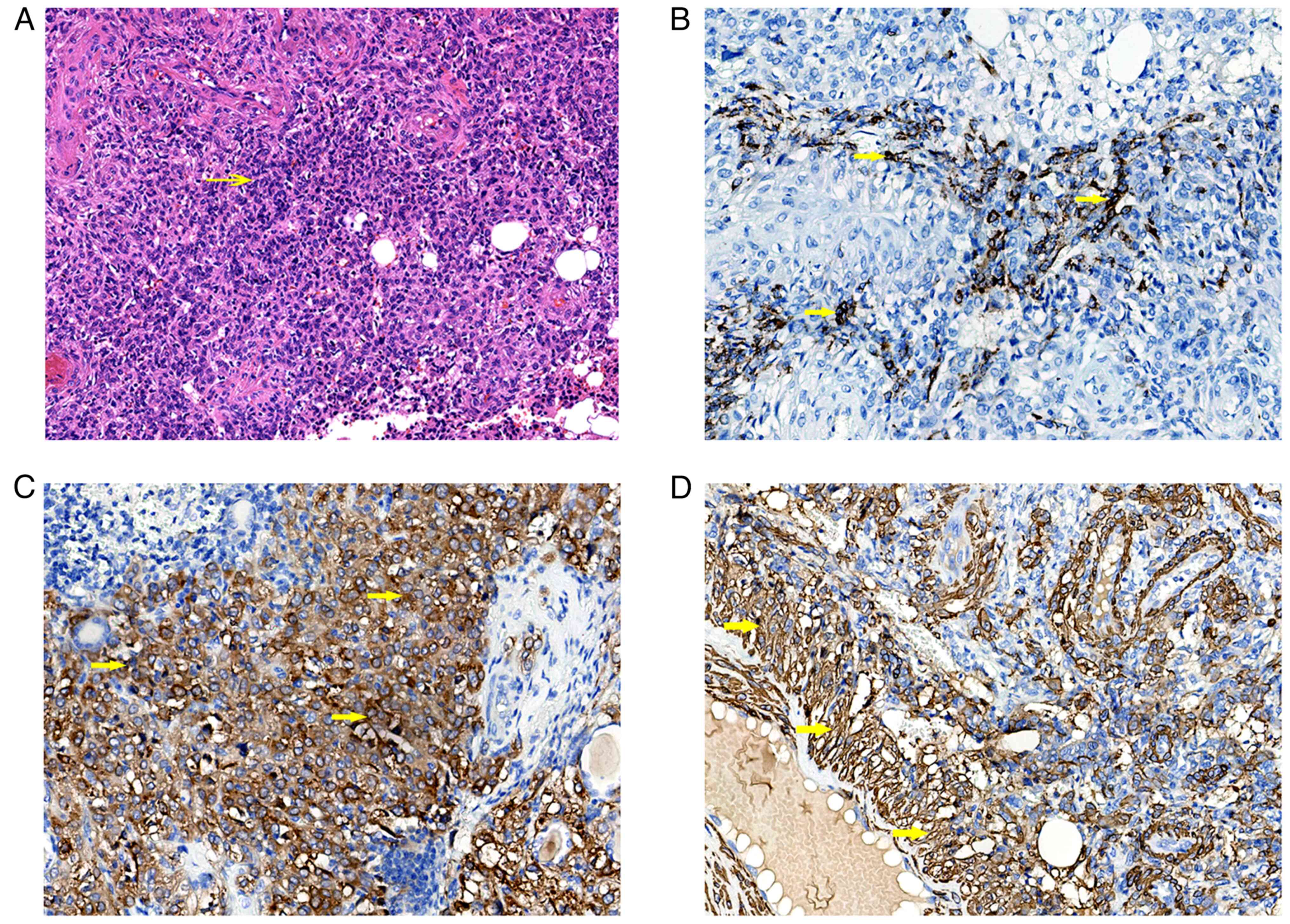
Histologic analysis of the tumor revealed that sheets of round and
polygonal epithelioid cells were arranged in close nests, which
were interspersed with stroma consisting of abundant blood vessels
(Fig. 4A). IHC staining revealed
that the tumor cells were strongly positive for HMB-45 (Fig. 4B), and diffusely positive for melan
A (Fig. 4C) and α-SMA (Fig. 4D). Histologically, both cases
exhibited mainly epithelial cells interspersed with smooth muscle
cells in two distinct patterns. A diffuse growth pattern was more
evident in case 1, with epithelial cells and plump spindle-shaped
cells arranged in diffuse sheets. The cytoplasm of tumor cells was
clear to granular or feathery with vesicular chromatin and
prominent nucleoli (Fig. 2A). A
carcinoma-like growth pattern was observed in case 2, in which
large polygonal cells with dense cytoplasm and atypical nuclei with
prominent nucleoli were arranged in cohesive nests with wide
alveoli (Fig. 4A).
Immunohistochemically, the epithelial cells expressed the
melanocyte maker HMB-45 and melanin A. The two cases were diagnosed
as EAML according to the WHO classification of tumors (12).
There was no evidence of recurrence or metastasis 4
years postoperatively (Fig. 3C).
The protocol for follow-up was the same as that for patient 1. The
present patient also had a favorable course of disease with routine
clinical evaluation and CT scans. At present, the right kidney of
the patient appears to be a normal size compared with the left
kidney and no atrophy has been observed. Written informed consent
was obtained from the patient for publication of the present
report.
Discussion
Classic renal AML is a benign mesenchymal tumor
composed of dysmorphic blood vessels, smooth muscle and adipose
tissue (14). Pathologically, AMLs
are considered to arise from perivascular epithelioid cells and are
often grouped together with other PEComas (15). EAML is a rare subtype of AML,
accounting for 4.6% of all AMLs (16), and is composed of epithelioid cells
arranged in sheets with a lack of abnormal vessels and adipocytes
(1). The 2016 World Health
Organization Classification of Renal Neoplasms defined EAML as a
potentially malignant mesenchymal neoplasm (12). Metastatic disease has been reported
in one-third of reported cases of EAML, including sporadic and
TSC-associated EAMLs (3,14,17).
Genetically, TSC is a group of autosomal dominant
genetic disorders caused by germline mutations in the TSC complex
subunit 1 (TSC1) or TSC complex subunit 2 (TSC2)
genes (18). The proteins hamartin
and tuberin are encoded by the TSC1 and TSC2 genes,
respectively (18). Among patients
with TSC, the prevalence of AML has been reported as 55–90%, with
an earlier presentation than sporadic cases (19,20).
Steiner et al (21) reported
that patients with TSC presented with AML at a mean age of 31.5
years (range, 17–62 years), while patients without TSC presented
with AML at a mean age of 53.6 years (range, 19–74 years).
TSC-associated AMLs typically present with multiple, bilateral and
symptomatic tumors of the kidney (20). As well as AMLs, patients with TSC
may develop renal cysts, RCC, oncocytoma, perirenal cysts and
polycystic kidney disease (20).
Histologically, EAML can resemble and be
misdiagnosed as sarcomatoid or high-grade RCC (22). However, EAML can be differentiated
from RCC by the presence of immunohistochemistry markers such as
melanosome-associated proteins (HMB-45 antigen and melan A) and
smooth muscle markers [actin monoclonal antibody (HHF-35), α-SMA
and caldesmon] (22,23). The presence of malignant potential
in EAML has been attributed to the following parameters: TSC and/or
concurrent AML, tumor size >7 cm, tumor necrosis, extrarenal
extension and/or involvement of the renal vein, and carcinoma-like
growth pattern. These parameters were used to stratify patients
into groups with low, intermediate and high risk for disease
progression, which had a risk of disease progression of 15, 64 and
100%, respectively (24). Brimo
et al (8) studied several
features that increased the likelihood of malignancy in renal EAML
with atypia. Based on these features, the authors developed a
predictive model of four atypical features that included ≥70%
atypical epithelioid cells, ≥2 mitotic figures per 10 high-power
fields, atypical mitotic figures and necrosis. The presence of
three or all of the features was predictive of malignant behavior.
This model accurately categorized 78% of clinically malignant EAMLs
with atypia and 100% of clinically benign EAMLs with atypia
(8).
It is difficult to distinguish EAMLs and fat-poor
AMLs from RCC based on CT scans since they do not contain
radiographically identifiable fat (9,25).
Radiologically, EAML typically presents as a large mass with
intratumoral hemorrhage and necrosis (26). Most EAMLs exhibit hyperattenuation
on unenhanced CT (typically >45 Hounsfield units) and T2
hypointensity due to their epithelioid muscle component (26). These findings cannot accurately
identify EAMLs preoperatively and differentiate them from RCC, as
seen in the present cases. Although the presence of necrosis or
cystic changes and the absence of fat have been found to be
independent predictors of EAML on CT imaging (10), most patients are treated as having a
presumed RCC (23). There are also
a few studies that have reported positron emission tomography (PET)
findings of AMLs (27–29). Fat-poor AMLs might mimic RCC on
18F-fluorodeoxyglucose (18F-FDG) PET because
of increased FDG uptake (27).
Increased tracer accumulation on 18F-FDG PET/CT imaging
was also observed in pure EAML (28) and local recurrence of EAML after
nephrectomy (29). Although it is
difficult to differentiate EAML from RCC on CT scans, these
radiographic findings may prompt the urologist to consider a
percutaneous biopsy if the suspicion of EAML is raised by CT
imaging (13). Percutaneous biopsy
may serve an important role in diagnosis in these cases, because a
core biopsy should be highly accurate in the diagnosis of AML with
minimal fat (30).
AML is the most common renal neoplasm associated
with spontaneous perirenal hemorrhage, closely followed by RCC
(31). In the two present cases,
the first presentation of the tumors was hemorrhage, leading to
acute flank pain. A previous study reported that hemorrhage was one
of the potential causes of heterogeneity on CT (32). From a pathological point of view,
both AML and EAML consist of thick-walled poorly organized blood
vessels without elastic fibers that can be observed under the
microscope, and thus, have a tendency to bleed, particularly in
large tumors (24). In the two
present cases, hemorrhage of the tumors might be the potential
causes of hyperattenuation on unenhanced CT, making them
undistinguishable from RCC. In both cases, spontaneous hemorrhage
of the tumor was suspected before surgeries, so AML with hemorrhage
was first considered, which should be treated by nephron-sparing
surgery (NSS) when technical feasible. In case 1, the tumor arose
from the renal sinus, where partial nephrectomy was challenging
with possible postoperative complications, so radical nephrectomy
was performed. In case 2, although percutaneous biopsy was carried
out, no tumor tissue was found, potentially due to necrosis or
hemorrhage of the mass. Subsequently, the patient was treated by
NSS to maximally preserve renal function. Definitive diagnosis of
EAML was confirmed by histologic analysis. Both cases exhibited
mainly epithelial cells in two distinct patterns, where a diffuse
growth pattern was more evident in case 1 and a carcinoma-like
growth pattern was more evident in case 2. Although preoperative
diagnosis was difficult to achieve, EAML should be included in the
differential diagnosis when a renal lesion is found and correct
histological diagnosis of this subtype of renal AML is crucial
because erroneous diagnosis of simple renal AML instead of EAML may
lead to insufficient postoperative management. If the tumors
described in the present case report were preoperatively considered
to be benign AMLs, they may have been treated conservatively, which
could have led to progression of the disease. At present, both
cases appear to have had a benign course of disease after surgery,
supporting the fact that the incidence of malignant behavior of
EAMLs is quite low (5.0%) (16);
however, close follow-up should be maintained due to the malignant
potential of EAML.
The management of EAML is controversial due to
uncertainty regarding the natural history of the condition.
However, because EAML has the potential for malignancy, it is often
managed as RCC. Surgery is the treatment of choice and curative in
most cases (33,34). Since CT imaging is seldom sufficient
to rule out RCC, resection is both diagnostic and therapeutic
(34). Treatment should be tailored
to the patient with the goal of renal function preservation
(34). When resection is chosen,
nephron-sparing approaches should be performed due to improved
renal function and decreased overall mortality when technically
feasible (35). Furthermore, in
patients with TSC, where multifocal, bilateral and recurring
lesions are almost universal, nephron sparing is paramount
(19). Since TSC is associated with
mutations that result in activation of the mTOR signaling pathway,
mTOR inhibitors have also been explored as systemic therapeutic
agents for managing this disease in patients with TSC (3). Everolimus has been assessed in a phase
III study of TSC- and lymphangioleiomyomatosis-associated AML. In
the study, the authors identified a response rate of 42%, with 80%
of patients achieving at least a 30% reduction in size.
Furthermore, no patient who had a tumor response exhibited
progression during follow-up (36).
Based on these results, everolimus was approved by the US Food and
Drug Administration for the treatment of AML in the setting of TSC
(37). There has also been a case
report of successful treatment of a patient with pulmonary
metastasis from EAML using everolimus. The authors concluded that
by identifying EAML and recognizing its high-risk features, which
have been described by Nese et al (24), the administration of mTOR inhibitors
might lead to improved clinical outcomes in patients with recurrent
metastatic EAMLs (3).
The preoperative distinction between EAML and RCC
may not be critical as both lesions are treated with surgical
resection. However, among consecutive resected EAMLs, the incidence
of malignant behavior is quite low (5.0%) (16), and some studies have reported that
mTOR inhibitors, such as sirolimus or temsirolimus, may represent
an improved treatment option for patients with EAML (38,39).
Therefore, the correct diagnosis of renal EAML can potentially
direct clinicians to a more effective chemotherapy, particularly in
patients with extensive disease. Therefore, the CT imaging-based
pre-operative diagnosis of this type of AML or biopsy may become
important in the future (25).
Although most reported cases of EAML, including the
two present cases, are unilateral, bilateral EAMLs have also been
reported in a patient with TSC, and metastatic lesions were
identified in the right lung, liver, diaphragm and mesentery of the
patient by autopsy (40).
Recurrence and metastasis have been reported in 17.2 and 48.5%,
respectively, of patients with EAMLs (24). Local recurrence was found after
prior renal surgeries (3,41,42),
so close follow-up was required (3,34).
Saoud et al (3) reported
that a large retroperitoneal mass was detected as a recurrence 9
months after left radical nephrectomy. Varma et al (41) reported local renal fossa recurrence
extending to the vena cava 9 years after right nephrectomy, with
metastasis to the liver, colon and lung. Late local, peritoneal and
systemic recurrence have also been reported 12 years after left
nephrectomy (42). Thus, a vigorous
follow-up even after curative surgery and complete remission is
warranted.
Mahajan et al (34) recommended that a 3-monthly follow-up
with clinical evaluation and relevant radiological investigations
for 2 years was imperative for EAML. Biannual follow-up up to 5
years must be undertaken, followed by annual follow-up (34). Others have recommended treating EAML
using the National Comprehensive Cancer Network guidelines
currently in place for the treatment of RCC (43), and implementing similar follow-up
protocols compared with those for RCC (44,45).
In summary, the present study reported two cases of
EAML, which were successfully treated by laparoscopic surgery.
Preoperative diagnosis was challenging. Definitive diagnosis was
not accomplished until histological analysis was performed. Due to
the malignant potential of EAML, surgical resection is the gold
standard treatment strategy for this neoplasm when technically
feasible (32). Although the
present cases had a favorable course of disease, it was considered
appropriate to continue close follow-up, similar to the follow-up
for RCC, due to approximately one third of EAMLs having been found
to present with malignant biological behavior (3–5,7). Thus,
long-term follow-up is required to detect any recurrence or
metastasis of the present patients. It is also recommended that all
similar cases should be subject to a similar, longer follow-up
period.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SH was the principal person responsible for the
study, and contributed to the conception and the design of the
study. HL, XZ and QZ obtained and analyzed the patient information,
and contributed to manuscript drafting and critical revisions of
the intellectual content, while JW performed analysis and
interpretation of CT imaging and MRI data. XY performed the
histological examination of the tumor. HL, XZ, QZ, JW, XY and SH
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
All procedures were approved by the ethics committee
of The First Affiliated Hospital of Shandong First Medical
University (approval no. 2023S348; Jinan, China). Closely adhering
to the principles of careful pre-, intra- and postoperative
preparation and management, the whole treatment procedure for the
two patients complied with our institutional standards. Written
informed consent for participation was obtained from the
patients.
Patient consent for publication
Written informed consent for publication of the
article was obtained from the patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mai KT, Perkins DG and Collins JP:
Epithelioid cell variant of renal angiomyolipoma. Histopathology.
28:277–280. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fujii Y, Ajima J, Oka K, Tosaka A and
Takehara Y: Benign renal tumors detected among healthy adults by
abdominal ultrasonography. Eur Urol. 27:124–127. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Saoud R, Kristof TW, Judge C, Chumbalkar
V, Antic T, Eggener S and Modi P: Clinical and pathological
features of renal epithelioid angiomyolipoma (PEComa): A single
institution series. Urol Oncol. 40:18–24. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tayal J, Doval DC, Kamboj M and
Suryavanshi M: Case report of everolimus-induced sustained partial
response in metastatic renal epithelioid angiomyolipoma. Turk J
Urol. 45 (Supp 1):S139–S142. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pea M, Bonetti F, Martignoni G, Henske EP,
Manfrin E, Colato C and Bernstein J: Apparent renal cell carcinomas
in tuberous sclerosis are heterogeneous: The identification of
malignant epithelioid angiomyolipoma. Am J Surg Pathol. 22:180–187.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kaneko K, Yoshida S, Yamamoto K, Arita Y,
Kijima T, Yokoyama M, Ishioka J, Matsuoka Y, Saito K and Fujii Y:
Renal epithelioid angiomyolipoma: Incidence in a Japanese cohort
and diagnostic utility of diffusion-weighted magnetic resonance
imaging. Int J Urol. 27:599–604. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun DZ and Campbell SC: Atypical
epithelioid angiomyolipoma: A rare variant with malignant
potential. Urology. 112:20–22. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brimo F, Robinson B, Guo C, Zhou M, Latour
M and Epstein JI: Renal epithelioid angiomyolipoma with atypia: A
series of 40 cases with emphasis on clinicopathologic prognostic
indicators of malignancy. Am J Surg Pathol. 34:715–722. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Potretzke AM, Potretzke TA, Bauman TM,
Knight BA, Park AM, Mobley JM, Figenshau RS and Siegel CL: Computed
tomography and magnetic resonance findings of fat-poor
angiomyolipomas. J Endourol. 31:119–128. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Luo C, Liu Z, Gao M, Hu Q, He X, Xi Y, Cai
F, Zhang R, Zeng X and Xiao N: Renal epithelioid angiomyolipoma:
Computed tomography manifestation and radiologic-pathologic
correlation depending on different epithelioid component
percentages. Abdom Radiol (NY). 47:310–319. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao Q, Zhang X, Yang X and Huang S:
Castleman's disease in the pelvic retroperitoneum: A case report.
Exp Ther Med. 24:6602022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Amin MB: Epithelioid angiomyolipoma. World
Health Organization classifification of tumors: Tumors of the
urinary system and male genital organs. Moch H, Humphrey PA,
Ulbright TM and Reuter VE: IARC Press; Lyon: pp. 65–66. 2016
|
|
13
|
Lebret T, Poulain JE, Molinie V, Herve JM,
Denoux Y, Guth A, Scherrer A and Botto H: Percutaneous core biopsy
for renal masses: Indications, accuracy and results. J Urol.
178:1184–1188. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nelson CP and Sanda MG: Contemporary
diagnosis and management of renal angiomyolipoma. J Urol.
168:1315–1325. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bissler JJ and Kingswood JC: Renal
angiomyolipomata. Kidney Int. 66:924–934. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
He W, Cheville JC, Sadow PM, Gopalan A,
Fine SW, Al-Ahmadie HA, Chen YB, Oliva E, Russo P, Reuter VE and
Tickoo SK: Epithelioid angiomyolipoma of the kidney: Pathological
features and clinical outcome in a series of consecutively resected
tumors. Mod Pathol. 26:1355–1364. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee KH, Tsai HY, Kao YT, Lin HC, Chou YC,
Su SH and Chuang CK: Clinical behavior and management of three
types of renal angiomyolipomas. J Formos Med Assoc. 118:162–169.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
European Chromosome 16 Tuberous Sclerosis
Consortium, . Identification and characterization of the tuberous
sclerosis gene on chromosome 16. Cell. 75:1305–1315. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Curatolo P, Bombardieri R and Jozwiak S:
Tuberous sclerosis. Lancet. 372:657–668. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lendvay TS and Marshall FF: The tuberous
sclerosis complex and its highly variable manifestations. J Urol.
169:1635–1642. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Steiner MS, Goldman SM, Fishman EK and
Marshall FF: The natural history of renal angiomyolipoma. J Urol.
150:1782–1786. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Eble JN, Amin MB and Young RH: Epithelioid
angiomyolipoma of the kidney: A report of five cases with a
prominent and diagnostically confusing epithelioid smooth muscle
component. Am J Surg Pathol. 21:1120–1130. 1997. View Article : Google Scholar
|
|
23
|
Park HK, Zhang S, Wong MK and Kim HL:
Clinical presentation of epithelioid angiomyolipoma. Int J Urol.
14:21–22. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nese N, Martignoni G, Fletcher CD, Gupta
R, Pan CC, Kim H, Ro JY, Hwang IS, Sato K, Bonetti F, et al: Pure
epithelioid PEComas (so-called epithelioid angiomyolipoma) of the
kidney: A clinicopathologic study of 41 cases: Detailed assessment
of morphology and risk stratifification. Am J Surg Pathol.
35:161–176. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jinzaki M, Silverman SG, Akita H,
Nagashima Y, Mikami S and Oya M: Renal angiomyolipoma: A
radiological classification and update on recent developments in
diagnosis and management. Abdom Imaging. 39:588–604. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tsukada J, Jinzaki M, Yao M, Nagashima Y,
Mikami S, Yashiro H, Nozaki M, Mizuno R, Oya M and Kuribayashi S:
Epithelioid angiomyolipoma of the kidney: Radiological imaging. Int
J Urol. 20:1105–1111. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Arnold RT and Myers DT: Visualization of
renal angiomyolipoma on F-18 FDG PET/CT. Clin Nucl Med. 34:539–540.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dong A, Wang Y and Zuo C: Synchronous pure
epithelioid angiomyolipoma of the kidney and retroperitoneal
schwannoma in the same patient on 18F-FDG PET/CT imaging. Clin Nucl
Med. 38:e98–e100. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Griffin A: 18F-FDG PET/CT of malignant
angiomyolipoma with tumor thrombus. Clin Nucl Med. 42:628–629.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Silverman SG, Mortele KJ, Tuncali K,
Jinzaki M and Cibas ES: Hyperattenuating renal masses: Etiologies,
pathogenesis, and imaging evaluation. Radiographics. 27:1131–1143.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang JQ, Fielding JR and Zou KH: Etiology
of spontaneous perirenal hemorrhage: A meta-analysis. J Urol.
167:1593–1596. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Delhorme JB, Fontana A, Levy A, Terrier P,
Fiore M, Tzanis D, Callegaro D, Dratwa C, Gronchi A and Bonvalot S:
Renal angiomyolipomas: At least two diseases. A series of patients
treated at two European institutions. Eur J Surg Oncol. 43:831–836.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Al Umairi R, Al Shamsi R, Kamona A, Al
Lawati F, Baqi SA, Kurian G and Al Kalbani J: Renal epithelioid
angiomyolipoma: A case report and review of literature. Oman Med J.
35:e1782020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mahajan D, Jain V, Agarwala S, Jana M and
Ramteke PP: Renal epithelioid angiomyolipoma in children. J Kidney
Cancer VHL. 8:20–26. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Thompson RH, Boorjian SA, Lohse CM,
Leibovich BC, Kwon ED, Cheville JC and Blute ML: Radical
nephrectomy for pT1a renal masses may be associated with decreased
overall survival compared with partial nephrectomy. J Urol.
179:468–473. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bissler JJ, Kingswood JC, Radzikowska E,
Zonnenberg BA, Frost M, Belousova E, Sauter M, Nonomura N,
Brakemeier S, de Vries PJ, et al: Everolimus for angiomyolipoma
associated with tuberous sclerosis complex or sporadic
lymphangioleiomyomatosis (EXIST-2): A multicentre, randomised,
double-blind, placebo-controlled trial. Lancet. 381:817–824. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Curatolo P, Nabbout R, Lagae L, Aronica E,
Ferreira JC, Feucht M, Hertzberg C, Jansen AC, Jansen F, Kotulska
K, et al: Management of epilepsy associated with tuberous sclerosis
complex: Updated clinical recommendations. Eur J Paediatr Neurol.
22:738–748. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wolff N, Kabbani W, Bradley T, Raj G,
Watumull L and Brugarolas J: Sirolimus and temsirolimus for
epithelioid angiomyolipoma. J Clin Oncol. 28:e65–e68. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shitara K, Yatabe Y, Mizota A, Sano T,
Nimura Y and Muro K: Dramatic tumor response to everolimus for
malignant epithelioid angiomyolipoma. Jpn J Clin Oncol. 41:814–816.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sato K, Ueda Y, Tachibana H, Miyazawa K,
Chikazawa I, Kaji S, Nojima T and Katsuda S: Malignant epithelioid
angiomyolipoma of the kidney in a patient with tuberous sclerosis:
An autopsy case report with p53 gene mutation analysis. Pathol Res
Pract. 204:771–777. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Varma S, Gupta S, Talwar J, Forte F and
Dhar M: Renal epithelioid angiomyolipoma: A malignant disease. J
Nephrol. 24:18–22. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
De Bree E, Stamatiou D, Chryssou E,
Michelakis D and Tzardi M: Late local, peritoneal and systemic
recurrence of renal angiomyolipoma: A case report. Mol Clin Oncol.
10:43–48. 2019.PubMed/NCBI
|
|
43
|
Motzer RJ, Jonasch E, Agarwal N, Alva A,
Baine M, Beckermann K, Carlo MI, Choueiri TK, Costello BA, Derweesh
IH, et al: Kidney cancer, version 3.2022, NCCN clinical practice
guidelines in oncology. J Natl Compr Canc Netw. 20:71–90. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Serrano Frago P, Del Agua Arias Camisón C,
Gil Sanz MJ, Allué López M, Gonzalvo Ibarra A, Plaza Mas L and
Rioja Sanz LA: Controversies related to epithelioid variant of
renal angiomyolipoma: A review of the literature. Urology.
67:846.e3–e5. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tsai HY, Lee KH, Ng KF, Kao YT and Chuang
CK: Clinicopathologic analysis of renal epithelioid angiomyolipoma:
Consecutively excised 23 cases. Kaohsiung J Med Sci. 35:33–38.
2019. View Article : Google Scholar : PubMed/NCBI
|