Introduction
Anaplastic thyroid cancer (ATC) is a very rare
disease with a remarkably poor prognosis. The Surveillance,
Epidemiology, and End Results database reported a frequency of 0.92
per million patients. The median survival was 3.16 months (1). As an orphan disease, and due to the
exceptionally high grade of malignancy, effective drug therapy has
not been established for ATC (2).
The American Thyroid Association guidelines recommend that genomic
analysis should be performed first after diagnosis and a
therapeutic drug should be the first choice of treatment if
available. In addition, antiangiogenic drugs have the risk of
bleeding in this patient population where disease often invades the
trachea, esophagus, and great vessels. Patients undergoing potent
antiangiogenic drug treatment should be warned about these
potential risks (3). In Japan,
lenvatinib is the only treatment approved by the health insurance
system, and we have reported its efficacy (4,5).
Previous reports revealed lenvatinib treatment results, including
patients previously treated with other agents (6,7).
Another report revealed results with lenvatinib in combination with
other drugs (8). Another report
revealed the results of lenvatinib treatment with the primary tumor
resected and the metastases targeted (9). Genomic analysis is time-consuming, ATC
disease progression is rapid, and death may occur while waiting for
the genomic analysis report. The American Thyroid Association
guidelines recommend that genomic analysis should be performed
first after diagnosis and a therapeutic drug should be the first
choice of treatment if available (3). Thus, this study aimed to report
lenvatinib outcomes as an initial and single-agent treatment in
unresectable ATC of the primary tumor and examine its safety and
efficacy. In addition, we reviewed the pathological findings in two
cases in which conversion surgery was feasible after response to
lenvatinib treatment and tumor shrinkage. Moreover, genomic
analysis results indicated the extent to which a therapeutic drug
for ATC might be found.
Materials and methods
Patients
The study was approved by our Institutional Review
Board (IRB), Kanagawa Cancer Center (Yokohama, Japan) and each
patient signed a comprehensive consent form and a treatment consent
form. This single-institution, retrospective cohort study evaluated
81 patients who were diagnosed with ATC and treated in the Kanagawa
Cancer Center, Japan from April 1, 2011 to July 31, 2022. Eligible
patients were aged >20 years, had at least one measurable target
lesion, and had pathologically confirmed ATC. The pathological
review was performed by three pathologists with experience in
thyroid pathology. This study protocol was reviewed and approved by
our IRB (#2019-34). Of the 81 cases of ATC in our department, four
with possible radical surgery upon initial diagnosis, 12 with
metastases that turned into ATC, and nine with anaplastic
transformation during lenvatinib treatment in differentiated
thyroid cancer (DTC) were excluded from the study. Hence, this
study included 56 patients with unresectable primary ATC. From 2011
to 2015, 12 patients were treated with weekly paclitaxel (PTX), 36
patients were treated with lenvatinib after 2015, and eight
patients, who refused drug treatment, were treated with best
supportive care (BSC). All patients were histopathologically
diagnosed as ATC by biopsy or surgery. This study is a
retrospective cohort study.
Drug treatment
Lenvatinib was started at 24 mg and PTX at 80
mg/m2 weekly (10). The
starting dose of lenvatinib was reduced for patients with diabetes
and/or hypertension, >80 years, weighed <40 kg, with chronic
kidney disease, or were poorly controlled. In addition, doses were
reduced or withdrawn during treatment depending on the patient's
condition. The actual lenvatinib treatment was performed as
reported according to DTC (11).
Renal function was evaluated by estimated glomerular filtration
rate (eGFR), and the treatment was withdrawn when the eGFR was
<30 ml/min/1.73 m2.
Efficacy
Time to treatment failure, progression-free survival
(PFS), and overall survival (OS) in the lenvatinib and PTX groups
were performed by the log-rank test. We evaluated the response rate
according to Response Evaluation Criteria in Solid Tumors (12) version 1.1 (13). A spider plot of the change in
maximum tumor diameter from the start of treatment to the time of
clinical progressive disease was shown in the lenvatinib treatment
group. The time of best response was determined based on these
results. Comprehensive genomic profiling (CGP) was performed on
cases after 2019 when insurance reimbursement became available.
Formalin-fixed paraffin-embedded tissue sections (FFPE specimens)
of biopsy or surgical specimens performed at diagnosis were
submitted to Foundation Medicine Inc. (FMI) for testing and
analyzed for 324 cancer-related genes.
Safety and tolerability
Safety parameters, including adverse events (AE),
hematology and clinical chemistry, urinalysis, vital signs, and
electrocardiograms, were assessed at the baseline and every visit
during the follow-up. AEs were graded from 1 to 5 according to the
Common Terminology Criteria for Adverse Events version 5.0
(http://www.jcog.jp/doctor/tool/ctcaev5.html), and the
maximum value was totaled for each patient.
Statistical analysis
Statistical analyses were performed using the EZR
version 1.37 (https://www.jichi.ac.jp/saitama-sct/SaitamaHP.files/download.html).
The Fisher's exact test was used to analyze nominal variables,
whereas the Kruskal-Wallis test was used to assess continuous
variables. Continuous variables are presented as medians with their
95% confidence intervals (CI), and categorical variables are
presented as numbers with percentages. The Kaplan-Meier method in
conjunction with the log-rank test was used to analyze the OS
curve. Univariate regression analysis, with calculated hazard
ratios and their 95% CI, was used to identify the clinical features
associated with PFS and OS. All P-values were two-sided, and
P<0.05 were considered statistically significant.
Results
Treatment outcomes
Two cases are currently alive, whereas 54 have died.
The mean survival was 4.34 months. Table I compares patient backgrounds in the
lenvatinib, PTX, and BSC treatment groups. No significant
differences were found in age, gender, weight, or TNM staging (AJCC
Cancer Staging Manual 8th Edition, 2017). In addition, no
significant differences were found in the percentage of
radiotherapy intervention, lung metastases, and maximum tumor size.
Survival was significantly longer in the lenvatinib group,
averaging 5.8 months (P=0.004). The PTX and BSC groups survived
1.98 and 1.20 months, respectively.
 | Table I.List of patient background in
lenvatinib, paclitaxel and best supportive care treatment
groups. |
Table I.
List of patient background in
lenvatinib, paclitaxel and best supportive care treatment
groups.
|
| Treatment |
|---|
|
|
|
|---|
| Factor | BSC | Lenvatinib | PTX | P-value |
|---|
| N | 8 | 36 | 12 |
|
| Age, years | 74.75 [62,87] | 72.33 [47,85] | 72.75 [61,86] | 0.812 |
| Sex (%) |
|
|
| 1.000 |
|
Female | 5 (62.5) | 19 (54.3) | 7 (58.3) |
|
| Male | 3 (37.5) | 16 (45.7) | 5 (41.7) |
|
| PS (%) |
|
|
|
|
| 0 | 3 (37.5) | 27 (75.0) | 9 (75.0) | 0.064 |
| 1 | 4 (50.0) | 9 (25.0) | 2 (16.7) |
|
| 2 | 1 (12.5) | 0 (0.0) | 1 (8.3) |
|
| Stage (%) |
|
|
| 0.864 |
| IVB | 1 (12.5) | 7 (19.4) | 1 (8.3) |
|
| IVC | 7 (87.5) | 29 (80.6) | 11 (91.7) |
|
| Lung metastasis
(%) | 7 (87.5) | 29 (80.6) | 11 (91.7) | 0.864 |
| Radiation therapy
(%) | 0 (0.0) | 9 (25.0) | 1 (8.3) | 0.228 |
| Body weight,
kg | 47.00 [40,58] | 56.27 [41,88] | 51.29 [37,70] | 0.0934 |
| Maximum diameter,
mm | 56.79 [37,85] | 48.78 [24,92] | 57.29 [30,92] | 0.177 |
| Overall survival,
months | 1.20 [0.3,2.2] | 5.83
[0.5,28.9] | 1.98 [0.2,4.9] |
0.0000264a |
Table II shows the
results of the 36 lenvatinib-treated patients with the best
response. The mean starting dose and treatment duration was 20.2 mg
and 4.89 months, respectively. Median PFS was 3.5 months (95% CI:
2.3–5.37). In contrast, the PTX group revealed no cases of PR, and
the mean duration of treatment was 1.65 months.
 | Table II.Comparison of lenvatinib and
paclitaxel outcomes. |
Table II.
Comparison of lenvatinib and
paclitaxel outcomes.
| Factor | Lenvatinib | PTX |
|---|
| N | 36 | 12 |
| ORR |
|
|
| Partial
Response (%) | 12 (33.3) | 0 |
| Stable
disease (%) | 19 (52.8) | 6 (50.0) |
|
Progressive disease (%) | 4 (11.1) | 3 (25.0) |
| Not
evaluated (%) | 1 (2.8) | 3 (25.0) |
| Mean starting dose,
mg (SD) | 20.2 (4.7) | -a |
| Mean duration of
treatment, months (SD) | 4.89 (5.0) | 1.65 (1.3) |
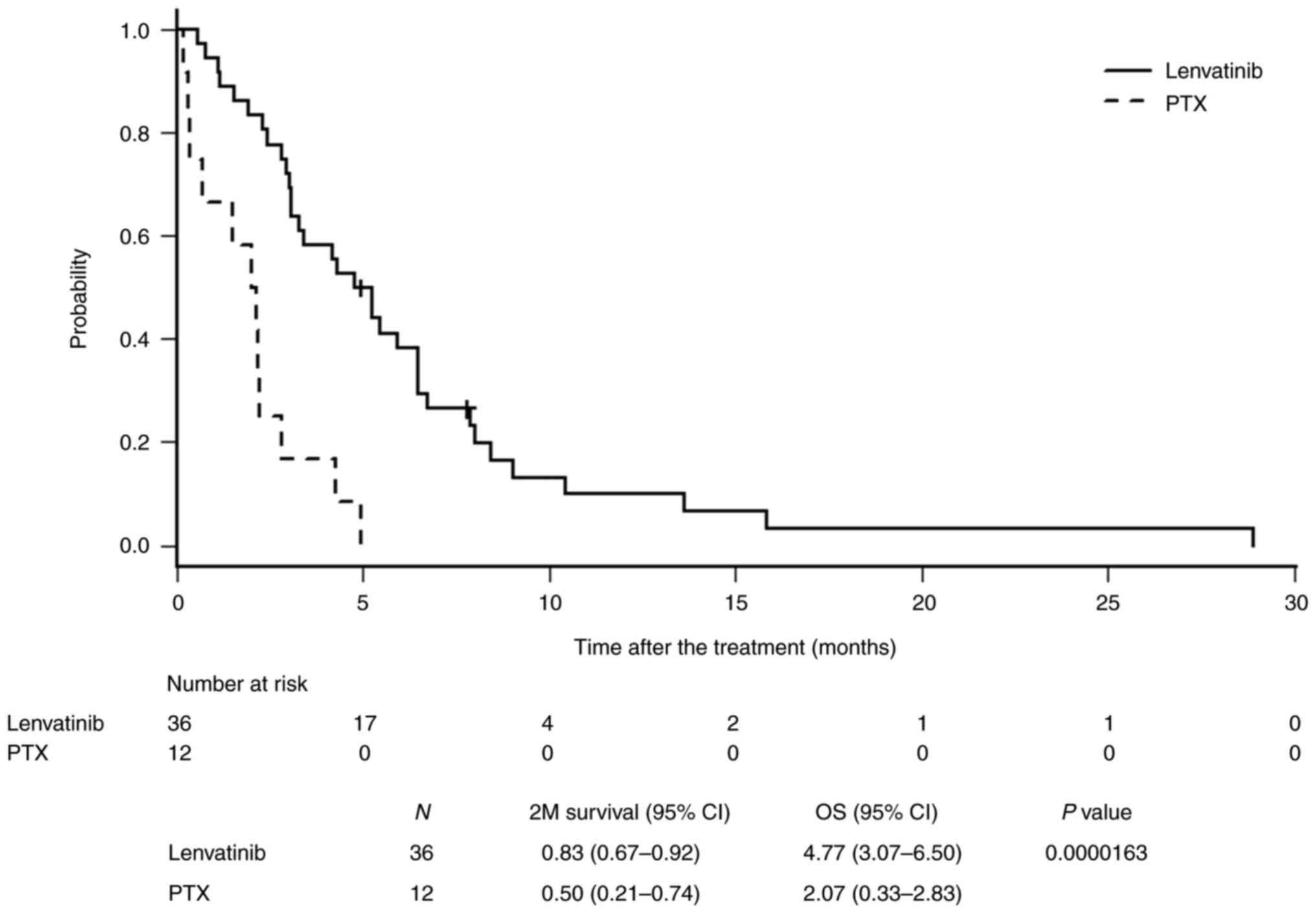
Fig. 1 shows the
comparison of OS between the lenvatinib and PTX groups. The median
OS was 4.77 and 2.07 months in the lenvatinib and PTX groups,
respectively, indicating a significant survival benefit
(P=0.0000163).
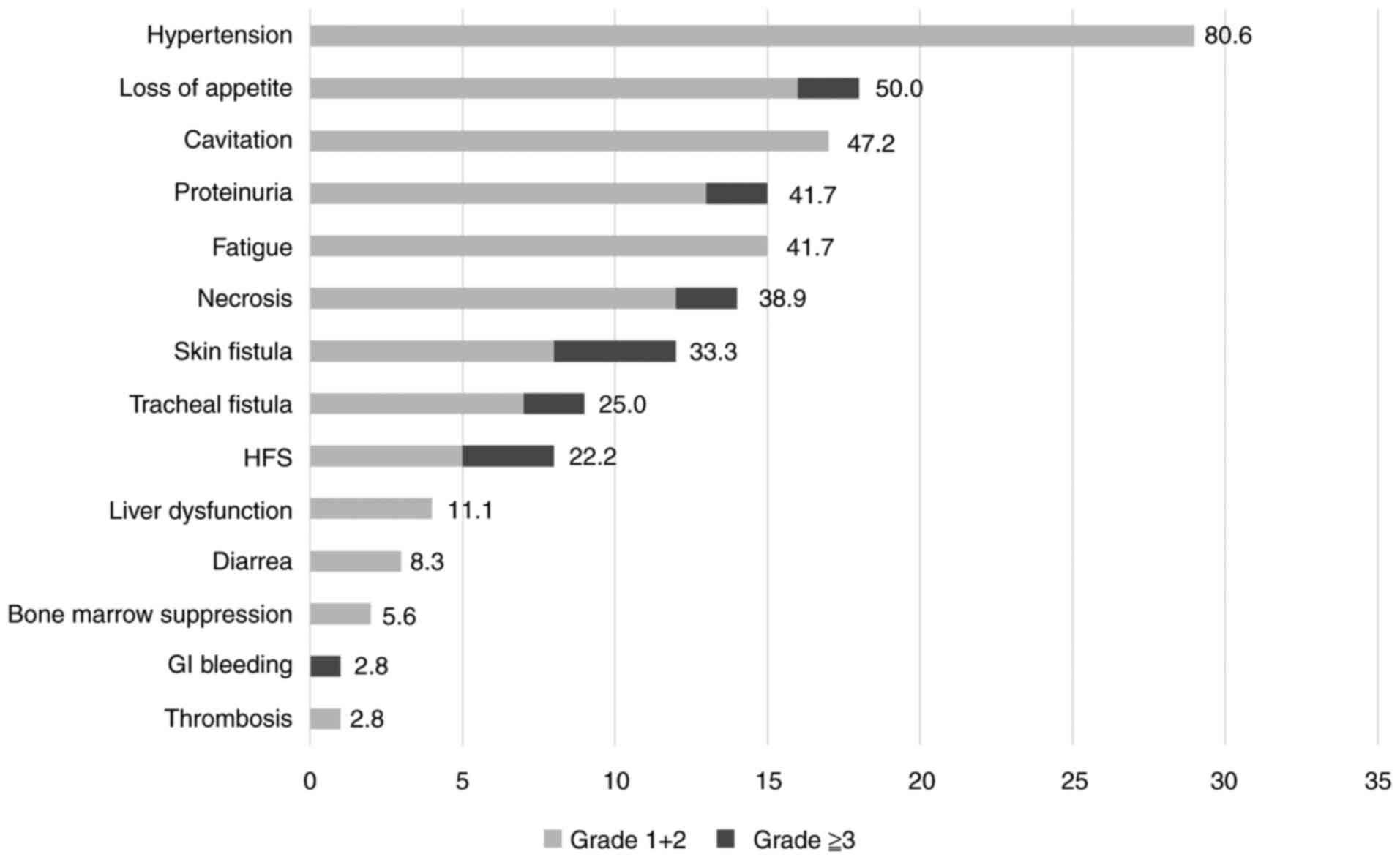
Adverse events of lenvatinib
The average lenvatinib treatment duration was 4.89
months, and Fig. 2 graphically
depicts the AEs that appeared during that time. The most common AE
was hypertension, which occurred in 29 (80.6%) patients, but there
were no Grade 3 or higher AEs that would interfere with continued
treatment. The next most common AEs were loss of appetite in 18
(50.0%), cavitation in 17 (47.2%), proteinuria and fatigue in 15
(41.7%), necrosis in 14 (38.9%), cutaneous fistula in 12 (33.3%),
and tracheal fistula (including pharyngoesophageal fistula) in 9
(25.0%), and hand-foot syndrome in 8 (22.2%) patients. Necrosis was
observed in two patients, who died of hemorrhage from the common
carotid artery. Two patients had Grade 3 loss of appetite and one
had a gastrointestinal hemorrhage, of whom treatment was
discontinued and the patient was treated with BSC.
Progress of the treatment
Surgical tracheotomy was performed upon initial
presentation to avoid asphyxia or for local control in five cases
in the lenvatinib group and in three cases in the other treatment
groups, all of which were resectable and positive for margins.
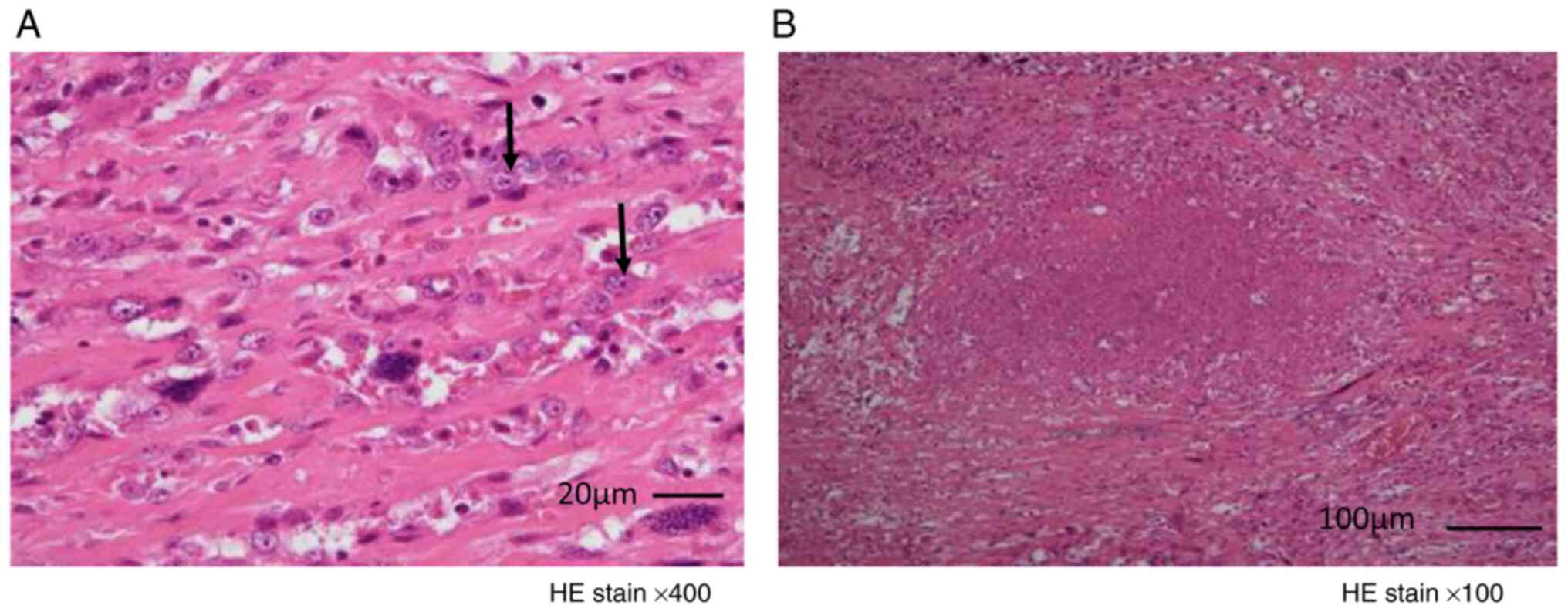
Conversion surgery was possible in two cases, and their
histopathological images are shown in Fig. 3. No residual tumor was observed on
gross examination in both cases; however, histology demonstrated
positive margins. Fibrosis without necrosis and viable tumor cells
were found in the tumor in case A and localized tumor necrosis was
found in case B; however, both specimens showed residual viable
cells. Local tumor necrosis was observed in case B; however, both
specimens showed residual viable cells. Radical surgery was
impossible although lenvatinib treatment reduced the tumor
size.
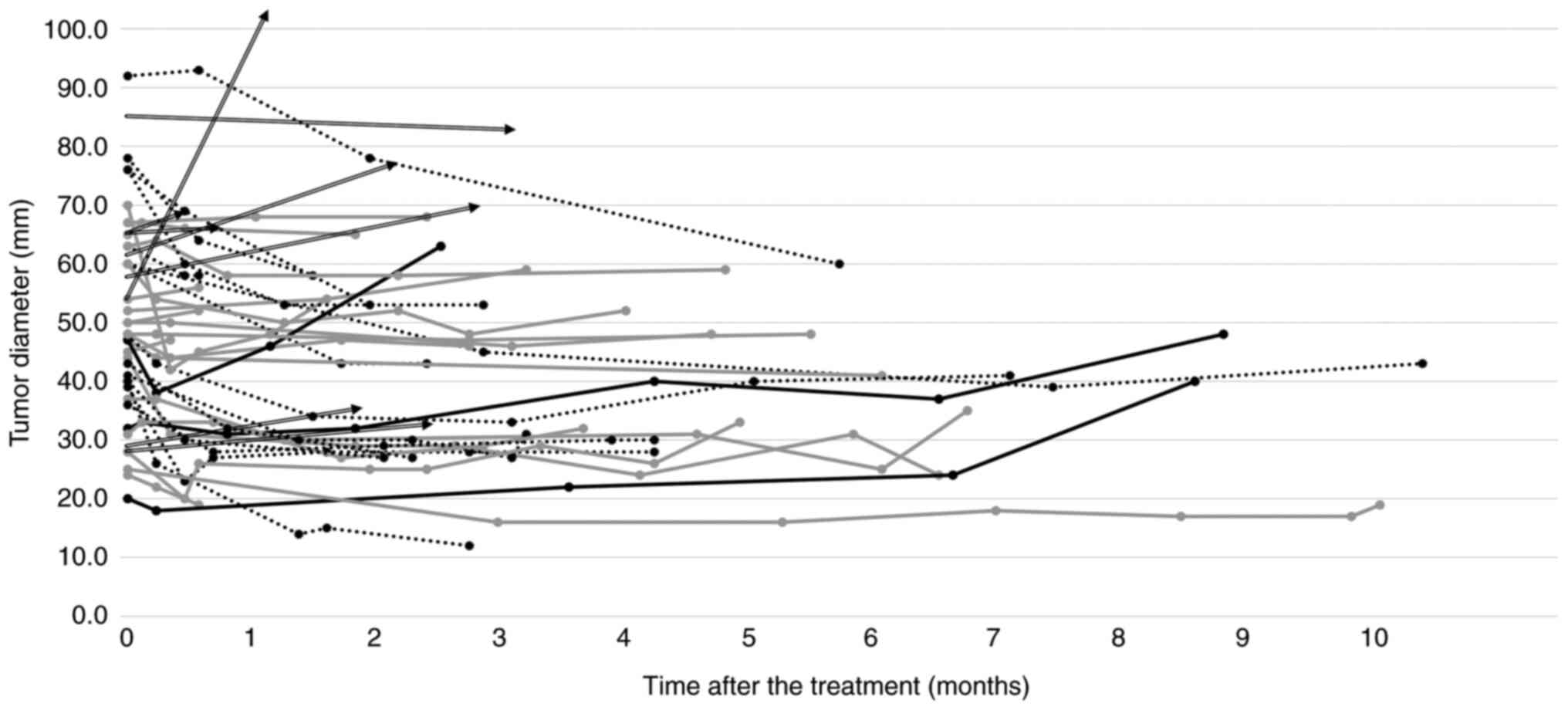
Fig. 4 shows the
evolution of the maximum tumor diameter after lenvatinib treatment.
The plots are shown until the final image evaluation at the end of
treatment. Tumor shrinkage is observed within 1–2 months of
treatment in most cases. Thereafter, treatment is maintained at the
current level and discontinued at 4 months. The longest period of
imaging evaluation is 9 months.
Genomic profiling
CGP results are summarized in Table III for the major cancer-related
gene abnormalities. All genetic abnormalities we observed are
included in Table SI. The CGP
results of 14 patients with ATC showed that 7 (50.0%) had
BRAF mutation, 4 (28.6%) had NRAS mutation, 11
(78.6%) had TERT mutation, and 10 (71.4%) had TP53
mutation. They were the most frequent mutations. These ATC cases
1–7 have BRAF mutations and can be treated with BRAF
inhibitor drugs. Case 8 has RET/PTC fusion and can be
treated with Selpercatinib. Case 3 has a high TMB and can be
treated with Pembrolizumab. These are thought to be of PTC origin,
which is consistent with the coexistence of PTC in the surgical
specimens in cases 1–3 and in biopsy tissue in case 7. Cases 9–12
are more likely to be of FTC origin due to NRAS mutations,
yet cannot be ruled out because PTC also has NRAS mutations,
although less frequently. Cases 9–14 have no available drugs.
 | Table III.Summarized results of genetic
analysis of anaplastic thyroid cancer. |
Table III.
Summarized results of genetic
analysis of anaplastic thyroid cancer.
|
|
Case
no. |
|---|
|
|
|
|---|
| Gene
abnormalitya | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
|---|
| BRAF | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| NRAS | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 |
| RET/PTC
fusion | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| TERT | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 |
| TP53 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| PIK3CA | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
|
CDKN2A/2B | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 |
| FGF | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| TMB
high | 0 | ND | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| PTEN | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Discussion
In the studies conducted by Wirth et al
(6) and Higashiyama et al
(7), the prior usage of anticancer
medications was reported to be 70 and 40.5%, respectively. However,
what distinguishes this current study is that it exclusively
involves treatment-naive participants, all experiencing their
initial disease onset. The median PFS and OS were 2.6 months (95%
CI: 1.4–2.8) and 3.2 months (95% CI: 2.8–8.2), respectively, which
may not be an effective ATC treatment. Lenvatinib treatment
resulted in disappointing survival for patients with unresectable
ATC. None of the results were satisfactory. A report describing the
treatment results of distant metastases in ATC in which the primary
lesion was resected (14) was shown
in relatively good results. The median PFS and OS were 7.4 months
(95% CI: 1.7–12.9) and 10.6 months (95% CI: 3.8–19.8),
respectively, and the objective response rate was 24%. This report
revealed that not all of the distant metastases have been
pathologically proven to be ATC. ATCs, in which at least the
primary tumor can be resected, have a better prognosis than
unresectable cases. Another real-world report (9) included patients with previously
treated cases or target lesions that include distant metastases.
The number of patients is small, but this is the only study that
investigated the effect of initial lenvatinib treatment,
pathologically proven, unresectable ATC only. Median PFS and OS
were 3.5 and 4.7 months, respectively. The result was slightly
better than (6). This may be
because patients were initially treated. However, CR with
lenvatinib alone is not possible considering the pathology of the
two cases of conversion surgery, and external irradiation or other
drug therapy must be sequentially added postoperatively. ATC
requires additional treatment, unlike the previously reported
conversion surgery for DTC, because residual lesions can quickly
enlarge and affect surrounding organs. This is not surprising given
the much lower frequency of CR in studies of lenvatinib for
RAI-refractory DTC (15).
The frequency of AEs themselves is less than
reported for DTC because of the short administration duration in
terms of safety (11). Grade 3 or
higher fistulas (cutaneous and tracheal fistulas) were observed in
three patients, and necrosis was observed in two patients, who died
of hemorrhage. In addition, two cases had Grade 3 loss of appetite
and one case had gastrointestinal bleeding. Eight of these patients
(21.1%) could not continue lenvatinib treatment due to serious AEs.
In contrast, 30 (78.9%) patients were able to continue treatment
until they recognized progressive disease. The most clinically
serious AEs of lenvatinib are fistula formation, rapid necrosis,
and hemorrhage, as previously reported (4,5). Two
deaths due to hemorrhage were caused by fistula formation and
aseptic abscess from necrosis, and local washing and continuous
lenvatinib treatment in the hope that the tumor would shrink.
Cutaneous fistulas are often fistulized at the site of needle
biopsy. Lenvatinib treatment can be continued if the fistula is
localized to the superficial skin, but lenvatinib should be
immediately discontinued if there is a risk of tracheal or
esophageal fistula and bleeding. Thus, we believe that safety can
be assured by carefully monitoring local findings once lenvatinib
therapy is initiated and preventing serious fistulas or bleeding
events.
There are more numerous reports of CGP results
(16), but our results revealed a
high number of BRAF mutations, which are PTC-derived ATCs,
indicating many PTC-derived ATCs, and FTC-derived ATCs inheriting
RAS mutations and de novo ATC, all of which are
consistent with previous reports. In addition, the high prevalence
of TP53 and TERT abnormalities as comorbid genetic
abnormalities is consistent with previous reports (17). Results of drugs targeting the driver
gene have been reported (18,19).
Another study reported the combination with immune checkpoint
inhibitors (8). These drugs could
not be used in Japan because it was not reimbursed by health
insurance. Therefore, this was a single-agent study of lenvatinib,
but it is significant as real-world data. The median PFS was 3.7
months, indicating an effective treatment to halt disease
progression for a little more than a month before the CGP results
are known. Patients with BRAF mutations should be treated
with BRAF inhibitors. Some reports are in combination with
an immune checkpoint inhibitor. A study of 36 ATCs, including
distant metastases, reported good results with median PFS and OS of
6.7 and 14.5 months, respectively (20). Drugs for RAS mutations may be
applied or drugs targeting other driver genes may be developed in
the future.
Lenvatinib treatment became available in 2015 and
CGP testing became available in 2019, so there is a bias in which
possible treatments and tests are selected depending on the timing
of availability, and the limitation of this study is that it is not
a random trial. Before 2015 in Japan, paclitaxel was the only
approved treatment for malignant thyroid tumors. However, since
2015, Lenvatinib has been specifically approved for treating
anaplastic thyroid cancer. As a result, the choice of treatment
options in this clinical research was constrained by considerations
of insurance approval. This limitation has, in turn, potentially
narrowed the scope of the study.
We evaluated the efficacy of lenvatinib in 36
patients with ATC with primary target organs. The response rate was
33%, and the median OS was 4.77 months. A safety review indicated
that lenvatinib should be used under the careful observation of
local findings. Two patients, who showed a reduction with
lenvatinib, underwent conversion surgery, which prolonged the
prognosis in terms of avoiding AEs, such as asphyxia, fistula, and
hemorrhage due to tumor growth. However, the resection specimens
were positive for margins, and CR was not possible even if the
reduction was observed. At present, a treatment strategy of
obtaining CGP results while the initial drug lenvatinib remained
effective and lead to effective drug therapy is appropriate.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Dr Tomoyuki Yokose
and Dr Youichiro Okubo (Department of Pathology, Kanagawa Cancer
Center, Yokohama, Japan), and Dr Hiroyuki Hayashi (Department of
Pathology, Yokohama City Hospital, Yokohama, Japan) for reviewing
the pathological diagnosis.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HI and ST designed the study. AT analyzed the data.
HI, ST, AT and KM contributed by performing the surgery and caring
for the patients. ST and KM confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The chemotherapy committee of Kanagawa Cancer Center
approved this regimen of lenvatinib for use in patients with ATC.
The cancer board of the hospital also approved lenvatinib
treatment, including surgery, for patients with ATC. Written
informed consent was obtained from all participants. All
experimental protocols were approved by the Institutional Review
Board of Kanagawa Cancer Center (approval no. #2019-34). We
confirmed that all methods were conducted in accordance with
relevant guidelines and regulations.
Patient consent for publication
All patients provided written informed consent prior
to their treatment. This form indicated that their personal data
could be utilized for academic or paper presentations, with the
assurance of maintaining absolute anonymity.
Authors' information
HI is an endocrine surgeon working at the Kanagawa
Cancer Center and has extensive experience in several surgeries for
ATC, as well as ATC treatment.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ATC
|
anaplastic thyroid cancer
|
|
IRB
|
Institutional Review Board
|
|
DTC
|
differentiated thyroid cancer
|
|
BSC
|
best supportive care
|
|
PTX
|
paclitaxel
|
|
eGFR
|
estimated glomerular filtration
rate
|
|
PFS
|
progression-free survival
|
|
OS
|
overall survival
|
|
AE
|
adverse events
|
|
CI
|
confidence intervals
|
|
CGP
|
comprehensive genomic profiling
|
|
FFPE
|
formalin-fixed paraffin-embedded
|
|
FMI
|
Foundation Medicine Inc.
|
|
TNM
|
Tumor, Node, Metastasis (a cancer
staging system)
|
|
AJCC
|
American Joint Committee on Cancer
|
|
PR
|
partial response
|
|
TMB
|
tumor mutational burden
|
|
PTC
|
papillary thyroid cancer
|
|
FTC
|
follicular thyroid cancer
|
|
RAI
|
radioactive iodine
|
|
EZR
|
Easy R (statistical software)
|
References
|
1
|
Lin B, Ma H, Ma M, Zhang Z, Sun Z, Hsieh
IY, Okenwa O, Guan H, Li J and Lv W: The incidence and survival
analysis for anaplastic thyroid cancer: A SEER database analysis.
Am J Transl Res. 11:5888–5896. 2019.PubMed/NCBI
|
|
2
|
Xia Q, Wang W, Xu J, Chen X, Zhong Z and
Sun C: Evidence from an updated meta-analysis of the prognostic
impacts of postoperative radiotherapy and chemotherapy in patients
with anaplastic thyroid carcinoma. Onco Targets Ther. 11:2251–2257.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bible KC, Kebebew E, Brierley J, Brito JP,
Cabanillas ME, Clark TJ Jr, Di Cristofano A, Foote R, Giordano T,
Kasperbauer J, et al: 2021 American thyroid association guidelines
for management of patients with anaplastic thyroid cancer. Thyroid.
31:337–386. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Iwasaki H, Yamazaki H, Takasaki H,
Suganuma N, Nakayama H, Toda S and Masudo K: Lenvatinib as a novel
treatment for anaplastic thyroid cancer: A retrospective study.
Oncol Lett. 16:7271–7277. 2018.PubMed/NCBI
|
|
5
|
Iwasaki H, Toda S, Suganuma N, Murayama D,
Nakayama H and Masudo K: Lenvatinib vs palliative therapy for stage
IVC anaplastic thyroid cancer. Mol Clin Oncol. 12:138–143.
2020.PubMed/NCBI
|
|
6
|
Wirth LJ, Brose MS, Sherman EJ, Licitra L,
Schlumberger M, Sherman SI, Bible KC, Robinson B, Rodien P, Godbert
Y, et al: Open-label, single-arm, multicenter, phase II trial of
lenvatinib for the treatment of patients with anaplastic thyroid
cancer. J Clin Oncol. 39:2359–2366. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Higashiyama T, Sugino K, Hara H, Ito KI,
Nakashima N, Onoda N, Tori M, Katoh H, Kiyota N, Ota I, et al:
Phase II study of the efficacy and safety of lenvatinib for
anaplastic thyroid cancer (HOPE). Eur J Cancer. 173:210–218. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dierks C, Seufert J, Aumann K, Ruf J,
Klein C, Kiefer S, Rassner M, Boerries M, Zielke A, la Rosee P, et
al: Combination of lenvatinib and pembrolizumab is an effective
treatment option for anaplastic and poorly differentiated thyroid
carcinoma. Thyroid. 31:1076–1085. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim M, Ahn J, Song DE, Yoon JH, Kang HC,
Lim DJ, Kim WG, Kim TY, Kim WB, Shong YK, et al: Real-world
experience of lenvatinib in patients with advanced anaplastic
thyroid cancer. Endocrine. 71:427–433. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Onoda N, Sugino K, Higashiyama T, Kammori
M, Toda K, Ito K, Yoshida A, Suganuma N, Nakashima N, Suzuki S, et
al: The safety and efficacy of weekly paclitaxel administration for
anaplastic thyroid cancer patients: A nationwide prospective study.
Thyroid. 26:1293–1299. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wirth LJ, Durante C, Topliss DJ, Winquist
E, Robenshtok E, Iwasaki H, Luster M, Elisei R, Leboulleux S and
Tahara M: Lenvatinib for the treatment of radioiodine-refractory
differentiated thyroid cancer: Treatment optimization for maximum
clinical benefit. Oncologist. 27:565–572. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bogaerts J, Ford R, Sargent D, Schwartz
LH, Rubinstein L, Lacombe D, Eisenhauer E, Verweij J and Therasse
P; RECIST Working Party, : Individual patient data analysis to
assess modifications to the RECIST criteria. Eur J Cancer.
45:248–260. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tahara M, Kiyota N, Yamazaki T, Chayahara
N, Nakano K, Inagaki L, Toda K, Enokida T, Minami H, Imamura Y, et
al: Lenvatinib for anaplastic thyroid cancer. Front Oncol.
7:252017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rendl G, Sipos B, Becherer A, Sorko S,
Trummer C, Raderer M, Hitzl W, Ardelt M, Gallowitsch HJ and Pirich
C: Real-world data for lenvatinib in radioiodine-refractory
differentiated thyroid cancer (relevant): A retrospective
multicentric analysis of clinical practice in Austria. Int J
Endocrinol. 2020:88341482020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu B, Fuchs T, Dogan S, Landa I, Katabi N,
Fagin JA, Tuttle RM, Sherman E, Gill AJ and Ghossein R: Dissecting
anaplastic thyroid carcinoma: A comprehensive clinical, histologic,
immunophenotypic, and molecular study of 360 cases. Thyroid.
30:1505–1517. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pozdeyev N, Gay LM, Sokol ES, Hartmaier R,
Deaver KE, Davis S, French JD, Borre PV, LaBarbera DV, Tan AC, et
al: Genetic analysis of 779 advanced differentiated and anaplastic
thyroid cancers. Clin Cancer Res. 24:3059–3068. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Subbiah V, Kreitman RJ, Wainberg ZA, Cho
JY, Schellens JHM, Soria JC, Wen PY, Zielinski C, Cabanillas ME,
Urbanowitz G, et al: Dabrafenib and trametinib treatment in
patients with locally advanced or metastatic BRAF V600-mutant
anaplastic thyroid cancer. J Clin Oncol. 36:7–13. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chintakuntlawar AV, Yin J, Foote RL,
Kasperbauer JL, Rivera M, Asmus E, Garces NI, Janus JR, Liu M, Ma
DJ, et al: A phase 2 study of pembrolizumab combined with
chemoradiotherapy as initial treatment for anaplastic thyroid
cancer. Thyroid. 29:1615–1622. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Subbiah V, Kreitman RJ, Wainberg ZA, Cho
JY, Schellens JHM, Soria JC, Wen PY, Zielinski CC, Cabanillas ME,
Boran A, et al: Dabrafenib plus trametinib in patients with BRAF
V600E-mutant anaplastic thyroid cancer: Updated analysis from the
phase II ROAR basket study. Ann Oncol. 33:406–415. 2022. View Article : Google Scholar : PubMed/NCBI
|