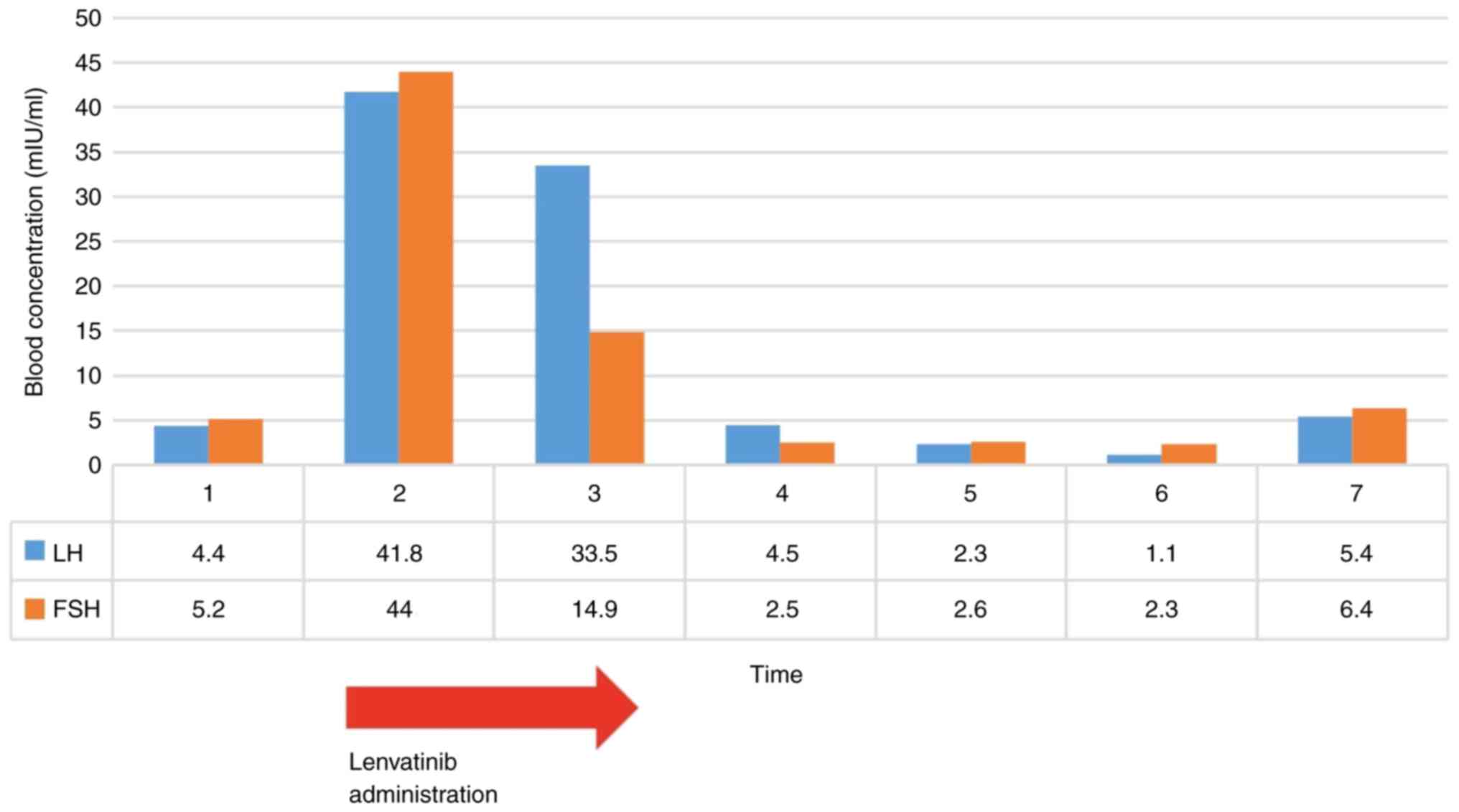
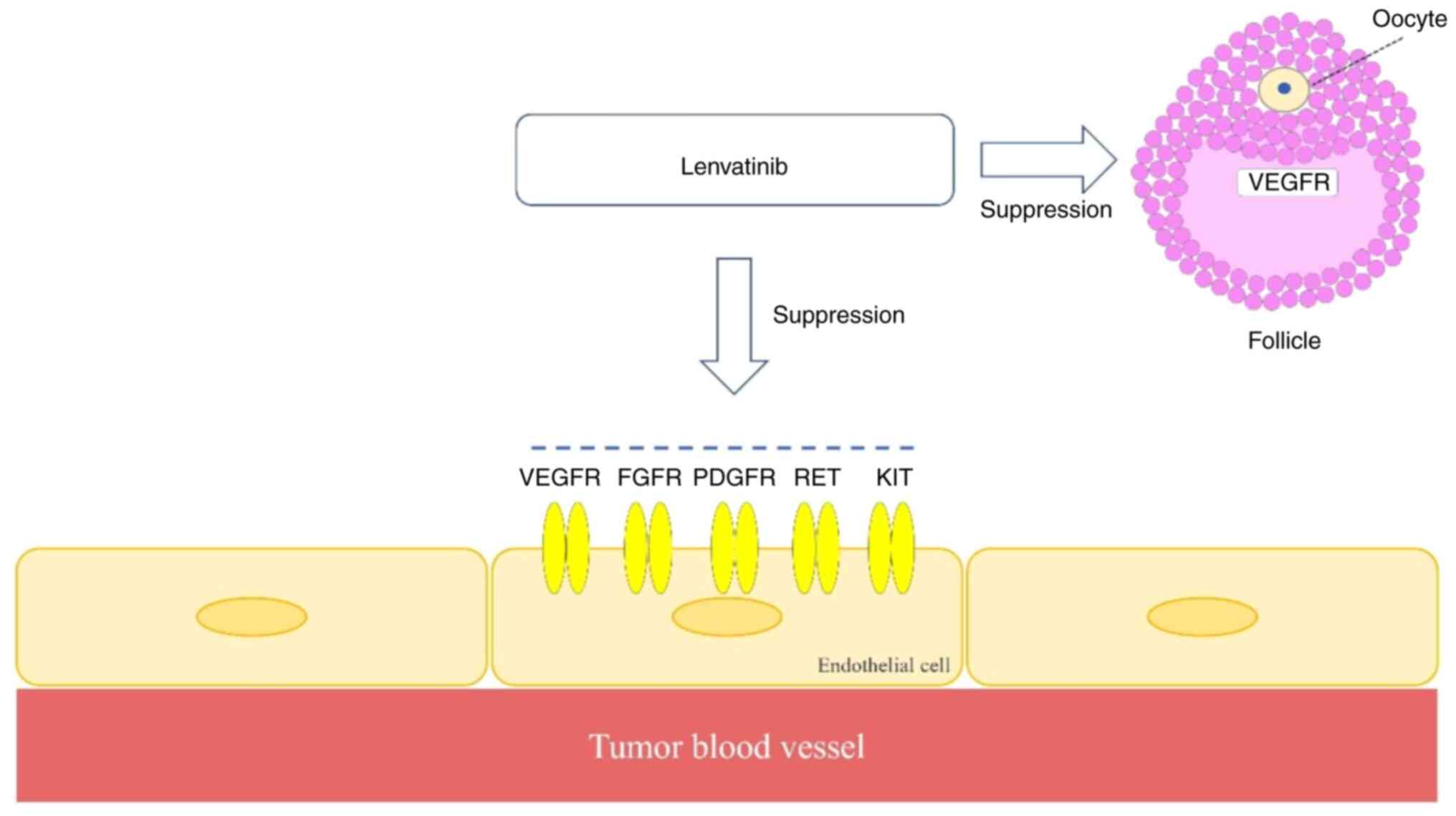
|
1
|
Santos R, Ursu O, Gaulton A, Bento AP,
Donadi RS, Bologa CG, Karlsson A, Al-Lazikani B, Hersey A, Oprea TI
and Overington JP: A comprehensive map of molecular drug targets.
Nat Rev Drug Discov. 16:19–34. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kao WH, Kuo CF, Chiou MJ, Liu YC, Wang CC,
Hong JH, Hsu JT, Chiang YJ and Chuang YF: Adverse birth outcomes in
adolescent and young adult female cancer survivors: A nationwide
population-based study. Br J Cancer. 122:918–924. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
El-Serag HB: Epidemiology of viral
hepatitis and hepatocellular carcinoma. Gastroenterology.
142:1264–1273. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Di Bisceglie AM: Natural history of
hepatitis C: Its impact on clinical management. Hepatology.
31:1014–1018. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McGlynn KA, Petrick JL and El-Serag HB:
Epidemiology of hepatocellular carcinoma. Hepatology. 73 (Suppl
1):S4–S13. 2021. View Article : Google Scholar
|
|
7
|
Lam CM, Chan AO, Ho P, Ng IO, Lo CM, Liu
CL, Poon RT and Fan ST: Different presentation of hepatitis
B-related hepatocellular carcinoma in a cohort of 1863 young and
old patients-implications for screening. Aliment Pharmacol Ther.
19:771–777. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tateishi R, Okanoue T, Fujiwara N, Okita
K, Kiyosawa K, Omata M, Kumada H, Hayashi N and Koike K: Clinical
characteristics, treatment, and prognosis of non-B, non-C
hepatocellular carcinoma: A large retrospective multicenter cohort
study. J Gastroenterol. 50:350–360. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu J, Tang B, Lv X, Meng M, Weng Q, Zhang
N, Li J, Fan K, Zheng L, Fang S, et al: Identifying
apoptosis-related transcriptomic aberrations and revealing clinical
relevance as diagnostic and prognostic biomarker in hepatocellular
carcinoma. Front Oncol. 10:5191802021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ye SL, Chen X, Yang J, Bie P, Zhang S, Liu
F, Liu L, Zhou J, Dou K, Yip CS and Yang X: Evaluation of sorafenib
in Chinese unresectable hepatocellular carcinoma patients with
prior surgery and portal vein tumor thrombosis: A subset analysis
of GIDEON study data. Tumour Biol. 39:10104283176950302017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kudo M, Finn RS, Qin S, Han KH, Ikeda K,
Piscaglia F, Baron A, Park JW, Han G, Jassem J, et al: Lenvatinib
versus sorafenib in first-line treatment of patients with
unresectable hepatocellular carcinoma: A randomised phase 3
non-inferiority trial. Lancet. 391:1163–1173. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matsui J, Yamamoto Y, Funahashi Y,
Tsuruoka A, Watanabe T, Wakabayashi T, Uenaka T and Asada M: E7080,
a novel inhibitor that targets multiple kinases, has potent
antitumor activities against stem cell factor producing human small
cell lung cancer H146, based on angiogenesis inhibition. Int J
Cancer. 122:664–671. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Harada K, Mizuguchi T, Katagiri Y,
Kawamoto M, Nakamura Y, Meguro M, Ota S, Sasaki S, Miyanishi K,
Sonoda T, et al: Area between the hepatic and heart curves of
(99m)Tc-galactosyl-human serum albumin scintigraphy represents
liver function and disease progression for preoperative evaluation
in hepatocellular carcinoma patients. J Hepatobiliary Pancreat Sci.
19:667–673. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tohyama O, Matsui J, Kodama K, Hata-Sugi
N, Kimura T, Okamoto K, Minoshima Y, Iwata M and Funahashi Y:
Antitumor activity of lenvatinib (e7080): An angiogenesis inhibitor
that targets multiple receptor tyrosine kinases in preclinical
human thyroid cancer models. J Thyroid Res. 2014:6387472014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Iesato A, Li S, Roti G, Hacker MR, Fischer
AH and Nucera C: Lenvatinib targets PDGFR-β pericytes and inhibits
synergy with thyroid carcinoma cells: Novel translational insights.
J Clin Endocrinol Metab. 106:3569–3590. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Glen H, Mason S, Patel H, Macleod K and
Brunton VG: E7080, a multi-targeted tyrosine kinase inhibitor
suppresses tumor cell migration and invasion. BMC Cancer.
11:3092011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yamamoto Y, Matsui J, Matsushima T,
Obaishi H, Miyazaki K, Nakamura K, Tohyama O, Semba T, Yamaguchi A,
Hoshi SS, et al: Lenvatinib, an angiogenesis inhibitor targeting
VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft
models associated with microvessel density and pericyte coverage.
Vasc Cell. 6:182014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gild ML, Bullock M, Robinson BG and
Clifton-Bligh R: Multikinase inhibitors: A new option for the
treatment of thyroid cancer. Nat Rev Endocrinol. 7:617–624. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Makker V, Colombo N, Herráez AC, Santin
AD, Colomba E, Miller DS, Fujiwara K, Pignata S, Baron-Hay S,
Ray-Coquard I, et al: Lenvatinib plus pembrolizumab for advanced
endometrial cancer. N Engl J Med. 386:437–448. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rimel BJ, Crane EK, Hou J, Nakayama J,
MacDonald J, Lutz K, Makker V and O'Cearbhaill RE: Tyrosine kinase
inhibitor toxicities: A society of gynecologic oncology review and
recommendations. Gynecol Oncol. 174:148–156. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hiraoka A, Kumada T, Kariyama K, Takaguchi
K, Atsukawa M, Itobayashi E, Tsuji K, Tajiri K, Hirooka M, Shimada
N, et al: Clinical features of lenvatinib for unresectable
hepatocellular carcinoma in real-world conditions: Multicenter
analysis. Cancer Med. 8:137–146. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Calo CA, Levine MD, Brown MD, O'Malley DM
and Backes FJ: Combination lenvatinib plus pembrolizumab in the
treatment of ovarian clear cell carcinoma: A case series. Gynecol
Oncol Rep. 46:1011712023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Backes FJ, Wei L, Chen M, Hill K,
Dzwigalski K, Poi M, Phelps M, Salani R, Copeland LJ, Fowler JM, et
al: Phase I evaluation of lenvatinib and weekly paclitaxel in
patients with recurrent endometrial, ovarian, fallopian tube, or
primary peritoneal cancer. Gynecol Oncol. 162:619–625. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Keizer RJ, Gupta A, Gillavry MR, Jansen M,
Wanders J, Beijnen JH, Schellens JH, Karlsson MO and Huitema AD: A
model of hypertension and proteinuria in cancer patients treated
with the anti-angiogenic drug E7080. J Pharmacokinet Pharmacodyn.
37:347–363. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu X, Wu S, Dahut WL and Parikh CR: Risks
of proteinuria and hypertension with bevacizumab, an antibody
against vascular endothelial growth factor: Systematic review and
meta-analysis. Am J Kidney Dis. 49:186–193. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu S, Chen JJ, Kudelka A, Lu J and Zhu X:
Incidence and risk of hypertension with sorafenib in patients with
cancer: A systematic review and meta-analysis. Lancet Oncol.
9:117–123. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu X, Stergiopoulos K and Wu S: Risk of
hypertension and renal dysfunction with an angiogenesis inhibitor
sunitinib: Systematic review and meta-analysis. Acta Oncol.
48:9–17. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Geva E and Jaffe RB: Role of vascular
endothelial growth factor in ovarian physiology and pathology.
Fertil Steril. 74:429–438. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stouffer RL, Martínez-Chequer JC,
Molskness TA, Xu F and Hazzard TM: Regulation and action of
angiogenic factors in the primate ovary. Arch Med Res. 32:567–575.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tamanini C and De Ambrogi M: Angiogenesis
in developing follicle and corpus luteum. Reprod Domest Anim.
39:206–216. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fraser HM, Wilson H, Morris KD, Swanston I
and Wiegand SJ: Vascular endothelial growth factor Trap suppresses
ovarian function at all stages of the luteal phase in the macaque.
J Clin Endocrinol Metab. 90:5811–5818. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Imai A, Ichigo S, Matsunami K, Takagi H
and Kawabata I: Ovarian function following targeted anti-angiogenic
therapy with bevacizumab. Mol Clin Oncol. 6:807–810. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ebrahimi M and Asbagh FA: Pathogenesis and
causes of premature ovarian failure: An update. Int J Fertil
Steril. 5:54–65. 2011.PubMed/NCBI
|
|
34
|
Rebar RW: Premature ovarian failure.
Obstet Gynecol. 113:1355–1363. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Coulam CB, Adamson SC and Annegers JF:
Incidence of premature ovarian failure. Obstet Gynecol. 67:604–606.
1986.PubMed/NCBI
|
|
36
|
Burness CB and Perry CM: Rivaroxaban: A
review of its use in the treatment of deep vein thrombosis or
pulmonary embolism and the prevention of recurrent venous
thromboembolism. Drugs. 74:243–262. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rodrigues VO, Soligo AGES and Pannain GD:
Antiphospholipid antibody syndrome and infertility. Rev Bras
Ginecol Obstet. 41:621–627. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yamakami LY, Serafini PC, de Araujo DB,
Bonfá E, Leon EP, Baracat EC and Silva CA: Ovarian reserve in women
with primary antiphospholipid syndrome. Lupus. 23:862–867. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Vega M, Barad DH, Yu Y, Darmon SK,
Weghofer A, Kushnir VA and Gleicher N: Anti-mullerian hormone
levels decline with the presence of antiphospholipid antibodies. Am
J Reprod Immunol. 76:333–337. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
De Sanctis R, Lorenzi E, Agostinetto E,
D'Amico T, Simonelli M and Santoro A: Primary ovarian insufficiency
associated with pazopanib therapy in a breast angiosarcoma patient:
A CARE-compliant case report. Medicine (Baltimore). 98:e180892019.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Verweij J and Sleijfer S: Pazopanib, a new
therapy for metastatic soft tissue sarcoma. Expert Opin
Pharmacother. 14:929–935. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nilsson EE, Detzel C and Skinner MK:
Platelet-derived growth factor modulates the primordial to primary
follicle transition. Reproduction. 131:1007–1015. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Long JP, Wan F, Zhang F, Zhou J and Don
LF: DTC chemotherapy regimen is associated with higher incidence of
premature ovarian failure in women of reproductive age with breast
cancer. Eur Rev Med Pharmacol Sci. 20:1087–1092. 2016.PubMed/NCBI
|
|
44
|
Yildiz C, Kacan T, Akkar OB, Karakus S,
Kacan SB, Ozer H and Cetin A: Effects of pazopanib, sunitinib, and
sorafenib, anti-VEGF agents, on the growth of experimental
endometriosis in rats. Reprod Sci. 22:1445–1451. 2015. View Article : Google Scholar : PubMed/NCBI
|