Introduction
Endometrial cancer is the 6th most common type of cancer in women globally, accounting for 417,367 new cancer cases and 97,370 cancer-associated deaths in 2020 (1). However, four different patients with endometrial cancer and histologically unusual mucinous differentiation of a gastric or/and intestinal phenotype were reported by Wong et al (2) in 2020. In the wake of the report by Wong et al (2), a new type of carcinoma, namely primary endometrial intestinal-type carcinoma, was added to the World Health Organization (WHO) classification of female genital tract malignancies in 2020 as part of a series of changes regarding the definition of endometrial carcinomas (3). Before this classification was established, this rare subtype was occasionally misdiagnosed as endometrioid adenocarcinoma with mucinous differentiation, which is not itself uncommon; one in 10 cases of endometrioid adenocarcinomas were diagnosed as the aforementioned subtype (4). In addition, the prognosis of this type of endometrioid adenocarcinoma was considered to be comparable with more ‘conventional’ subtypes of endometrial carcinomas, such as endometrioid adenocarcinomas (2,4). According to the four cases described by Wong et al (2), the prognosis of endometrial intestinal-type mucinous carcinoma was dismal compared with endometrioid adenocarcinoma with mucinous differentiation or classical mucinous (Müllerian type) carcinomas of the endometrium (2). Therefore, this rare histological type should be diagnosed correctly according to the diagnostic criteria described by Wong et al (2). Apart from the four cases described by Wong et al (2), there have been only 11 cases in the literature reporting the features of this endometrial intestinal-type mucinous carcinoma after the establishment of its definitive classification (5), where the findings remained ambiguous (6). Therefore, further studies, including case reports, are necessary to deepen the understanding of this rare histological subtype. In the present report, a rare case of intestinal type endometrial mucinous carcinoma along with clinical and pathological findings was documented. In addition, a literature review on this malignancy was performed.
Case report
The patient was an 80-year-old gravida 4 para 3 female who had experienced menopause around the age of 50 years. The patient had undergone an appendectomy at age 13 and surgery for rhino polyps at age 74. In addition, the patient had received surgery and radiotherapy for vulvar Paget's disease at the Department of Dermatology of Osaka Metropolitan University Hospital (Osaka, Japan) at age 79. Regarding the family history of the patient, the brother of the patient had gastric cancer. After the surgery (tumorectomy) and radiotherapy (radiation dose, 60 Gy; fractionation, 30) for vulvar Paget's disease, the patient was followed up at the outpatient department of the Department of Dermatology, where a uterine tumor was accidentally identified by CT (Aquilion ONE; Canon, Inc.) 1 year after surgery (Fig. 1A). Therefore, the patient was referred to the Gynecological Department of Osaka Metropolitan University Hospital (Osaka, Japan). The patient experienced no subjective symptoms, such as abnormal genital bleeding or abdominal pain. A vaginal examination revealed uterine bleeding that was unremarkable and not abnormal. Transvaginal ultrasonography (Aplio a450; Canon, Inc.) showed a high-intensity mass in the uterine cavity (Fig. 1B). Tumor markers revealed that the serum levels of carcinoembryonic antigen (CEA) measured using CEA Abbott Alinity G06290R04, carbohydrate antigen (CA)19-9 measured using CA19-9 XR Abbott Alinity G06327R03, CA125 measured using the CA125 II Abbott Alinity G06330R04 (all from Abbott Molecular Inc.) and sialyl-Tn measured using the STN Otsuka RIA kit (Otsuka Assay Laboratories) (all measurements taken according to the manufacturer's instructions) were within normal ranges as follows: CEA <1.7 ng/ml (normal range, ≤5.0 ng/ml); CA19-9, 3 U/ml (normal range, ≤37.0); CA125, 16 U/ml (normal range, <35.0 U/ml), sialyl-Tn, 16.0 U/ml (normal range, ≤45.0), with blood tests indicating no other abnormal findings. MRI revealed a 25×15-mm mass, which was of low intensity in the tumor (T)2-weighed images, but of high intensity in the T1-weighted images (version 3.0; Philips Ingenia). In addition, the mass of the patient had high intensity in the diffusion-weighted images, but low intensity in the apparent diffusion coefficient map, which suggested the presence of a malignant tumor in the uterine cavity. There were no abnormal lesions in the uterine cervix, suggesting that there was no cervical invasion or malignancies of cervical origin (Fig. 2).
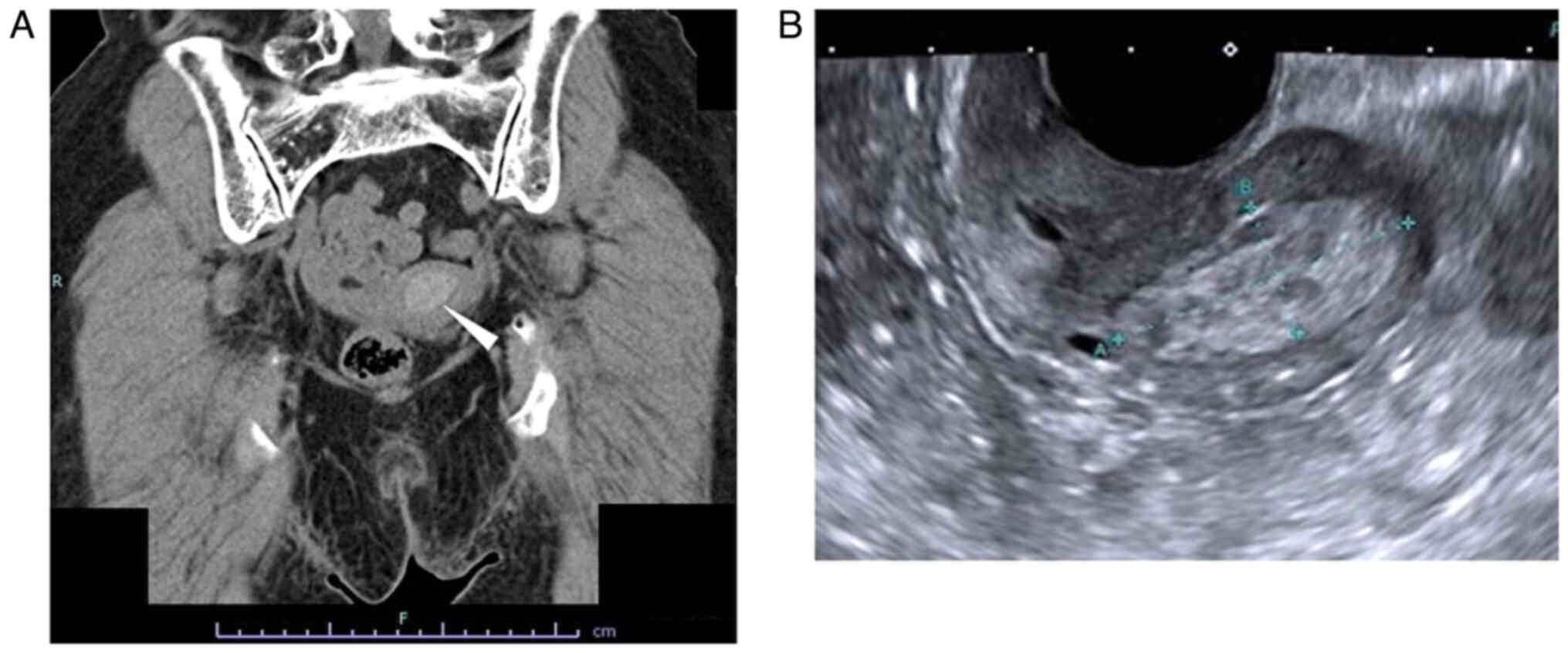 |
Figure 1.
Images of CT scan and trans-vaginal ultrasonography. (A) CT scan. A 25.1×15.2-mm, high-density lesion was detected in the uterine cavity indicated by the white arrowhead (120 kilovoltage peak; quality reference mAsec, 114/200). (B) Trans-vaginal ultrasonography. A 31.2×13.3-mm, high-echoic lesion was detected in the uterine cavity (dynamic range, 65; gain, 89; frame rate, 18 frames per sec).
|
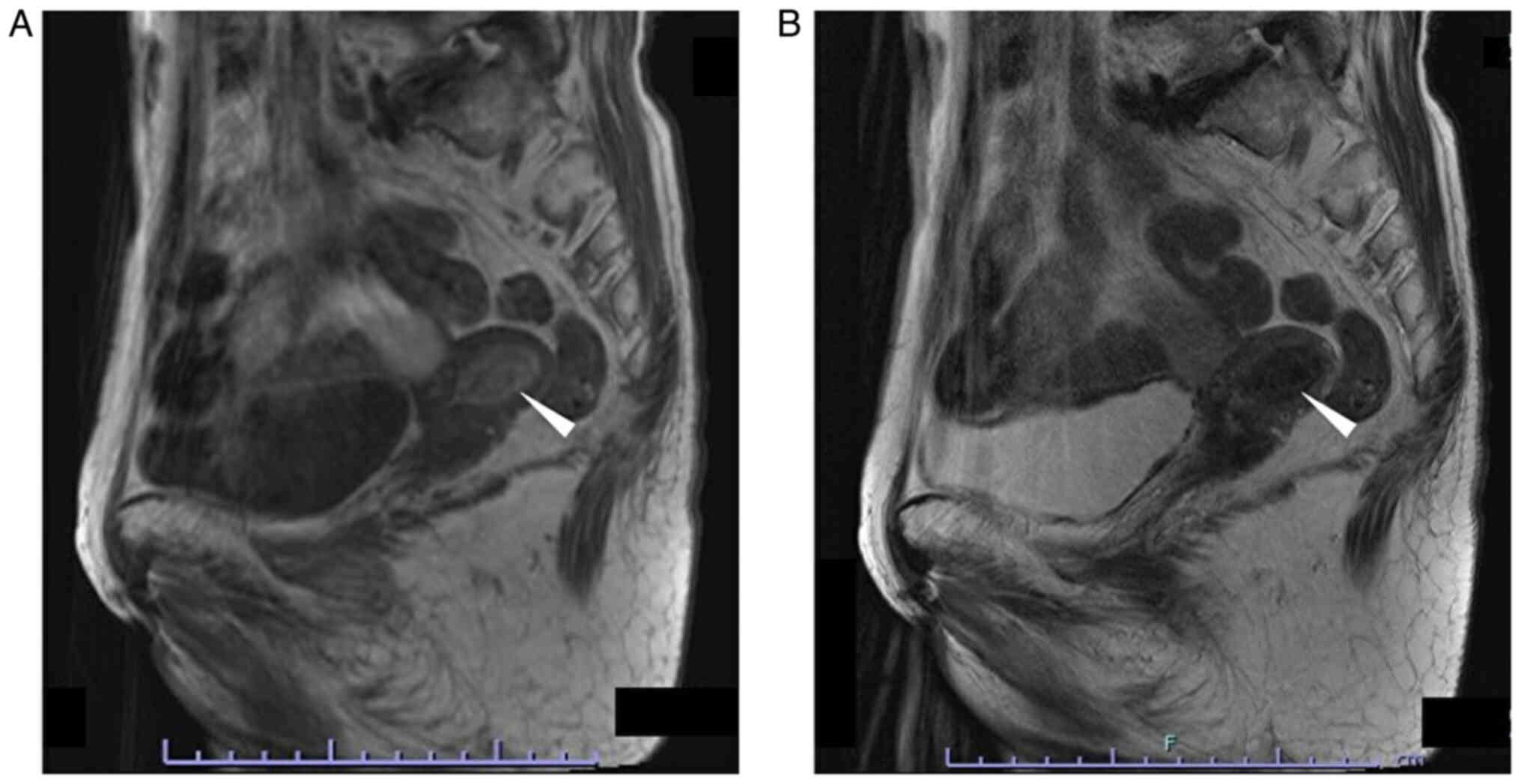 |
Figure 2.
MRI. (A) T1-weighted image. A 26.6×16.6-mm, high-intensity lesion was detected in the uterine cavity (white arrowhead). Repetition time, 500 msec; echo time, 12 msec; flip angle; 150°. (B) T2-weighted image. A 26.6×16.6-mm sized low-intensity lesion was detected in the uterine cavity (white arrowhead). Repetition time, 4,000 msec; echo time, 97 msec; flip angle, 140°. T, tumor.
|
Subsequent endometrial cytology, which was obtained trans-uterine cervically, yielded negative results, whereas endometrial histology analysis, for which hematoxylin and eosin staining was performed in an automated staining instrument (Tissue-Tek® Prisma™ Plus; Sakura Finetek USA, Inc.) according to the manufacturer's instructions and in line with standard protocols using Gill's hematoxylin V solution (Muto Pure Chemicals Co., Ltd.) and pure eosin (Muto Pure Chemicals Co., Ltd.) at the Department of Pathology at Osaka Metropolitan University Hospital, identified mild nuclear atypia and certain cell polarity disorders, which were not sufficient for a definitive diagnosis (Fig. 3A). Therefore, a hysteroscopy and endometrial biopsy were performed. Hysteroscopy revealed a mass lesion of irregular surface on the posterior wall of the uterus (Fig. 3B). Simultaneous endometrial histological examination revealed adenocarcinoma, which had an intestinal-type glandular epithelium with mild nuclear atypia. Immunohistochemical staining using paraffin-embedded tissues revealed that the tissue was positive for mucin (MUC)2 and caudal type homeobox (CDX)2 staining, but negative for estrogen receptor (ER) staining (Fig. 4). Immunohistochemical staining was performed in an automated staining instrument (HISTSTAINER 48A; Nichirei Bioscience Inc.) according to manufacturer's instructions and in line with standard protocols using monoclonal mouse anti-human MUC2 (cat. no. M7313; 50-fold dilution; DAKO), anti-CDX2 antibody (cat. no. ab76541; 500-fold dilution; Abcam) and monoclonal rabbit anti-human ERα (cat. no. IR084; 1.5-fold dilution; DAKO) as the primary antibodies and EnVision FLEX/HRP (cat. no. K8024; DAKO) as the secondary antibody for all, at the Department of Pathology. The patient underwent a simple hysterectomy, bilateral salpingo-oophorectomy, pelvic lymph node and para-aortic lymph node dissection and partial omentectomy. The excised surgical specimen was a 1.6-cm polypoidal exophytic tumor that was grossly confined to the uterus with a stalk (Fig. 5A and B). Microscopic histological images showed a proliferating intestinal-type epithelium, with cysts of various sizes and the majority of mucinous epithelia had polarity. However, severe structural atypia was also observed in part (Fig. 5C-E; arrows). The lesion did not show any myometrial invasion or extramural extension, and there were no metastases in either the bilateral adnexa, pelvic lymph nodes, para-aortic lymph nodes or omentum. This finding led to a diagnosis of intestinal-type mucinous endometrial carcinoma, specifically The International Federation of Gynecology and Obstetrics (version 2008) (7) stage IA (pT1aN0M0). The patient received three cycles of adjuvant chemotherapy consisting of carboplatin (area under the curve=6 mg/ml/min) and 175 mg/m2 paclitaxel. As of the time of writing of the present study, the patient is alive without any evidence of recurrence 6 months after surgery.
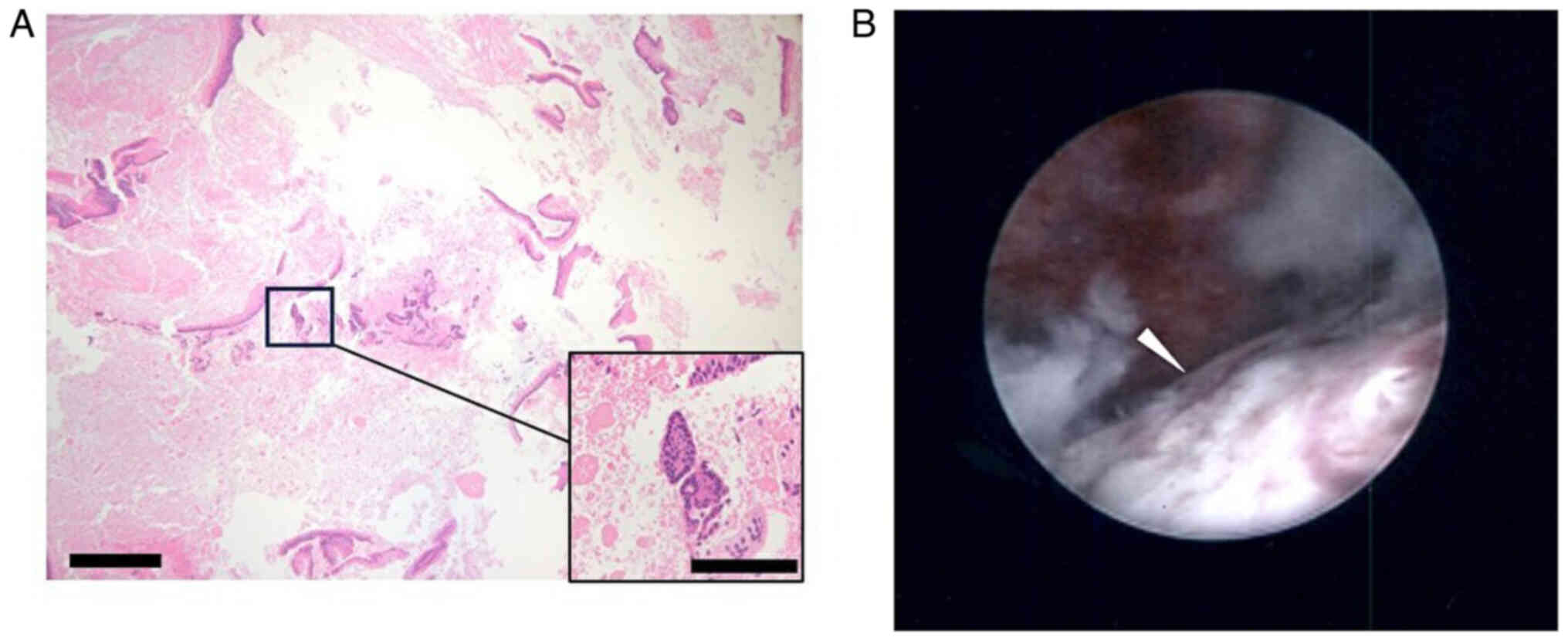 |
Figure 3.
Images of endometrial histology and hysteroscopy. (A) Microscopic image of endometrial histology. In ~50% of the specimen, necrosis may be observed. Although most of the epithelium shows mild atypia, the structure is complex; therefore, malignancy must be assumed (scale bar, 200 and 50 µm in the main window and the magnified window, respectively; hematoxylin and eosin staining). (B) Image of hysteroscopy. An irregular surface mass was detected on the posterior wall of the uterine cavity (white arrowhead).
|
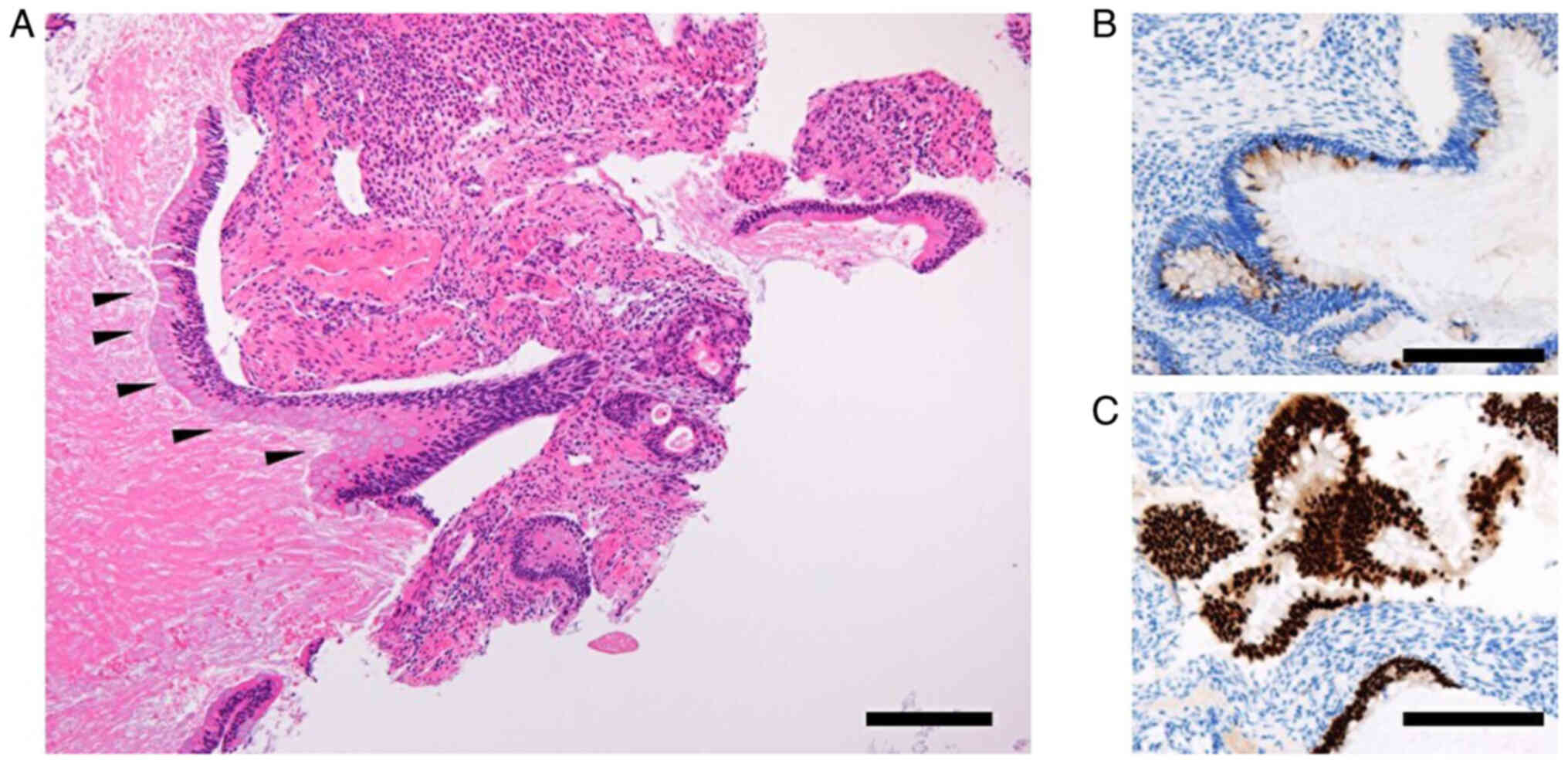 |
Figure 4.
Histology/immunohistochemistry images of the tumor obtained by endometrial biopsy. (A) Endometrial intestinal-type mucinous carcinoma with goblet cells (black arrowheads) (hematoxylin and eosin staining). Positive immunohistochemical staining for (B) mucin 2 and (C) caudal type homeobox 2 (scale bar, 200 µm).
|
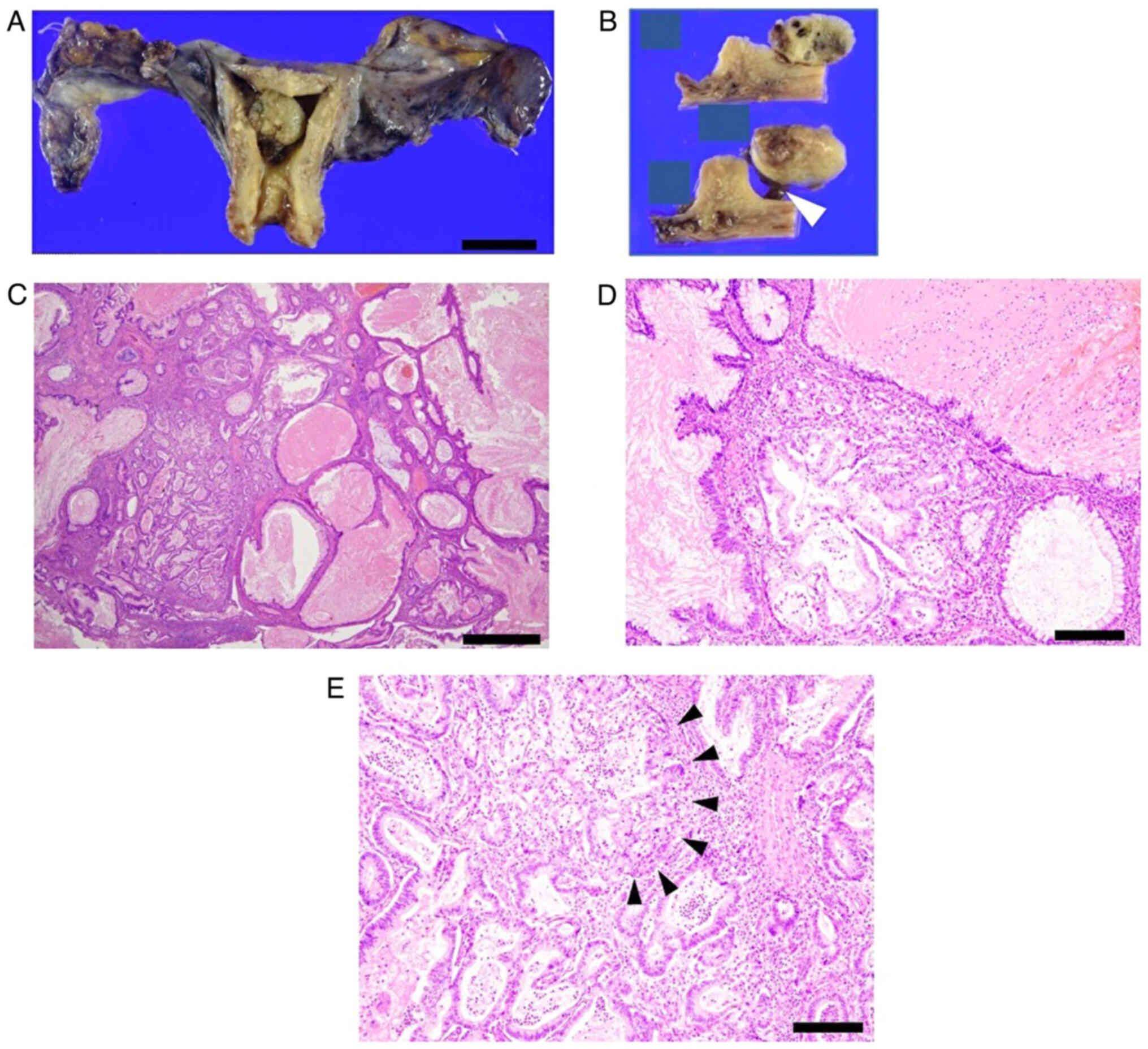 |
Figure 5.
Macroscopic and histology images of the tumor obtained by surgery. (A) Macroscopic image shows a 1.6-cm polypoidal exophytic tumor (scale bar, 5 cm). (B) Cross-section image of the tumor grossly confined to the uterus with a stalk. The stalk is indicated by the white arrowhead. (C) Proliferating intestinal type epithelium shows cysts of various sizes (hematoxylin and eosin staining; scale bar, 1 mm). (D) The majority of the mucinous epithelium has polarity (Hematoxylin and eosin staining); however, (E) severe structural atypia is present (black arrowheads; hematoxylin and eosin staining; scale bar, 200 µm).
|
Discussion
Dridi et al (5) previously found only 11 published cases of endometrial intestinal-type carcinoma that met the diagnostic criteria established by Wong et al (2). Wong et al (2) proposed the following criteria for diagnosing endometrial intestinal-type carcinoma: The tumor i) presents with gastric-type morphology and/or intestinal differentiation, accompanied with goblet cells in an endometrial carcinoma excluding metastasis from another primary site; ii) does not have cervical glandular or stromal involvement; iii) has positive staining for ≥1 gastrointestinal markers, such as CDX2, cytokeratin 20 and MUC6, according to immunohistochemical staining; and iv) has no or minimal (<5%) ER expression according to immunohistochemical staining.
This rare tumor may be misdiagnosed as Müllerian-type endometrial mucinous carcinoma, endocervical adenocarcinoma, metastasis of a gastro-intestinal tract adenocarcinoma or gastric (gastro-intestinal) type mucinous metaplasia (5). The presence of mucin production in both non-neoplastic and neoplastic endometrium is common (6). Therefore, Müllerian-type endometrial mucinous carcinoma should be distinguished from endometrial intestinal-type carcinoma (5). Müllerian-type endometrial mucinous carcinoma frequently exhibits a typical endometrioid component, and shows strong and diffuse positivity for ER and progesterone receptor, which is not typical for endometrial intestinal-type carcinoma (8,9). In addition, MUC2 expression may be useful, as it has been previously shown to be expressed in a notable percentage of cervical intestinal-type adenocarcinoma cases (10). However, confusion between endometrial intestinal-type carcinoma and a direct extension from an endocervical carcinoma should be avoided (5). It is not possible to differentiate whether the intestinal-type adenocarcinoma originated from the endocervical region or the endometrium based on morphological or immunohistochemical features (5). Confirming the endocervical origin of an intestinal-type carcinoma can be facilitated by the detection of high-risk human papillomavirus (5). Overall, sampling the entire cervix appears to be the most effective approach for ruling out direct extension of cervical carcinoma (5). Although the endometrium is less susceptible to metastasis compared with other organs in the female genital tract, such as the ovaries, colonic carcinomas are among the most commonly reported metastatic carcinomas affecting the uterine corpus (11). When considering endometrial intestinal-type carcinoma, it is important to rule out the possibility of metastasis from a gastrointestinal tract carcinoma (12). Medical history of extra-uterine carcinoma is key for diagnosis of metastasis from another organ (12). Furthermore, the WHO classification proposed a method for differentiating between primary endometrial carcinoma and metastatic carcinoma (6). It specifically states that metastatic tumors demonstrate ‘widespread replacement of endometrial stroma while sparing benign endometrial glands, along with the disproportional involvement of serosa or outer myometrium’ (5). Although mucinous metaplasia in the non-neoplastic endometrium is relatively common, and can be found in 8% of all benign endometria, there is limited information on the description of gastric-type and/or intestinal-type metaplasia in the endometrium (2,13–19). Gastric-type and intestinal-type metaplasia of non-malignant lesions should be distinguished from endometrial intestinal-type carcinoma (5). Positive expression of ER, the presence in an endometrial polyp or inside the endometrial mucosa and the absence of atypia are factors that can aid in distinguishing non-atypical gastrointestinal metaplasia from endometrial intestinal-type carcinoma (2,5).
Regarding the present case, all diagnostic criteria described by Wong et al (2) were met. The present case showed intestinal differentiation with goblet cells in an endometrial carcinoma, and the patient did not have any evidence of another primary site of mucinous carcinoma that influenced the endometrium according to CT and MRI. The patient had a medical history of vulvar Paget's disease, but the histological morphology of this endometrial tumor was distinguished from that of vulvar Paget's disease, meaning that this tumor originated from the endometrium. This tumor was a polypoidal exophytic tumor that was confined to the uterus with a stalk, where the lesion did not show myometrial invasion or extramural extension, suggesting the lack of cervical glandular or stromal involvement. Immunohistochemical staining revealed that the tumor was positive for MUC2 and CDX2 expression, which are gastrointestinal markers, but negative for ER. Therefore, these features led to the diagnosis of endometrial intestinal-type carcinoma.
To conclude, the present report described a rare case of intestinal-type mucinous carcinoma of the endometrium, which showed a polypoidal exophytic form. According to a number of previous reports (2,5,6), endometrial intestinal-type carcinoma appears to have a dismal prognosis compared with that of classical mucinous Müllerian type carcinomas of the endometrium (5,6). The present case did not present with any myometrial invasion or extramural extension due to its polypoidal exophytic form, which may have contributed to its favorable prognosis. Given its recent inclusion and definition in the new WHO classification, it is necessary to document further cases to deepen the understanding of and characterize this rare malignancy.
Acknowledgements
Not applicable.
Funding
The present study was funded by The Osaka Medical Research Foundation for Intractable Diseases (grant no. 29-1-46; Osaka, Japan).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Authors' contributions
CN, TF and TS conceived and designed the study. CN, TF, ST, TN, EU, YA, RT, KI, MY, TI, TY and TS acquired, analyzed and interpreted the data. CN, TF and TS drafted and revised the manuscript. TF and TS confirm the authenticity of all the raw data. ST reviewed the pathological specimens. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the patient for the publication of the case details and associated images.
Competing interests
The authors declare that they have no competing interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wong RW, Ralte A, Grondin K, Talia KL and McCluggage WG: Endometrial gastric (gastrointestinal)-type mucinous lesions: Report of a series illustrating the spectrum of benign and malignant lesions. Am J Surg Pathol. 44:406–419. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McCluggage WG, Singh N and Gilks CB: Key changes to the world health organization (WHO) classification of female genital tumours introduced in the 5th edition (2020). Histopathology. 80:762–778. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rauh-Hain JA, Vargas RJ, Clemmer J, Clark RM, Bradford LS, Growdon WB, Goodman A, Boruta DM II, Schorge JO and del Carmen MG: Mucinous adenocarcinoma of the endometrium compared with endometrioid endometrial cancer: A SEER analysis. Am J Clin Oncol. 39:43–48. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dridi M, Peoc'h M and Karpathiou G: Primary endometrial gastric (gastro-intestinal)-type carcinoma: A practical approach. Pathol Res Pract. 241:1542712023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Karpathiou G, Chauleur C, Mathonat L, Dridi M, Picot T, Devouassoux-Shisheboran M and Peoc'h M: Morphologic diversity of the endometrial gastrointestinal-type adenocarcinoma. Pathol Res Pract. 230:1537592022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pecorelli S: Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J gynaecol Obstet. 105:103–104. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shabani N, Mylonas I, Jeschke U, Thaqi A, Kuhn C, Puchner T and Friese K: Expression of estrogen receptors alpha and beta, and progesterone receptors A and B in human mucinous carcinoma of the endometrium. Anticancer Res. 27:2027–2033. 2007.PubMed/NCBI
|
|
9
|
Lax SF, Pizer ES, Ronnett BM and Kurman RJ: Comparison of estrogen and progesterone receptor, Ki-67, and p53 immunoreactivity in uterine endometrioid carcinoma and endometrioid carcinoma with squamous, mucinous, secretory, and ciliated cell differentiation. Hum Pathol. 29:924–931. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mikami Y, Kiyokawa T, Hata S, Fujiwara K, Moriya T, Sasano H, Manabe T, Akahira J, Ito K, Tase T, et al: Gastrointestinal immunophenotype in adenocarcinomas of the uterine cervix and related glandular lesions: A possible link between lobular endocervical glandular hyperplasia/pyloric gland metaplasia and ‘adenoma malignum’. Mod Pathol. 17:962–972. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Karpathiou G, Chauleur C, Hathroubi S and Peoc'h M: Secondary tumors of the gynecologic tract: A clinicopathologic analysis. Int J Gynecol Pathol. 38:363–370. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ronquillo N and Pinto A: Gynaecological or gastrointestinal origin? Recognising Müllerian neoplasms with gastrointestinal phenotype and determining the primary site in selected entities. Pathology. 54:207–216. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rubio A, Schuldt M, Guarch R, Laplaza Y, Giordano G and Nogales FF: Pseudomyxoma-type invasion in gastrointestinal adenocarcinomas of endometrium and cervix: A report of 2 cases. Int J Gynecol Pathol. 35:118–122. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Trippel M, Imboden S, Papadia A, Mueller MD, Mertineit N, Härmä K, Nicolae A, Vassella E and Rau TT: Intestinal differentiated mucinous adenocarcinoma of the endometrium with sporadic MSI high status: A case report. Diagn Pathol. 12:392017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Buell-Gutbrod R, Sung CJ, Lawrence WD and Quddus MR: Endometrioid adenocarcinoma with simultaneous endocervical and intestinal-type mucinous differentiation: Report of a rare phenomenon and the immunohistochemical profile. Diagn Pathol. 8:1282013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zheng W, Yang GC, Godwin TA, Caputo TA and Zuna RE: Mucinous adenocarcinoma of the endometrium with intestinal differentiation: A case report. Hum Pathol. 26:1385–1388. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
McCarthy WA, Makhijani R, Miller K, Rojas K, Beffa L, Mathews C, Robison K and Quddus MR: Gastric-type endometrial adenocarcinoma: Report of two cases in patients from the United States. Int J Surg Pathol. 26:377–381. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hino M, Yamaguchi K, Abiko K, Yoshioka Y, Hamanishi J, Kondoh E, Koshiyama M, Baba T, Matsumura N, Minamiguchi S, et al: Magnetic resonance imaging findings and prognosis of gastric-type mucinous adenocarcinoma (minimal deviation adenocarcinoma or adenoma malignum) of the uterine corpus: Two case reports. Mol Clin Oncol. 4:699–704. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Abiko K, Baba T, Ogawa M, Mikami Y, Koyama T, Mandai M and Konishi I: Minimal deviation mucinous adenocarcinoma (‘adenoma malignum’) of the uterine corpus. Pathol Int. 60:42–47. 2010. View Article : Google Scholar : PubMed/NCBI
|



















