Introduction
Neoadjuvant therapy (NAT) is an increasingly used
treatment modality for human epidermal growth factor receptor 2
(HER2)-positive early-stage breast cancer (1). It has several advantages: it allows
for downstaging the tumour size and provides access to an important
prognostic factor, pathological complete response (pCR), defined as
no invasive cancer left in the breast or axillary lymph nodes.
Achieving pCR increases the incidence of favourable survival
outcomes (2–6), whereas patients who do not obtain pCR
benefit from the escalation of adjuvant treatment, such as
trastuzumab emtansine in the HER2-positive subtype (7).
Discovering novel predictive factors and the
identification of patients who do not benefit from conventional NAT
are important topics of research. HER2-positive and hormone
receptor negative breast cancers respond better to NAT on average
(2,6). The use of targeted agents against
HER2-positive cancer improves treatment results even further: a
meta-analysis by Pathak et al (8) including 491 patients in five
randomised, controlled trials concluded that the addition of
trastuzumab more than doubled the achieved pCR rates (Risk Ratio
(RR) 2.20; 95% confidence interval (CI) 1.62–2.99) compared to
other targeted therapies. Dual-HER2-blockade seems to be even more
effective in this regard. Meta-analyses have shown that combining
trastuzumab with either lapatinib or pertuzumab increases pCR rates
clinically meaningfully in breast and axillary lymph nodes,
independent of the concurrent chemotherapy used (9,10).
In the present observational study, we
systematically collected the clinical data of patients with
HER2-positive breast cancer receiving modern NAT in a single centre
in Finland. Since some of the patients still have poor prognoses
after NAT, we aimed to identify the factors contributing to a
higher probability of obtaining pCR and a longer survival.
Materials and methods
Patients and data
The clinical data was collected retrospectively from
patients with HER2-positive breast cancer receiving NAT in Helsinki
University Hospital Comprehensive Cancer Centre, Finland, during
2005–2022. The patient cohort was collected from the Helsinki
University Hospital database using the free-text search term
‘neoadj*’ along with a pathologist's diagnosis of HER2-positivity.
This cohort was manually processed to ensure they met the
eligibility criteria (i.e., HER2-positive breast cancer patients
receiving NAT with a curative intention). The decision to use NAT
for each patient was discussed in a multidisciplinary meeting at
the Helsinki University Hospital. Patients with distant metastases
were excluded, but lymph node metastases above the collarbone level
or in the internal mammary chain were accepted in select cases if
the patient had been evaluated as being suitable for NAT with a
potentially curative intention.
The pathological characteristics of the tumours
(Table I) were determined before
the onset of NAT by core needle biopsy samples of the tumour, or,
if not available, by fine needle aspiration samples of the lymph
nodes (8 patients; 7.6%). HER2-positivity was assessed by
immunohistochemistry (IHC), and positive results were confirmed in
all cases using chromogenic in situ hybridization (CISH)
according to the American Society of Clinical Oncology/College of
American Pathologists guidelines (11). Two patients with negative IHC but
positive CISH were also included. The HER2 status had to be
positive preoperatively to be suitable for the study. Postoperative
histopathological characteristics were determined either from the
residual tumour samples or the lymph nodes, if no breast tumour was
available.
 | Table I.Tumour characteristics before the
initiation of NAT and in postoperative samples. |
Table I.
Tumour characteristics before the
initiation of NAT and in postoperative samples.
| Variables | Before NAT, n
(%) | Postoperative, n
(%) | P-value |
|---|
| Histology |
|
| 0.00026 |
| Invasive
ductal carcinoma | 92 (76.0) | 35 (28.9) |
|
| Invasive
lobular carcinoma | 4 (3.3) | 3 (2.5) |
|
| Other
invasive carcinoma | 9 (7.4) | 10 (8.3) |
|
| DCIS, no
invasive carcinoma | 0 (0.0) | 12 (9.9) |
|
| No DCIS,
no invasive carcinoma | 0 (0.0) | 61 (50.4) |
|
|
Missing/unavailable/pCR | 16 (13.2) | 0 (0.0) |
|
| Histopathological
grade |
|
| >0.99 |
| Grade
1 | 0 (0.0) | 5 (4.1) |
|
| Grade
2 | 14 (11.6) | 21 (17.4) |
|
| Grade
3 | 75 (62.0) | 24 (19.8) |
|
|
Missing/unavailable/pCR | 32 (26.4) | 71 (58.7) |
|
| ER expression, % |
|
| 0.76 |
| 0-9 | 55 (45.5) | 13 (10.7) |
|
|
10-29 | 5 (4.1) | 3 (2.5) |
|
|
30-59 | 7 (5.8) | 0 (0.0) |
|
|
>59 | 54 (44.6) | 31 (25.6) |
|
|
Missing/unavailable/pCR | 0 (0.0) | 74 (61.2) |
|
| PgR expression,
% |
|
| 0.21 |
| 0-9 | 85 (70.2) | 34 (28.1) |
|
|
10-29 | 11 (9.1) | 6 (5.0) |
|
|
30-59 | 10 (8.3) | 1 (0.8) |
|
|
>59 | 14 (11.6) | 6 (5.0) |
|
|
Missing/unavailable/pCR | 1 (0.8) | 74 (61.2) |
|
| HER2
immunohistochemistry |
|
| 0.0080 |
|
Negative | 2 (1.7) | 4 (3.3) |
|
| 1+ | 0 (0.0) | 3 (2.5) |
|
| 2+ | 20 (16.5) | 23 (19.0) |
|
| 3+ | 99 (81.8) | 16 (13.2) |
|
|
Missing/unavailable/pCR | 0 (0.0) | 75 (62.0) |
|
| Ki-67 expression,
% |
|
| 0.001 |
|
<10 | 0 (0.0) | 12 (9.9) |
|
|
10-20 | 13 (10.7) | 10 (8.3) |
|
|
21-30 | 22 (18.2) | 6 (5.0) |
|
|
>30 | 82 (67.8) | 18 (14.9) |
|
|
Missing/unavailable/pCR | 4 (3.3) | 75 (62.0) |
|
| Preoperative lymph
node cytology |
|
|
|
|
Malignant | 93 (76.9) |
|
|
|
Non-malignant | 3 (2.5) |
|
|
|
Missing/unavailable | 25 (21.7) |
|
|
| Extracapsular lymph
node extension in metastatic lymph nodes |
|
|
|
|
Present |
| 13 (10.7) |
|
| Not
present |
| 22 (18.2) |
|
|
Missing/unavailable/pCR |
| 86 (71.1) |
|
Evaluation of responses to NAT
The responses to NAT were evaluated by using
ultrasound, magnetic resonance imaging, or, in rare cases, computed
tomography. Radiological CR was defined as the disappearance of
clearly enhanced lesions before surgery. Pathological response was
assessed from the surgical breast samples, and pCR was defined as
no invasive cancer left in breast or lymph nodes.
Statistical analysis
Statistical analyses were conducted using SPSS
version 28.0.0.0 for Mac (IBM Corporation, Armonk, NY, USA).
Associations were calculated with Fisher's exact test, except for
oestrogen receptor (ER), progesterone receptor (PgR), and Ki-67,
which were assessed as continuous factors with a range of 0–100%.
For continuous variables, Mann Whitney U test was used when
evaluating their association with pCR. Wilcoxon Signed Rank test
was used when comparing the expression of ER, PgR and Ki-67 between
the pre-NAT and postoperative samples. Clinical and pathological
baseline factors were tested against pCR with two-sided
χ2 test, with the exception of histological type, for
which Fisher's exact test had to be used. Survival was analysed
using the Kaplan-Meier method and the log-rank test. RRs with 95%
confidence intervals (CI) were calculated using Cox regression.
Disease-free survival (DFS) was defined as the time between surgery
and the time of the first local or distant recurrence. Breast
cancer-specific survival (BCSS) was defined as the time between
diagnosis and confirmed death due to breast cancer. Overall
survival (OS) was calculated from the date of surgery to the time
of death or the end of the follow-up. P-values < 0.05 were
considered significant.
Results
Of the 121 tumours (119 patients, two with bilateral
HER2-positive breast cancer) treated with NAT, 63 (52.1%) had pCR.
The median age of the patients was 54 years (range 22–81 years) and
the median follow-up time was 42.0 months. One hundred patients
(82.6%) were diagnosed during 2016–2020. The dataset included one
male patient.
Table II summarises
the treatment regimens used. The median number of neoadjuvant
cycles was seven (range 3–13), and trastuzumab was included in all
treatments except for one patient. Only one patient received three
chemotherapy courses (treatment discontinued because of
radiological CR), while the other patients received at least five
cycles of NAT. Mastectomy and axillary lymph node evacuation was
the most common type of surgery. 87.6% of the patients were also
treated with trastuzumab in the adjuvant setting, 9.9% with some
other adjuvant therapy, and 2.5% with no adjuvant therapy at all.
Nine (7.6%) patients received trastuzumab emtansine
postoperatively. The median number of postoperative trastuzumab
cycles was 12 (range 0–17). Most of the patients (96.7%) received
postoperative radiotherapy. Sixty-nine (58.0%) patients received
adjuvant endocrine treatment.
 | Table II.Oncological and surgical treatments
received by the patients in the study cohort. |
Table II.
Oncological and surgical treatments
received by the patients in the study cohort.
| Treatment | Value |
|---|
| Neoadjuvant
therapy, n (%) |
|
|
Docetaxel + trastuzumab +
pertuzumab followed by anthracycline + cyclophosphamide | 39 (32.2) |
|
Docetaxel + trastuzumab
followed by anthracycline + cyclophosphamide | 2 (1.7) |
|
Trastuzumab + taxane (no
anthracycline) | 28 (23.1) |
|
Trastuzumab + pertuzumab +
taxane (no anthracycline) | 23 (19.0) |
| Other
trastuzumab-containing treatment | 28 (23.1) |
| Other
non-trastuzumab-containing treatment | 1 (0.8) |
| Median number of
NAT cycles (range) | 7 (3–13) |
| Type of breast
surgery, n (%) |
|
|
Resection + axillary lymph
node evacuation | 32 (26.4) |
|
Mastectomy + axillary lymph
node evacuation | 75 (62.0) |
|
Resection + sentinel lymph
node biopsy | 9 (7.4) |
|
Mastectomy + sentinel lymph
node biopsy | 5 (4.1) |
| Adjuvant therapy, n
(%) |
|
|
None | 3 (2.5) |
|
Trastuzumab | 106 (87.6) |
|
Other | 12 (9.9) |
| Median
postoperative trastuzumab cycles (range) | 12 (0–17) |
| Postoperative
radiotherapy, n (%) |
|
|
Yes | 117 (96.7) |
| No | 4 (3.3) |
| Endocrine adjuvant
therapya, n (%) |
|
|
None | 2 (2.9) |
|
Tamoxifen | 13 (18.8) |
|
Aromatase inhibitor | 31 (44.9) |
| LHRH
analogue + tamoxifen | 11 (15.9) |
| LHRH
analogue + aromatase inhibitor | 5 (7.2) |
|
Other | 7 (10.1) |
In the last radiographical assessment during the
neoadjuvant treatment, 56 tumours (46.3%) were in CR. Radiological
progression was observed in only three (2.5%) of the tumours (in
two patients, one with bilateral HER2-positive breast cancer
progression). All three progressive tumours had several factors in
common: Ki-67 level of 90%, 3–5% ER positivity, PgR negative, and
high (3+) IHC HER2-positivity in the core needle biopsy before the
initiation of the NAT. One of these patients was a frail, elderly
woman who was treated with vinorelbine and trastuzumab, while the
other patient received six cycles of a combination of docetaxel,
trastuzumab and pertuzumab, followed by three cycles of doxorubicin
and cyclophosphamide. In one (0.8%) case, the best response for NAT
was categorised as a stable disease. In the postoperative
pathological assessment in the patients with no pCR, the median
size of all invasive cancer foci was 21 millimetres (range 2–341
millimetres). The median number of removed lymph nodes was 23
(range 12–30) in the patients with no pCR, while the median number
of malignant lymph nodes was 2 (range 1–19). The median size of the
malignant lymph nodes after surgery was 6 millimetres (range 1–45
millimetres).
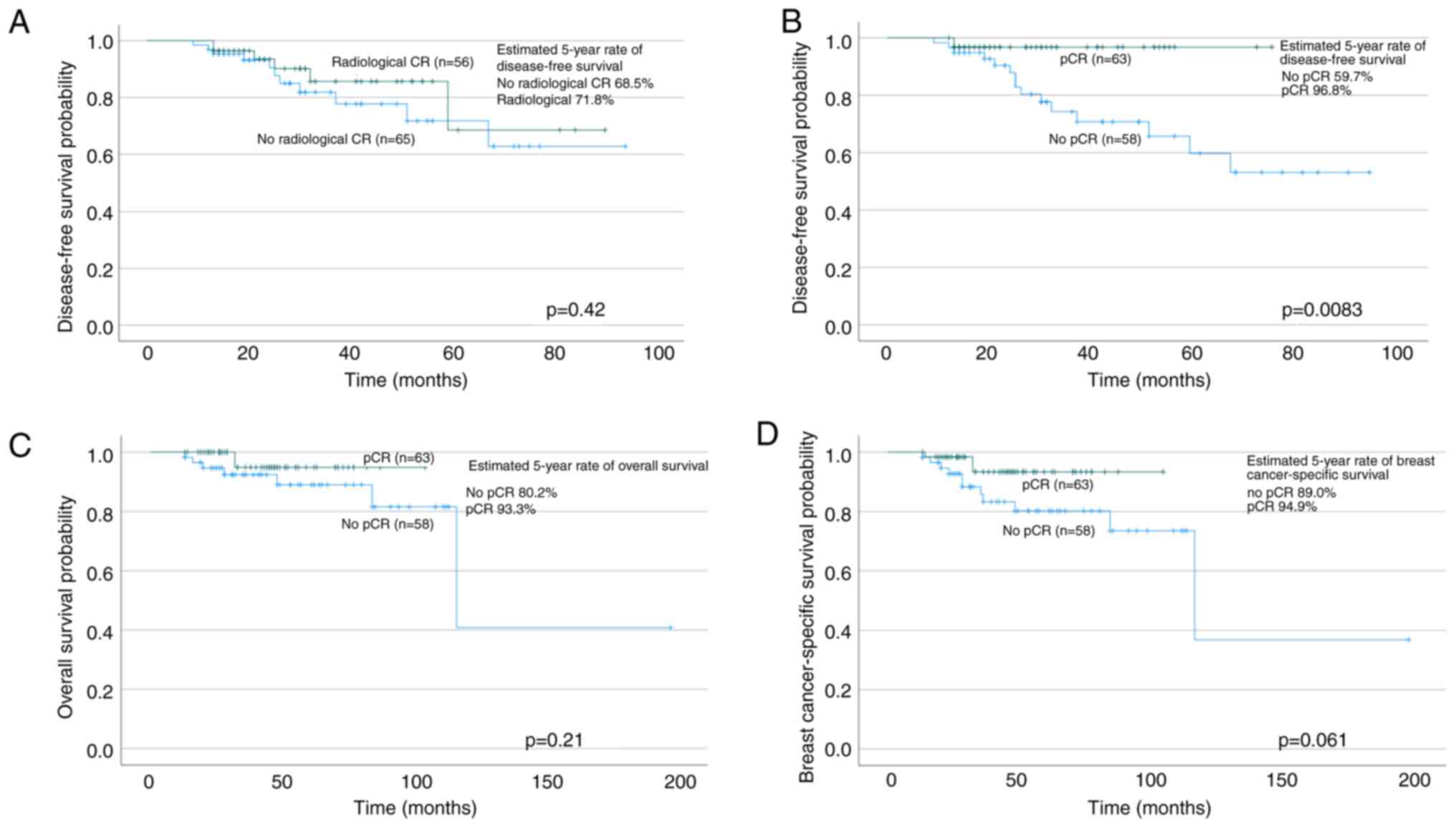
Radiological CR was associated with pCR (P=0.00033).
Even so, 30.4% of the patients with radiological CR still had
invasive disease in the pathological assessment, and 36.9% of the
patients with pCR had no radiological CR. The association between
the radiological CR and pCR was more obvious in ER-negative tumours
(ER expression below 10%; P=0.0030) than in ER-positive tumours
(P=0.043).
Next, we analysed how clinical and pathological
baseline factors associated with pCR (compared with no pCR). The
patients with high (3+) IHC expression of HER2 had a higher chance
of achieving pCR than those with moderate (2+) HER2 IHC expression
(pCR rates 57.6 and 25%, respectively; Fisher's exact test
P=0.0078). Other categorical variables, grade, menopausal status,
histological type, or the presence of extracapsular extension in
lymph node metastasis, did not predict the possibility of pCR.
From the continuous variables, which were analysed
with the Mann-Whitney U test, both low baseline ER expression
(P=0.0011) and low baseline PgR expression (P=0.00087) were
associated with a pCR (vs. no pCR). Other continuous variables, age
at diagnosis, or Ki-67 expression were not associated with
achieving pCR.
Postoperative samples had a lower Ki-67 expression
(P=0.001), and lower immunohistochemical HER2-positivity (P=0.0080)
than preoperative samples (Table
I). In postoperative analysis, HER2 status as defined by ISH
assay changed from positive to negative in 13.2% of the evaluable
cases (n=38), and similarly in both of the HER2 IHC 2+ and 3+
groups.
In the whole study population, the DFS rate at five
years was 71.8%, OS was 86.6%, and BCSS was 91.8%, respectively.
The patients with pCR had a longer DFS than those with invasive
cancer remaining after surgery (survival at five years 96.8 and
59.7%, respectively; log-rank P=0.0083; Cox regression RR 0.17; 95%
CI 0.039–0.76) (Fig. 1). OS was not
significantly longer in the patients with pCR after NAT (log-rank
P=0.061; Cox regression RR 0.31; 95% CI 0.084–1.1). In BCSS, no
difference was observed between the pCR and non-pCR groups
(log-rank P=0.21; Cox regression RR 0.37; 95% CI 0.072–1.9).
Radiological CR was not associated with any of the survival
endpoints.
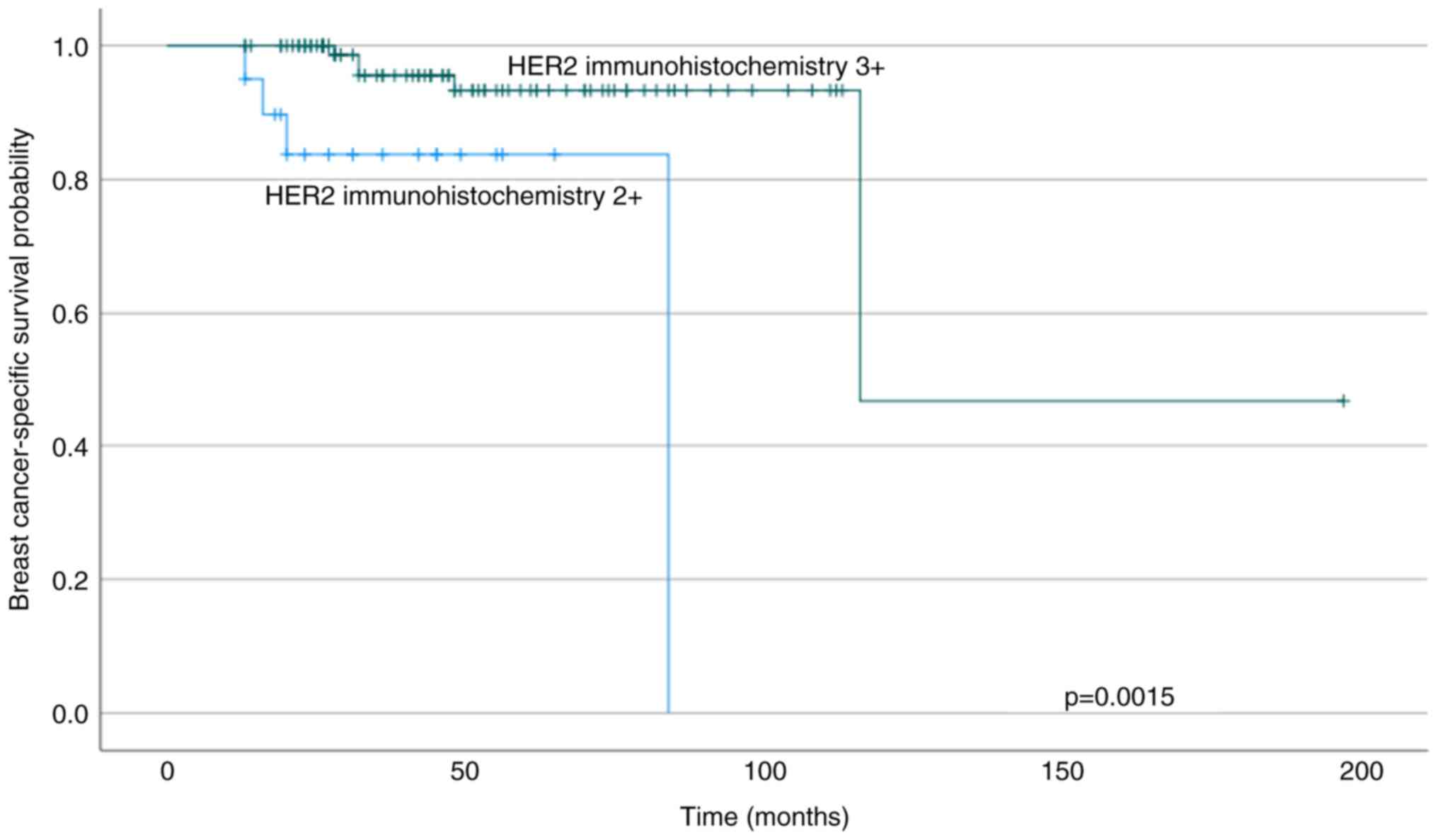
The patients who had tumours with high (3+) HER2
immunostaining preoperatively had longer BCSS than those with
moderate HER2+ immunostaining (log-rank P=0.0015; Cox regression RR
0.14; 95% CI 0.035–0.58) (Fig. 2).
A trend of shorter DFS and OS was seen in the group with moderate
HER2 immunostaining compared with strong staining (for DFS log-rank
P=0.062; Cox regression RR 0.38; 95% CI 0.13–1.1 and for OS
log-rank P=0.057; Cox regression RR 0.34; 95% CI 0.10–1.1,
respectively). Preoperative ER, PgR or Ki-67 expression, grade, or
the presence of extracapsular extension in lymph node metastases
did not predict survival. Multivariate analyses were considered
unreliable due to the low number of events and were thus not
performed.
Discussion
This is the first study to show that patients with
only moderate immunohistochemical HER2 expression, irrespective of
positive ISH status, had a lower chance of reaching pCR and also
experienced worse survival rates. Another main finding was that
only a moderate correlation between radiological and pathological
CR existed. Finally, this study confirmed an excellent long-term
outcome of the patients with HER2-positive breast cancer, in spite
of large primary tumours and lymph node-involved diseases.
pCR was achieved in 52.1% of the ISH-confirmed
HER2-positive tumours in our cohort, which is in accordance with
previous observational evidence. For example, a study with 776
patients reported pCR rates of 51% when pCR was defined as ypT0/is
ypN0 (12). Notably, most of our
patients had locally advanced cancers with preoperatively large
inoperable tumours and/or biopsy-confirmed multiple lymph node
metastases. Consequently, in our study, those with residual cancer
had a substantial cancer mass in both the breast and axilla.
Radiological CR was moderately associated with pCR,
although approximately one-third of the tumours with radiological
CR had invasive disease left in the pathological assessment, and
more than one third of the tumours with pCR had no radiological CR.
For a small number of patients, this could be due to the
heterogenous imaging modalities used in evaluating the responses to
NAT, as some of the patients' responses were assessed with
ultrasonography, as well as the challenges in the evaluation of the
contrast medium intensity. The correlation between imaging and the
pathological evaluation was notably more accurate in ER-negative
tumours. This correlation can result from the higher likelihood of
false-negative results if the pCR rate is lower, or if there is
non-mass or diffuse enhancement, as often observed in ER-positive
tumours (13–15). These results are still consistent
with the previous findings estimating the accuracy of MRI in
HER2-positive breast cancer, which have shown radiological CR
corresponding to a pCR in 70–73% of the patients, with the
proportion increasing to 88% in hormone receptor-negative subgroup
(16,17).
Achieving pCR after HER2-positive breast cancer NAT
is known to be associated with improved survival, and our DFS
results with RR of 0.17 (95% CI 0.039–0.76) were consistent with
this previous evidence (2–4,6). The
five-year DFS of only 59.7% in the non-pCR group reflects both the
nature of large tumours and also the inherent drug resistance of
HER2-positive and ER-positive tumours for the available treatments.
Both the trend for shorter OS (RR 0.31; 95% CI 0.084–1.1) and BCSS
(RR 0.37; 95% CI 0.072–1.9) in the patients with non-pCR were
statistically non-significant, which may result from the limited
statistical power in our cohort. A recent real-world study by
LeVasseur et al (18) with a
median follow-up of 7.5 years revealed a trend for improved BCSS
and OS in patients with HER2-positive cancer obtaining pCR,
although the finding did not reach statistical significance. In
another retrospective study, combining pertuzumab to a
trastuzumab-based neoadjuvant chemotherapy was observed to improve
five-year BCSS and OS in early HER2-positive breast cancer,
especially in patients younger than 50 years old (19). It is worth noting, that there is no
randomised study showing that pertuzumab would improve overall
survival when administered as neoadjuvant therapy, but the evidence
is relying only applies to improved pCR rates in a phase II study
(20). There were only two patients
(three breast cancers) with progressive diseases in our material,
all with almost absent ER expression, a total lack of PR
expression, and a very high proliferation rate. Additionally, one
of these patients only received an oncologically suboptimal
combination of vinorelbine and trastuzumab due to her
fragility.
There is previous observational evidence of strong
HER2 IHC staining predicting improved pCR rates. In our material
with HER2 status confirmed with ISH in all cases, we also found
that the HER2 IHC result of 3+ more than doubled the chances for
pCR compared with the IHC result of 2+. In an observational study,
IHC 3+ tumours treated with neoadjuvant trastuzumab had pCR rates
(ypT0 ypN0) of 46.0%, whereas IHC 2+/FISH-positive tumours had pCR
rates of 25.0% (21). Wang et
al (22) reported pCR rates of
55.1% in IHC 3+ group and only 17.6% on IHC 2+/ISH-positive groups,
although ISH was not performed in all IHC 3+ cases. However, to the
best of our knowledge, this is the first time when a high IHC score
of 3+ has also been associated with improved BCSS. One possible
explanation for this is the higher HER2 heterogeneity in HER2 2+
tumours which has been associated with worse outcomes (23–25).
Although in the present study, the number of patients with HER2
immunostaining of 2+ was limited (n=20), these results still
suggest that treatment intensification strategies should be
investigated in this patient population and encourage more
intensive surveillance after NAT.
As a single-site, retrospective study, this study
contains several inherent limitations. The number of the events was
too small to conduct reliable multivariate analysis. At the time of
the treatments, trastuzumab emtansine was rarely used as an
adjuvant therapy, although it is now routinely used in non-pCR
HER2-positive patients in high-income countries and could have
improved DFS (but not OS) rates (7). Still, most of the patients received
pertuzumab as part of their NAT, and all but one patient were
treated with trastuzumab. As the breast imaging interval was not
standardised, this variation could have led to the underestimation
of the radiological CR rates. Similarly, the residual cancer burden
was not standardized in the pathology reports.
We conclude that in this study of patients, most
with locally advanced HER2-positive breast cancers and treated with
contemporary procedures, pCR after NAT still served as a precise
predictor of excellent prognosis. In addition, as a novel finding,
the patients with only moderate IHC HER2 expression, irrespective
of positive ISH status, seemed to have a lower chance of reaching
pCR. Since they also seem to carry a higher risk of breast
cancer-related death, novel treatments, such as trastuzumab
deruxtecan in the treatment of metastatic HER2-low breast cancer
(26), would be needed to improve
their prognosis.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PK and JM conceptualized and supervised the study,
and oversaw the project administration, resources, software used
and methodology. The data were collected by ENH, who also wrote the
original draft. The data were analysed by ENH and PK and
interpreted by all authors. All authors participated in the writing
and editing process of the manuscript. ENH and PK confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was based on secondary data and did not
involve human participants. Therefore, ethics committee approval or
patient consent for participation was not required.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Loibl S, Poortmans P, Morrow M, Denkert C
and Curigliano G: Breast cancer. Lancet. 397:1750–1769. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cortazar P, Zhang L, Untch M, Mehta K,
Costantino JP, Wolmark N, Bonnefoi H, Cameron D, Gianni L,
Valagussa P, et al: Articles Pathological complete response and
long-term clinical benefit in breast cancer: The CTNeoBC pooled
analysis. Lancet. 384:164–172. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Davey MG, Browne F, Miller N, Lowery AJ
and Kerin MJ: Pathological complete response as a surrogate to
improved survival in human epidermal growth factor
receptor-2-positive breast cancer: Systematic review and
meta-analysis. BJS Open. 6:zrac0282022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu H, Lv L, Gao H and Cheng M: Pathologic
complete response and its impact on breast cancer recurrence and
patient's survival after neoadjuvant therapy: A comprehensive
meta-analysis. Comput Math Methods Med. 2021:75450912021.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Harbeck N: Neoadjuvant and adjuvant
treatment of patients with HER2-positive early breast cancer.
Breast. 62 (Suppl 1):S12–S16. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Broglio KR, Quintana M, Foster M, Olinger
M, McGlothlin A, Berry SM, Boileau JF, Brezden-Masley C, Chia S,
Dent S, et al: Association of pathologic complete response to
neoadjuvant therapy in HER2-Positive breast cancer with long-term
outcomes: A meta-analysis. JAMA Oncol. 2:751–760. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
von Minckwitz G, Huang CS, Mano MS, Loibl
S, Mamounas EP, Untch M, Wolmark N, Rastogi P, Schneeweiss A,
Redondo A, et al: Trastuzumab emtansine for residual invasive
HER2-Positive breast cancer. N Engl J Med. 380:617–628. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pathak M, Dwivedi SN, Deo SVS, Thakur B,
Sreenivas V and Rath GK: Effectiveness of added targeted therapies
to neoadjuvant chemotherapy for breast cancer: A systematic review
and meta-analysis. Clin Breast Cancer. 19:e690–e700. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bria E, Carbognin L, Furlanetto J, Pilotto
S, Bonomi M, Guarneri V, Vicentini C, Brunelli M, Nortilli R,
Pellini F, et al: Impact of neoadjuvant single or dual HER2
inhibition and chemotherapy backbone upon pathological complete
response in operable and locally advanced breast cancer:
Sensitivity analysis of randomized trials. Cancer Treat Rev.
40:847–856. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nakashoji A, Hayashida T, Yokoe T, Maeda
H, Toyota T, Kikuchi M, Watanuki R, Nagayama A, Seki T, Takahashi
M, et al: The updated network meta-analysis of neoadjuvant therapy
for HER2-positive breast cancer. Cancer Treat Rev. 62:9–17. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wolff AC, Hammond MEH, Allison KH, Harvey
BE, Mangu PB, Bartlett JMS, Bilous M, Ellis IO, Fitzgibbons P,
Hanna W, et al: Human epidermal growth factor receptor 2 testing in
breast cancer: American society of clinical oncology/college of
American pathologists clinical practice guideline focused update.
Arch Pathol Lab Med. 142:1364–1382. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takada M, Ishiguro H, Nagai S, Ohtani S,
Kawabata H, Yanagita Y, Hozumi Y, Shimizu C, Takao S, Sato N, et
al: Survival of HER2-positive primary breast cancer patients
treated by neoadjuvant chemotherapy plus trastuzumab: A multicenter
retrospective observational study (JBCRG-C03 study). Breast Cancer
Res Treat. 145:143–153. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
von Minckwitz G, Untch M, Nüesch E, Loibl
S, Kaufmann M, Kümmel S, Fasching PA, Eiermann W, Blohmer JU, Costa
SD, et al: Impact of treatment characteristics on response of
different breast cancer phenotypes: Pooled analysis of the German
neo-adjuvant chemotherapy trials. Breast Cancer Res Treat.
125:145–156. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Santamaría G, Bargalló X, Fernández PL,
Farrús B, Caparrós X and Velasco M: Neoadjuvant systemic therapy in
breast cancer: Association of Contrast-enhanced MR imaging
findings, diffusion-weighted imaging findings, and tumor subtype
with tumor response. Radiology. 283:663–672. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mukhtar RA, Yau C, Rosen M, Tandon VJ and
Hylton N: I-SPY 1 TRIAL and ACRIN 6657 Investigators; Hylton N and
Esserman LJ: Clinically meaningful tumor reduction rates vary by
prechemotherapy MRI phenotype and tumor subtype in the I-SPY 1
tRIAL (CALGB 150007/150012; ACRIN 6657). Ann Surg Oncol.
20:3828–3830. 2013. View Article : Google Scholar
|
|
16
|
van Ramshorst MS, Loo CE, Groen EJ,
Winter-Warnars GH, Wesseling J, van Duijnhoven F, Peeters MTV and
Sonke GS: MRI predicts pathologic complete response in
HER2-positive breast cancer after neoadjuvant chemotherapy. Breast
Cancer Res Treat. 164:99–106. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
De Los Santos JF, Cantor A, Amos KD,
Forero A, Golshan M, Horton JK, Hudis CA, Hylton NM, McGuire K,
Meric-Bernstam F, et al: Magnetic resonance imaging as a predictor
of pathologic response in patients treated with neoadjuvant
systemic treatment for operable breast cancer. Translational breast
cancer research consortium trial 017. Cancer. 119:1776–1783. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
LeVasseur N, Sun J, Gondara L, Diocee R,
Speers C, Lohrisch C and Chia S: Impact of pathologic complete
response on survival after neoadjuvant chemotherapy in early-stage
breast cancer: A population-based analysis. J Cancer Res Clin
Oncol. 146:529–536. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
van der Voort A, Liefaard MC, van
Ramshorst MS, van Werkhoven E, Sanders J, Wesseling J, Scholten A,
Vrancken Peeters MJTFD, de Munck L, Siesling S and Sonke GS:
Efficacy of neoadjuvant treatment with or without pertuzumab in
patients with stage II and III HER2-positive breast cancer: A
nationwide cohort analysis of pathologic response and 5-year
survival. Breast. 65:110–115. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gianni L, Pienkowski T, Im YH, Tseng LM,
Liu MC, Lluch A, Starosławska E, de la Haba-Rodriguez J, Im SA,
Pedrini JL, et al: 5-year analysis of neoadjuvant pertuzumab and
trastuzumab in patients with locally advanced, inflammatory, or
early-stage HER2-positive breast cancer (NeoSphere): A multicentre,
open-label, phase 2 randomised trial. Lancet Oncol. 17:791–800.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen HL, Chen Q and Deng YC: Pathologic
complete response to neoadjuvant anti-HER2 therapy is associated
with HER2 immunohistochemistry score in HER2-positive early breast
cancer. Medicine (Baltimore). 100:e276322021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Y, Singh K, Dizon D, Graves T, Amin A
and Yakirevich E: Immunohistochemical HER2 score correlates with
response to neoadjuvant chemotherapy in HER2-positive primary
breast cancer. Breast Cancer Res Treat. 186:667–676. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Andersson J, Linderholm B, Bergh J and
Elmberger G: HER-2/neu (c-erbB-2) Evaluation in primary breast
carcinoma by fluorescent in situ hybridization and
immunohistochemistry with special focus on intratumor heterogeneity
and comparison of invasive and in situ components. Appl
Immunohistochem Mol Morphol. 12:14–20. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Öhlschlegel C, Zahel K, Kradolfer D, Hell
M and Jochum W: HER2 genetic heterogeneity in breast carcinoma. J
Clin Pathol. 64:1112–1116. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Seol H, Lee HJ, Choi Y, Lee HE, Kim YJ,
Kim JH, Kang E, Kim SW and Park SY: Intratumoral heterogeneity of
HER2 gene amplification in breast cancer: Its clinicopathological
significance. Mod Pathol. 25:938–948. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Modi S, Jacot W, Yamashita T, Sohn J,
Vidal M, Tokunaga E, Tsurutani J, Ueno NT, Prat A, Chae YS, et al:
Trastuzumab deruxtecan in previously treated HER2-Low advanced
breast cancer. N Engl J Med. 387:9–20. 2022. View Article : Google Scholar : PubMed/NCBI
|