Introduction
Primary adult retroperitoneal tumors include a wide
range of neoplasms, of which mesenchymal tumors are the most common
histotype (1,2). Soft-tissue sarcomas are rare
malignancies accounting for approximately 1% of all adult tumors
(3) with more than 50 different
histologic subtypes (2).
Liposarcomas (LPS) are among the most frequent
malignant retroperitoneal neoplasms of adipose origin (0.9/100,000
people/year) (4–6). Histologically, they are divided into
well-differentiated LPS (WDLPS), dedifferentiated LPS (DDLPS),
myxoid LPS, pleomorphic LPS, and myxoid pleomorphic LPS (2).
The most frequent retroperitoneal sarcoma (RPS) is
DDLPS (incidence of 0.1/1,000,000 per year) (7), which mainly affects patients in their
sixth-seventh decade (8). Due to
the retroperitoneum's anatomic characteristics, such lesions can
grow considerably and involve multiple contiguous structures before
the diagnosis is carried out (5,6).
Symptoms are often vague and even large neoplasms are usually
diagnosed incidentally (9). As per
the majority of solid tumors (10–12),
the main treatment of non-metastatic retroperitoneal RPS and DDLPS
is radical surgery with the inclusion of involved structures,
accompanied or not by radiotherapy in some specialized centers
(3,5,13–16),
while the advanced or metastatic disease can only benefit from
systemic chemotherapy and molecular targeted agents or debulking,
when possible (3,13–18).
Among the organs most frequently
involved/infiltrated by retroperitoneal giant DDLPS we find the
kidneys. Although in these cases nephrectomy is sometimes performed
for technical reasons, its validity from an oncological point of
view is still debated.
The pandemic had a strong impact on health resources
and medical activity, making hospitals and diagnostic services less
accessible to the general population. Outpatient prevention and
screening services have suffered many limitations due to the
relocation of health personnel to COVID departments or services,
significantly lengthening the waiting lists for all the other
services (19–21). Patients were also reluctant to
attend health facilities as they feared contagion. An increase in
late-stage diagnoses of malignant diseases, especially breast
(22,23), prostate (24), and colon cancer (25) has been recorded worldwide, and Italy
was one of the most strongly affected countries. Moreover, many
Centers experienced significant delays in the surgical treatment of
oncologic diseases, due to operating theatres' schedule
rearrangements (26).
Herein, we describe two successful radical surgical
removals of giant retroperitoneal DDLPS (a one-time primitive tumor
resection and a two-step surgical resection), treated with/without
kidney sparing, and describe the available evidence in the
literature.
Case report
Case 1
A 56-year-old woman complaining of vague digestive
disorders in September 2021 was prescribed an abdominal ultrasound
and a gastroscopy, which were not performed due to both the
considerable lengthening of the waiting lists and the fear of
contracting COVID at the healthcare facilities. Following the
worsening of her symptoms, after almost two years and the reduction
of the COVID emergency, she finally performed an abdominal
ultrasound in May 2022 (General Electrics Ultrasound System, model
Logiq S8 XDclear (equipped with 3.5, 5, and 7.5 MHz probes) at our
Institution that revealed a giant inhomogeneous abdominal mass. At
admission, the patient reported weight loss (7 kg in the previous 2
months) and severe abdominal discomfort. Her medical history was
not significant, her blood chemistry tests showed a slight
elevation of LDH (250 U/l, normal range 125–243 U/l) and a mild
iron deficiency anemia (25 mcg/dl, normal range 50–170 mcg/dl),
while all the tumoral and viral markers were negative.
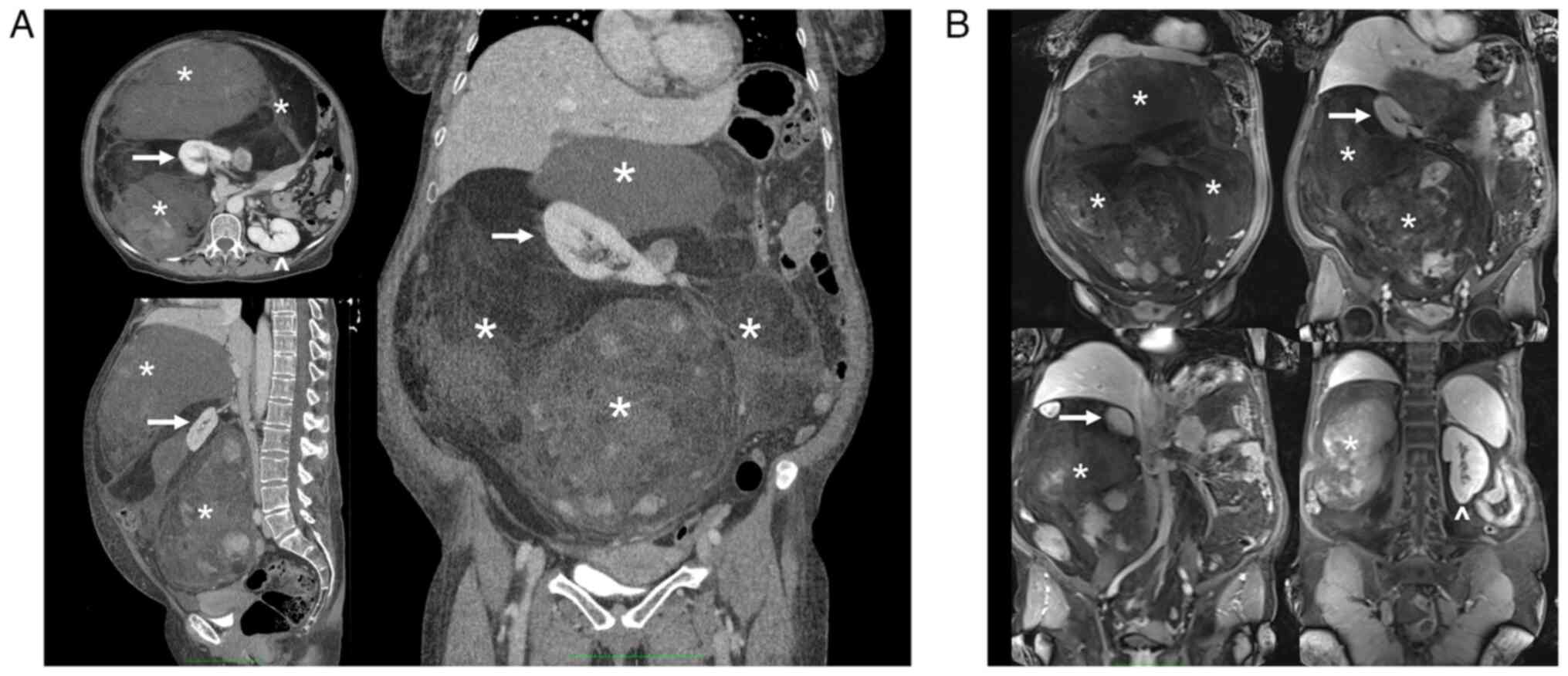
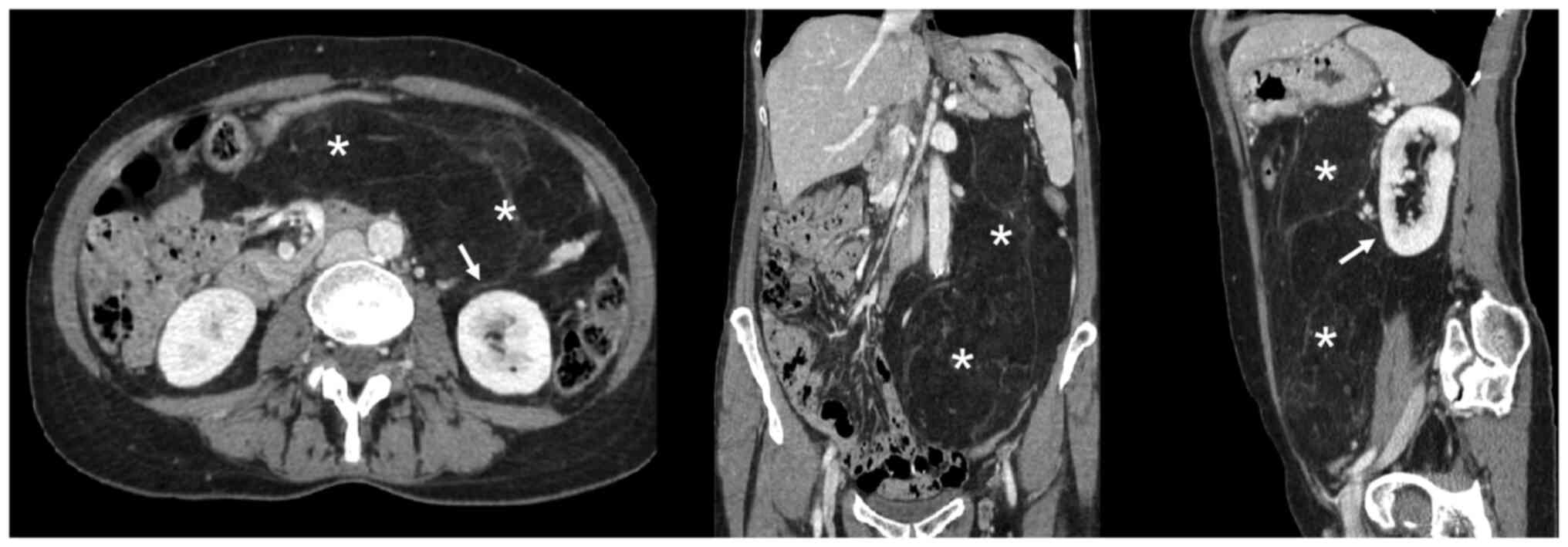
A total body CT scan, MRI, and PET confirmed the
presence of the voluminous (max diam. 31 cm) and inhomogeneous mass
with intra-lesion cystic components and excluded the presence of
distant lesions consistent with an RPS (Figs. 1 and 2).
For the CT scan we used a Toshiba Aquilion 64
four-phase CT scan (Toshiba Aquilion 64, Japan) with a protocol
including basal and post contrast medium IV injection (30–35 sec
after injection for the arterial phase; 75–80 sec after contrast
injection to obtain the venous phase; 3–5 min after injection for
the delayed phase).
For the MRI we used Siemens Magnetom Trio High-field
MRI (Magnetom Trio 3T, Siemens Medical Imaging, Erlangen, Germany)
with a protocol including a T1 and T2 weighted sequences, also with
TE opposed-phase and fat suppression, completed with DWI study and
acquisition before, during and after e.v. injection of paramagnetic
contrast media.
For the CT-PET we used a Philips Ingenuity 64 and
the exam was performed after intravenous injection of (18F) FDG.
Images of the isotope distribution were acquired in 15 min. PET
images were reconstructed with 4 mm thick tomographic slices. The
CT exam was performed with tomographic sections of 2 mm (120 kV)
and dose modulation in relation to the patient's build.
An oncologic consultation suggested a CT-PET-guided
biopsy, which was oriented toward a mesenchymal tumor. Finally, the
patient underwent surgical removal of the mass.
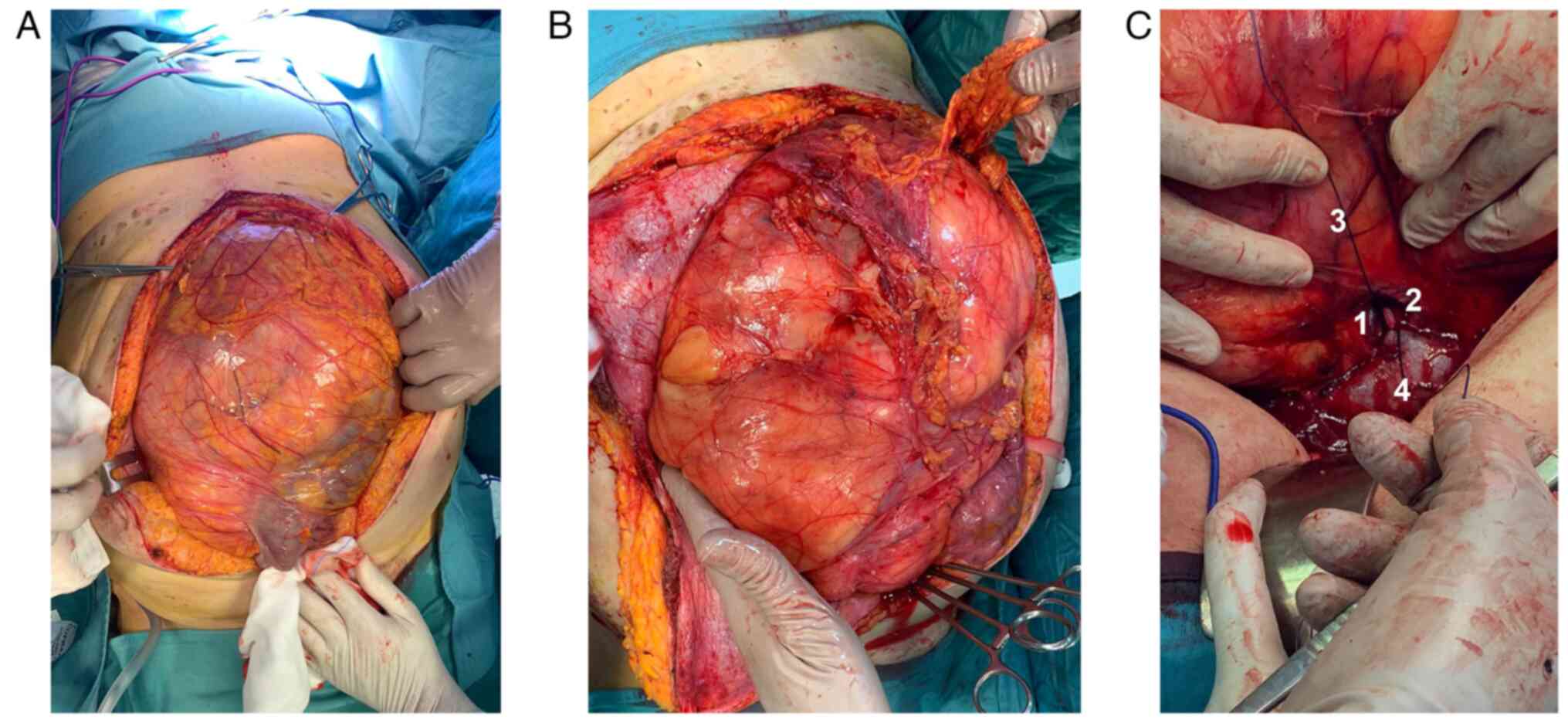
Ureteral stents were placed bilaterally, and a
xipho-pubic incision with supra-umbilical right lateral split was
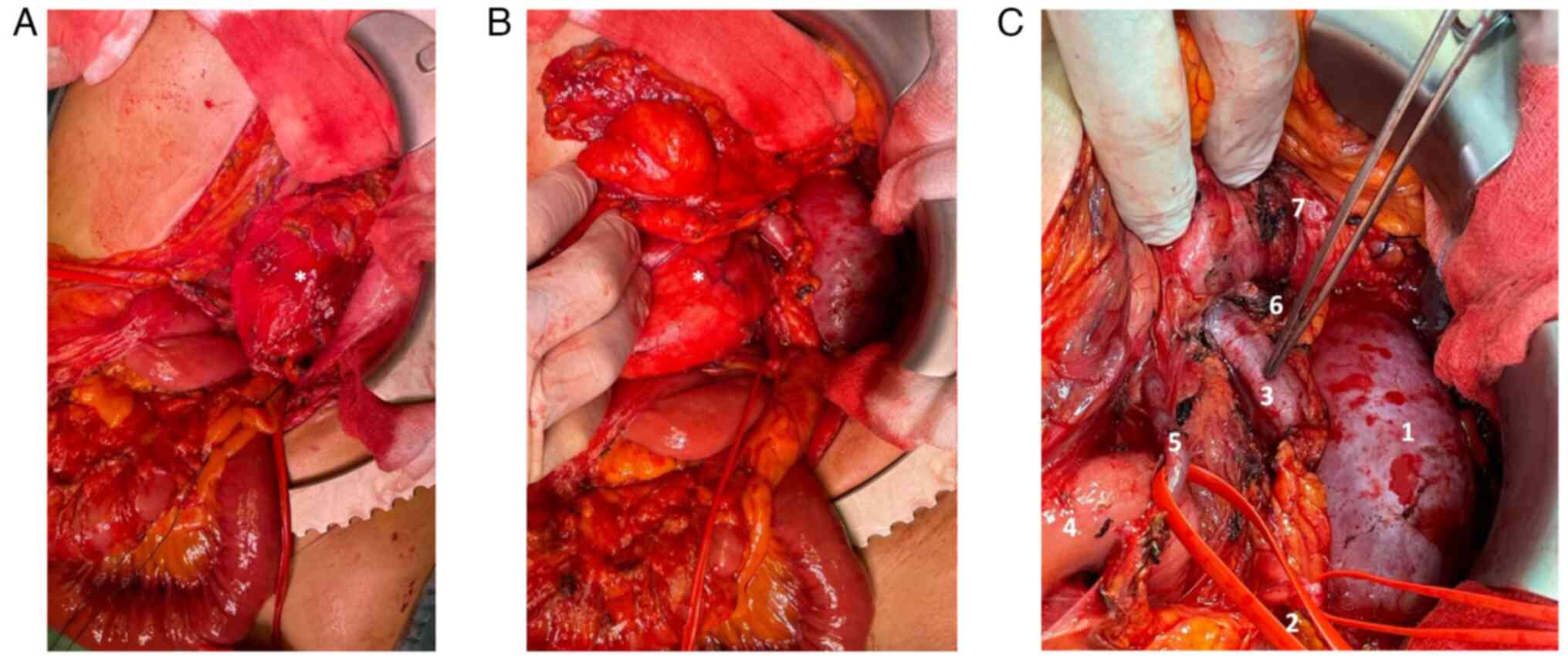
performed. The lesion significantly protruded out of the abdomen,
compressing all the viscera, and determining important abdominal
hypertension. However, it appeared non-infiltrating and
well-defined from the surrounding tissues (Fig. 3A and B). The vena cava was used as a
landmark to isolate the renal veins: the ureters were running in
parallel, and the right kidney was completely embedded and
undetachable from the mass: therefore, a right nephrectomy was
mandatory (Fig. 3C). After the
exposure of the retroperitoneal plane and the diaphragm, the mass
was removed. An inter-aorto-caval lymph-node picking, right
adrenalectomy, and cholecystectomy were performed.
Following surgery two PRBCs were infused due to mild
anemia. The further postoperative course was uneventful. The
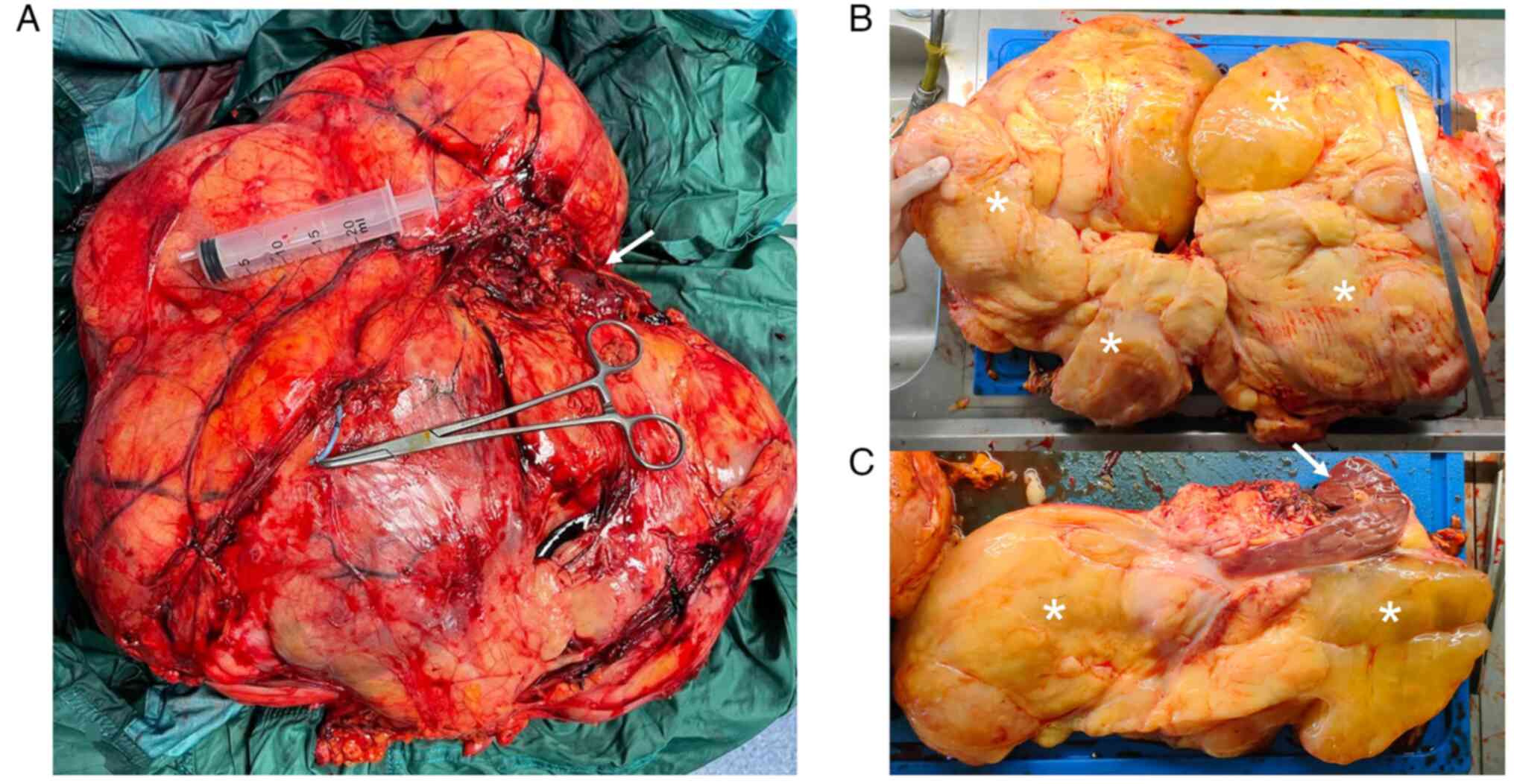
lesion's diameters were 41×36×16 cm and it weighed 13.56 Kg
(Fig. 4), with yellow, lobulated,
and partly gelatinous sections, and a focal bone metaplastic area
of 3.5 cm. No liquid and/or cystic areas were observed. The right
renal parenchyma appeared twisted and deformed, but not
infiltrated. Surgical margins were negative (R0) and none of the
isolated lymph nodes and intraoperative specimens showed neoplastic
features (N0, M0).
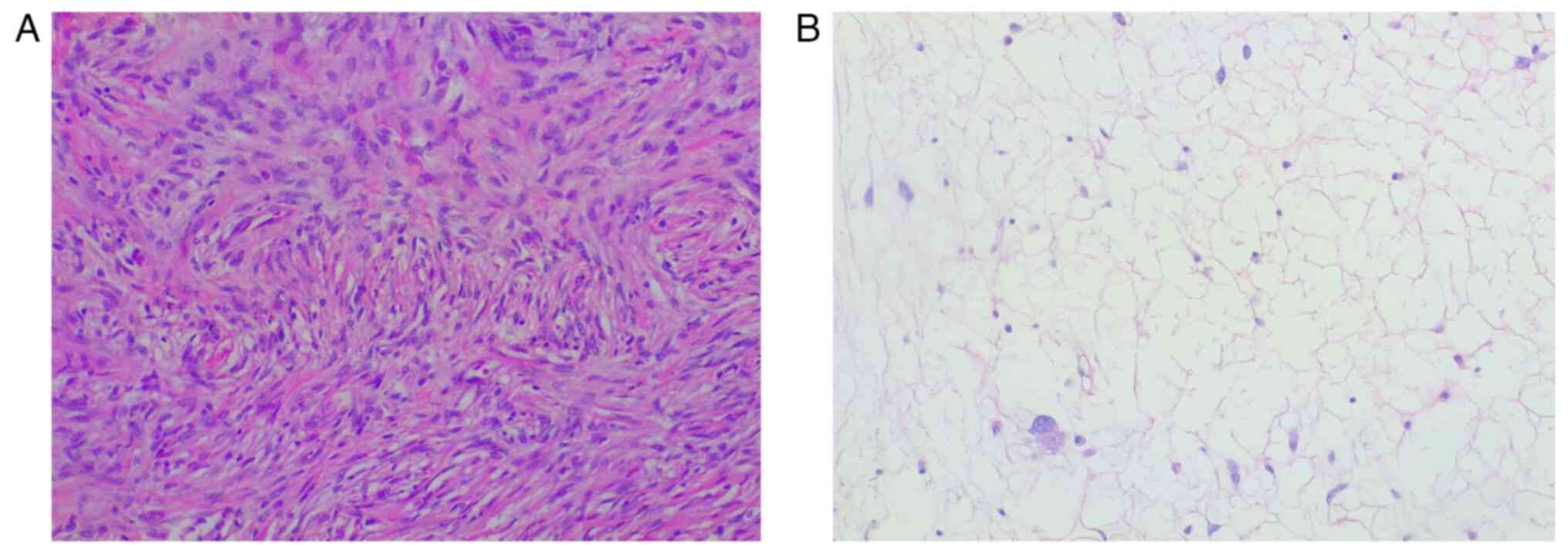
The histological examination was performed through a
hematoxylin and eosin staining, carried out on 4-µm thick sections
from formalin fixed, paraffin embedded tissues. The slides
underwent an automated processing step (Fig. 5). Immunohistochemical staining was
not performed due to the peculiar characteristics of the lesion,
that suggested a DDLPS diagnosis: the sections showed morphological
features typical of dedifferentiated liposarcoma, such as the
presence of areas of atypical lipomatous tumor (ALT), with mature
adipocytes in which substantial variation in cell size is
appreciated, and areas of non-lipogenic sarcoma with low grade
dedifferentiation showing fibroblastic spindle cells with moderate
nuclear atypia, organized in a fascicular architectural pattern,
and exhibiting medium to high cellularity. Transition between the
two areas was abrupt. Hence, immunohistochemical staining was
considered not necessary for this diagnosis. The histological
features, together with the outcome of the imaging exams, the
clinical trend and the intraoperative findings were all compatible
with the diagnosis of DDLPS, Stage IIIb (larger than 10 cm, not
spread to nearby lymph nodes or to distant sites) (27). The patient started adjuvant
chemotherapy: the schedule consisted of doxorubicin (70
mg/m2) intravenously for 20 min and ifosfamide (4
g/m2) with mesna intravenously for 24 h at day 1, and
pegfilgrastim subcutaneously, starting 24 h after completing
ifosfamide. Four cycles were given at 3 week intervals (28,29).
The control CT scan performed on POD8 and a control MRI at eight
months showed no signs of recurrence; at discharge, LDH was 290 U/l
and iron levels were at 30 mcg/dl, thus iron supplementation was
prescribed. Even if the patient was referred to nephrological
follow-up, apparently the nephrectomy and the subsequent
chemotherapy had no reflections on the renal function, and the left
kidney fully compensated for the loss of the contralateral organ:
the creatinine value at the last follow-up was 1.1 mg/dl (normal
range: 0.6–1.1 mg/dl). The next check-up at our outpatient clinic
is set at 12 months after surgery.
Case 2
With the same timing and due to the same
COVID-related delays of the first patient, a 61-year-old woman
complaining about worsening general abdominal discomfort for two
years finally underwent an abdominal US in February 2022 at another
hospital (although the first indication to perform this diagnostic
test was given in January 2021), that revealed a giant abdominal
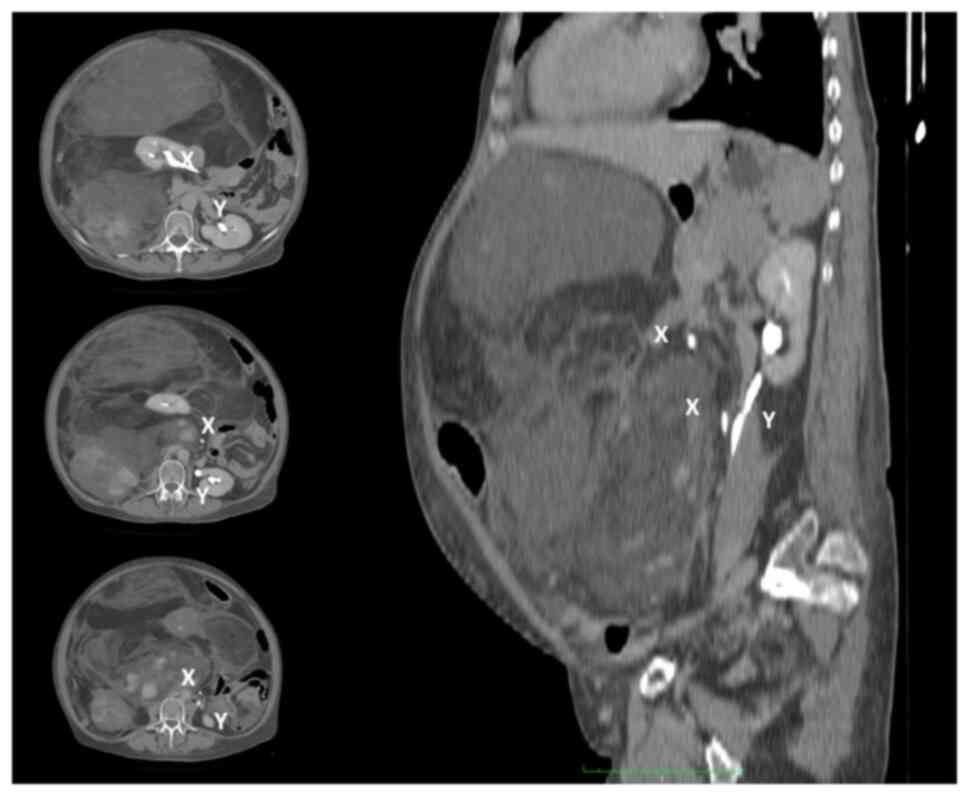
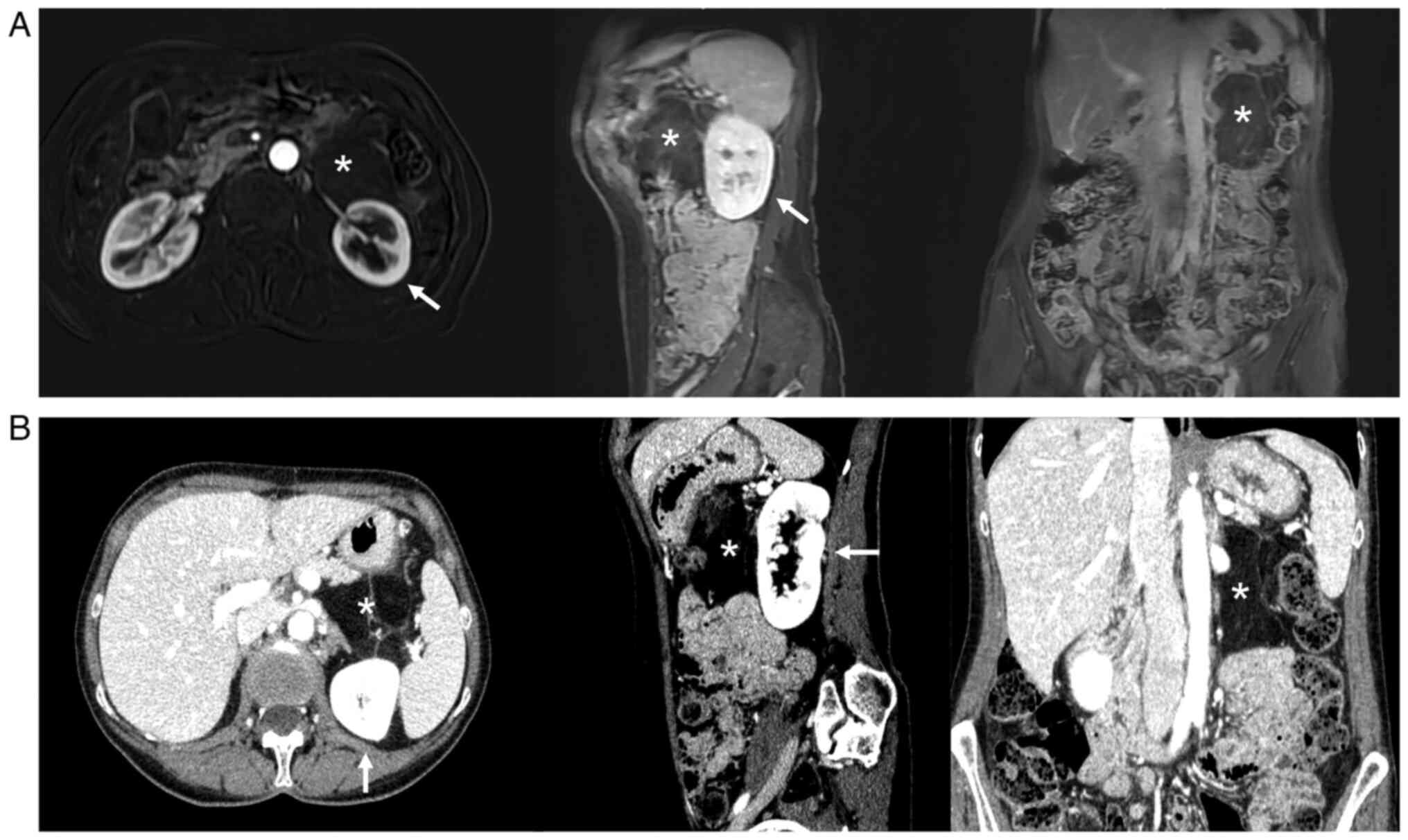
inhomogeneous mass. A preoperative CT scan showed a giant
inhomogeneous non-infiltrating retroperitoneal lesion (23×17×6.5
cm) (Fig. 6).
Blood chemistry tests were the norm, as well as
viral and tumoral markers. Given the worsening of her symptoms, the
patient underwent laparoscopic excision of the mass in April 2022,
in another Institute. When approaching the anterior aspect of the
pancreas and the III–IV duodenal portion the resection resulted
difficult, so excision of this level was interrupted, resulting in
an incomplete resection (R2). The postoperative course was
complicated by acute pancreatitis. Histologically, the lesion
appeared to be a DDLPS with a maximum diameter of 19 cm.
As soon as postoperative pancreatitis subsided in
May 2022, a control US, MRI, and PET (performed at our Institution,
with the same equipment and protocols described above) (Fig. 7) revealed the presence of a
recurrence/residue of disease (9×6.5×4.6 cm), close to the Treitz
ligament and anteriorly to the left kidney, but the absence of
distant lesions. The presence of disappearing fat in the
fat-suppressor sequences, the constant isointensity of the solid
component with that of neighboring muscle structures, the
inhomogeneous uptake/absence of uptake of the contrast
medium/beta-emitting tracer in some areas of the mass, and the
presence of necrotic areas all made us suspect a DDLPS (Fig. 7).
The patient was admitted to our Institution
reporting weight loss (5 Kg in 3 months) and was enlisted for
radical resection, scheduled in June 2022. After a median incision
and a wide adhesions ablation, the mass appeared non-infiltrative
and detachable from the left kidney, pancreatic tail, left colic
flexure, and splenic hilum. After the individuation of a resection
plane, the mass was removed en bloc without resecting other
structures (Fig. 8).
The postoperative course was uneventful, and also
this patient underwent adjuvant chemotherapy with the same protocol
as in case 1. Histological and immunohistochemical examination
confirmed the previously stated diagnosis of DDLPS. Hematoxylin and
eosin staining was performed as described above, but the results
were not as decisive as in the first case so to better define the
diagnosis we performed other tests. Thus, immunohistochemical
analysis was performed, using 4-µm-thick formalin-fixed,
paraffin-embedded tissue sections. Sections were stained for
immunohistochemical analysis by using standard techniques and the
antibodies' dilutions were performed according to the instructions
predefined by the manufacturer. Briefly, consecutive parallel
sections were stained with the following antibodies: anti-s100
(rabbit polyclonal antibody anti-s100 protein, CONFIRM, catalog
Number: 760-2523), anti-p16 protein (mouse monoclonal anti-p16
antibody, clone E6H4, CINtec, catalog Number: 705-4793),
anti-Vimentin (mouse monoclonal antibody, clone V9, VENTANA,
catalog Number: 790-2917), anti-SMA (mouse monoclonal Primary
Antibody, clone 1A4, VENTANA, catalog number: 760-2833). Our sample
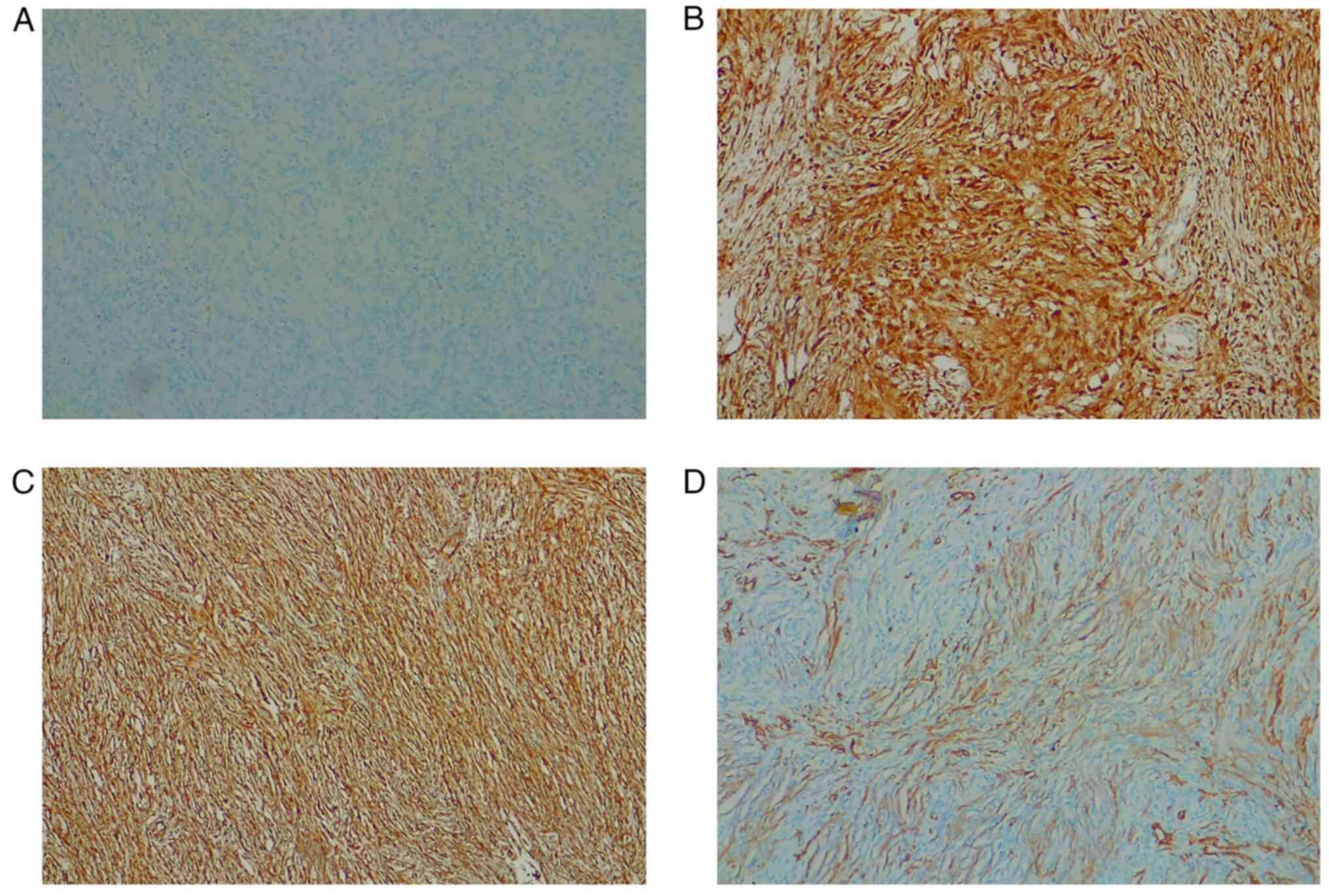
was S-100-negative, p16-positive, Vimentin positive, and
SMA-positive (Fig. 9); CDK4, MDM2
and STAT6 immunohistochemical analysis were not performed due to
the lack of antibodies. Overall, the clinical and instrumental data
were all compatible with the diagnosis of DDLPS, Stage IIIa (larger
than 5 cm but not more than 10 cm, not spread to nearby lymph nodes
or to distant sites) (27). Two
postoperative CT scans at one week and 7 months revealed no signs
of recurrence, and a good creatinine value (0.8 mg/dl) was found at
the last follow-up. The next check-up at our outpatient clinic is
set at 12 months after surgery.
Discussion
Surgical resection is the gold standard treatment of
RPS, as several guidelines (3,13–15)
and case series (30–32) confirm. Some papers including one of
the largest series to date (11,33),
comprising 8653 patients undergoing surgery for primary RPS, advise
against the routine use of chemotherapy since the currently
available regimens do not confer a significant survival advantage.
However, the superiority of a radical excision comprising
surrounding organs vs. the resection of the tumor alone is still
under debate (15,31,34–38).
Given their rarity and heterogeneity, the available
literature often groups all RPS in cohorts spanning a considerable
number of years. In a 28-year study, a single-centered series of
675 patients (31), determined that
R1 resection (along with histologic type, age, and tumor size) was
independently associated with local recurrence. Moreover, some
histological types, such as liposarcomas and leiomyosarcomas, were
associated with recurrence also until 15 years from diagnosis, and
with lower disease-specific overall survival (OS) (31,32).
RPS is associated with a considerable recurrence
rate, accounting for 70.5% of a recent multicentric series of 567
RPS patients undergoing surgery (39). The 5-years OS depends on the
recurrence site (local, distal, or both), the possibility of
undergoing a second resection, and its timing. MacNeill et
al (30) reported the
post-relapse outcomes for 1007 RPS surgical patients: recurrence is
predictive of high mortality and a new surgical treatment should be
considered when radicality is possible, as we did for our second
case.
Surgical series of DDLPSs are scarce, given the
rarity of these tumors and the tendency of grouping them with other
RPSs. A recent cohort of 32 patients undergoing resection (18
DDLPSs vs. 14 WDLPS) showed a significant difference in
recurrence-free survival times at 2 years (26% vs. 59%,
respectively; P=0.005), but could not confirm a difference in OS
(40). Local recurrence of DDLPS
develops four times more frequently compared with WDLPS (38), especially if the dedifferentiated
component is found at the resection margin (15,35–37,41).
All these considerations gain even more validity for bigger lesions
and giant tumors (5,42–45).
The definition of giant tumors is vague (5,42–44),
but guidelines and literature reports support surgery as a valid
treatment for both small and giant DDLPSs (1,3,5,6,13,15,34,36).
However, despite being the optimal therapy, the surgical removal of
giant DDLPSs presents a great degree of difficulty and a low R0
rate (5,44).
DDLPSs are the most frequent histotype of RPS. They
appear as de novo lesions in 90% of cases, while the
remaining DDLPSs progress after a previous well-differentiated RPS
develops recurrence, most likely in a time-dependent
dedifferentiation process (2). For
this reason, giant DDLPSs often present de-differentiated areas
(4,13,31,38).
DDLPSs appear as predominantly non-lipomatous neoplasms that are
adjacent, inside or encompass a fatty mass (46).
Imaging is crucial in the diagnosis and preoperative
management of DDLPSs (9,14,46).
All the investigations carried out preoperatively were fundamental
for the selection of the correct treatment: the absence of
infiltration of large retroperitoneal vessels and the lack of
distant metastases led to choosing an aggressive treatment instead
of palliation (3,15,17,18,37).
At the same time, the finding of morphological and functional
characteristics at the operating table (absence of cystic areas and
larger-than-expected dimensions) did not negatively affect surgical
removal, as we observed in our first case.
The biopsy's goal is to evaluate both surgical and
medical management and eventually perform neoadjuvant therapy, as
recommended by international guidelines (3,15,47).
In our first case, given the primitive nature of the lesion, a
preoperative biopsy was performed percutaneously, as surgical
biopsy could favor abdominal wall seeding due to accidental tumor
rupture (1,15,47).
When deemed necessary, given their common inhomogeneity, we suggest
a TC-PET-guided core needle biopsy of the lesions to obtain a more
substantial and contributive tissue sample. However, neoadjuvant
chemotherapy is not routinely recommended (16) and, in the case of symptomatic giant
masses, there is no possibility of waiting for the chemotherapy's
effects. Moreover, the presence of distant lesions had already been
excluded by preoperative imaging. Therefore, a biopsy could be
avoided in symptomatic upfront resectable giant RPS.
Our patients were not candidates for neoadjuvant
radiotherapy, due to the extension of the lesions, the onset of
symptoms, and their proximity to radiosensitive organs (3,14,15).
For this reason, a more invasive but radical surgical approach was
selected.
Radiologic embolization can be considered a
preoperative treatment whenever a giant mass has a clear vascular
axis, both to shrink the mass and to reduce intraoperative blood
loss (3). This procedure was taken
into consideration for both patients but was finally excluded due
to the absence of a single clear tributary vessel. Moreover, a
preoperative kidney embolization could be considered when an
organ-preserving resection is not possible (45) and the removal of the kidney is
certain. In our first patient, although the kidney was enveloped by
the tumor, renal vessels' embolization was not contemplated because
of the preoperative uncertainty of being able to take away all the
mass containing the kidney, while the second patient did not show a
clear renal involvement. Moreover, preoperative embolization of RPS
is scarcely reported in the literature and can lead to severe
complications (i.e.: post-embolization syndrome).
Giant lesions' invasiveness and boundaries are
assessed mostly intraoperatively, and, even after an extensive
preoperative discussion, it is often difficult to anticipate the
surgical management in detail (5,9,36,42).
This is particularly true for unexpected vascular invasion, which
has a worse prognosis by definition and could hamper the margins'
radicality. Vascular resections, especially when retroperitoneal
vessels are involved, are difficult to couple with an en bloc
resection, as they often require an incomplete R1 debulking before
reaching optimal exposure, leading to a higher rate of peritoneal
seeding (37,42). Our first case benefited from an
en-bloc resection without vascular reconstruction, allowing
a no-touch technique and avoiding seeding. Although visceral
or vascular invasion after recurrence or incomplete resection is
common (30,36,39)
our second patient benefited from an early and prompt radical
second look without the need for organ removals or vascular
reconstruction. This reinforces our belief that patients with
recurrence or previous R1 resections should be thoroughly studied
and evaluated for surgery, when possible. Both cases showed the
lesions' confinement to the surgical field and surgery was
performed without the exposure of tumoral tissue; however, after
surgical manipulation, adjuvant chemotherapy and a strict follow-up
are necessary (32,33).
Our first patient is one of the few giant
retroperitoneal DDLPS reported in the literature that could be
resected with negative margins and no vascular resections, despite
being >40 cm of maximum diameter in a non-obese patient
(43,44,48).
Incidental primitive lesions in otherwise healthy patients are even
rarer (43,48).
In both cases, surgery was performed respecting the
lesion's plane without needing bowel resections or reconstruction
of major structures. Mortality from RPLS usually results from local
recurrence, underlining the importance of an R0 resection,
sometimes hard to reach for the difficulty in differentiating the
liposarcoma from adjacent normal fat, and for the absence of an
evident anatomical vascular peduncle (49). Although this, the prognosis from RPS
following R0 resection also depends on its histologic type, indeed
the 5-year survival rate for well-differentiated subtypes is 90,
75% for dedifferentiated, 60–90% for myxoid/round cell, and 30–50%
for pleomorphic subtypes (50). It
has also been demonstrated that tumor burden and nephrectomy are
not associated with disease-specific survival (51).
Due to the tumor's location, en bloc resection of
close organs together with RLPS is not always avoidable. The
necessity of multi-visceral resection (≥3 organs) has been
correlated to a higher local recurrence rate and worse survival
rate (15,31,36,37).
However, a recent systematic review seems to dismiss this
suggestion (35). In case of kidney
invasion, it is necessary to balance the benefit of free margin
resection against medical complications and quality of life loss.
In the first patient, a nephrectomy was unavoidable due to the
complete embedding of the right kidney into the lesion.
The second patient benefited from a nephron-sparing
resection thanks to an early diagnosis that possibly favored the
presence of an adequate cleavage plane and the absence of
infiltration.
These two cases show the duality of kidney resection
in the radical surgical treatment of giant DDLPSs: the first case
was a first presentation of DDLPs and unfortunately the kidney was
removed for technical reasons as it was surrounded by the tumor
even if this organ was not infiltrated. At the same time, in the
second case we treated a large DDLPS recurrence, that often
requires contextual extensive organs removals, through a
kidney-sparing procedure, achieving nonetheless a radical
resection. Our cases showed how nephrectomy should be evaluated
case by case and, in selected cases it is still possible achieve
radical surgical removal of a DDLPs through nephron-sparing
surgery, even in case of a recurrence.
So in conclusion the surgical aggression of a
recurrence does not necessarily have to foresee the removal of the
kidney as well as the presence of a giant liposarcoma involving but
not infiltrating the kidney can basically foresee the removal of
the mass saving the organ.
In a case series of 228 patients undergoing surgery
for RLPS, the group of those not requiring multi-organ resection
had a higher 10-year survival rate as opposed to those requiring
multi-organ resection (35% vs. 26%) (52). This suggests that, although invasion
to renal parenchyma and the possibility of future recurrence from
that site, the choice of performing a nephron-sparing resection
must be balanced on the tumor's histologic type, patient's age, and
increased morbidity from resection (53).
Despite the existence of reports describing excision
through a minimally-invasive approach (54,55),
our experience and literature data suggest performing an open
approach when dealing with a giant retroperitoneal mass since the
retroperitoneal plane is often narrow and movements could be
restricted by the mass, abdominal hypertension and visceral
dislocation (5,43,56).
The proximity with important vessels and anatomic structures must
be assessed carefully, and severe intraoperative complications are
possible when an adequate surgical field is difficult to attain
(57,58). However, to reach definitive
statements on the matter it is necessary to wait for case series
with a substantial number of cases and a long-term follow-up.
As well as because of mild symptoms, the late
diagnosis could also be attributed to the COVID pandemic, due to
the patient's resistance to going through a diagnostic process at
the first warning signs. Moreover, the accessibility of diagnostic
services was limited due to the overload of hospitalized COVID
patients, which caused important delays and undertreatment of many
non-respiratory disease patients and bore a dramatic impact on
oncologic disease patients (21,59).
The COVID-19 pandemic has resulted in a massive
backlog of the elective case as pointed out in a retrospective
case-control study; anonymized case-related routine data of a
Germany-wide voluntary hospital association (CLINOTEL association)
(60).
Delays in the provision of ‘elective’ surgical care
have dire consequences for the patient: progression of disease and
comorbidities, higher complication rates, and lower overall
survival. Delays in elective surgery also have significant
consequences for the healthcare system: added emotional strain on
healthcare workers, reduction in training opportunities, rising
costs, and increased inequality in health-service provision
(61). The COVID-19 pandemic led to
disruptions in the delivery of cancer treatments and the most
relevant factors associated with this delay are subjective patient
issues and local healthcare organizations (62).
This was particularly important in Italy, which
suffered among the strongest restrictions in Europe at that time
(19,22,24).
Healthcare workflow was heavily affected as well since oncologic
multidisciplinary discussions could only be performed via online
meetings and outpatient visits were reduced to the minimum,
following necessary anti-COVID tests (20,25,26).
To the best of our knowledge, our cases represent the first report
of surgically treated giant DDLPS during the COVID era. Moreover,
these two cases were discovered and treated in a short time span
(two months). Although there are no studies comparing the stage of
disease at diagnosis in pre- and post-COVID era, given the growth
pattern of DDLPS (2) and the recent
delay of medical attention to non-respiratory diseases, an
increased number of RPS and DDLPS cases could be expected
shortly.
In conclusion, the treatment of giant DDLPS is still
challenging and requires multidisciplinary treatment as well as,
when possible, radical surgical removal. The lack of tissue
infiltration and the avoidance of major organs' excision or
reconstruction could lead to an easier postoperative course and a
better prognosis. In the current state of knowledge for the
treatment of giant DDLPS, an open approach is preferable over
minimally invasive techniques. In the same way, considering the
technical and oncological point of view, the excision of the
kidneys together with the neoplastic mass should be avoided and the
surgical management of recurrences or incompletely resected masses
must be pursued. As the COVID pandemic caused a limited
medicalization of many population groups and a late diagnosis of
other oncologic diseases, an increased number of DDLPSs could be
expected in the next future.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FP, MLS, NC and VD participated in the
multidisciplinary meetings prior to surgeries, operated the
patients, conceived and designed the manuscript, acquired and
confirmed the authenticity of all the raw data, drafted the final
version of the manuscript, and provided the surgical details
described in the manuscript. DS, LC, MI and CGM performed the
radiologic imaging of all patients in the present study, and
provided radiologic advice and the machinery details described in
the present study. LI and RA performed histologic analysis of the
specimens and provided all pathologic details described in the
present study. MG provided oncologic advice and treated both
patients, providing all details of the adjuvant treatment described
in the present study. AS, LP, NC and AJ performed a critical
literature review, contributed to the acquisition, analysis and
interpretation of data, contributed to the drafting of the
Introduction and Discussion, and provided critical surgical advice.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Given the retrospective nature of the present study,
ethics committee approval was not necessary.
Patient consent for publication
The patients signed a specific informed consent that
included the acquirement of clinical data and pictures in an
anonymous form for publication purposes.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Improta L, Tzanis D, Bouhadiba T,
Abdelhafidh K and Bonvalot S: Overview of primary adult
retroperitoneal tumours. Eur J Surg Oncol. 46:1573–1579. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sbaraglia M, Bellan E and Dei Tos AP: The
2020 WHO Classification of Soft Tissue Tumours: News and
perspectives. Pathologica. 113:70–84. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
von Mehren M, Randall RL, Benjamin RS,
Boles S, Bui MM, Ganjoo KN, George S, Gonzalez RJ, Heslin MJ, Kane
JM, et al: Soft tissue sarcoma, version 2.2018, NCCN clinical
practice guidelines in oncology. J Natl Compr Canc Netw.
16:536–563. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ducimetière F, Lurkin A, Ranchère-Vince D,
Decouvelaere AV, Péoc'h M, Istier L, Chalabreysse P, Muller C,
Alberti L, Bringuier PP, et al: Incidence of sarcoma histotypes and
molecular subtypes in a prospective epidemiological study with
central pathology review and molecular testing. PLoS One.
6:e202942011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bachmann R, Eckert F, Gelfert D, Strohäker
J, Beltzer C and Ladurner R: Perioperative strategy and outcome in
giant retroperitoneal dedifferentiated liposarcoma-results of a
retrospective cohort study. World J Surg Oncol. 18:2962020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hassan I, Park SZ, Donohue JH, Nagorney
DM, Kay PA, Nasciemento AG, Schleck CD and Ilstrup DM: Operative
management of primary retroperitoneal sarcomas: A reappraisal of an
institutional experience. Ann Surg. 239:244–250. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hirata M, Asano N, Katayama K, Yoshida A,
Tsuda Y, Sekimizu M, Mitani S, Kobayashi E, Komiyama M, Fujimoto H,
et al: Integrated exome and RNA sequencing of dedifferentiated
liposarcoma. Nat Commun. 10:56832019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nishio J, Nakayama S, Nabeshima K and
Yamamoto T: Biology and management of dedifferentiated liposarcoma:
State of the art and perspectives. J Clin Med. 10:32302021.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Messiou C, Moskovic E, Vanel D, Morosi C,
Benchimol R, Strauss D, Miah A, Douis H, van Houdt W and Bonvalot
S: Primary retroperitoneal soft tissue sarcoma: Imaging
appearances, pitfalls and diagnostic algorithm. Eur J Surg Oncol.
43:1191–1198. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Capece M, Creta M, Calogero A, La Rocca R,
Napolitano L, Barone B, Sica A, Fusco F, Santangelo M, Dodaro C, et
al: Does physical activity regulate prostate carcinogenesis and
prostate cancer outcomes? A narrative review. Int J Environ Res
Public Health. 17:14412020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Strocchi E, Iaffaioli RV, Facchini G,
Mantovani G, Ricci S, Cavallo G, Tortoriello A, D'Angelo R, Formato
R, Rosato G, et al: Stop-flow technique for loco-regional delivery
of high dose chemotherapy in the treatment of advanced pelvic
cancers. Eur J Surg Oncol. 30:663–670. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Santangelo M, Esposito A, Tammaro V,
Calogero A, Criscitiello C, Roberti G, Candida M, Rupealta N,
Pisani A and Carlomagno N: What indication, morbidity and mortality
for central pancreatectomy in oncological surgery? A systematic
review. Int J Surg. 28 (Suppl 1):S172–S176. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kilpatrick SE: Dedifferentiated
liposarcoma: A comprehensive historical review with proposed
evidence-based guidelines regarding a diagnosis in need of further
clarification. Adv Anatomic Pathol. 28:426–438. 2021.PubMed/NCBI
|
|
14
|
Baldini EH, Wang D, Haas RL, Catton CN,
Indelicato DJ, Kirsch DG, Roberge D, Salerno K, Deville C,
Guadagnolo BA, et al: Treatment guidelines for preoperative
radiation therapy for retroperitoneal sarcoma: Preliminary
consensus of an international expert panel. Int J Radiat Oncol Biol
Phys. 92:602–612. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Casali P, Abecassis N, Aro HT, Bauer S,
Biagini R, Bielack S, Bonvalot S, Boukovinas I, Bovee JVMG,
Brodowicz T, et al: Soft tissue and visceral sarcomas: ESMO-EURACAN
Clinical Practice Guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 29 (Suppl 4):iv51–iv67. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Callegaro D, Raut CP, Keung EZ, Kim T, Le
Pechoux C, Martin-Broto J, Gronchi A, Swallow C and Gladdy R:
Strategies for care of patients with gastrointestinal stromal tumor
or soft tissue sarcoma during COVID-19 pandemic: A guide for
surgical oncologists. J Surg Oncol. 123:12–23. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Keung EZ, Lazar AJ, Torres KE, Wang WL,
Cormier JN, Ashleigh Guadagnolo B, Bishop AJ, Lin H, Hunt KK, Bird
J, et al: Phase II study of neoadjuvant checkpoint blockade in
patients with surgically resectable undifferentiated pleomorphic
sarcoma and dedifferentiated liposarcoma. BMC Cancer. 18:9132018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Roland CL, Keung EZ-Y, Lazar AJ, Torres
KE, Wang WL, Guadagnolo A, Bishop AJ, Lin HY, Hunt Y, Feig BW, et
al: Preliminary results of a phase II study of neoadjuvant
checkpoint blockade for surgically resectable undifferentiated
pleomorphic sarcoma (UPS) and dedifferentiated liposarcoma (DDLPS).
Am Soc Clin Oncol. 38:115052020. View Article : Google Scholar
|
|
19
|
Ferrara G, De Vincentiis L,
Ambrosini-Spaltro A, Barbareschi M, Bertolini V, Contato E,
Crivelli F, Feyles E, Mariani MP, Morelli L, et al: Cancer
diagnostic delay in northern and central Italy during the 2020
lockdown due to the coronavirus disease 2019 pandemic. Am J Clin
Pathol. 155:64–68. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Battisti F, Falini P, Gorini G, Sassoli de
Bianchi P, Armaroli P, Giubilato P, Giorgi Rossi P, Zorzi M,
Battagello J, Senore C, et al: Cancer screening programmes in Italy
during the COVID-19 pandemic: An update of a nationwide survey on
activity volumes and delayed diagnoses. Ann Ist Super Sanita.
58:16–24. 2022.PubMed/NCBI
|
|
21
|
Bracale U, Podda M, Castiglioni S,
Peltrini R, Sartori A, Arezzo A, Corcione F and Agresta F; CLOUD-19
Collaborative Group, : Changes in surgicaL behaviOrs dUring the
CoviD-19 pandemic. The SICE CLOUD19 Study. Updates Surg.
73:731–744. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mentrasti G, Cantini L, Vici P, D'Ostilio
N, La Verde N, Chiari R, Paolucci V, Crocetti S, De Filippis C,
Pecci F, et al: Rising incidence of late stage breast cancer after
COVID-19 outbreak. Real-world data from the Italian COVID-DELAY
study. Breast. 65:164–171. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Piscitelli P, Santoriello A, Buonaguro FM,
Di Maio M, Iolascon G, Gimigliano F, Marinelli A, Distante A,
Serravezza G, Sordi E, et al: Incidence of breast cancer in Italy:
Mastectomies and quadrantectomies performed between 2000 and 2005.
J Exp Clin Cancer Res. 28:862009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pepe P, Pepe L, Pennisi M and Fraggetta F:
Prostate cancer diagnosis and management during one year of the
COVID-19 pandemic. Anticancer Res. 41:3127–3130. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ricciardiello L, Ferrari C, Cameletti M,
Gaianill F, Buttitta F, Bazzoli F, Luigi de'Angelis G, Malesci A
and Laghi L: Impact of SARS-CoV-2 pandemic on colorectal cancer
screening delay: Effect on stage shift and increased mortality.
Clin Gastroenterol Hepatol. 19:1410–1417.e9. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lazzerini M, Barbi E, Apicella A,
Marchetti F, Cardinale F and Trobia G: Delayed access or provision
of care in Italy resulting from fear of COVID-19. Lancet Child
Adolesc Health. 4:e10–e11. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Amin MB, Greene FL, Edge SB, Compton CC,
Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and
Winchester DP: The Eighth edition AJCC Cancer Staging Manual:
Continuing to build a bridge from a population-based to a more
‘personalized’ approach to cancer staging. CA Cancer J Clin.
67:93–99. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Woll PJ, Reichardt P, Le Cesne A, Bonvalot
S, Azzarelli A, Hoekstra HJ, Leahy M, Van Coevorden F, Verweij J,
Hogendoorn PC, et al: Adjuvant chemotherapy with doxorubicin,
ifosfamide, and lenograstim for resected soft-tissue sarcoma (EORTC
62931): A multicentre randomised controlled trial. Lancet Oncol.
13:1045–1054. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gronchi A, Miah AB, Dei Tos AP, Abecassis
N, Bajpai J, Bauer S, Biagini R, Bielack S, Blay JY, Bolle S, et
al: Soft tissue and visceral sarcomas: ESMO-EURACAN-GENTURIS
Clinical Practice Guidelines for diagnosis, treatment and
follow-up(★). Ann Oncol. 32:1348–1365. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
MacNeill AJ, Miceli R, Strauss DC,
Bonvalot S, Hohenberger P, Van Coevorden F, Rutkowski P, Callegaro
D, Hayes AJ, Honoré C, et al: Post-relapse outcomes after primary
extended resection of retroperitoneal sarcoma: A report from the
Trans-Atlantic RPS Working Group. Cancer. 123:1971–1978. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tan MC, Brennan MF, Kuk D, Agaram NP,
Antonescu CR, Qin LX, Moraco N, Crago AM and Singer S:
Histology-based classification predicts pattern of recurrence and
improves risk stratification in primary retroperitoneal sarcoma.
Ann Surg. 263:593–600. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao X, Li P, Huang X, Chen L, Liu N and
She Y: Prognostic factors predicting the postoperative survival
period following treatment for primary retroperitoneal liposarcoma.
Chin Med J. 128:85–90. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Miura JT, Charlson J, Gamblin TC, Eastwood
D, Banerjee A, Johnston FM and Turaga KK: Impact of chemotherapy on
survival in surgically resected retroperitoneal sarcoma. Eur J Surg
Oncol. 41:1386–1392. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Villano AM, Zeymo A, Nigam A, Chan KS,
Shara N, Unger KR and Al-Refaie WB: Radical excision for
retroperitoneal soft tissue sarcoma: A national propensity-matched
outcomes analysis. Surgery. 168:831–837. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Guo Q, Zhao J, Du X and Huang B: Survival
outcomes of surgery for retroperitoneal sarcomas: A systematic
review and meta-analysis. PLoS One. 17:e02720442022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bonvalot S, Miceli R, Berselli M, Causeret
S, Colombo C, Mariani L, Bouzaiene H, Le Péchoux C, Casali PG, Le
Cesne A, et al: Aggressive surgery in retroperitoneal soft tissue
sarcoma carried out at high-volume centers is safe and is
associated with improved local control. Ann Surg Oncol.
17:1507–1514. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bonvalot S, Rivoire M, Castaing M,
Stoeckle E, Le Cesne A, Blay JY and Laplanche A: Primary
retroperitoneal sarcomas: A multivariate analysis of surgical
factors associated with local control. J Clin Oncol. 27:31–37.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Singer S, Antonescu CR, Riedel E and
Brennan MF: Histologic subtype and margin of resection predict
pattern of recurrence and survival for retroperitoneal liposarcoma.
Ann Surg. 238:358–371. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
van Houdt WJ, Fiore M, Barretta F,
Rutkowski P, Blay JY, Lahat G, Strauss D, Gonzalez RJ, Ahuja N,
Grignani G, et al: Patterns of recurrence and survival probability
after second recurrence of retroperitoneal sarcoma: A study from
TARPSWG. Cancer. 126:4917–4925. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Osuna-Soto J, Caro Cuenca T, Sanz-Zorrilla
A, Torrecilla-Martínez A, Ortega Salas R and Leiva-Cepas F:
Prognosis and survival of patients diagnosed with
well-differentiated and dedifferentiated retroperitoneal
liposarcoma. Cir Esp (Engl Ed). 100:622–628. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dehner CA, Hagemann IS and Chrisinger JSA:
Retroperitoneal dedifferentiated liposarcoma. Am J Clin Pathol.
156:920–925. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fodor M, Maglione M, Kogler P,
Kafka-Ritsch R, Ofner D and Perathoner A: Challenges in the
treatment of a giant retroperitoneal liposarcoma. Ann Ital Chir.
9:S2239253X20033162. 2020.PubMed/NCBI
|
|
43
|
Inoue K, Higaki Y and Yoshida H: Giant
retroperitoneal liposarcoma. Int J Urol. 12:220–222. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kanthala L, Ray S, Aurobindo Prasad Das S,
Nundy S and Mehta N: Recurrent giant retroperitoneal liposarcoma:
Review of literature and a rare case report. Ann Med Surg.
65:1023292021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Galatola R, Stanzione A, Sirignano C,
Mainolfi C, Guadagno E, Carlomagno N, Insabato L, Santangelo M and
Maurea S: Giant Epithelioid Angiomyolipoma: An imaging-related
differential diagnosis among Fat-containing renal masses. Clin
Genitourin Cancer. 18:e5–e9. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Czeyda-Pommersheim F, Menias C, Boustani A
and Revzin M: Diagnostic approach to primary retroperitoneal
pathologies: What the radiologist needs to know. Abdom Radiol (NY).
46:1062–1081. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Trans-Atlantic RPS Working Group, :
Management of recurrent retroperitoneal sarcoma (RPS) in the adult:
A consensus approach from the Trans-Atlantic RPS Working Group. Ann
Surg Oncol. 23:3531–3540. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Dubois-Silva A and Barbagelata-Lopez C:
Retroperitoneal dedifferentiated liposarcoma. Intern Emerg Med.
14:619–620. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Nijhuis PH, Sars PR, Plaat BE, Molenaar
WM, Sluiter WJ and Hoekstra HJ: Clinico-pathological data and
prognostic factors in completely resected AJCC stage I–III
liposarcomas. Ann Surg Oncol. 7:535–543. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Karadayi K, Yildiz C, Karakus S, Kurt A,
Bozkurt B, Soylu S, Cicekli AA, Egilmez R and Cetin A:
Well-differentiated abdominal liposarcoma: Experience of a tertiary
care center. World J Surg Oncol. 13:1662015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tseng WW, Madewell JE, Wei W, Somaiah N,
Lazar AJ, Ghadimi MP, Hoffman A, Pisters PW, Lev DC and Pollock RE:
Locoregional disease patterns in well-differentiated and
dedifferentiated retroperitoneal liposarcoma: Implications for the
extent of resection? Ann Surg Oncol. 21:2136–2143. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Stilidi IS, Nikulin MP, Nered SN, Davydov
MM, Bolotskiĭ VI and Gubina GI: Combined operations by
retroperitoneal liposarcomas. Khirurgiia (Mosk). 20–25. 2013.(In
Russian). PubMed/NCBI
|
|
53
|
Argadjendra M, Napitupulu R, Yudadi R,
Hoetama S and Wibowo HS: Kidney sparing giant retroperitoneal
liposarcoma: Case report and literature review. Int J Surg Case
Rep. 56:70–73. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Agrusa A, Di Buono G, Buscemi S, Randisi
B, Gulotta L, Sorce V, Badalamenti G, Albano D, Galia M, Romano G
and Gulotta G: Dedifferentiated retroperitoneal large liposarcoma
and laparoscopic treatment: Is it possible and safe? The first
literature case report. Int J Surg Case Rep. 57:113–117. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Xiao J, Liu J, Chen M, Liu W and He X:
Diagnosis and prognosis of retroperitoneal liposarcoma: A single
Asian center cohort of 57 Cases. J Oncol. 2021:75940272021.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ferrarese A, Pozzi G, Borghi F, Marano A,
Delbon P, Amato B, Santangelo M, Buccelli C, Niola M, Martino V and
Capasso E: Malfunctions of robotic system in surgery: Role and
responsibility of surgeon in legal point of view. Open Med (Wars).
11:286–291. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Liu H, Hu T, Li Y, Yue Z, Zhang F and Fu
J: Successful intraoperative management in patients with abdominal
compartment syndrome induced by giant liposarcomas: Two case
reports. Medicine. 99:e225752020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Santangelo M, De Rosa P, Spiezia S,
Spinosa G, Grassia S, Zuccaro M and Renda A: Healing of surgical
incision in kidney transplantation: A single transplant center's
experience. Transplant Proc. 38:1044–1046. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Effect of COVID-19 pandemic lockdowns on
planned cancer surgery for 15 tumour types in 61 countries: An
international, prospective, cohort study. Lancet Oncol.
22:1507–1517. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Hunger R, König V, Stillger R and Mantke
R: Impact of the COVID-19 pandemic on delays in surgical procedures
in Germany: A multi-center analysis of an administrative registry
of 176,783 patients. Patient Saf Surg. 16:222022. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Nel D: Strategies for recovery of a
surgical service in the COVID-19 era. S Afr J Surg. 60:154–159.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Mullangi S, Aviki EM, Chen Y, Robson M and
Hershman DL: Factors associated with cancer treatment delay among
patients diagnosed with COVID-19. JAMA Netw Open. 5:e22242962022.
View Article : Google Scholar : PubMed/NCBI
|