Introduction
Malignant melanoma is a tumor derived from
melanocytes that is normally located in the basal layer of the
epidermis and dermis of the skin where DNA is protected from
ultraviolet light-induced damage. Malignant melanoma accounted for
~3% of all tumors in the United States in 1998 (1) and may be caused by the malignant
transformation of dysplastic nevi, junctional nevi and benign
melanocytic nevi (2,3). Based on their location, malignant
melanomas are generally classified into cutaneous malignant
melanomas (skin and acra; 90%), mucosal malignant melanomas (mucosa
of head, neck, gastrointestinal, conjunctiva and genital areas;
8–15%), uveal malignant melanomas (uvea and ciliary body; 3–5%) and
central nervous system (CNS) malignant melanomas (dopaminergic
neurons in the substantia nigra and locus coeruleus; 1%) (4–6).
Although CNS melanomas are rare, primary malignant melanomas in the
spinal canal are even more rare, with only a few cases reported
thus far (7,8). The majority of previous studies have
reported the diagnosis, radiographic features and gross total
resection of primary malignant melanomas originating in the spinal
canal, however, the prognosis and ideal treatment of patients with
residual tumors remain elusive and the efficacy of postoperative
radiotherapy or chemotherapy is controversial (7,8). The
present study reported an unusual case of primary malignant
melanoma originating from the thoracic spinal canal in a patient
without a history of irradiation exposure. Furthermore, this
patient had an incompletely resected tumor. To our knowledge, the
patient described in this report appears to represent the first
case of primary malignant melanoma originating from the spinal
canal with a residual tumor and a good prognosis.
Case report
A 21-year-old female attended First Affiliated
Hospital of Kunming Medical University in Kunming, China on June
2020 for gradually aggravated lower back pain for 3 months and
progressive numbness in both lower limbs over 2 weeks. These
symptoms were associated with activity and abated after rest. There
was no significant past medical history, no history of tumors or
any previous radiation exposure and no positive family history of
tumors. Neurological examination indicated that the pain and
numbness were accompanied by a muscle strength of grade 4 in the
right lower limb and grade 4 in the left lower limb. A general
examination of the patient, including physical and radiological
examinations, did not find any subcutaneous nodules or skin
lesions. In addition, no mental status, motor or sensory
impairments were detected in the patient.
The following descriptions of the imaging
examinations were based on reports by 2 or 3 independent
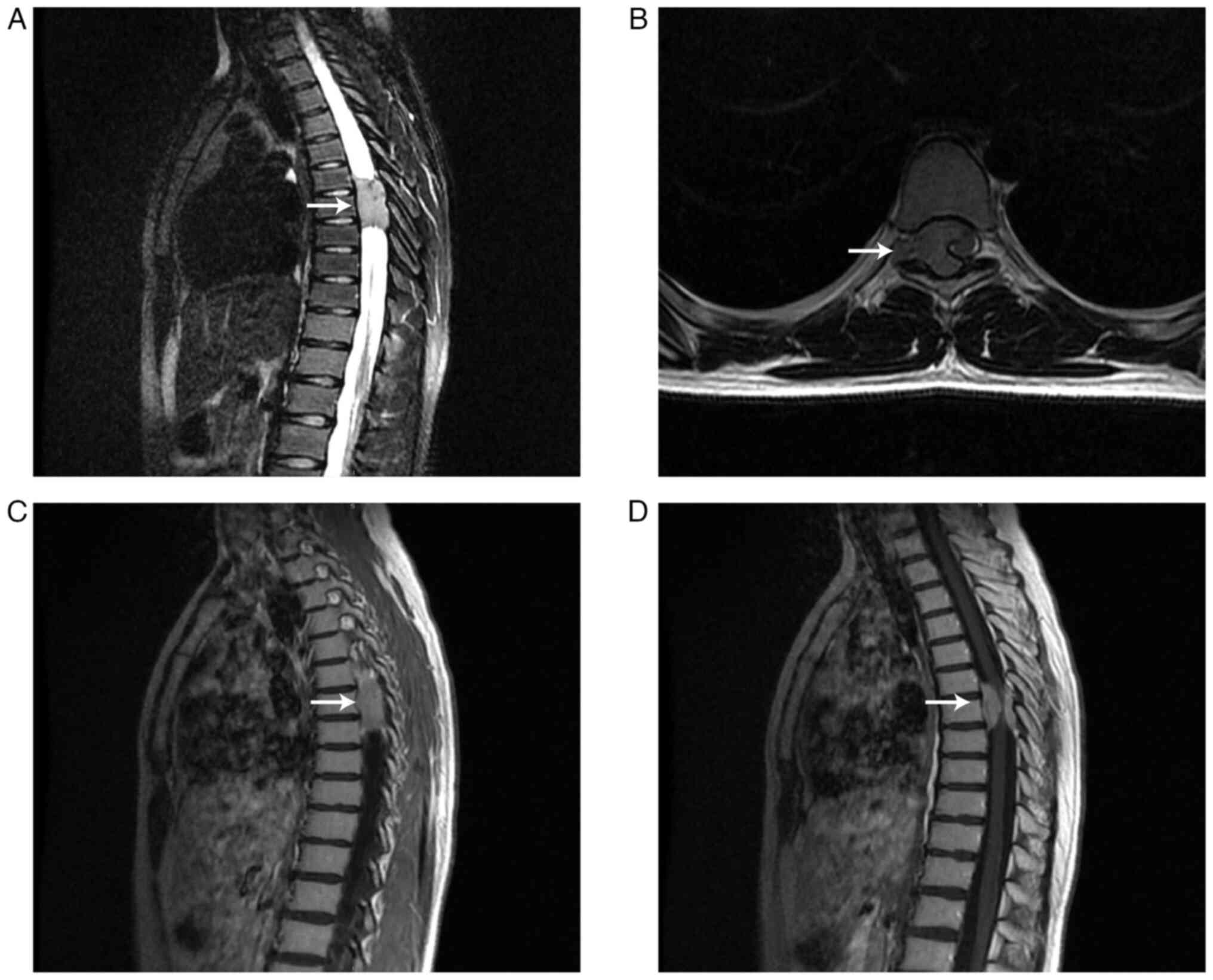
radiologists. Magnetic resonance imaging (MRI) examination
demonstrated an oval mass (~1.7×2.1×4.0 cm3; front-back
× left-right × up-down) in the extraspinal subdural space and the
right side of the intervertebral foramen in the T6-8 spinal canal.
The mass exhibited high signal intensity on T2-weighted images and
isointensity on T1-weighted images. The signal intensity
demonstrated uneven enhancement in the lesion after contrast
enhancement. At the same level, spinal stenosis and intervertebral
foramen enlargement were observed and the spinal cord appeared
significantly compressed and displaced to the left side (Fig. 1). An MRI diagnosis of neurogenic
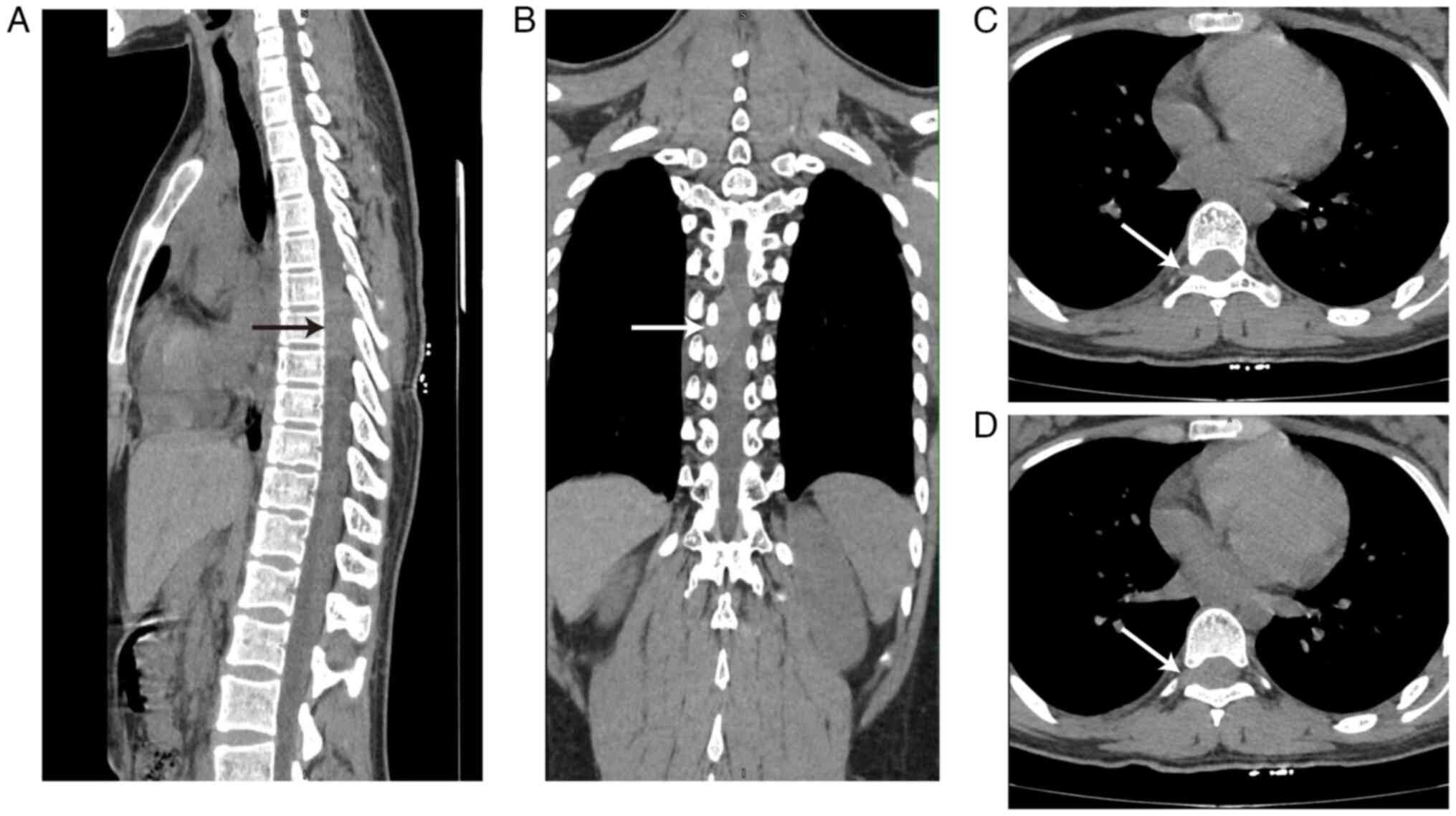
tumor was considered. Non-contrast CT imaging demonstrated a mass
~1.5×2.3×3.4 cm3 in the T6-8 plane with intervertebral
foramen enlargement and the spinal cord appeared significantly
compressed and displaced to the left side (Fig. 2). Neurogenic tumors were considered
based on CT examination. No obvious abnormalities were demonstrated
upon hematological examination of the patient.
Due to the difficulty of intraspinal diagnostic
biopsy and the possibility of tumor spread and metastasis, surgery
to remove as much of the lesion as possible was considered the best
option for the patient. The surgery was performed by orthopedic
surgeons and neurosurgeons and the description of the operation in
the present report was based on the operation records written by
the surgeons. After imaging and hematology tests, the patient
underwent thoracolumbar posterior focal lesion exploration and
excisional biopsy, laminectomy and orthopedic internal fixation
under general anesthesia. The tumor was located in both the
epidural and subdural regions and appeared red and black because of
the plentiful supply of blood. In addition, the tumor demonstrated
aggressive and invasive growth and indistinct borders from the
surrounding tissues. After piecemeal resection of the epidural
tumor tissue, the subdural object was excised following cutting of
the retrodural space of Okada and release of the cerebrospinal
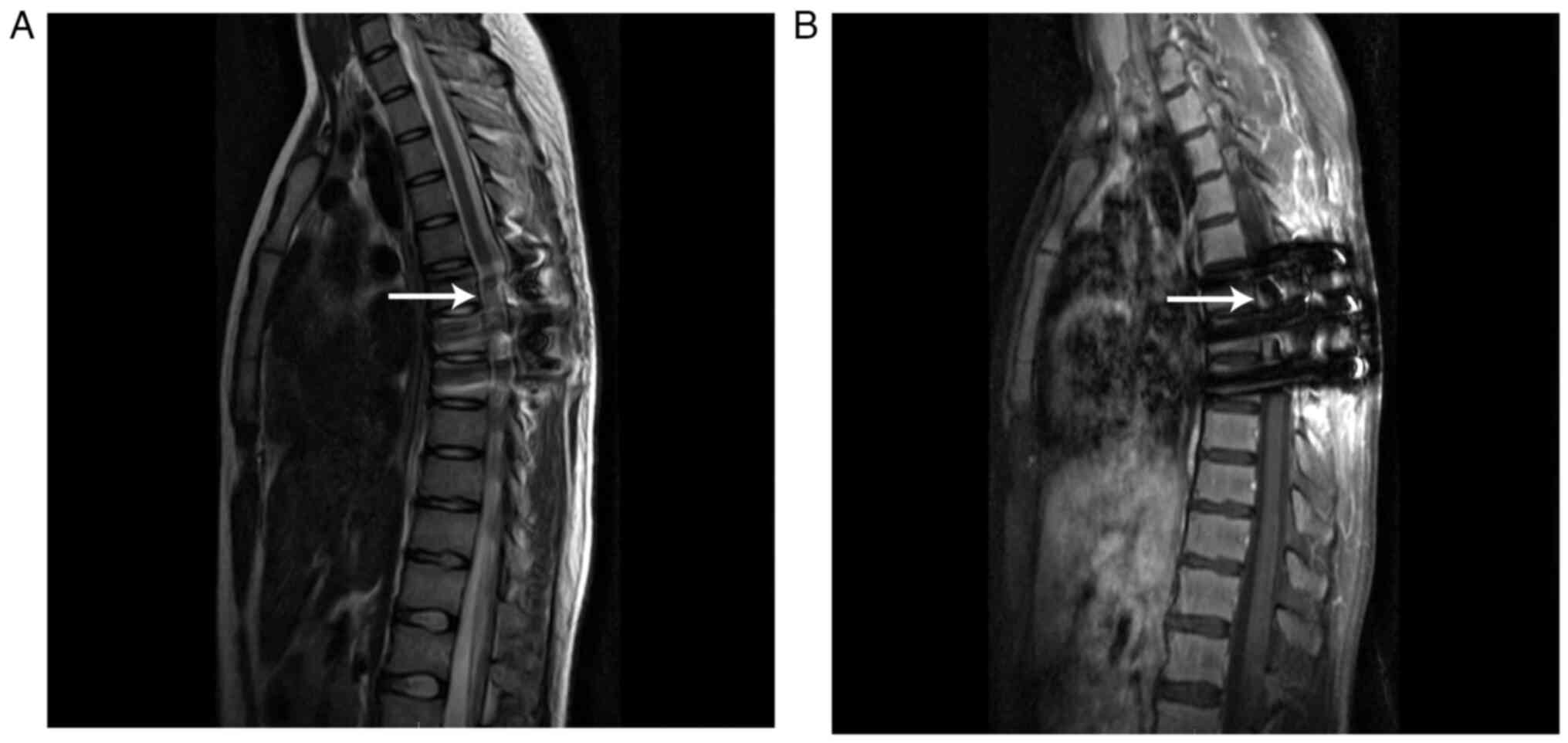
fluid (CSF). Postoperative MRI demonstrated a poorly defined,
residual T2 hyperintense mass and a T1 isointense or hypointense
mass in the T6-8 spinal canal, and the signal intensity
demonstrated obvious enhancement in the lesion after contrast
enhancement (Fig. 3).
Histology is critical for the differential diagnosis
of atypical primary malignant melanoma and neurogenic tumors
originating in the spinal canal. The descriptions of the
pathological findings were based on the pathological records
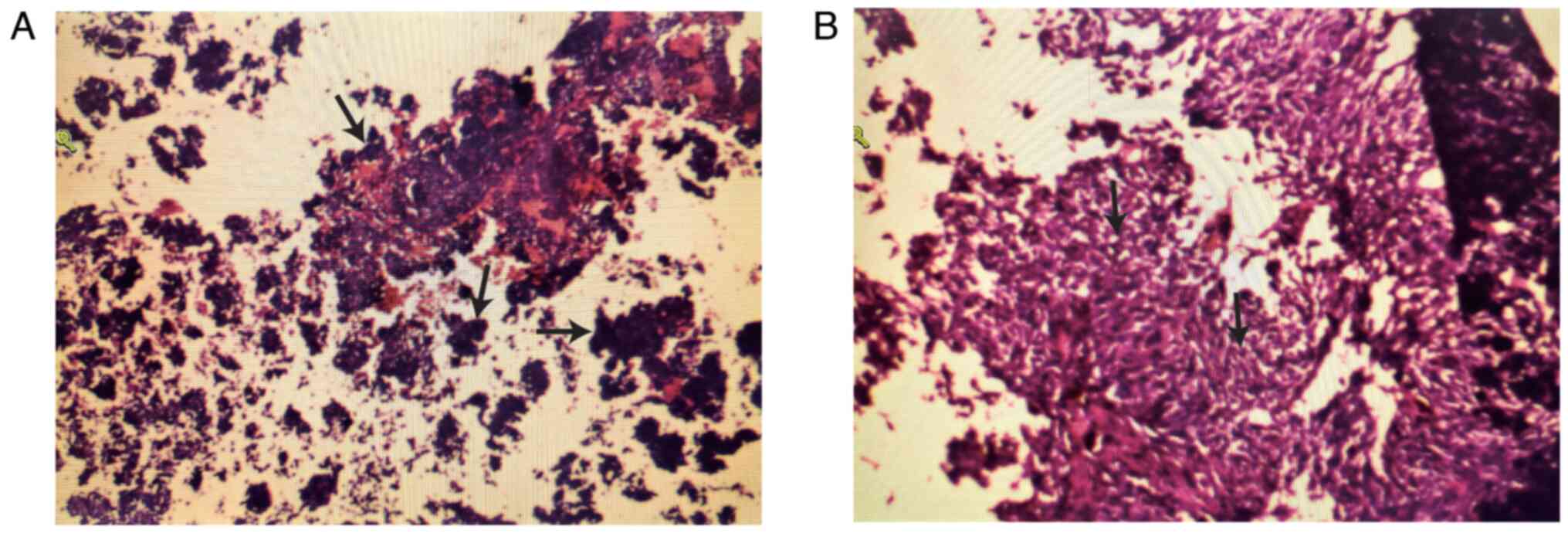
written by 2 independent pathologists. Intraoperatively, biopsies
of the lesion from the T6-8 spinal canal were taken for frozen and
permanent pathology samples. Histopathological examination after
hematoxylin and eosin staining according to standard procedures
(9) of the surgical specimen
demonstrated spindle cell features and melanin pigmentation
(Fig. 4). Immunohistochemistry
staining according to standard procedures (9) demonstrated that the positive rate of
the Ki-67 (marker of proliferation) index was >5%, and
positivity for Vimentin, S-100 (S100 calcium binding protein A1),
HMB45 (premelanosome protein), MelanA and SOX-10 was observed.
However, staining for P53, CK (cytokeratin), EMA (mucin 1), PR
(progesterone receptor), MBP (myelin basic protein), CD34, GFAP
(glial fibrillary acidic protein) and CD57 were negative (data not
shown) (the above antibodies were purchased from Beijing Zhongshan
Jinqiao Biotechnology Co., LTD.). Thus, the histological diagnosis
was primary malignant melanoma of the T6-8 spinal canal. Currently,
there is no test available for tumor-infiltrating lymphocytes
(TILs).
The surgeons and physicians agreed that the patient
had residual tumor and was at high risk for tumor dissemination
along the CSF; therefore, postoperative treatment was necessary.
Concurrent chemoradiotherapy was performed for four weeks
post-surgery with a dose of 40 Gy in 20 fractions at the T5-9
spinal canal level using volumetric modulated arc therapy plus
continuous daily temozolomide [75 mg/m2 of body surface
area (BSA)/day for 42 days]. Subsequently, the patient received
five cycles of adjuvant temozolomide (150–200 mg/m2 BSA
for five days during each 28-day cycle of treatment) plus
bevacizumab every three weeks (7.5 mg/kg of body weight) for six
cycles and then entered the follow-up phase of treatment.
Throughout chemotherapy plus bevacizumab, the patient experienced
mild gastrointestinal events and no grade 3–4 adverse events were
reported.
Follow-up was performed every three months in the
first two years following completion of adjuvant chemotherapy. At
each follow-up, the patient underwent clinical examination, quality
of life test, hematological and imaging examination including tumor
markers, chest CT, and head and pyramidal MRI. A whole-body bone
scan was performed every six months. To date, the patient has
demonstrated no evidence of progression or tumor recurrence for
>2.5 years, has reported having no issues and has been studying
and working normally. Further follow-up will continue.
Discussion
All types of cancer have the potential to invade the
CNS as primary or metastatic tumors (2,3).
Clinically, the most common type of cancer to invade the CNS is
malignant melanoma metastatic to the CNS, whilst primary malignant
melanoma of the CNS is rare (10).
Primary melanoma of the CNS originates from aberrant changes in
melanocytes of the neural crest or melanocytic elements of the pia
mater during early embryonic development (7,11,12).
Primary spinal malignant melanomas are also rare, with only several
cases reported to date (7,8).
Primary melanoma of the CNS is diagnosed using the
criteria devised by Hayward (13)
as follows: No malignant melanoma outside the CNS, no presence of
the lesion in any other region of the CNS and histological
confirmation of the melanoma (8).
Regarding the symptoms of primary spinal malignant melanoma,
patients often present with intracranial hypertension and focal
neurological deficits due to spinal blocking; thus, the diagnosis
of spinal malignant melanomas is difficult and requires imaging
examinations, pathological examination and exclusion of other CNS
diseases and systemic melanoma (14,15). A
diagnosis of cutaneous melanoma should also be ruled out, as
cutaneous melanoma have a higher incidence rate combined with a
high propensity to metastasize to the CNS, with CNS involvement
reported in ~10% of patients with cutaneous melanoma in previous
clinical studies (16,17). In addition to the skin, primary
melanomas may originate from less visible locations, such as nail
beds, mucosal surfaces of the genitals, sinonasal cavity and
gastrointestinal tract and the uveal tract (4–6).
Therefore, before concluding that spinal melanoma is a primary
tumor, a thorough clinical examination, as well as histological
revision of previously removed melanocytic tumors, should be
performed.
Primary spinal melanoma should also be
differentiated from schwannoma. Schwannoma frequently occurs in
association with cranial nerves, spinal nerve roots and the
paraspinal sympathetic chain, thereby showing overlap with primary
spinal melanoma (18,19). Melanotic schwannoma is a rare type
of schwannoma that is composed of melanin-producing cells and has
the characteristics of Schwann cells under electron microscopy,
intracytoplasmic melanosomes and is reactive for melanoma markers
(20). Primary spinal melanoma and
melanotic schwannomas are neural crest-derived and are often
positive for S100 and other melanocytic markers, such as MelanA and
HMB-45 (18,19). Thus, both tumor types have
histological overlap and histopathologic discrimination between
primary spinal melanoma and melanotic schwannoma in individual
patients is often difficult; therefore, immunohistochemistry may
not be useful. However, the psammomatous body and the autosomal
dominantly inherited Carney complex are associated with melanotic
schwannoma and are useful for differentiating between the two tumor
types (21,22). Carney complex is a rare multiple
neoplasia syndrome characterized by multiple types of skin tumors,
cardiac myxomas, pigmented lesions and endocrine tumors, including
pigmented nodular adrenocortical disease (23). Melanin schwannomas are highly
specific to Carney syndrome, rarely occur in patients who do not
have Carney syndrome and usually involve peripheral nerves
(21–23). In the present case study, imaging,
pathological examination and exclusion of melanotic schwannoma and
systemic melanoma were performed prior to patient diagnosis.
CNS melanomas have an unpredictable clinical course
and total resection is associated with improved prognosis (5). In a previous review of 67 cases of
solitary primary intracranial melanomas with reported therapy, the
mean survival after gross total resection was 19.58 months compared
with 9.30 months after biopsy and subtotal resection and 3.40
months in patients who did not receive surgery (20,24–26).
Therefore, total resection may be associated with longer survival
than no or partial resection. However, the efficacy of
postoperative radiotherapy or chemotherapy is controversial.
Previous studies recommend postoperative radiotherapy in all cases
and long-term survival has been reported with combined surgery and
radiotherapy (10,24,27).
Radiotherapy is also reported to have potential as a primary
therapy in disseminated CNS melanomas or as a postoperative
prophylactic adjuvant to prevent progression or recurrence
(5). Chemotherapy has been used for
primary CNS melanomas with variable response rates (5). Puyana et al (5) reported that gross total resection plus
radiotherapy with or without chemotherapy significantly improved
patient survival compared with gross total resection plus
chemotherapy. Currently approved chemotherapeutics for malignant
melanoma include dacarbazine, temozolomide and fotemustine;
however, these drugs have demonstrated low efficacy as single
therapeutic agents (26,28,29).
Thus, chemotherapy may be paired with gross total resection and
radiation, which may increase patient survival.
Biotherapeutic options are a promising, more
targeted alternative to chemotherapy. These agents commonly include
BRAF and MAPK kinase inhibitors that target the MAPK pathway, such
as dabrafenib and vemurafenib (5,26,30). A
previous study on the use of immunotherapy, such as programmed
death-1 inhibitors and a cytotoxic T lymphocyte antigen-4
inhibitor, reported improved outcomes in patients with advanced
melanoma (31). It has previously
been established that melanoma growth and progression depend on
angiogenesis (32). Antiangiogenic
therapy targeting the VEGF-A pathway has been reported as a
possible strategy to prevent melanoma relapse and spread (33). Adjuvant treatment with the
anti-VEGF-A antibody bevacizumab after resection of high-risk
melanoma improved the disease-free interval but not overall
survival in a large multicenter randomized phase 3 trial in
patients with melanoma with high a risk of recurrence (34,35).
Radiotherapy or chemotherapy combined with
bevacizumab has achieved promising results in the treatment of
melanomas, particularly CNS tumors, because in addition to
conferring antitumor effects, bevacizumab reduces peritumor edema
and thus reduces the need for steroids (36–40).
These findings suggest that bevacizumab may be warranted as an
adjuvant treatment to minimize the risk of nerve function deficits.
In addition, bevacizumab and temozolomide have been included in the
Chinese Society of Clinical Oncology (41) and National Comprehensive Cancer
Network (NCCN) (30) guidelines for
the second- or third-line therapy of advanced melanoma.
In summary, primary malignant melanomas in the
spinal canal are rare tumors with a high potential for recurrence
and a high mortality rate if residual tumor is present; thus, total
resection is essential for patients. However, depending on the
tumor location, complete resection may be difficult, highlighting
the need for more efficacious systemic treatment regimens and
potential integration of newer biological agents. To our knowledge,
the present study reported the first case of a primary malignant
melanoma originating from the thoracic spinal cord with a residual
tumor that responded well to a combination of chemoradiotherapy and
bevacizumab. Thus, the present study provided new insight into the
diagnosis and treatment of intraspinal tumors in which the spinal
cord with a residual tumor responded to a combination of
chemoradiotherapy and bevacizumab. Further studies are needed to
investigate the mechanisms driving the development and treatment
response of this type of tumor.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Joint Special Funds for
the Department of Science and Technology of Yunnan Province-Kunming
Medical University (grant no. 202001AY070001-141) and the PhD
Research Fund project of the First Affiliated Hospital of Kunming
Medical University (grant no. 2019BS003).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZe, YZh and QF identified and selected this case.
YZe and YZh drafted the manuscript. RL, JZ, YZe and QF reviewed and
edited the manuscript. WJ, JZ and QF were involved in the patient's
clinical management. YZh, YZe, RL and QF analyzed and interpreted
data. RL and QF confirm the authenticity of all the raw data. All
authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written informed consent for
publication of patient data and associated images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chang AE, Karnell LH and Menck HR: The
national cancer database report on cutaneous and noncutaneous
melanoma: A summary of 84,836 cases from the past decade. The
American college of surgeons commission on cancer and the American
cancer society. Cancer. 83:1664–1678. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fujimoto M, Matsuzaki I, Nishitsuji K,
Yamamoto Y, Murakami D, Yoshikawa T, Fukui A, Mori Y, Nishino M,
Takahashi Y, et al: Adipophilin expression in cutaneous malignant
melanoma is associated with high proliferation and poor clinical
prognosis. Lab Invest. 100:727–737. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mastoraki A, Schizas D, Giannakodimos I,
Rebakos A, Margaris I, Katsaros I, Vagios I, Vassiliu P and
Pikoulis E: Malignant melanoma of the breast: Controversies in the
diagnosis and therapeutic management of a rare nosologic entity.
Int J Dermatol. 59:1057–1064. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Amalinei C, Grigoraș A, Lozneanu L,
Căruntu ID, Giușcă SE and Balan RA: The interplay between tumour
microenvironment components in malignant melanoma. Medicina
(Kaunas). 58:3652022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Puyana C, Denyer S, Burch T, Bhimani AD,
Mcguire LS, Patel AS and Mehta AI: Primary malignant melanoma of
the brain: A population-based study. World Neurosurg.
130:e1091–e1097. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang K, Oak ASW, Slominski RM, Brożyna AA
and Slominski AT: Current molecular markers of melanoma and
treatment targets. Int J Mol Sci. 21:35352020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shi YF, Chen YQ, Chen HF and Hu X: An
atypical primary malignant melanoma arising from the cervical nerve
root: A case report and review of literture. World J Clin Cases.
10:381–387. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Iga T, Iwanami A, Funakoshi T, Mikami S,
Tsuji O, Nagoshi N, Okada E, Fujita N, Yagi M, Watanabe K, et al:
Multifocal primary melanoma of the cervical spinal cord
successfully treated by tumorectomy: A case report. Spinal Cord Ser
Cases. 4:242018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fu QF, Liu Y, Fan Y, Hua SN, Qu HY, Dong
SW, Li RL, Zhao MY, Zhen Y, Yu XL, et al: Alpha-enolase promotes
cell glycolysis, growth, migration, and invasion in non-small cell
lung cancer through FAK-mediated PI3K/AKT pathway. J Hematol Oncol.
8:222015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liubinas SV, Maartens N and Drummond KJ:
Primary melanocytic neoplasms of the central nervous system. J Clin
Neurosci. 17:1227–1232. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pappenheim E and Bhattacharji SK: Primary
melanoma of the central nervous system. Clinical-pathological
report of a case, with survey and discussion of the literature.
Arch Neurol. 7:101–113. 1962. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamasaki T, Kikuchi H, Yamashita J, Asato
R and Fujita M: Primary spinal intramedullary malignant melanoma:
Case report. Neurosurgery. 25:117–121. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hayward RD: Malignant melanoma and the
central nervous system. A guide for classification based on the
clinical findings. J Neurol Neurosurg Psychiatry. 39:526–530. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fujimori K, Sakai K, Higashiyama F, Oya F,
Maejima T and Miyake T: Primary central nervous system malignant
melanoma with leptomeningeal melanomatosis: A case report and
review of the literature. Neurosurg Rev. 41:333–339. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rodriguez y Baena R, Gaetani P, Danova M,
Bosi F and Zappoli F: Primary solitary intracranial melanoma: Case
report and review of the literature. Surg Neurol. 38:26–37. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cohn-Cedermark G, Mansson-Brahme E,
Rutqvist LE, Larsson O, Johansson H and Ringborg U: Central nervous
system metastases of cutaneous malignant melanoma-a
population-based study. Acta Oncol. 37:463–470. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
de Vries E and Coebergh JWW: Melanoma
incidence has risen in Europe. BMJ. 331:6982005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang HY, Park N, Erlandson RA and
Antonescu CR: Immunohistochemical and ultrastructural comparative
study of external lamina structure in 31 cases of cellular,
classical, and melanotic schwannomas. Appl Immunohistochem Mol
Morphol. 12:50–58. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Küsters-Vandevelde HVN, van Engen-van
Grunsven IACH, Küsters B, van Dijk MR, Groenen PJTA, Wesseling P
and Blokx WAM: Improved discrimination of melanotic schwannoma from
melanocytic lesions by combined morphological and GNAQ mutational
analysis. Acta Neuropathol. 120:755–764. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Küsters-Vandevelde HVN, Küsters B, van
Engen-van Grunsven ACH, Groenen PJTA, Wesseling P and Blokx WAM:
Primary melanocytic tumors of the central nervous system: A review
with focus on molecular aspects. Brain Pathol. 25:209–226. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Carney JA: Psammomatous melanotic
schwannoma. A distinctive, heritable tumor with special
associations, including cardiac myxoma and the Cushing syndrome. Am
J Surg Pathol. 14:206–222. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rodriguez FJ, Stratakis CA and Evans DG:
Genetic predisposition to peripheral nerve neoplasia: Diagnostic
criteria and pathogenesis of neurofibromatoses, Carney complex, and
related syndromes. Acta Neuropathol. 123:349–367. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rothenbuhler A and Stratakis CA: Clinical
and molecular genetics of Carney complex. Best Pract Res Clin
Endocrinol Metab. 24:389–399. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Balakrishnan R, Porag R, Asif DS, Satter
AMR, Taufiq M and Gaddam SSK: Primary intracranial melanoma with
early leptomeningeal spread: A case report and treatment options
available. Case Rep Oncol Med. 2015:2938022015.PubMed/NCBI
|
|
25
|
Salpietro FM, Alafaci C, Gervasio O, La
Rosa G, Baio A, Francolini DC, Batolo D and Tomasello F: Primary
cervical melanoma with brain metastases. Case report and review of
the literature. J Neurosurg. 89:659–666. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Troya-Castilla M, Rocha-Romero S,
Chocrón-González Y and Marquez-Rivas FJ: Primary cerebral malignant
melanoma in insular region with extracranial metastasis: Case
report and review literature. World J Surg Oncol. 14:2352016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang J, Guo ZZ, Wang YJ, Zhang SG and Xing
DG: Microsurgery for the treatment of primary malignant
intracranial melanoma: A surgical series and literature review. Eur
J Surg Oncol. 40:1062–1071. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ma Y, Gui Q and Lang S: Intracranial
malignant melanoma: A report of 7 cases. Oncol Lett. 10:2171–2175.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Young GJ, Bi WL, Wu WW, Johanns TM, Dunn
GP and Dunn IF: Management of intracranial melanomas in the era of
precision medicine. Oncotarget. 8:89326–89347. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Swetter SM, Thompson JA, Albertini MR,
Barker CA, Baumgartner J, Boland G, Chmielowski B, DiMaio D, Durham
A, Fields RC, et al: NCCN guidelines® insights:
Melanoma: Cutaneous, version 2.2021. J Natl Compr Canc Netw.
19:364–376. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Han CH and Brastianos PK: Genetic
characterization of brain metastases in the era of targeted
therapy. Front Oncol. 7:2302017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jour G, Ivan D and Aung PP: Angiogenesis
in melanoma: An update with a focus on current targeted therapies.
J Clin Pathol. 69:472–483. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ferrara N, Hillan KJ, Gerber HP and
Novotny W: Discovery and development of bevacizumab, an anti-VEGF
antibody for treating cancer. Nat Rev Drug Discov. 3:391–400. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Corrie PG, Marshall A, Dunn JA, Middleton
MR, Nathan PD, Gore M, Davidson N, Nicholson S, Kelly CG, Marples
M, et al: Adjuvant bevacizumab in patients with melanoma at high
risk of recurrence (AVAST-M): Preplanned interim results from a
multicentre, open-label, randomised controlled phase 3 study.
Lancet Oncol. 15:620–630. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Corrie PG, Marshall A, Nathan PD, Lorigan
P, Gore M, Tahir S, Faust G, Kelly CG, Marples M, Danson SJ, et al:
Adjuvant bevacizumab for melanoma patients at high risk of
recurrence: Survival analysis of the AVAST-M trial. Ann Oncol.
30:2013–2014. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ascierto PA, Mandalà M, Ferrucci PF,
Guidoboni M, Rutkowski P, Ferraresi V, Arance A, Guida M, Maiello
E, Gogas H, et al: Sequencing of ipilimumab plus nivolumab and
encorafenib plus binimetinib for untreated BRAF-mutated metastatic
melanoma (SECOMBIT): A randomized, three-arm, open-label phase II
trial. J Clin Oncol. 41:212–221. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bai X and Zhou M: The benefit of
bevacizumab therapy in patients with refractory vasogenic edema
caused by brain metastasis from lung and colon cancers. Front
Oncol. 12:8386702022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Banks PD, Lasocki A, Lau PKH, Sandhu S,
Mcarthur G and Shackleton M: Bevacizumab as a steroid-sparing agent
during immunotherapy for melanoma brain metastases: A case series.
Health Sci Rep. 2:e1152019. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Markovic SN, Suman VJ, Javed A, Reid JM,
Wall DJ, Erickson LA, Ernstoff M and Anderson DM: Sequencing
ipilimumab immunotherapy before or after chemotherapy
(Nab-paclitaxel and bevacizumab) for the treatment of BRAFwt (BRAF
wild-type) metastatic malignant melanoma: Results of a study of
academic and community cancer research United (ACCRU) RU261206I. Am
J Clin Oncol. 43:115–121. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shields CL, Dalvin LA, Chang M, Mazloumi
M, Fortin P, McGarrey M, Martin A, Yaghy A, Yang X, Vichitvejpaisal
P, et al: Visual outcome at 4 years following plaque radiotherapy
and prophylactic intravitreal bevacizumab (every 4 months for 2
years) for uveal melanoma: Comparison with nonrandomized historical
control individuals. JAMA Ophthalmol. 138:136–146. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chinese guidelines for diagnosis and
treatment of melanoma 2018 (English version). Chin J Cancer Res.
31:578–585. 2019. View Article : Google Scholar : PubMed/NCBI
|