Introduction
Gastric cancer (GC) is the fifth most common cancer
in the world, with ~952,000 newly diagnosed cases per year and
1,089,103 diagnosed cases in 2020 (1). It is the third leading cause of
cancer-associated mortality, with ~723,000 patients dying from the
disease each year. Although the morbidity of GC is higher in East
Asian countries compared with Western countries, the histological
types of GC are similar in Asian and Western countries, with
adenocarcinoma being the most common (2–4).
Regardless of stage, the 5-year overall survival (OS) rate in the
United States (US) is <30%. Known risk factors for developing GC
include high salt intake, smoking, Helicobacter pylori
infection (2,3) and advanced age (5,6). The
risk index of GC survival outcome involves age, sex, clinical tumor
size, body mass index, histology, clinical stages and tumor
location (7–9).
According to the 2020 Chinese Society of Clinical
Oncology Guidelines for diagnosis and treatment of GC (10), second- or third-line drugs for
advanced metastatic or recurrent gastric or gastroesophageal
junction adenocarcinomas have poor efficacy and limited options.
The benefit of chemotherapy for advanced GC is limited (11). With the launch of biological
products such as programmed cell death protein 1 (PD-1), programmed
death-ligand 1 (PD-L1) monoclonal antibodies and anti-angiogenic
factors, more and more patients with GC benefit from immunotherapy
(12,13). Therefore, apart from biomarkers,
investigations into parameters or indexes that are independent risk
factors that affect the prognosis of immunotherapy need to be
performed. For example, the number of patients receiving
second-line immunotherapy varies in clinical trials in
CHECKMATE-649 (14); the proportion
of patients receiving second-line immunotherapy is 8%, and in the
KEYNOTE-062 (15) clinical trial,
the proportion of patients receiving second line immunotherapy is
15%. Whether immunotherapy intervention be performed after the
failure of first-line and second-line standard treatments remains
to be investigated, as the time point of immunotherapy intervention
may be an independent risk factor for the survival and prognosis of
patients receiving immunotherapy. According to CHECKMATE-649
(14) or KEYNOTE-061/062 (15), there is little data on Asian
patients with GC. The analysis of prognosis after immunotherapy is
insufficient, therefore, there is a lack of systematic tools for
predicting individual survival outcomes in patients with GC before
immunotherapy.
At present, the common clinical prognostic
evaluation is mainly based on the American Joint Committee on
Cancer (AJCC) staging guidelines, which mainly include the depth of
tumor invasion, lymph node metastasis data, hematogenous
metastasis, location of the tumor in the stomach, histological
grade and lymphovascular invasion (16,17).
However, factors such as age, sex, marital status, degree of tumor
differentiation, number of primary tumors and immunotherapy cycle,
which may be of great significance for the prediction of individual
survival, have not yet been fully considered. Therefore, its
guiding value for the individualized prognosis of patients is
limited to a certain extent. One study has stated that the
prognosis is improved in younger patients (18), whereas another has reported it being
improved in the elderly (19).
Women are more likely to have cancer of certain organs such as the
breast, uterine corpus, colon and rectum (20), while similar can be said in men for
some carcinomas, such as prostate and bladder cancer (21). Similarly, degree of tumor
differentiation and number of primary tumors have their own varying
significance (22). Prognostic
factors and survival models for advanced patients with GC
undergoing immunotherapy are to be explored.
Therefore, the present study was designed to develop
a novel GC nomogram, which was built using only clinically
available variables, to determine the clinical prognosis of
patients with GC receiving immunotherapy through risk prediction.
In addition to further exploring whether there are novel targets
that could be used to guide further immunotherapy.
Materials and methods
Study cohorts
From the perspective of evidence-based medicine,
aiming at the aforementioned problems, prospective methods were
used to establish the prognosis model of GC. The prognostic model
was based on a cohort study and cluster sampling method (Fig. 1).
As the goal of the prediction model research is an
application, the study objects have as few exclusion criteria as
possible in addition to the inclusion criteria, so as to ensure
that the study population is as consistent as possible with the
target population. Although randomized controlled trials (RCTs) are
typical representatives of high-quality research data, too high a
number of exclusion criteria in the RCT scheme leads to
inconsistency between the research population and the population
served in clinical practice. Selective bias leads to inconsistency
between the prediction model and the real situation, a poor
prediction effect and low research quality (23). Therefore, the data sources in the
present scheme were patients with advanced gastric or
gastroesophageal junction tumors who received immunotherapy at two
clinical sites (Fig. 1).
Research subjects
To develop a nomogram, the development cohort
consisted of patients with GC who received immunotherapy between
January 2018 and June 2022, in The First Affiliated Hospital of
Nanjing Medical University (Nanjing, China), which was a tertiary
teaching hospital and ranked in the top 20 in Science and
Technology Evaluation Metrics China. The mean age was 62.27
(SD±11.27) years and 366 (73.05%) patients were male. Patients in
the validation cohort were selected from Drum Tower Hospital
(Nanjing, China), another high-level clinical facility (Fig. 1). The mean age was 62.11 (SD±11.51)
years and 364 (71.09%) patients were male.
The inclusion criteria were as follows: Patients
with clinically confirmed advanced gastric cancer, adenocarcinomas
of the stomach or gastroesophageal junction (based on pathology,
cytology and imaging diagnosis) who received immunotherapy between
January 2018 and June 2022. The immunotherapy was anti-PD-1 and
anti-PD-L1 monoclonal antibodies, including Camrelizuma,
Tislelizumab, Sintilimab, Toripalimab, Nivolumab, Pembrolizumab,
Penpulimab and Envafolimab for injection. The exclusion criteria
were as follows: i) Patients with critical organ dysfunction, such
as heart failure, respiratory failure, liver failure and renal
failure; and ii) pregnant women.
Predictive indicators
i) Demographics, including age and marital
status.
ii) Disease characteristics. Histological grade and
primary stage of the tumor at first diagnosis according to the
AJCC. Tumor node metastasis (TNM), including primary tumor (T),
regional lymph node (N) and distant metastasis (M). The clinical
stage was based on the CT and histopathology findings, tumor
location, macroscopic type and histological differentiation based
on endoscopic biopsy. The primary tumor T stages were classified as
T1, T2, T3, T4a and T4b, and the clinical N stages were classified
as N0 and Nx.
iii) Pathological features. Microsatellite stable
(MSS), microsatellite unstable (MSI) or mismatch repair deficient
(dMMR); human herpesvirus type 4; Epstein-Barr virus; human
epidermal growth factor receptor-2 (HER2); PD-L1. Previous and
current treatment regimens were collected, as well as the survival
status of the subjects after immunotherapy. For a dichotomous
variable, all negative data records were ‘0’ and all positive data
records were ‘1’.
Survival follow-up. The cut-off point for follow-up
was September 2022. A telephone follow-up was conducted to verify
the post-discharge treatment, genetic testing results and OS
conditions. The data of patients who could not be contacted was
eliminated.
Ethical approval
This research was approved by Ethic Review Board of
The First Affiliated Hospital of Nanjing Medical University. All
the necessary formalities for the informed consent of the patients
were fulfilled according to the local regulation and Declaration of
Helsinki.
Statistical analysis
A Cox proportional hazards regression model (Cox
model) was performed to estimate the OS risk and predictor using
stepwise regression method for variable selection; sls=0.1 and
sle=0.1 indicate that both inclusion and exclusion criteria are
0.1. The Kaplan-Meier method combined with the two-stage test was
used for survival analysis with survival curves. The development
cohort was divided into two groups by sex and by immunotherapy
manufacture separately. For the comparison of baseline categorical
variables, the wald χ2 statistic for hypothesis testing
of regression parameters in the COX model. The survival prediction
model used a nomogram based on the results of regression modeling.
To evaluate the internal and external discrimination performance of
the nomogram, bootstrapping validation was performed on both the
developmental and validation cohorts with the concordance index
(Harrell's C-index and Uno C). The criteria of discrimination,
calibration and decision curve analysis were used to evaluate the
clinical utility of the prediction model. All analyses were
performed using R software version 4.1.1 (24). P<0.05 was considered to indicate
a statistically significant difference.
Results
Patients and demographics
Both datasets consisted of patients who underwent
immunotherapy for GC, and the therapeutic strategy was determined
by the appropriate protocol and guidelines. Patients in both
datasets were followed up regularly by physical examinations,
laboratory tests, endoscopy and CT. Table I presents the descriptive statistics
of both the developmental (n=501) and validation (n=512) cohorts.
As displayed in Table I, the
majority of patients in the two cohorts were in Stage III–IV
(n=410, 90.11%; and n=389, 75.98%). The mean ages were 62.27
(SD±11.27) and 62.11 (SD±11.51). A total of 366 men (73.05%) in the
development set and 364 men (71.09%) in the validation set were
included. A total of 235 (48.55%) and 290 (60.54%) patients
underwent surgery. The number of patients who received all three
interventions in the sequence were 177 (43.07%) and 174 (52.73%);
the number of patients treated with chemotherapy as first- or
second-line and immunotherapy + chemotherapy after were 173
(42.09%) and 103 (31.21%); and the number of patients treated with
immunotherapy + chemotherapy only were 61 (14.84%) and 53 (16.06%)
in the development and validation sets, respectively. The mean
number of immunotherapy cycles patients received was 4.34 (SD±4.03)
vs. 4.91 (SD±4.26) in the development and validation sets. A total
of six tumor markers, including HER2, MSS/MSI, MMR, PD-L1, TMB and
EBER, were designed as independent indices, but most of them have
not been tested based on their medical records.
 | Table I.Clinicopathological characteristics of
the development and validation sets. |
Table I.
Clinicopathological characteristics of
the development and validation sets.
| Variables | Development set
(n=501) | Validation set
(n=512) |
|---|
| Age, years |
|
|
| Mean (SD) | 62.27 (11.27) | 62.11 (11.51) |
|
Median | 63.87 | 64.59 |
| Q1,
Q3 | 56.02,70.29 | 55.94,69.44 |
| Min,
Max | 24.74,120.90 | 21.65,84.82 |
| Sex, n (%) |
|
|
| Male | 366 (73.05) | 364 (71.09) |
|
Female | 135 (26.95) | 148 (28.91) |
| Marital status, n
(%) |
|
|
|
Unmarried | 3 (0.68) | 196 (38.28) |
|
Married | 484 (96.61) | 311 (60.74) |
|
Divorced | 10 (2.26) | 2 (0.39) |
|
Widowed | 4 (0.90) | 3 (0.59) |
| History of chronic
diseases, n (%) |
|
|
| 1 | 218 (46.88) | 203 (41.86) |
| 0 | 247 (48.41) | 282 (58.14) |
| Histological grade, n
(%) |
|
|
| Highly
differentiated | 18 (4.69) | 4 (0.88) |
| Medium
differentiation | 125 (32.55) | 200 (43.86) |
| Low
differentiation | 241 (62.76) | 252 (55.26) |
| Borrmann, n (%) |
|
|
| I | 9 (2.01) | 9 (2.24) |
| II | 48 (10.71) | 30 (7.46) |
| III | 114 (25.45) | 256 (63.68) |
| IV | 277 (61.83) | 107 (26.62) |
| LAUREN, n (%) |
|
|
|
Intestinal type | 89 (34.63) | 119 (40.48) |
| Diffuse
type | 99 (38.52) | 105 (35.71) |
| Mixed
type | 69 (26.85) | 70 (23.81) |
| Primary tumor
stage, n (%) |
|
|
| T1 | 16 (3.19) | 24 (4.69) |
| T2 | 22 (4.39) | 24 (4.69) |
| T3 | 91 (18.16) | 112 (21.88) |
| T4 | 206 (41.12) | 162 (31.64) |
| Tx | 166 (33.13) | 192 (37.5) |
| Lymph node
metastasis, n (%) |
|
|
| N0 | 34 (11.18) | 50 (19.61) |
| N1 | 50 (16.45) | 34 (12.94) |
| N2 | 77 (25.33) | 55 (21.57) |
| N3 | 144 (28.74) | 177 (22.85) |
| Nx | 196 (39.12) | 257 (22.85) |
| Distant metastasis,
n (%) |
|
|
| M0 | 155 (34.91) | 197 (43.97) |
| M1 | 289 (65.09) | 251 (56.03) |
| Stage, n (%) |
|
|
| I | 9 (1.98) | 18 (3.52) |
| II | 36 (7.91) | 48 (9.38) |
|
III | 109 (23.96) | 138 (26.95) |
| IV | 301 (66.15) | 251 (49.02) |
| Surgery, n (%) |
|
|
| 0 | 249 (51.45) | 186 (38.83) |
| 1 | 235 (48.55) | 290 (60.54) |
| Chemotherapy, n
(%) |
|
|
| 0 | 107 (22.86) | 148 (33.18) |
| 1 | 361 (77.14) | 297 (66.59) |
| Immunotherapy
cycles, n |
|
|
| Mean
(SD) | 4.34 (4.03) | 4.91 (4.26) |
|
Median | 3 | 4 |
|
Q1,Q3 | 2.6 | 2.6 |
| Min,
Max | 1.27 | 1.36 |
| Treatment, n
(%) |
|
|
| Surgery
+ Chemo + (Immuno + Chemo) | 177 (43.07) | 174 (52.73) |
| Chemo +
(Immuno + Chemo) | 173 (42.09) | 103 (31.21) |
| Immuno
+ Chemo | 61 (14.84) | 53 (16.06) |
| HER2, n (%) |
|
|
| 0 | 118 (20.81) | 182 (50.14) |
| 1+ | 50 (22.52) | 77 (21.21) |
| 2+ | 36 (16.22) | 53 (14.60) |
| 3+ | 18 (8.11) | 51 (14.05) |
| Signet ring cell, n
(%) |
|
|
| 0 | 449 (89.62) | 458 (89.11) |
| 1 | 52 (10.38) | 56 (10.89) |
| Immunotherapy
manufacturers, n (%) |
|
|
|
Indigenous | 356 (72.95) | 305 (59.45) |
|
Multinational | 132 (27.05) | 208 (40.55) |
| Line of first
immunotherapy, n (%) |
|
|
| 1 | 211 (42.12) | 302 (58.98) |
| 2 | 241 (48.1) | 147 (28.71) |
| 3 | 42 (8.38) | 34 (6.64) |
| 4 | 2 (0.40) | 14 (2.73) |
Risk factors for survival outcomes and
nomogram construction
A total of 167 (33.33%) patients from the
development cohort and 158 (30.85%) from the validation cohort died
during the observation period. Cox proportional hazards regression
had been used to select independent risk factors for survival. To
estimate the lifetime of the development cohort, the Kaplan-Meier
method was conducted with a mean OS of 677.53 (SD±21.88) days. The
survival status for the development set was 342 (77.38%) during the
observation period. The predicted probability of OS was shown in
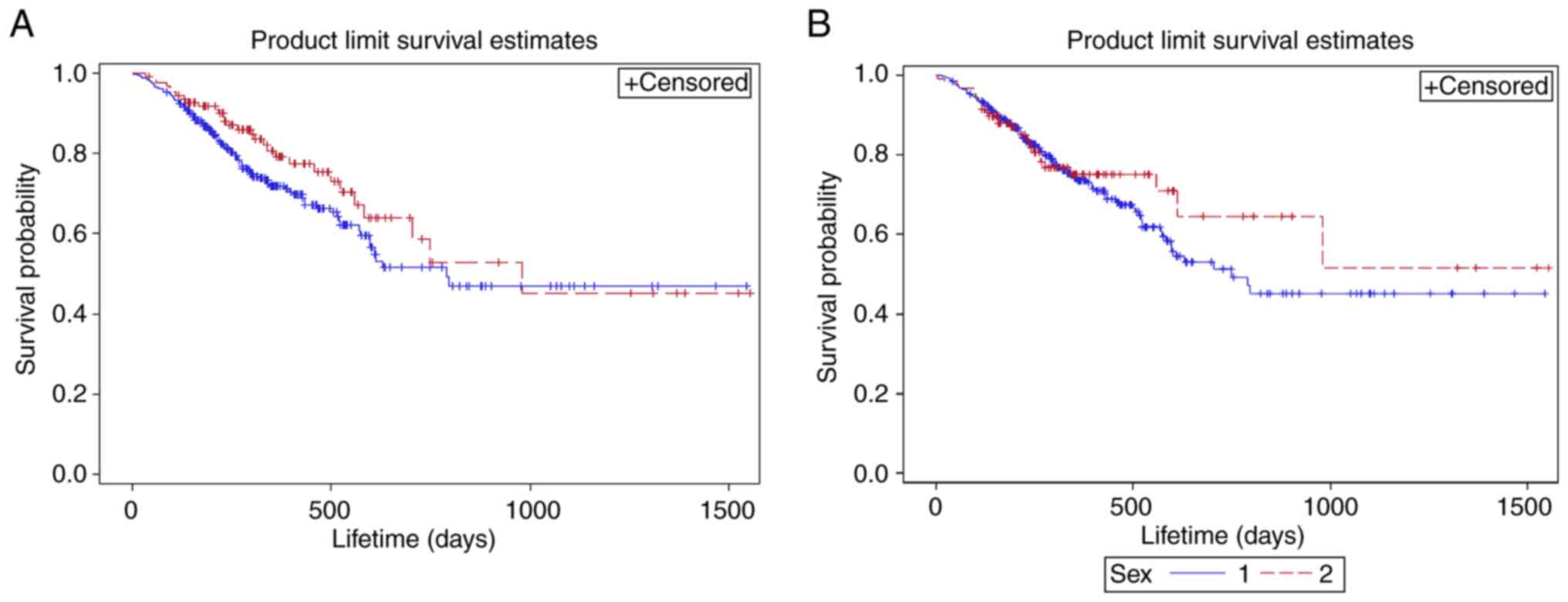
Fig. 1. Overall, 50% of patients
remained alive in 794 days. According to Fig. 2A, the mean survival outcome of
patients with domestic immunotherapy was 570.28 (SD±18.55) days
inferior to the imported immunotherapy group with a mean OS of 720
(SD±40.65). The median survival outcome of patients with
indigenously manufactured medicine immunotherapy was 789 (95% CI,
597-NA) days, which was lower compared with the multinational
medicine immunotherapy group with a median OS of 980 (95% CI,
582-NA) days. Fig. 2B revealed that
the mean OS of female patients was 739.12 (SD±43.27), which was
superior compared with the male patients 577.02 (SD±17.56). Median
OS of female patients was 980 (95% CI, 613-NA), which was greater
compared with the male patients 748 (95% CI, 597-NA). However, no
statistical significance was found
The hazard ratio (HR) of all the variables are
plotted in Table II. All
predictors were entered into the model after multivariate analysis:
The difference between the two groups of immunotherapy
manufacturers (HR=1.419; P=0.0078), Borrmann (P=0.0287),
histological grade (HR=1.395; P=0.0151), immunotherapy cycles
(HR=0.932; P=0.028), the line of first immunotherapy (HR=1.693,
P=0.0003) and age (HR=1.012; P=0.0245). However, only histological
grade, immunotherapy cycle, line of first immunotherapy and age
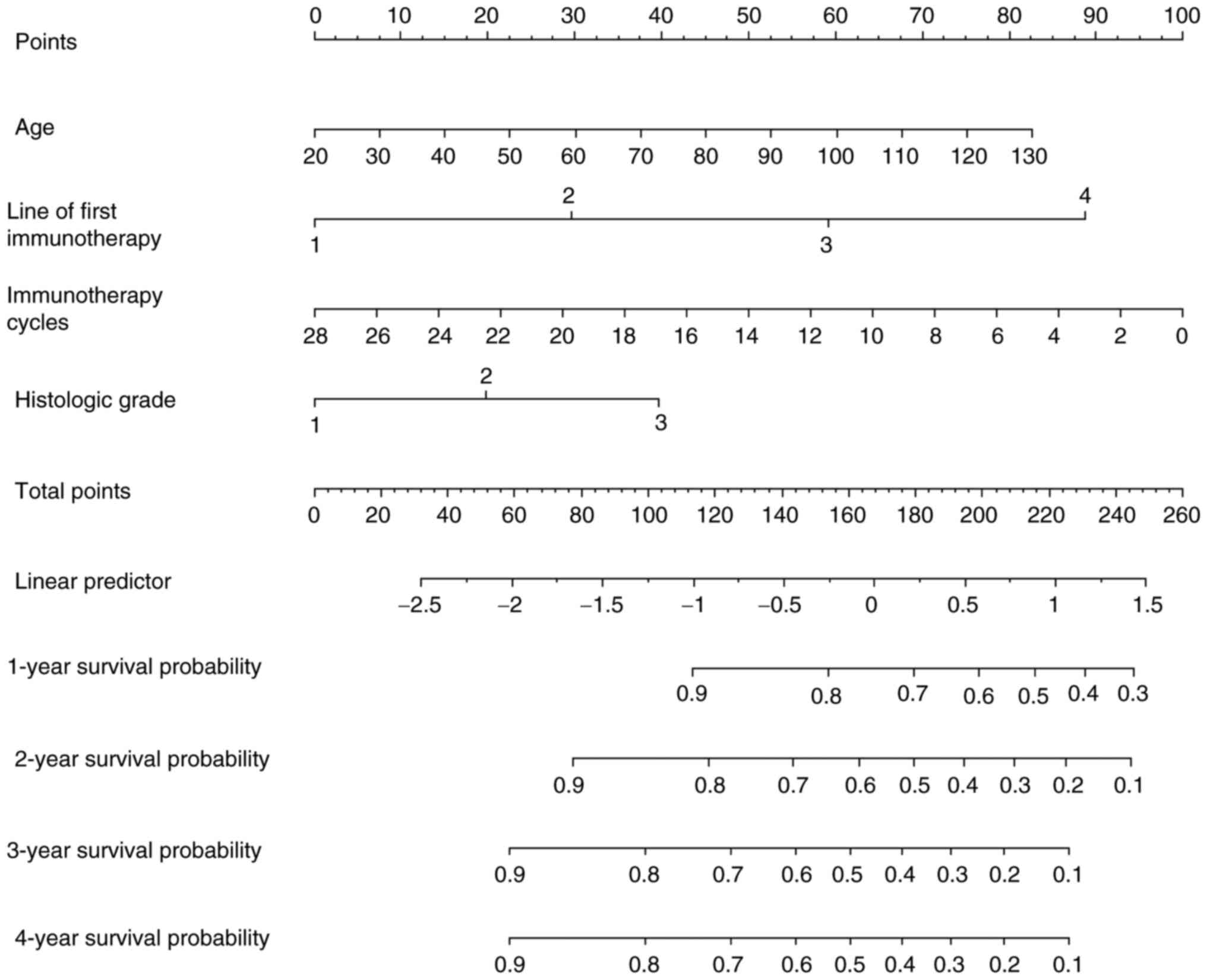
entered the model and may be the predictors (Fig. 3). With the potential prediction
index, a nomogram (Fig. 3) was
used, allowing clinicians to discuss future treatmet options with
individual patients based on their previous medical records. It
appears that patients who received immunotherapy 10 cycles earlier
as the first-line treatment and whose tumors were highly
differentiated at a younger age led to an increase in survival time
(Fig. 3).
 | Table II.Multi-variable cox proportion hazards
model of the development cohort. |
Table II.
Multi-variable cox proportion hazards
model of the development cohort.
|
|
|
|
| 95% CI |
|
|---|
|
|
|
|
|
|
|
|---|
| Variables | Wald
χ2 | Hazard ratio | d.f. | Lower | Upper |
Pr>χ2 |
|---|
| Age | 5.0568 | 1.012 |
| 0.996 | 1.029 | 0.0245 |
| Sex | 0.6009 |
| 1 |
|
| 0.4382 |
|
Male | 0.6009 | 1.178 |
| 0.790 | 1.814 |
|
|
Female |
|
|
| Reference |
|
|
| History of chronic
diseases | 0.0493 | 1.042 | 1 |
|
| 0.8242 |
|
Yes |
|
|
| 0.725 | 1.493 |
|
| No |
|
|
| Reference |
|
|
| Histological
grade | 3.1897 | 1.395 | 2 |
|
| 0.0151 |
| Highly
differentiated | 0.3057 | 0.774 |
| 0.271 | 1.736 |
|
| Medium
differentiation | 4.4034 | 0.611 |
| 0.378 | 0.954 |
|
| Low
differentiation |
|
|
| Reference |
|
|
| LAUREN | 3.6707 |
| 2 |
|
| 0.1596 |
|
Intestinal type | 0.0014 | 1.013 |
| 0.528 | 1.995 | 0.9701 |
| Diffuse
type | 2.2786 | 1.611 |
| 0.883 | 3.076 | 0.1312 |
| Mixed
type |
|
|
| Reference |
|
|
| Borrmann | 9.0417 |
| 3 |
|
| 0.0287 |
| I | 0.6659 | 1.521 |
| 0.463 | 3.669 | 0.4145 |
| II | 1.2826 | 0.704 |
| 0.364 | 1.242 | 0.2574 |
|
III | 7.2571 | 0.487 |
| 0.279 | 0.801 | 0.0071 |
| IV |
|
|
| Reference |
|
|
| Primary tumor
stage | 4.0937 |
| 4 |
|
| 0.3935 |
| T1 | 0.0005 | 0.989 |
| 0.298 | 2.433 | 0.9825 |
| T2 | 0.0075 | 1.036 |
| 0.427 | 2.149 | 0.9309 |
| T3 | 2.9429 | 0.608 |
| 0.334 | 1.050 | 0.0863 |
| T4 | 0.1113 | 1.069 |
| 0.722 | 1.591 | 0.7386 |
| Tx |
|
|
| Reference |
|
|
| Lymph node
metastasis | 6.5785 |
| 4 |
|
| 0.1599 |
| N0 | 4.1054 | 0.390 |
| 0.136 | 0.877 | 0.0427 |
| N1 | 0.0023 | 1.014 |
| 0.544 | 1.765 | 0.9617 |
| N2 | 0.0000 | 1.001 |
| 0.588 | 1.633 | 0.9980 |
| N3 | 2.6430 | 0.689 |
| 0.434 | 1.068 | 0.1040 |
| Nx |
|
|
| Reference |
|
|
| Distant
metastasis | 1.8407 |
| 1 |
|
| 0.1749 |
| M0 | 1.8407 | 0.768 |
| 0.519 | 1.115 | 0.1749 |
| M1 |
|
|
|
|
|
|
| P-stage | 2.6580 |
| 3 |
|
| 0.4474 |
| I | 0.2012 | 0.726 |
| 0.119 | 2.296 | 0.6538 |
| II | 1.5306 | 0.615 |
| 0.258 | 1.235 | 0.2160 |
|
III | 1.3421 | 0.769 |
| 0.483 | 1.180 | 0.2467 |
| IV |
|
|
|
| Reference |
|
| Signet ring
cell | 1.2166 |
|
|
|
| 0.2700 |
| 0 | 1.2166 | 0.737 |
| 0.443 | 1.321 | 0.2700 |
| 1 |
|
|
| Reference |
|
|
| HER2 | 0.6298 |
| 3 |
|
| 0.8896 |
| 0 | 0.0007 | 1.013 |
| 0.425 | 2.989 |
|
| 1+ | 0.1376 | 1.210 |
| 0.472 | 3.711 |
|
| 2+ | 0.2218 | 1.302 |
| 0.448 | 4.248 |
|
| 3+ |
|
|
| Reference |
|
|
| Surgery | 1.8390 |
| 1 |
|
| 0.1751 |
| 0 | 1.8390 | 1.275 |
| 0.899 | 1.819 | 0.1751 |
| 1 |
|
|
| Reference |
|
|
| Chemotherapy | 1.3038 |
| 1 |
|
| 0.2535 |
| 0 | 1.3038 | 0.759 |
| 0.460 | 1.193 | 0.2535 |
| 1 |
|
|
| Reference |
|
|
| Treatment | 0.7154 |
|
|
|
| 0.6993 |
| Surgery
+ Chemo + Immuno | 0.0218 | 1.046 |
| 0.590 | 1.976 | 0.8827 |
| Chemo +
Immuno | 0.4121 | 1.216 |
| 0.688 | 2.293 | 0.5209 |
|
Immuno |
|
|
|
|
|
|
| Line of first
immunotherapy | 10.9980 |
| 3 |
|
| 0.0117 |
| 1 | 0.0007 | 52219.83 |
| 0.154 |
| 0.9791 |
| 2 | 0.0007 | 62171.62 |
| 0.184 |
| 0.9788 |
| 3 | 0.0008 | 120253.2 |
| 0.348 |
| 0.9775 |
| 4 |
|
|
| Reference |
|
|
| Immunotherapy
manufacturers | 7.0682 | 1.419 | 1 |
|
| 0.0078 |
|
Indigenous | 2.2802 | 1.372 |
| 0.922 | 2.102 | 0.1310 |
|
Multinational |
|
|
| Reference |
|
|
| Immunotherapy
cycles | 4.8301 | 0.932 | 1 | 0.885 | 0.975 | 0.028 |
Nomogram discrimination and
calibration
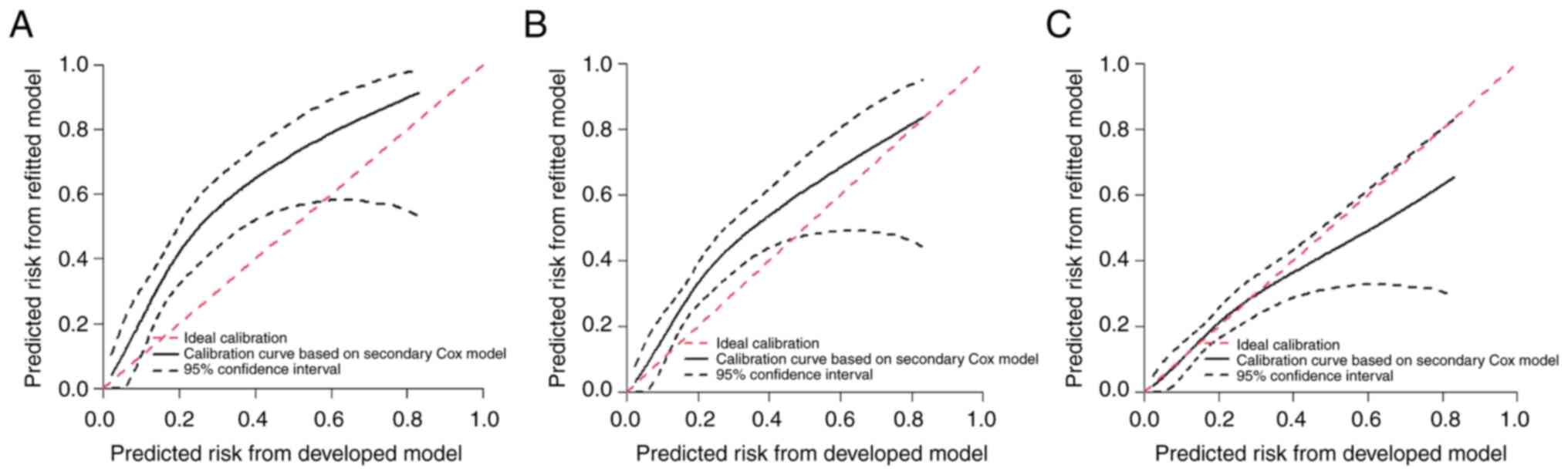
The Harrel C-index for the present nomogram was 0.64
(95% CI 0.58–0.7), and the bias-corrected Harrell C-index was 0.62;
Uno C was 0.61 after bootstrapping (n=1,013) internal validation.
In the external validation cohort, the C-identification power
calibration plots with external verification for the developmental
set were 0.67 (95% CI, 0.63–0.72), indicating that the nomogram
maintained a certain discrepancy as presented by each year in
Fig. 4. The mean calibration of
1-year survival was observed expected ratio (OE)=0.95 (95% CI,
0.77–1.16), as revealed in Fig. 4C.
The mean calibration of 2-year survival was OE=0.86 (95% CI,
0.72–1.03), as shown in Fig. 5. The
3-year mean calibration survival was OE=0.86 (95% CI, 0.72–1.02) in
Fig. 4A. To combine all the
timelines, the mean calibration in the large was slope was 0.95,
and the interquartile range was (0.798–1.12). The present monogram
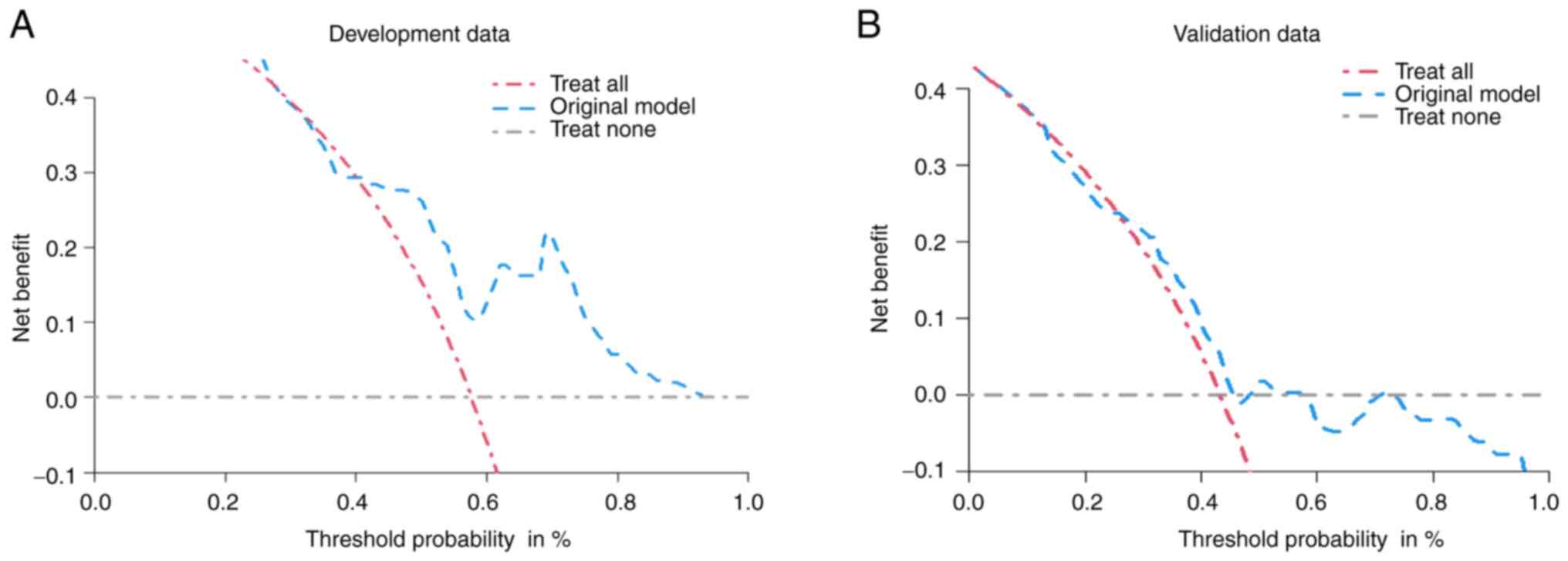
consistently showed favorable net benefits across a wide range of
threshold probabilities in both the developmental and validation
cohorts (Fig. 5).
Discussion
The present study developed a novel nomogram for
survival assessment and prediction of immunotherapy for patients
with advanced GC. There have been numerous nomograms to assess the
survival of patients with GC. Previously, Eom et al
(25) developed a nomogram for
undergoing curative resection for GC, predicting that age, tumor
size, lymphovascular invasion, depth of invasion and metastatic
lymph nodes were significant prognostic factors for OS. Han et
al (26) selected patients with
GC after D2 gastrectomy to predict the long-term survival outcome.
The multivariate Cox model identified age at diagnosis, sex,
location, depth of invasion, number of metastatic lymph nodes and
number of examined lymph nodes as covariates to be associated with
survival. Hou et al (27)
established a prognostic model of liver metastasis in GC based on
the SEER database. Ethnicity, grade, marital status, tumor size,
TNM stage, T stage and M stage are independent risk factors for GC,
and GC bone metastasis is an independent risk factor that affects
the prognosis of patients with GC. Song et al (17) built a survival prediction model for
radical surgery that only included patients with lymph node
metastases. Shin et al (28)
used preoperative data to select the high-risk patients without
considering the treatment they received; eight independent
predictors, including age, sex, clinical tumor size, macroscopic
features, body mass index, histology, clinical stages and tumor
location, were considered for the preoperative nomogram of patients
with GC. The present study has different research subjects and
prospective cohorts.
The current study developed a novel nomogram by
considering the most common and significant parameters as
aforementioned that could help identify high-risk patients before
immunotherapy and help clinicians make appropriate decisions for
patients. In the present study, the depth of invasion, called the T
stage, and metastatic lymph nodes, also known as the N stage, did
not enter the model due to their progressive status distribution.
Survival time increased with an increase in patient age, and vice
versa. The aforementioned studies reveal that as the age of the
patient and the age of diagnosis rise, the survival period falls.
The present study showed that the higher number of cycles of
immunotherapy taken by the patients and the higher the
immunotherapy intervention, the higher the survival. The
histological grade is also a risk factor for GC; patients with a
poorly differentiated tumour are associated with a lower survival
rate. As a result, immunotherapy should be applied in advance as
the first-line treatment for stage III–IV patients with cancer with
chemotherapy.
The present study developed a novel nomogram and
validated it both internally and externally using a data set from
multicenter studies. There have been numerous nomograms to assess
the survival of patients with GC, but they cannot be applied to
advanced patients with GC who have received immunotherapy.
The current study had several strengths. First, all
the factors used in the nomogram are easy and convenient to obtain
and are objective; thus, they could be widely applicable to
physicians. Additionally, based on careful statistical calculation,
a novel significant indicator was identified: The line of
first-line immunotherapy in both the developmental and validation
datasets, elevating early intervention in immunotherapy with
chemotherapy as the first-line treatment. And no matter which line
of immunotherapy was involved, the more cycles patients received,
their survival condition improved; thus, they could be widely
applicable to physicians. Therefore, more careful clinical
consideration may be required to select a therapeutic approach.
Moreover, the difference in GC survival outcome between domestic
immunotherapy and imported immunotherapy had no statistical
significance. It seems female OS tended to be higher compared with
male OS in immunotherapy; however, longer observation is needed.
Signet ring cells are usually considered to have a poor prognosis
for GC; however, they were not included in the present
nomogram.
The current study demonstrated not so good
discrimination but a good calibration, and there were several
limitations. First, the present nomogram was developed and
validated only in Asian patients who underwent immunotherapy;
therefore, further validation is needed for the application of our
nomogram in a more diverse population. Secondly, the present study
attempted to search for as much preoperative information as
possible; however, there were unavoidable missing values that
needed to be imputed using statistical methods, such as tumor
markers, which patients would not be required to test. In addition,
patients without surgery lack data on Borrmann and Lauren grades.
Thirdly, in cases of diffuse types of cancer, such as Borrmann type
4, more careful application would be needed. Finally, the present
nomogram did not include frailty or patient psychology-related
variables, such as the Self-Rating Anxiety Scale. Frailty and
psychological conditions are important clinical factors and are not
included in the nomogram. This could be a potential confounding
source. Despite advances in treatment techniques, there is no
recommended method to establish a risk factor system for
immunotherapy patients.
Acknowledgements
Not applicable.
Funding
This study was supported by 2021 Science and Technology
Development Plan funded by Nanjing Municipal Bureau of Science and
Technology (grant no. 2021SX00-000213-202100545).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MS and JZ carried out the conception and design of
this study. Acquisition of data was conducted by MS and YY.
MS conducted the statistical analysis, data
interpretation and drafted the manuscript, that was later revised
by JZ. The funding was obtained by JZ. MS, JZ and YY confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethical approval and consent to
participate
This research was approved by Ethic Review Board of
The First Affiliated Hospital with Nanjing Medical University. All
the necessary formalities for the informed consent of the patients
were fulfilled according to the local regulation and Declaration of
Helsinki.
Patient consent for publication
All the patients involved in this study provided
written informed consent for the publication of any data and/or
accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide forb36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nicholls RJ, Zinicola R and Haboubi N:
Extramural Spread of rectal cancer and the AJCC Cancer Staging
Manual 8thedition, 2017. J Ann Oncol. 30:1394–1395. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Japanese Gastric Cancer Association, .
Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric
Cancer. 20:1–19. 2017. View Article : Google Scholar
|
|
5
|
Lordick F, Mariette C, Haustermans K,
Obermannová R and Arnold D; ESMO Guidelines Committee, :
Oesophageal cancer: ESMO Clinical Practice guidelines for
diagnosis, treatment and follow-up. Ann Oncol. 27:50–57. 2016.
View Article : Google Scholar
|
|
6
|
Uemura N, Okamoto S, Yamamoto S, Matsumura
N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N and Schlemper RJ:
Helicobacter pylori infection and the development of gastric
cancer. N Engl J Med. 345:784–793. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lu J, Xu BB, Zheng CH, Li P, Xie JW, Wang
JB, Lin JX, Chen QY, Truty MJ and Huang CM: Development and
external validation of a nomogram to predict recurrence free
survival after Ro resection for Stage II/III gastric cancer: An
International Multicenter Stud. Front Oncol. 10:5746112020.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Song Z, Wu Y, Yang J, Yang D and Fang X:
Progress in the treatment of advanced gastric cancer. Tumour Biol.
39:10104283177146262017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Edge SB, Byrd DR, Carducci MA, Greene FL
and Trotti A: AJCC Cancer Staging Manual. pp. 649Springer; New
York: 2010
|
|
10
|
Wang FH, Zhang XT, Li YF, Tang L, Qu XJ,
Ying JE, Zhang J, Sun LY, Lin RB, Qiu H, et al: The Chinese Society
of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis
and treatment of gastric cancer, 2021. Cancer Commun (Lond).
41:747–795. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bang YJ, Ruiz EY, Van Cutsem E, Lee KW,
Wyrwicz L, Schenker M, Alsina M, Ryu MH, Chung HC, Evesque L, et
al: Phase III, randomised trial of avelumab versus physician's
choice of chemotherapy as third-line treatment of patients with
advanced gastric or gastro-oesophageal junction cancer: Primary
analysis of JAVELIN Gastric 300. Ann Oncol. 29:2052–2060. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Song Y, Fu Y, Xie Q, Zhu B, Wang J and
Zhang B: Anti-angiogenic agents in combination with immune
checkpoint inhibitors: A promising strategy for cancer treatment.
Front Immunol. 11:19562020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hack SP, Zhu AX and Wang Y: Augmenting
anticancer immunity through combined targeting of angiogenic and
PD-1/PD-L1 pathways: Challenges and opportunities. Front Immunol.
11:5988772020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Janjigian YY, Shitara K, Moehler M,
Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T,
Campos Bragagnoli A, et al: First-line nivolumab plus chemotherapy
versus chemotherapy alone for advanced gastric, gastro-oesophageal
junction, and oesophageal adenocarcinoma (CheckMate 649): A
randomised, open-label, phase 3 trial. Lancet. 398:27–40. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shitara K, Özgüroğlu M, Bang YJ, Di
Bartolomeo M, Mandalà M, Ryu MH, Fornaro L, Olesiński T, Caglevic
C, Chung HC, et al: Pembrolizumab versus paclitaxel for previously
treated, advanced gastric or gastro-oesophageal junction cancer
(KEYNOTE-061): A randomised, open-label, controlled, phase 3 trial.
Lancet. 392:123–133. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kattan MW, Karpeh MS, Mazumdar M and
Brennan MF: Postoperative nomogram for disease-specific survival
after an Ro resection for gastric carcinoma. J Clin Oncol.
21:3647–3650. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Song KY, Park YG, Jeon HM and Park CH: A
nomogram for predicting individual survival of patients with
gastric cancer who underwent radical surgery with extended lymph
node dissection. Gastric Cancer. 17:287–293. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lacy PD, Piccirillo JF, Merritt MG and
Zequeira MR: Head and neck squamous cell carcinoma: Better to be
young. Otolaryngol Head Neck Surg. 122:253–258. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bollschweiler E, Plum P, Mönig SP and
Hölscher AH: Current and future treatment options for esophageal
cancer in the elderly. Expert Opin Pharmacother. 18:1001–1010.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Miller KD, Nogueira L, Mariotto AB,
Rowland JH, Yabroff KR, Alfano CM, Jemal A, Kramer JL and Siegel
RL: Cancer treatment and survivorship statistics, 2019. CA Cancer J
Clin. 69:363–385. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shariat SF, Sfakianos JP, Droller MJ,
Karakiewicz PI, Meryn S and Bochner BH: The effect of age and
gender on bladder cancer: A critical review of the literature. BJU
Int. 105:300–308. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nicholls RJ, Zinicola R and Haboubi N:
Extramural spread of rectal cancer and the AJCC Cancer Staging
Manual 8th edition, 2017. J Ann Oncol. 30:1394–1395. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bacigalupo R, Cudd P, Littlewood C,
Bissell P, Hawley MS and Buckley Woods H: Interventions employing
mobile technology for overweight and obesity: An early systematic
review of randomized controlled trials. Obes Rev. 14:279–2791.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wainana SM, Karomo JN, Kyalo R and Mutai
N: Using data mining techniques and R software to analyze crime
data in Kenya. Int J Data Sci Analysis. 6:202020. View Article : Google Scholar
|
|
25
|
Eom BW, Ryu KW, Nam BH, Park Y, Lee HJ,
Kim MC, Cho GS, Kim CY, Ryu SW, Shin DW, et al: Survival nomogram
for curatively resected Korean gastric cancer patients: Multicenter
retrospective analysis with external validation. PLoS One.
10:01196712015. View Article : Google Scholar
|
|
26
|
Han DS, Suh YS, Kong SH, Lee HJ, Choi Y,
Aikou S, Sano T, Park BJ, Kim WH and Yang HK: Nomogram predicting
long-term survival after d2 gastrectomy for gastric cancer. J Clin
Oncol. 30:3834–3874. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hou S, Xie X, Peng Q, Zhou G, Li L, Zhou
H, Zhang G and Zhou T: Prognostic factor analysis and prognostic
model of liver metastasis of gastric cancer based on SEER database.
Chin J Gen Surg. 29:1212–1223. 2020.(In Chinese).
|
|
28
|
Shin HJ, Choi YO, Roh CK, Son SY, Hur H
and Han SU: Prediction of survival outcomes based on preoperative
clinical parameters in gastric cancer. Ann Surg Oncol.
28:7027–7037. 2021. View Article : Google Scholar : PubMed/NCBI
|