Introduction
Liver cancer, the major subtype of primary liver
cancer, is the fourth leading cause of cancer-related mortality
worldwide (1,2). Although the precise pathophysiological
mechanism of liver cancer remains obscure, some factors (such as
viral infection, metabolism dysregulation, alcohol consumption)
have been identified to be associated with the risk of liver
cancer, thus improving the prevention and screening of liver cancer
(3–6). However, most patients with liver
cancer are diagnosed at an intermediate or advanced stage which
renders radical treatment unavailable, resulting in limited
curative options and a poor survival (7). Therefore, it is essential to reveal
novel and valid molecular mechanisms of liver cancer progression to
identify new treatment targets.
Keratin 15 (KRT15) is a type I intermediate filament
that was originally identified in the basal cells of the epidermis
and stratified squamous epithelia (8). According to previous studies, the
carcinogenic property of KRT15 in several cancers has been
identified (9–11). For instance, a previous study showed
that KRT15 facilitated colorectal cancer cell migration and
invasion in a β-catenin-dependent manner (9). Another study revealed that
KRT15-positive breast ductal progenitors have similar
transcriptomic signatures with basal-like breast cancer (10). In addition, a previous animal study
reported that KRT15-positive crypt cells may originate intestinal
cancer formation and are resistant to radiation (11). Clinically, KRT15 demonstrated the
ability to predict prognosis in several gastrointestinal carcinomas
including esophageal, gastric and colorectal cancer (12–14).
However, the mechanism involved with regard to the function of
KRT15 in liver cancer remains poorly elucidated.
Therefore, the aim of the present study was to
explore the effect of KRT15 knockdown on liver cancer viability,
mobility and chemoresistance, as well as its interaction with the
β-catenin pathway.
Materials and methods
Cell lines
The normal liver cell line THLE-2 (Cat. No.
iCell-h38) cell lines were provided by the iCell Bioscience Inc.
and liver cancer cell lines including Huh7 (Cat. No. CL-0120), PLC
(Cat. No. CL-0415), Hep3B (Cat. No. CL-0102), HepG2 (Cat. No.
CL-0103), and Li-7 (Cat. No. CL-0139) were obtained from Procell
Life Science & Technology Co., Ltd. Cells of THLE-2 were
maintained in BEGM (Lonza Group, Ltd.), cells including PLC, Hep3B,
HepG2 and Li-7 were maintained in RPMI-1640 medium (Procell Life
Science & Technology Co., Ltd.) and Huh7 cells were maintained
in DMEM (Procell Life Science & Technology Co., Ltd. The medium
was supplemented with 10% fetal bovine serum (FBS; Sigma-Aldrich;
MilliporeSigma) and 1% penicillin/streptomycin (Procell Life
Science & Technology Co., Ltd.). All cells were cultured in a
humidity incubator with 5% CO2 at 37°C. The expression
level of KRT15 in these cells was assessed using reverse
transcription-quantitative PCR (RT-qPCR) and western blot
analyses.
KRT15 regulation
The KRT15 small interfering (si)RNA (si-KRT15, 5′
AGGAGTACAAGATGCTGCTTGACAT 3′) and negative control (si-NC, scramble
siRNA, 5′ AGGATACGATACGGTTCGTAGACAT 3′), KRT15 overexpression
plasmid (oe-KRT15, pcDNA3.1 vector containing the cDNA of KRT15,
NM_002275.4) and negative control overexpression plasmid (oe-NC,
empty pcDNA3.1 vector) were acquired from GenScript. Huh7 and HepG2
cells were plated and transfected with 50 pM si-NC or si-KRT15, 0.8
µg oe-NC or oe-KRT15 in the presence of Lipofectamine®
3000 (Sigma-Aldrich; MilliporeSigma) according to the
manufacturer's protocol at 37°C for 6 h. Untransfected cells served
as the control group. After 72 h of culture, cells were collected
for RT-qPCR and western blotting. Subsequently, Cell Counting Kit-8
(CCK-8), Annexin V/Propidium iodide (AV/PI), and Transwell assays
were performed to assess the effect of KRT15 on the viability,
apoptosis and invasion of liver cancer cells. The doxorubicin (Dox)
sensitivity assay was carried out using the CCK-8 method.
CHIR-99021 treatment
CHIR-99021, an agonist of the β-catenin pathway, was
used to assess the regulation of the β-catenin pathway by KRT15 in
liver cancer cells. Huh7 and HepG2 cells were transfected as
aforementioned. The 5×105 transfected cells were then
cultured for 24 h with or without CHIR-99021 treatment (5 µM;
MedChemExpress) at 37°C. In addition, cells without transfection
and CHIR-99021 treatment served as the control group. The
concentration of CHIR-99021 was determined according to a previous
study (15) and a preliminary
experiment performed in the present study. Western blotting, CCK-8,
AV/PI, Transwell, and drug sensitivity assays were performed.
RT-qPCR
Briefly, total RNA of THLE-2 or liver cancer cells
was extracted using Beyozol (Beyotime Institute of Biotechnology).
The RT-qPCR assays were carried out using a One-Step SYBR Green
RT-qPCR Kit (Wuhan Servicebio Technology Co., Ltd.) according to
the manufacturer's protocol. KRT15 gene expression was assessed
using the 2−ΔΔCq method (16). The thermal cycles for qPCR were as
followings: 98°C for 2 min (min), 1 cycle; 98°C for 15 sec (sec),
61°C for 20 sec, 40 cycles. The sets of primers were as follows:
KRT15 forward, 5′-AGGACTGACCTGGAGATGCAGA-3′ and reverse,
5′-TGCGTCCATCTCCACATTGACC-3′; GAPDH forward,
5′-GAGTCCACTGGCGTCTTCAC-3′ and reverse,
5′-ATCTTGAGGCTGTTGTCATACTTCT-3′.
Western blotting
Briefly, THLE-2 or liver cancer cells were lysed
with RIPA buffer (Wuhan Servicebio Technology Co., Ltd.) and the
concentration of total protein was measured using a BCA Protein
Assay kit (Cat. No. ml095490; Shanghai Enzyme-Linked Biotechnology
Co., Ltd.). The 20 µg protein was then separated by 4–20% SDS-PAGE
and transferred to a nitrocellulose membrane (Beyotime Institute of
Biotechnology). After being blocked with 5% BSA (Wuhan Servicebio
Technology Co., Ltd.) for 90 min at 37°C, the membranes were
incubated with primary antibodies at the recommended dilution
ratio, including KRT15 (1:10,000; Cat. No. ab52816; Abcam),
β-catenin (1:10,000; Cat. No. ab32572; Abcam), cyclin D1 (1:2,000;
Cat. No. ab239794; Abcam), c-Myc (1:1,000; Cat. No. ab32072;
Abcam), and GAPDH (1:10,000; Cat. No. ab8245; Abcam) at 4°C
overnight. Subsequently, the membranes were incubated using diluted
HRP linked secondary antibody (1:20,000; Cat. No. ab6721; Abcam) at
37°C for 1.5 h and detected using an ECL kit (Wuhan Servicebio
Technology Co., Ltd.). The grey value was analyzed by Image J (1.0,
NIH).
Cell viability assay
Briefly, the 3×103 treated Huh7 and HepG2
cells were plated and cultured for 24 h at 37°C. The CCK-8 mixture
(10 µl/well; APeXBIO Technology LLC) was then used, and the cells
were incubated for 2 h at 37°C. The optical density (OD) values
were read using a microplate reader (Rayto Life and Analytical
Sciences Co., Ltd.). The viability of Huh7 and HepG2 cells was
evaluated according to the OD values.
Cell apoptosis assay
The apoptosis of treated cells was evaluated using
an Annexin V-FITC/PI Kit (Nanjing Jiancheng Bioengineering
Institute). Huh7 and HepG2 cells were collected, washed, and
resuspended in Annexin V binding reagent. Subsequently, an Annexin
V-FITC and PI mixture was added to the cells and incubated for 20
min at 37°C. Finally, the cells were analyzed using BD FACSCanto
(BD Biosciences) and Flowjo 10 (BD Biosciences).
Transwell assay
Following treatment, the 4×104 Huh7 and
HepG2 cells were resuspended in FBS-free medium (DMEM for Huh7
cells, RPMI-1640 for HepG2) and seeded into the upper chamber of a
Matrigel-coated Transwell plate (37°C for 1 h; Corning, Inc.).
Complete medium (10% FBS-containing DMEM or RPMI-1640) was added to
the lower chambers. After being cultured for 24 h at 37°C, the
invasive cells were stained using crystal violet (Wuhan Servicebio
Technology Co., Ltd.) for 20 min at room temperature and counted
under an inverted light microscope (Leica Microsystems, Inc.;
magnification was X200.
Drug sensitivity assay
A total of 5×104 treated Huh7 and HepG2
cells were plated and stimulated with 0, 1, 2, 4, 8, 16, and 32 µM
Dox (MedChemExpress) according to a previous study (17) and a preliminary experiment performed
in the present study. After 48 h of incubation at 37°C, a CCK-8
assay was carried out as aforementioned, and the half maximal
inhibitory concentration (IC50) of Dox was analyzed by a
sigmoidal dose-response function curve (18).
Statistical analysis
The data (in triplets) were represented as mean ±
standard deviation. The comparisons were performed using one-way
ANOVA with Tukey's or Dunnett's post hoc test. All analyses were
performed using GraphPad Prism (V.7.0; GraphPad Software, Inc.;
Dotmatics). P<0.05 was considered to indicate a statistically
significant difference.
Results
KRT15 expression among different cell
lines
KRT15 gene expression was enhanced in liver cancer
cell lines (including Huh7, PLC, Hep3B and HepG2 cells) compared
with normal liver cell line, THLE-2 cells (all P<0.01; Fig. 1A). Moreover, KRT15 protein
expression was also increased in Huh7, PLC, Hep3B, and HepG2 cells
compared with THLE-2 cells (all P<0.05; Fig. 1B and C). Subsequently, Huh7 cells
and HepG2 cells were selected for the following functional
experiments, since they had the highest and second highest
expression level of KRT15 among the cell lines. si-KRT15 was then
transfected into Huh7 cells and HepG2 cells, leading to a decrease
in KRT15 gene expression (both P<0.001; Fig. 1D) as well as protein expression
(both P<0.001; Fig. 1E and
F).
Effect of si-KRT15 on viability,
apoptosis, invasion, and chemosensitivity in liver cancer cell
lines
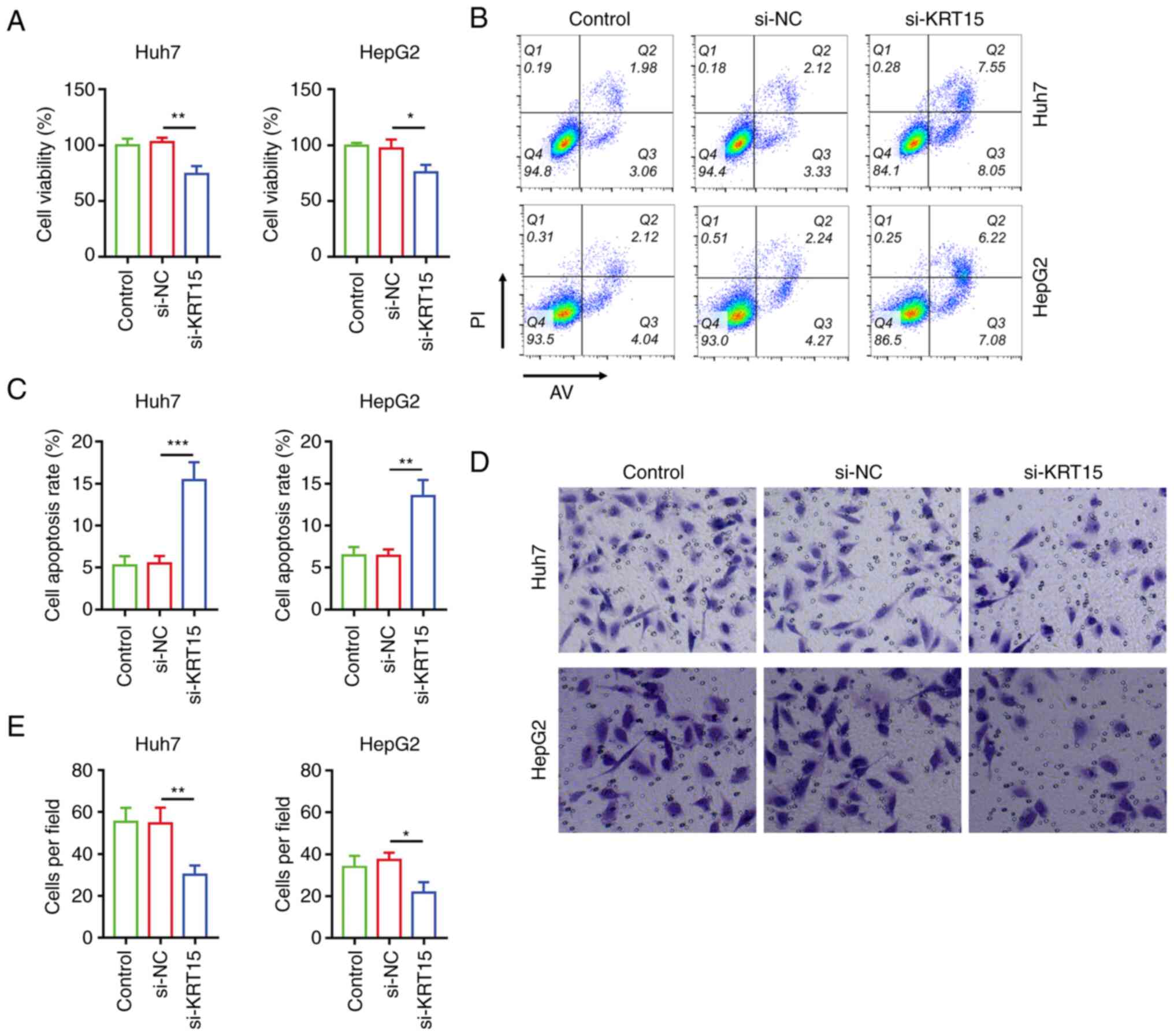
Following transfection, si-KRT15 reduced the
viability of Huh7 cells (P<0.01) and HepG2 cells (P<0.05)
compared with si-NC (Fig. 2A).
Moreover, si-KRT15 enhanced the cell apoptosis rate in HuH7 cells
(P<0.001) and HepG2 cells (P<0.01; Fig. 2B and C). In addition, si-KRT15 also
reduced the invasive cell count in HuH7 cells (P<0.01) and HepG2
cells (P<0.05; Fig. 2D and E).
Subsequently, the effect of si-KRT15 on chemosensitivity to Dox in
liver cancer cell lines was explored. It was observed that si-KRT15
reduced the IC50 value of Dox in Huh7 cells (P<0.01)
as well as in HepG2 cells (P<0.05) compared with si-NC (Fig. 3A and B), suggesting the enhancement
of chemosensitivity to Dox.
Regulatory function of si-KRT15 on the
β-catenin pathway
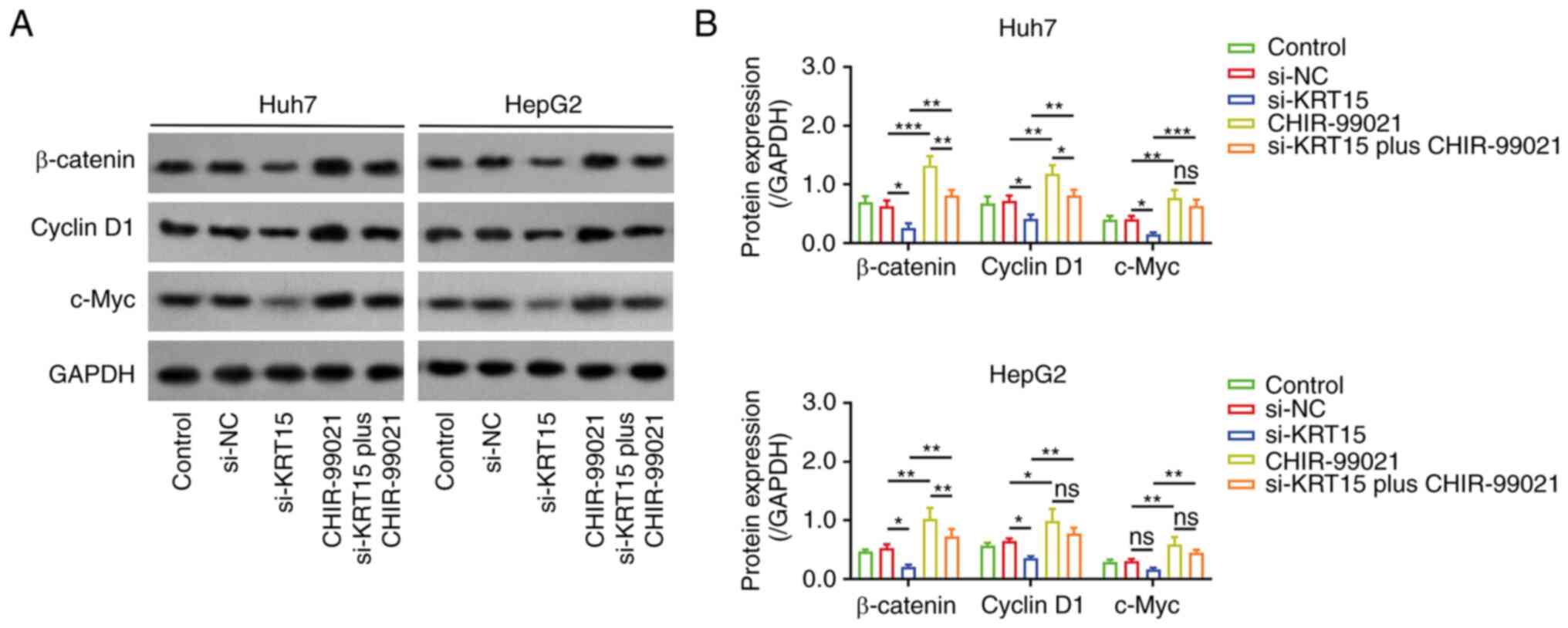
Subsequently, western blot analysis was performed to
determine the effect of si-KRT15 on the β-catenin pathway. Notably,
it was observed that si-KRT15 downregulated the expression of
β-catenin (P<0.01), cyclin D1 (P<0.01), and c-Myc (P<0.05)
compared with si-NC in Huh7 cells (Fig.
4A and B). si-KRT15 also decreased the expression of β-catenin
(P<0.05) and cyclin D1 (P<0.05), while it did not
significantly affect the expression of c-Myc in HepG2 cells.
Effect of CHIR-99021 on
si-KRT15-induced β-catenin inactivation
CHIR-99021 (an agonist of β-catenin) was added for
subsequent experiments. CHIR-99021 upregulated the expression
levels of β-catenin, cyclin D1, and c-Myc (all P<0.05) compared
with si-NC in liver cancer cell lines (Fig. 5A and B). Notably, CHIR-99021
attenuated the effect of si-KRT15 on regulating the β-catenin
pathway. Furthermore, it was also observed that oe-KRT15
upregulated the expression levels of β-catenin, cyclin D1, and
c-Myc, but si-KRT15 downregulated them, in the liver cancer cell
lines (Fig. S1A and B).
Effect of CHIR-99021 on
si-KRT15-modified liver cancer cell functions
CHIR-99021 treatment increased the viability of Huh7
cells (P<0.01) and HepG2 cells (P<0.05; Fig. 6A) compared with si-NC. However,
CHIR-99021 treatment decreased the cell apoptosis rate in Huh7
cells (P<0.01) and HepG2 cells (P<0.05; Fig. 6B and C). Moreover, CHIR-99021
treatment enhanced the invasive cell count in Huh7 cells and HepG2
cells (both P<0.01; Fig. 6D and
E). Notably, CHIR-99021 treatment attenuated the effect of
si-KRT15 on mediating cell viability, the apoptosis rate, and
invasion in Huh7 cells and HepG2 cells (all P<0.01; Fig. 6A-E).
 | Figure 6.CHIR-99021 attenuates
si-KRT15-induced viability and invasion of liver cancer cell lines.
(A) Comparison of cell viability among si-NC, si-KRT15, CHIR-99021,
and si-KRT15 plus CHIR-99021 groups in liver cancer cell lines. (B)
Flow cytometric analysis and (C) quantitative comparison of the
cell apoptosis rate among si-NC, si-KRT15, CHIR-99021, and si-KRT15
plus CHIR-99021 groups in liver cancer cell lines. (D) Images of
Transwell cell invasion (200 X) and (E) quantitative comparison of
invasive cells among si-NC, si-KRT15, CHIR-99021, and si-KRT15 plus
CHIR-99021 groups in liver cancer cell lines. *P<0.05,
**P<0.01 and ***P<0.001. si-, small interfering RNA; KRT15,
keratin 15; NC, negative control; ns, not significant. |
Subsequently, it was also demonstrated that
CHIR-99021 treatment greatly increased the IC50 value of
Dox in Huh7 cells (P<0.001) and HepG2 cells (P<0.01; Fig. 7A and B). In addition, CHIR-99021
alleviated the effect of si-KRT15 on regulating chemosensitivity to
Dox in liver cancer cell lines (both P<0.01).
Discussion
In the clinical field, KRT15 exhibits diagnostic
value and prognostic value in gastric, colorectal, endometrial, and
breast invasive cancer (13,14,19,20).
Regarding its application in patients with liver cancer, one
previous bioinformatic study reported that KRT15 is an upregulated
gene in patients with liver cancer with viral infection compared
with controls (19). The present
study detected KRT15 mRNA and protein expression in liver cancer
cell lines and a normal liver cell line, and then observed an
upregulation of KRT15 expression at both the mRNA and protein
levels in liver cancer cell lines. The possible explanation was
that KRT15 reflected cell malignant growth and stemness, which were
obviously elevated in the liver cancer cell lines versus the normal
cell line; therefore, KRT15 demonstrated a higher trend in the
liver cancer cell lines.
In addition, KRT15 was demonstrated to promote
colorectal cancer cell mobility (9), and to be associated with tumor size,
invasive degree, lymph-node metastasis, and increased TNM stage in
patients with esophageal carcinoma and colorectal cancer (12,14).
These previous studies indicate its implication in regulating
gastrointestinal carcinoma malignant behaviors. Furthermore,
loss-of-function experiments revealed that KRT15 knockdown impaired
viability and invasion, while it promoted the apoptosis rate in
liver cancer cell lines, which could be explained as follows: i)
KRT15 was associated with cancer stemness, the latter closely
involved in the invasion of cancer cells (21,22),
therefore, KRT15 knockdown reduced liver cancer cell invasion; and
ii) KRT15 knockdown inactivated the β-catenin pathway (9), to repress the cell viability of liver
cancer. These findings suggested that KRT15 serves as an oncogene
in liver cancer pathogenesis, and its silencing could be a
therapeutic option for liver cancer management.
Dox is a common type of anthracycline that exerts an
antitumor effect in a wide spectrum of solid tumors (23). Regarding the application of Dox in
treating patients with liver cancer, it is usually loaded during
the transarterial chemoembolization (TACE) procedure which is a
cornerstone for intermediate-stage liver cancer treatment (24,25).
However, resistance to Dox may occur after several TACE procedures
in patients with liver cancer, which further impairs their response
and survival (26,27). Some aspects have been revealed to be
involved in the chemoresistance of liver cancer, such as stemness,
DNA damage repair, ATP binding cassette, etc. (28,29).
In addition, identifying molecules that are involved in acquiring
chemoresistance to Dox may serve as one crucial method to enhance
chemosensitivity and therapeutic outcomes for liver cancer
management. In the present study, the viability of liver cancer
cell lines transfected with si-KRT15 under different Dox
concentrations was determined, and then IC50 values of
doxorubicin were also calculated. It was then revealed that KRT15
knockdown enhanced the chemosensitivity to Dox in liver cancer cell
lines, indicating the potential therapeutic value of KRT15
knockdown for enhancing the response to Dox. The possible
explanation was that KRT15 knockdown weakened the cancer stemness,
thus restoring the sensitivity of chemotherapeutics (21,22,30).
However, Dox-resistant liver cancer cell lines were not established
in the present study, which served as a limitation for
comprehensively analyzing the effect of KRT15 on chemoresistance.
Besides, The lack of in vivo validation is also another
limitation of the study.
The β-catenin pathway is a well-established
oncogenic pathway in liver cancer (31). The canonical β-catenin pathway
involves binding of Wnt to its membrane receptors followed by the
upregulation of β-catenin, which further forms the destruction
complex and translocates to the nucleus to initiate the expression
of cell division-related transcription factors (such as c-Myc and
cyclin D1) for carcinogenesis (31–33).
In a previous study, KRT15 was shown to promote the
β-catenin-mediated MMP7 pathway in colorectal cancer (9). In line with this previous study, KRT15
knockdown inactivated the β-catenin pathway in liver cancer in the
present study. In addition, CHIR-99021, an agonist for β-catenin
that activates the β-catenin pathway in endodermal differentiation,
follicle development, and cerebral organoids (34–36),
was added for subsequent compensative experiments in the present
study. It was discovered that CHIR-99021 enhanced viability and
invasion while reducing chemosensitivity to Dox in liver cancer
cell lines, which could be due to activation of the β-catenin
pathway (37,38). Furthermore, the present study also
observed that the administration of CHIR-99021 alleviated the
effect of KRT15 knockdown on cell viability, apoptosis, invasion
and Dox chemosensitivity in liver cancer cell lines, which further
confirmed the implication of the β-catenin pathway in the effect of
KRT15 on liver cancer malignant behaviors. However, other types of
β-catenin agonists could be applied in future studies to further
validate the effect of β-catenin in KRT15-mediated liver cancer
pathogenesis, and si-NC plus CHIR-99021 could be added to further
confirm the effect.
In conclusion, KRT15 knockdown impairs viability and
mobility while it enhances chemosensitivity to Dox by inactivating
the β-catenin pathway in liver cancer, implying its potency as a
treatment target of liver cancer. However, further validation is
warranted.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JW and GZ contributed to study conception and
design. Material preparation, data collection and analysis were
performed by JW and GZ. JW wrote the first draft of the manuscript.
JW and GZ confirm the authenticity of all the raw data. Both
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vogel A, Meyer T, Sapisochin G, Salem R
and Saborowski A: Hepatocellular carcinoma. Lancet. 400:1345–1362.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chidambaranathan-Reghupaty S, Fisher PB
and Sarkar D: Hepatocellular carcinoma (HCC): Epidemiology,
etiology and molecular classification. Adv Cancer Res. 149:1–61.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
D'Souza S, Lau KC, Coffin CS and Patel TR:
Molecular mechanisms of viral hepatitis induced hepatocellular
carcinoma. World J Gastroenterol. 26:5759–5783. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang C, Huang X, Liu Z, Qin W and Wang C:
Metabolism-associated molecular classification of hepatocellular
carcinoma. Mol Oncol. 14:896–913. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sasaki-Tanaka R, Ray R, Moriyama M, Ray RB
and Kanda T: Molecular changes in relation to alcohol consumption
and hepatocellular carcinoma. Int J Mol Sci. 23:96792022.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Torimura T and Iwamoto H: Treatment and
the prognosis of hepatocellular carcinoma in Asia. Liver Int.
42:2042–2054. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Moll R, Divo M and Langbein L: The human
keratins: Biology and pathology. Histochem Cell Biol. 129:705–733.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen W and Miao C: KRT15 promotes
colorectal cancer cell migration and invasion through
β-catenin/MMP-7 signaling pathway. Med Oncol. 39:682022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kohler KT, Goldhammer N, Demharter S,
Pfisterer U, Khodosevich K, Rønnov-Jessen L, Petersen OW, Villadsen
R and Kim J: Ductal keratin 15+ luminal progenitors in
normal breast exhibit a basal-like breast cancer transcriptomic
signature. NPJ Breast Cancer. 8:812022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Giroux V, Stephan J, Chatterji P, Rhoades
B, Wileyto EP, Klein-Szanto AJ, Lengner CJ, Hamilton KE and Rustgi
AK: Mouse intestinal Krt15+ crypt cells are radio-resistant and
tumor initiating. Stem Cell Reports. 10:1947–1958. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin JB, Feng Z, Qiu ML, Luo RG, Li X and
Liu B: KRT 15 as a prognostic biomarker is highly expressed in
esophageal carcinoma. Future Oncol. 16:1903–1909. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang C, Liang Y, Ma MH, Wu KZ and Dai DQ:
KRT15, INHBA, MATN3, and AGT are aberrantly methylated and
differentially expressed in gastric cancer and associated with
prognosis. Pathol Res Pract. 215:893–899. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rao X, Wang J, Song HM, Deng B and Li JG:
KRT15 overexpression predicts poor prognosis in colorectal cancer.
Neoplasma. 67:410–414. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li Y, Liu Y, Liu B, Wang J, Wei S, Qi Z,
Wang S, Fu W and Chen YG: A growth factor-free culture system
underscores the coordination between Wnt and BMP signaling in
Lgr5+ intestinal stem cell maintenance. Cell Discov.
4:492018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lei KF, Liu TK and Tsang NM: Towards a
high throughput impedimetric screening of chemosensitivity of
cancer cells suspended in hydrogel and cultured in a paper
substrate. Biosens Bioelectron. 100:355–360. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tian S, Lou L, Tian M, Lu G, Tian J and
Chen X: MAPK4 deletion enhances radiation effects and triggers
synergistic lethality with simultaneous PARP1 inhibition in
cervical cancer. J Exp Clin Cancer Res. 39:1432020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang H, Li A, Li A, Zhao F and Zhang T:
Upregulated keratin 15 links to the occurrence of lymphovascular
invasion, stromal cervical invasion as well as unfavorable survival
profile in endometrial cancer patients. Medicine (Baltimore).
101:e296862022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhong P, Shu R, Wu H, Liu Z, Shen X and Hu
Y: Low KRT15 expression is associated with poor prognosis in
patients with breast invasive carcinoma. Exp Ther Med. 21:3052021.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zekri AN, El-Sisi ER, Abdallah ZF, Ismail
A and Barakat Barakat A: Gene expression profiling of circulating
CD133+ cells of hepatocellular carcinoma patients
associated with HCV infection. J Egypt Natl Canc Inst. 29:19–24.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Abbas O, Richards JE, Yaar R and
Mahalingam M: Stem cell markers (cytokeratin 15, cytokeratin 19 and
p63) in in situ and invasive cutaneous epithelial lesions. Mod
Pathol. 24:90–97. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alam Khan S and Jawaid Akhtar M:
Structural modification and strategies for the enhanced doxorubicin
drug delivery. Bioorg Chem. 120:1055992022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chang Y, Jeong SW, Young Jang J and Jae
Kim Y: Recent updates of transarterial chemoembolilzation in
hepatocellular carcinoma. Int J Mol Sci. 21:81652020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Manjunatha N, Ganduri V, Rajasekaran K,
Duraiyarasan S and Adefuye M: Transarterial chemoembolization and
unresectable hepatocellular carcinoma: A narrative review. Cureus.
14:e284392022.PubMed/NCBI
|
|
26
|
Al-Malky HS, Al Harthi SE and Osman AM:
Major obstacles to doxorubicin therapy: Cardiotoxicity and drug
resistance. J Oncol Pharm Pract. 26:434–444. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Buschauer S, Koch A, Wiggermann P, Müller
M and Hellerbrand C: Hepatocellular carcinoma cells surviving
doxorubicin treatment exhibit increased migratory potential and
resistance to doxorubicin re-treatment in vitro. Oncol Lett.
15:4635–4640. 2018.PubMed/NCBI
|
|
28
|
Marin JJG, Macias RIR, Monte MJ, Romero
MR, Asensio M, Sanchez-Martin A, Cives-Losada C, Temprano AG,
Espinosa-Escudero R, Reviejo M, et al: Molecular bases of drug
resistance in hepatocellular carcinoma. Cancers (Basel).
12:16632020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ceballos MP, Rigalli JP, Cere LI, Semeniuk
M, Catania VA and Ruiz ML: ABC transporters: Regulation and
association with multidrug resistance in hepatocellular carcinoma
and colorectal carcinoma. Curr Med Chem. 26:1224–1250. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nagaraju GP, Farran B, Luong T and
El-Rayes BF: Understanding the molecular mechanisms that regulate
pancreatic cancer stem cell formation, stemness and
chemoresistance: A brief overview. Semin Cancer Biol. 88:67–80.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
He S and Tang S: WNT/β-catenin signaling
in the development of liver cancers. Biomed Pharmacother.
132:1108512020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schaefer KN, Bonello TT, Zhang S, Williams
CE, Roberts DM, McKay DJ and Peifer M: Supramolecular assembly of
the beta-catenin destruction complex and the effect of Wnt
signaling on its localization, molecular size, and activity in
vivo. PLoS Genet. 14:e10073392018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pfister AS and Kühl M: Of Wnts and
ribosomes. Prog Mol Biol Transl Sci. 153:131–155. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ma Y, Ma M, Sun J, Li W, Li Y, Guo X and
Zhang H: CHIR-99021 regulates mitochondrial remodelling via
β-catenin signalling and miRNA expression during endodermal
differentiation. J Cell Sci. 132:jcs2299482019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Feng Z, Mabrouk I, Msuthwana P, Zhou Y,
Song Y, Gong H, Li S, Min C, Ju A, Duan A, et al: In ovo injection
of CHIR-99021 promotes feather follicles development via activating
Wnt/β-catenin signaling pathway during chick embryonic period.
Poult Sci. 101:1018252022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Delepine C, Pham VA, Tsang HWS and Sur M:
GSK3ß inhibitor CHIR 99021 modulates cerebral organoid development
through dose-dependent regulation of apoptosis, proliferation,
differentiation and migration. PLoS One. 16:e02511732021.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xu C, Xu Z, Zhang Y, Evert M, Calvisi DF
and Chen X: β-Catenin signaling in hepatocellular carcinoma. J Clin
Invest. 132:e1545152022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Leung RWH and Lee TKW: Wnt/β-Catenin
signaling as a driver of stemness and metabolic reprogramming in
hepatocellular carcinoma. Cancers (Basel). 14:54682022. View Article : Google Scholar : PubMed/NCBI
|