Introduction
Prostate cancer (PC) is the second most common
malignancy after lung cancer and one of the leading causes of death
in males worldwide. Notably, there are ~1,200,000 new cases and
~350,000 PC-associated deaths annually (1). In addition, the incidence of PC has
risen by 2.75% in China over the past three decades (2,3).
Although patients with metastatic PC are often treated with
androgen deprivation therapy (ADT) and achieve initial treatment
response, 10–20% of patients develop metastatic
castration-resistant PC (mCRPC) (4–6).
Abiraterone is the first-line anti-androgen therapy
in patients with mCRPC (7,8). However, the prognosis of
abiraterone-treated patients with mCRPC remains suboptimal and
mCRPC management is complex due to heterogeneity among patients
(9). Thus, the identification of
novel potential biomarkers is required for predicting survival in
abiraterone-treated patients with mCRPC.
Circulating tumor cells (CTCs) originate from
primary or metastatic tumor sites and enter the bloodstream,
playing a key role in the formation of metastases (10,11).
Alterations of specific biomarkers, such as breast cancer
susceptibility gene 2 (BRCA2) and ezrin, in CTCs provide novel
perspectives for tumor recurrence, metastasis, therapeutic efficacy
and prognosis in patients with mCRPC (12,13).
In addition, stem cell markers are abnormally expressed in CTCs
(14,15). Notably, due to their direct origin
from the tumor, CTCs may share similar characteristics with the
tumor. Therefore, determination of biomarkers in CTCs may exhibit
potential in predicting prognosis of patients with mCRPC in
clinical practice (16).
Octamer-binding transcription factor 4 (OCT4),
located on chromosome 6p21 in the human genome, is a stem cell
marker that serves a crucial role in the carcinogenesis of several
types of cancer, including pancreatic cancer, ovarian cancer and
breast cancer (17–20). Previous studies demonstrated the
prognostic value of OCT4-positive (OCT4+) CTCs in
patients with cancer (16,21). Notably, results of a previous study
demonstrated that OCT4+ CTCs are associated with
advanced stage and distant metastasis in patients with
non-small-cell lung cancer (21).
Another study indicated that prevalence of OCT4+ CTCs is
increased in patients with pathologically confirmed muscle invasive
bladder cancer compared with patients with non-muscle invasive
bladder cancer (22). Moreover,
previous studies demonstrated that OCT4 facilitates therapeutic
resistance to ADT in PC; thus, OCT4 may exhibit potential as a
biomarker for predicting survival in abiraterone-treated patients
with mCRPC (23–25). To the best of our knowledge,
however, research surrounding the clinical role of OCT4+
CTCs in patients with mCRPC treated with abiraterone + prednisone
is limited.
The present prospective study aimed to explore the
prevalence of OCT4+ CTCs and the potential association
of OCT4+ CTCs with clinical features and prognosis of
patients with mCRPC treated with abiraterone + prednisone
therapy.
Patients and methods
Subjects
From May 2018 to December 2021, 70 patients with
mCRPC (aged from 55–89 years old) treated with abiraterone +
prednisone were enrolled from Shanghai Songjiang District Sijing
Hospital, Shanghai, China. The inclusion criteria were as follows:
i) Diagnosed with PC via histological examination; ii) confirmation
of CRPC. The CRPC diagnosis was according to the previous study
(26); iii) confirmation of mPC via
imaging technology; iv) aged >18 years and v) treated with
abiraterone + prednisone. The following patient exclusion criteria
were used: i) Presence of other primary malignant tumors; ii)
absence of adequate organ and bone marrow function and iii) Eastern
Cooperative Oncology Group performance status (ECOG PS) score >1
(27). The present study was
approved by the Ethics Committee of Shanghai Songjiang District
Sijing Hospital (approval no. 20180314sjyy01). All patients
provided written informed consent.
Collection and detection of clinical
features and samples
Clinical characteristics, such as age, therapeutic
history, Gleason (28),
International Society of Urological Pathology (ISUP) (29) and ECOG PS score, metastasis status
and levels of prostate-specific antigen (PSA), alkaline phosphatase
(ALP) and lactate dehydrogenase (LDH) were obtained from all
patients; the level of PSA was detected by electrochemiluminescence
immunoassay analyzer (cat. no. E-170; Roche Diagnostics), and the
levels of ALP and LDH were detected by automatic biochemical
analyzer (cat. no. AU54000; Olympus Corporation). In addition, 10
ml peripheral blood samples were obtained from patients with mCRPC
prior to treatment initiation. CTC counts in the peripheral blood
samples were detected via a Canpatrol system, as previously
described (30). CTC count ≥1 in 5
ml peripheral blood was defined as CTC-positive (CTC+);
CTC count <1 was defined as CTC-negative (CTC−)
(31,32). RNA in situ hybridization was
used for determining OCT4 expression in CTC+ samples
(21). The capture probe sequences
for OCT4 gene were the same as a previous study (21). Briefly, after washing three times
with PBS, the probes for epithelial cell adhesion molecule (EpCAM;
green color) and OCT4 (red color) were added and allowed to
hybridize for 3 h at 40°C. After washing 3 times with 0.1X SSC
buffer (MilliporeSigma), CTCs were incubated with 0.5 fmol
preamplification probes in the preamplification buffer (30% horse
serum; 1.5% sodium dodecyl sulfate; 3-mM Tris-HCl; pH 8.0) for 30
min. at 40°C. After washing with 0.1X SSC buffer, CTCs were
incubated with 1 fmol amplification probes (sequences shown in
Table SI). After washing, nuclei
were stained with 4′,6′-diamidino-2-phenylindole (DAPI;
MilliporeSigma) for 5 min. The cells were observed and images
captured under a fluorescence microscope at ×400 magnification and
counted by the clinicians. CTC+/OCT4+ was
defined as ≥1 CTC expressing OCT4 and
CTC+/OCT4− was defined as no OCT4 expression
observed in CTCs.
Treatment, follow-up and
evaluation
Patients with mCRPC were treated with 28-day cycles
of abiraterone + prednisone (abiraterone, 1,000 mg/day; prednisone,
10 mg/day). Treatment was discontinued following clinical disease
progression, severe toxicity or death. Patients underwent follow-up
once every 2 months in the first 6 months, then once every 3
months. The median and mean follow-up durations were 17.9 and 19.6
months, respectively, ranging from 2.1 to 42.5 months. The last
follow-up date was August 2022. Based on follow-ups, radiographic
progression was evaluated via modified Response Evaluation Criteria
in Solid Tumors (RECIST) version 1.1 for soft tissue sites
(33) or Prostate Cancer Clinical
Trials Working Group 2 criteria for bone sites (34). The criteria of radiographic
progression were as follows: i) appearance of ≥2 new lesions; ii)
first observation of progression by bone scan and iii) progression
of soft tissue lesions by computed tomography or magnetic resonance
imaging (33,35). Radiographic progression-free
survival (rPFS) and overall survival (OS) rates were
determined.
Statistical analysis
SPSS (version 26.0; IBM Corp.) was used for data
analysis and GraphPad Prism (version 7.01; GraphPad Software, Inc.;
Dotmatics) was used for figure construction. The mean ± standard
deviation and median (interquartile range) were used to show normal
distribution continuous variables and skewed distribution
continuous variables, respectively. The number (percentage) was
used to show counting variables. Wilcoxon rank sum, χ2
or Fisher's exact test was used for comparison. Kaplan-Meier curves
were constructed to determine rPFS and OS and log-rank or
Tarone-Ware tests were used. The small vertical lines in the
Kaplan-Meier curve represented censored data, defined as patients
who had an event during follow-up and those who had no event by the
end of follow-up. All clinical characteristics were included in the
Cox models. Factors associated with rPFS and OS were determined
using univariate and forward-multivariate Cox regression analysis.
In addition, multivariate Cox regression models with backward
elimination methods were performed for validation. P<0.05 was
considered to indicate a statistically significant difference.
Results
Clinical characteristics
The mean age of patients was 71.0±8.8 years
(Table I). In total, 2 (2.8%), 27
(38.6%) and 41 (58.6%) patients were evaluated as Gleason score ≤6,
7 and ≥8 at initial diagnosis, respectively. A total of 2 (2.8%),
13 (18.6%), 14 (20.0%) 16 (22.9%), and 25 (35.7%) patients were
assessed as ISUP grade 1, 2, 3, 4 and 5, respectively. A total of
46 (65.7%) patients were evaluated as ECOG PS score 0 and the
remaining 24 (34.3%) patients were assessed as ECOG PS score 1. In
addition, 63 (90.0%), 39 (55.7%), 11 (15.7%) and 10 (14.3%)
patients experienced bone, lymph node, soft tissue and visceral
metastasis, respectively. The median [interquartile range (IQR)]
PSA, ALP, and LDH were 32.1 (16.9–90.5) ng/ml, 88.6 (64.9–147.6)
IU/l, and 217.7 (170.0–402.0) IU/l, accordingly.
 | Table I.Clinical characteristics of patients
with mCRPC. |
Table I.
Clinical characteristics of patients
with mCRPC.
| Characteristic | Patients with mCRPC
(N=70) |
|---|
| Mean age, years, ±
SD | 71.0±8.8 |
| History of
prostatectomy (%) |
|
| No | 36.0 (51.4) |
|
Yes | 34.0 (48.6) |
| History of
radiotherapy (%) |
|
| No | 29.0 (41.4) |
|
Yes | 41.0 (58.6) |
| History of hormone
therapy (%) |
|
| No | 0.0 (0.0) |
|
Yes | 70.0 (100.0) |
| History of other
therapy (%) |
|
| No | 62.0 (88.6) |
|
Yes | 8.0 (11.4) |
| Gleason score at
initial diagnosis (%) |
|
| ≤6 | 2.0 (2.8) |
| 7 | 27.0 (38.6) |
| ≥8 | 41.0 (58.6) |
| ISUP grade (%) |
|
| 1 | 2.0 (2.8) |
| 2 | 13.0 (18.6) |
| 3 | 14.0 (20.0) |
| 4 | 16.0 (22.9) |
| 5 | 25.0 (35.7) |
| ECOG PS score
(%) |
|
| 0 | 46.0 (65.7) |
| 1 | 24.0 (34.3) |
| Bone metastasis
(%) |
|
| No | 7.0 (10.0) |
|
Yes | 63.0 (90.0) |
| Lymph node
metastasis (%) |
|
| No | 31.0 (44.3) |
|
Yes | 39.0 (55.7) |
| Soft tissue
metastasis (%) |
|
| No | 59.0 (84.3) |
|
Yes | 11.0 (15.7) |
| Visceral metastasis
(%) |
|
| No | 60.0 (85.7) |
|
Yes | 10.0 (14.3) |
| Median PSA, ng/ml
(IQR) | 32.1
(16.9–90.5) |
| Median ALP, IU/l
(IQR) | 88.6
(64.9–147.6) |
| Median LDH, IU/l
(IQR) | 217.7
(170.0–402.0) |
CTC count and OCT4+ CTC
quantification
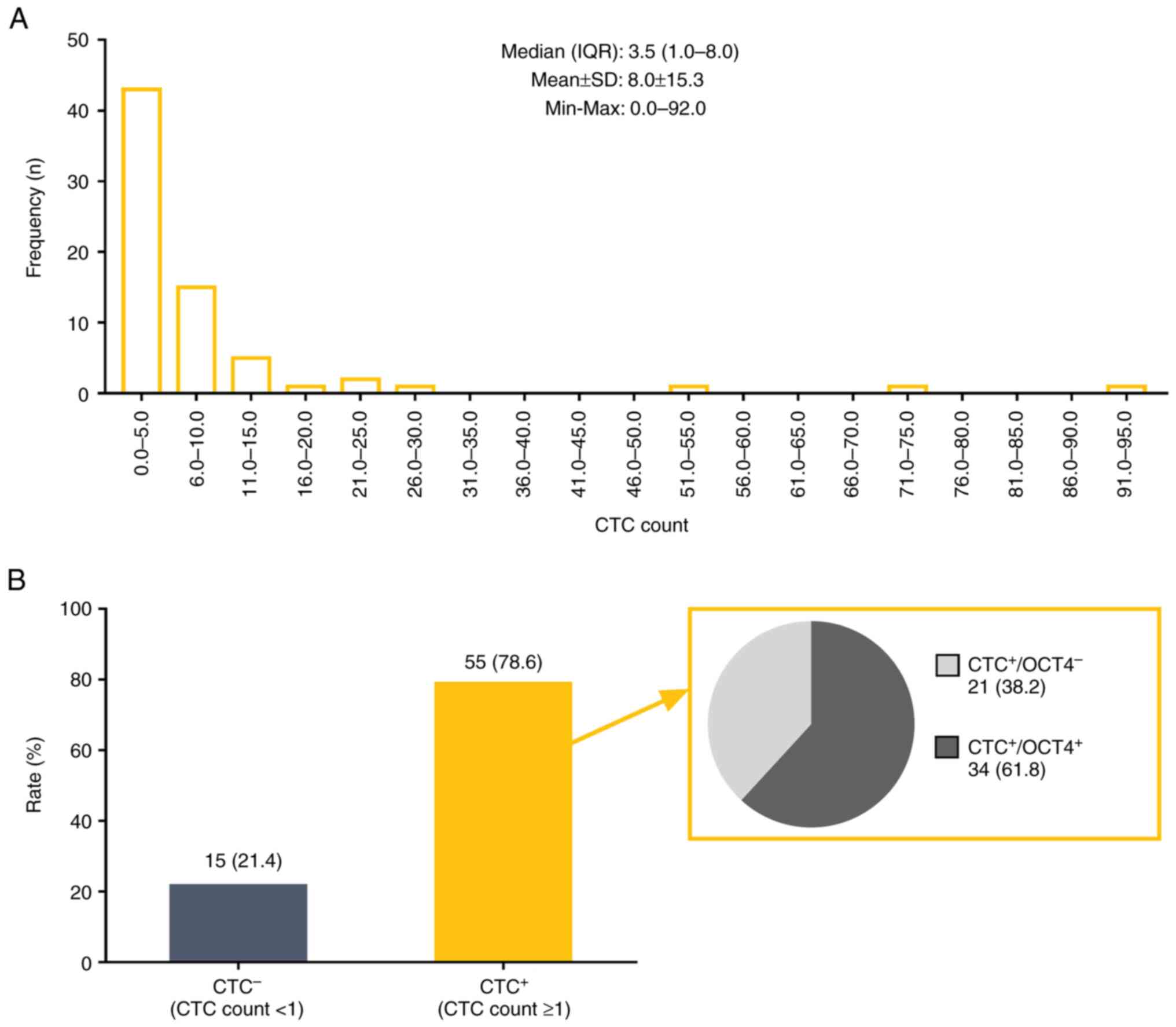
CTC count of patients is displayed in Fig. 1A. The median (IQR) CTC count was 3.5
(1.0–8.0) and the mean CTC count was 8.0±15.3. Moreover, 55 (78.6%)
and 15 (21.4%) patients were assessed as CTC+ and
CTC−, respectively. Among the 55 CTC+
patients, 34 (61.8%) patients were evaluated as
CTC+/OCT4+ and the remaining 21 (38.2%)
patients were identified as CTC+/OCT4−
(Fig. 1B). The in situ
hybridization images were presented in Fig. S1A and B.
Association between CTC count and
OCT4+ CTCs with patient characteristics
Elevated CTC count was associated with lymph node
(P=0.011) and visceral metastasis (P=0.003), high levels of PSA
(P=0.041) and low levels of LDH (P=0.026; Table II). CTC count was not associated
with age, history of prostatectomy, radiotherapy or other
therapies, Gleason score at initial diagnosis, ISUP grade, ECOG PS
score, bone and soft tissue metastasis or ALP (all P>0.05;
Table II).
 | Table II.Comparison of CTC count in metastatic
castration-resistant prostate cancer patients with different
characteristics. |
Table II.
Comparison of CTC count in metastatic
castration-resistant prostate cancer patients with different
characteristics.
| Characteristic | Median CTC count
(IQR) | P-value |
|---|
| Age, years |
| 0.619 |
|
<70 | 3.0 (1.0–7.0) |
|
|
≥70 | 4.0 (1.0–10.0) |
|
| History of
prostatectomy |
| 0.303 |
| No | 3.5 (1.0–9.8) |
|
|
Yes | 3.5 (0.0–7.3) |
|
| History of
radiotherapy |
| 0.112 |
| No | 3.0 (0.0–6.5) |
|
|
Yes | 4.0 (1.0–10.0) |
|
| History of other
therapy |
| 0.662 |
| No | 4.0 (1.0–7.3) |
|
|
Yes | 1.0 (0.3–13.8) |
|
| Gleason score at
initial diagnosis |
| 0.272 |
| ≤7 | 2.0 (0.0–7.0) |
|
|
>7 | 4.0 (1.0–9.5) |
|
| ISUP grade |
| 0.278 |
| 1 | 3.5 (2.0-NA) |
|
| 2 | 2.0 (0.0–7.0) |
|
| 3 | 2.0 (0.0–8.5) |
|
| 4 | 3.5 (1.0–9.5) |
|
| 5 | 4.0 (1.5–9.5) |
|
| ECOG PS score |
| 0.276 |
| 0 | 3.0 (0.8–7.0) |
|
| 1 | 6.0 (1.0–11.5) |
|
| Bone
metastasis |
| 0.324 |
| No | 1.0 (0.0–10.0) |
|
|
Yes | 4.0 (1.0–8.0) |
|
| Lymph node
metastasis |
| 0.011 |
| No | 2.0 (0.0–6.0) |
|
|
Yes | 5.0 (1.0–13.0) |
|
| Soft tissue
metastasis |
| 0.655 |
| No | 3.0 (1.0–7.0) |
|
|
Yes | 4.0 (1.0–15.0) |
|
| Visceral
metastasis |
| 0.003 |
| No | 2.5 (1.0–6.0) |
|
|
Yes | 11.5
(6.0–28.5) |
|
| PSA |
| 0.041 |
|
Low | 2.0 (0.0–7.0) |
|
|
High | 4.0 (1.0–10.0) |
|
| ALP |
| 0.054 |
|
Low | 2.0 (0.0–8.0) |
|
|
High | 5.0 (2.0–9.0) |
|
| LDH |
| 0.026 |
|
Low | 2.0 (0.0–6.0) |
|
|
High | 6.0 (2.0–9.0) |
|
CTC+/OCT4+ was associated with
visceral metastasis (P=0.009) and high levels of LDH (P=0.032;
Table III). Moreover, there was
no association between CTC+/OCT4+ and patient
characteristics, such as age, history of prostatectomy,
radiotherapy or other therapies, Gleason score at initial
diagnosis, ISUP grade, ECOG PS score, bone, lymph node or soft
tissue metastasis and PSA or ALP levels (all P>0.050; Table III).
 | Table III.Comparison of OCT4 expression in CTC
in meta- static castration-resistant prostate cancer patients with
different characteristics. |
Table III.
Comparison of OCT4 expression in CTC
in meta- static castration-resistant prostate cancer patients with
different characteristics.
| Characteristic |
CTC+/OCT4− (%) |
CTC+/OCT4+ (%) | P-value |
|---|
| Age, years |
|
| 0.348 |
|
<70 | 12.0 (57.1) | 15.0 (44.1) |
|
|
≥70 | 9.0 (42.9) | 19.0 (55.9) |
|
| History of
prostatectomy |
|
| 0.304 |
| No | 10.0 (47.6) | 21.0 (61.8) |
|
|
Yes | 11.0 (52.4) | 13.0 (38.2) |
|
| History of
radiotherapy |
|
| 0.663 |
| No | 8.0 (38.1) | 11.0 (32.4) |
|
|
Yes | 13.0 (61.9) | 23.0 (67.6) |
|
| History of other
therapy |
|
| 0.664 |
| No | 18.0 (85.7) | 31.0 (91.2) |
|
|
Yes | 3.0 (14.3) | 3.0 (8.8) |
|
| Gleason score at
initial diagnosis |
|
| 0.431 |
| ≤7 | 9.0 (42.9) | 11.0 (32.4) |
|
|
>7 | 12.0 (57.1) | 23.0 (67.6) |
|
| ISUP grade |
|
| 0.630 |
| 1 | 0.0 (0.0) | 2.0 (5.9) |
|
| 2 | 5.0 (23.8) | 4.0 (11.8) |
|
| 3 | 4.0 (19.0) | 5.0 (14.7) |
|
| 4 | 4.0 (19.0) | 10.0 (29.4) |
|
| 5 | 8.0 (38.1) | 13.0 (38.2) |
|
| ECOG PS score |
|
| 0.834 |
| 0 | 13.0 (61.9) | 22.0 (64.7) |
|
| 1 | 8.0 (38.1) | 12.0 (35.3) |
|
| Bone
metastasis |
|
| 1.000 |
| No | 2.0 (9.5) | 3.0 (8.8) |
|
|
Yes | 19.0 (90.5) | 31.0 (91.2) |
|
| Lymph node
metastasis |
|
| 0.052 |
| No | 11.0 (52.4) | 9.0 (26.5) |
|
|
Yes | 10.0 (47.6) | 25.0 (73.5) |
|
| Soft tissue
metastasis |
|
| 1.000 |
| No | 18.0 (85.7) | 28.0 (82.4) |
|
|
Yes | 3.0 (14.3) | 6.0 (17.6) |
|
| Visceral
metastasis |
|
| 0.009 |
| No | 21.0 (100.0) | 25.0 (73.5) |
|
|
Yes | 0.0 (0.0) | 9.0 (26.5) |
|
| PSA |
|
| 0.212 |
|
Low | 11.0 (52.4) | 12.0 (35.3) |
|
|
High | 10.0 (47.6) | 22.0 (64.7) |
|
| ALP |
|
| 0.304 |
|
Low | 11.0 (52.4) | 13.0 (38.2) |
|
|
High | 10.0 (47.6) | 21.0 (61.8) |
|
| LDH |
|
| 0.032 |
|
Low | 13.0 (61.9) | 11.0 (32.4) |
|
|
High | 8.0 (38.1) | 23.0 (67.6) |
|
Prognostic value of CTC count and
OCT4+ CTCs
A total of 43 (61.4%) patients had PSA progression
(defined as the first rise in PSA of 2 ng/ml and 25% above the
lowest point). Among them, 36 patients had PSA and radiographic
progression and seven patients had PSA progression alone.
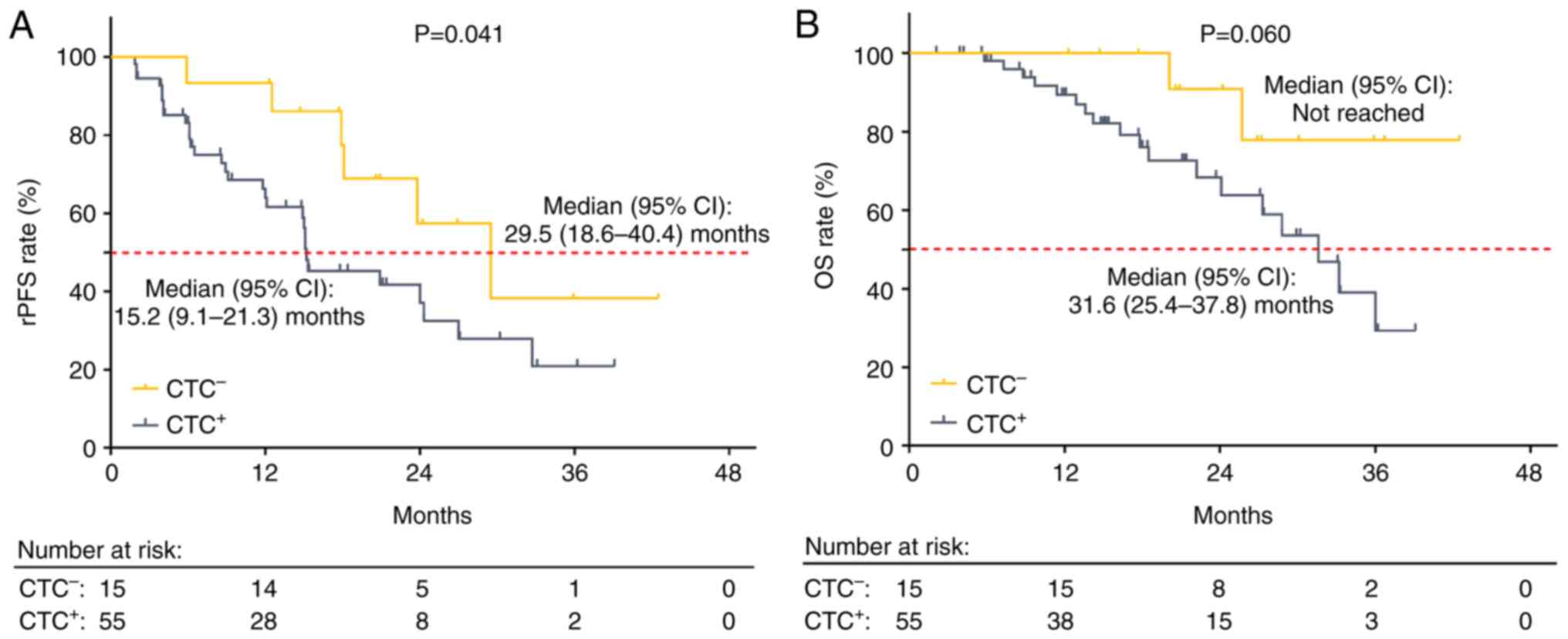
CTC+ patients exhibited reduced rPFS compared with
CTC− patients (P=0.041). Notably, the median [95%
confidence interval (CI)] rPFS of CTC+ and
CTC− patients was 15.2 (9.1–21.3) and 29.5 (18.6–40.4)
months, respectively (Fig. 2A). OS
was decreased in CTC+ compared with CTC−
patients but this result was not statistically significant
(P=0.060). Specifically, the median (95% CI) OS was 31.6
(25.4–37.8) months in CTC+ and not reached in
CTC− patients (Fig.
2B).
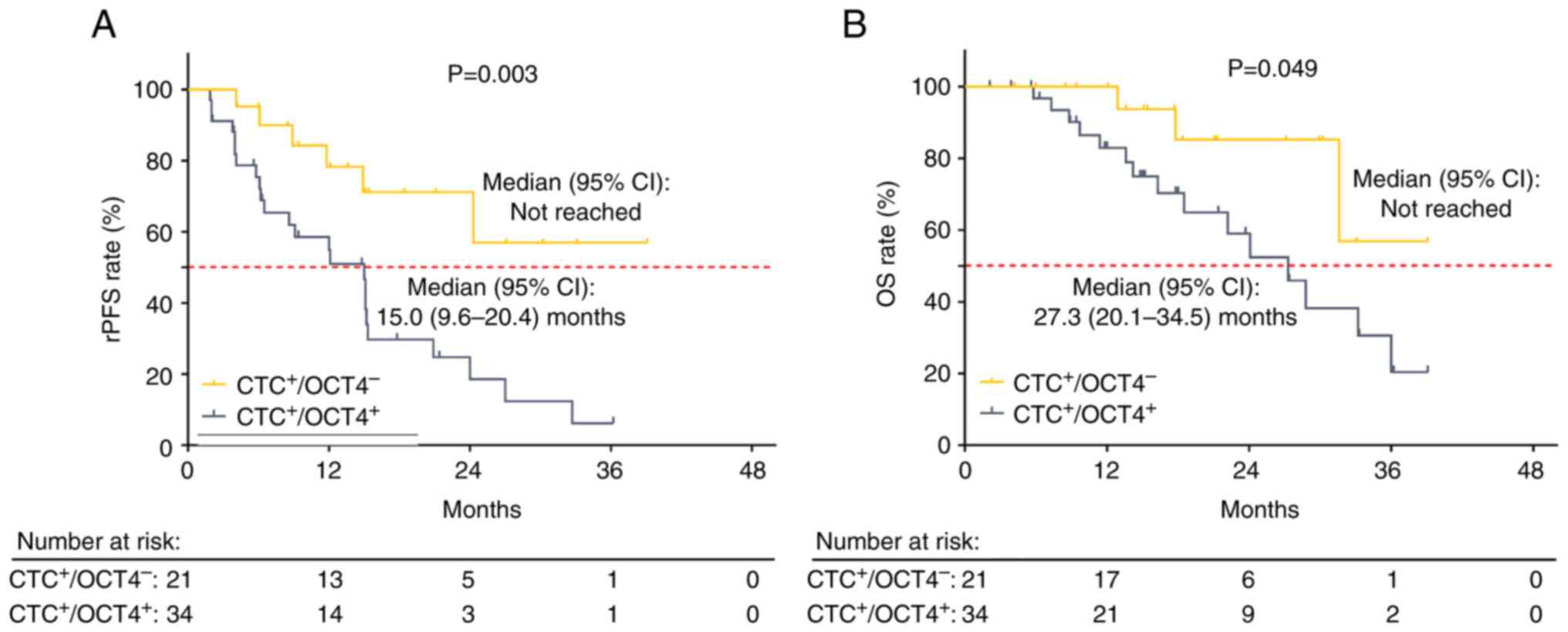
Compared with CTC+/OCT4−, rPFS
was decreased in CTC+/OCT4+ patients
(P=0.003). Median (95% CI) rPFS was 15.0 (9.6–20.4) months in
CTC+/OCT4+ patients, and not reached in
CTC+/OCT4− patients (Fig. 3A). In addition, OS was decreased in
CTC+/OCT4+ compared with
CTC+/OCT4− patients (P=0.049). Specifically,
the median (95% CI) OS of CTC+/OCT4+ and
CTC+/OCT4− patients was 27.3 (20.1–34.5)
months and not reached, respectively (Fig. 3B).
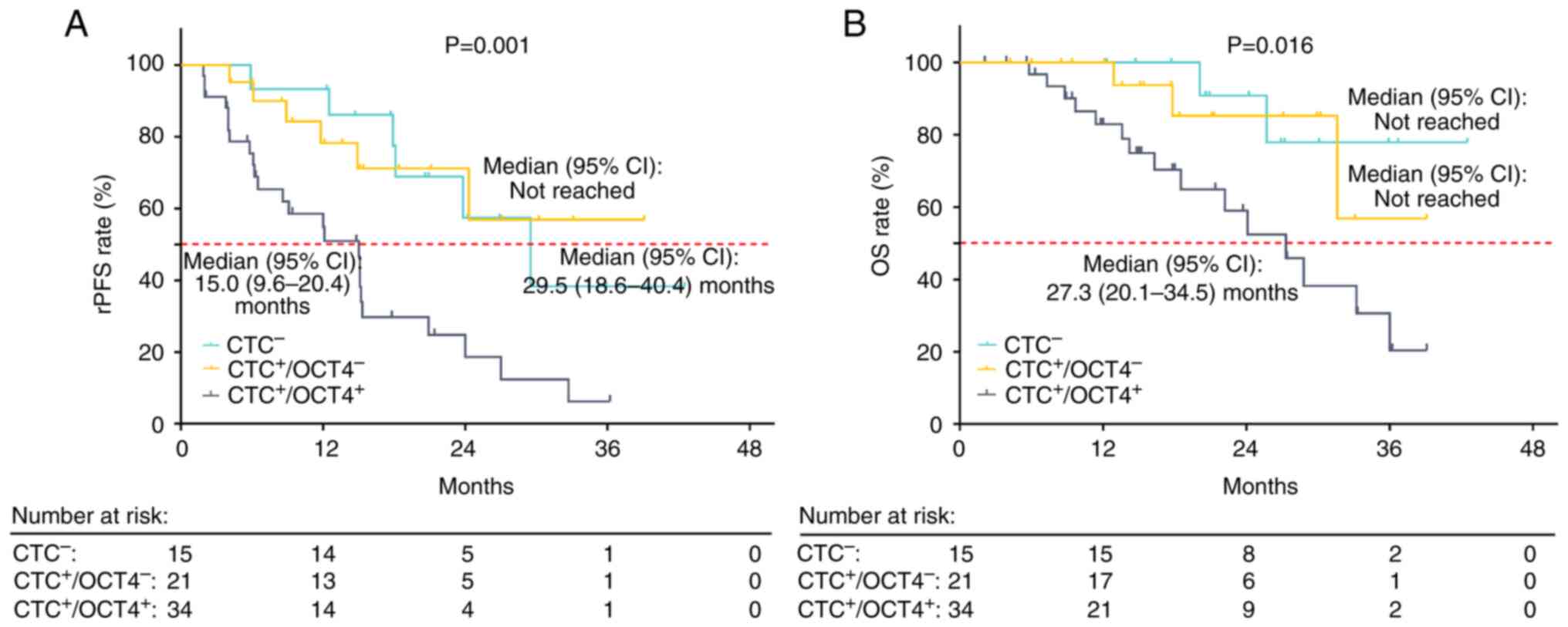
rPFS (P=0.001; Fig.
4A) and OS (P=0.016; Fig. 4B)
were decreased in CTC+/OCT4+ compared with
CTC− and CTC+/OCT4− patients.
Independent risk factors for rPFS and
OS
Forward-multivariate Cox regression models
demonstrated that CTC+/OCT4+ (vs.
CTC+/OCT4− or CTC−) was
independently associated with decreased rPFS [hazard ratio (HR),
3.833; P<0.001] and OS (HR, 3.938; P=0.008). ECOG PS score (1
vs. 0) was also independently associated with reduced rPFS (HR,
2.163; P=0.033) and OS (HR, 2.750; P=0.032; Table IV).
 | Table IV.Multivariate Cox regression models of
rPFS and OS in patients with metastatic castration-resistant
prostate cancer. |
Table IV.
Multivariate Cox regression models of
rPFS and OS in patients with metastatic castration-resistant
prostate cancer.
| A, rPFS |
|---|
|
|---|
|
|
|
| 95% CI |
|---|
|
|
|
|
|
|---|
| Variable | P-value | HR | Lower | Upper |
|---|
|
CTC+/OCT4+ vs.
CTC+/OCT4− or CTC− | <0.001 | 3.833 | 1.887 | 7.784 |
| ECOG PS score, 1
vs. 0 | 0.033 | 2.163 | 1.063 | 4.402 |
|
| B, OS |
|
|
|
|
| 95% CI |
|
|
|
|
|
|
Variable | P-value | HR | Lower | Upper |
|
|
CTC+/OCT4+ vs.
CTC+/OCT4− or CTC− | 0.008 | 3.938 | 1.428 | 10.858 |
| ECOG PS score, 1
vs. 0 | 0.032 | 2.750 | 1.090 | 6.935 |
Further multivariate models were established to
validate the findings of the forward-multivariate Cox model.
Multivariate model 1 included factors with P<0.05 in the
univariate model; CTC+/OCT4+ vs.
CTC+/OCT4− or CTC− (P=0.005) was
an independent risk factor, while LDH (P=0.127) was not an
independent risk factor for decreased rPFS (Table SII). Multivariate model 2 included
factors with P<0.1 in the univariate model;
CTC+/OCT4+ vs.
CTC+/OCT4− or CTC− (P=0.006) and
ECOG PS score 1 vs. 0 (P=0.048) were independently associated with
decreased rPFS. However, LDH was not an independent risk factor for
decreased rPFS (P=0.180; Table
SII). Multivariate model 3 included all factors and used a
backward elimination method; CTC+/OCT4+ vs.
CTC+/OCT4− or CTC− (P<0.001)
and ECOG PS score 1 vs. 0 (P=0.033) were independently associated
with decreased rPFS (Table SII).
Concerning OS, multivariate models 1, 2 and 3 all showed that
CTC+/OCT4+ vs.
CTC+/OCT4− or CTC− and ECOG PS
score 1 vs. 0 were independently associated with shorter OS (all
P<0.050; Table SIII).
Discussion
According to a previous study, OCT4, as a cancer
stem cell marker, is elevated in PC compared with normal prostate
and benign prostatic hyperplasia tissue, indicating its cancer
specificity in PC (23). Previous
studies have quantified CTCs and demonstrated value of CTCs in
predicting survival for patients with mCRPC (36–39).
Results of the present study demonstrated that elevated CTC count
was associated with lymph node and visceral metastasis and high
levels of PSA and LDH in patients with mCRPC treated with
abiraterone + prednisone. Further Kaplan-Meier curves demonstrated
that CTC+ was associated with decreased rPFS in patients
with mCRPC treated with abiraterone + prednisone. This may be
because CTCs in the blood may reflect the ability of cancer cells
to detach from primary or metastatic sites to new sites,
exacerbating the progression of PC (40). Lymph node and visceral metastasis
and high levels of PSA and LDH may result in a worse survival
(11). Therefore, CTC+
was associated with shortened rPFS in patients with mCRPC treated
with abiraterone + prednisone. Patients with lymph node metastasis
or visceral metastasis are more likely to exhibit CTC+
and CTC+/OCT4+ (21), but in fact, in situ
hybridization images are quite similar in patients with
CTC+ and CTC+/OCT4+ no matter what
the metastasis status.
Previous studies have demonstrated a potential
association between OCT4 and disease features and prognosis of
patients with PC (23,41). For example, increased OCT4 levels
are associated with elevated TNM stage and distant metastasis in
patients with PC (23). In
addition, a previous study used OCT4+ tumors from
palliative transurethral resection prostate specimens and the
results demonstrated that increased tumor OCT4 was associated with
increased T stage and PSA recurrence in post-docetaxel-treated
patients with mCRPC (41). Since
blood samples are more convenient to obtain (compared with
radiology) and CTCs as a marker in blood-based liquid biopsy have
been extensively explored (10,42),
it would be helpful for providing a monitoring option for cancer
prognosis to identify the clinical role of OCT4+ CTC in
patients with mCRPC treated with abiraterone + prednisone, which
has yet to be reported. Here, CTC+/OCT4+ was
associated with visceral metastasis and high levels of LDH in
patients with mCRPC treated with abiraterone + prednisone. In
addition, CTC+/OCT4+ vs.
CTC+/OCT4− or CTC− was
independently associated with reduced rPFS and OS. OCT4 may play a
role in promoting stemness, epithelial-mesenchymal transition,
proliferation and metastasis of tumor cells via numerous signaling
pathways (such as PI3K/AKT/mTOR pathway and notch signaling
pathway), further promoting tumor progression (20,43,44).
Moreover, OCT4 promotes malignancy and drug resistance of cancer
cells, leading to disease progression (17). Thus,
CTC+/OCT4+ vs.
CTC+/OCT4− or CTC− was
independently associated with reduced rPFS and OS in patients with
mCRPC treated with abiraterone + prednisone. As PSA progression is
less accurate than radiographic progression in patients with mCRPC
(45), the present study only
utilized rPFS to investigate the prognostic value of
OCT4+ CTCs. rPFS did not differ between CTC−
and CTC+/OCT4− patients. The present results
may have been biased, as rPFS rates were initially higher in
CTC− compared with CTC+/OCT4−
patients and one CTC− patient died in the 30th month.
The present study was limited by short follow-up duration and the
censored data in the CTC+/OCT4− group led to
a high rPFS rate. Thus, rPFS did not differ between CTC−
and CTC+/OCT4− patients. Further
investigation with a larger sample size and longer follow-up period
are required to validate the findings of the present study.
Notably, OCT4+ CTC levels were determined
prior to treatment initiation but OCT4+ CTC levels
following abiraterone treatment are yet to be elucidated. Further
in vivo and in vitro investigations are required to
determine the mechanisms underlying OCT4 in regulating drug
resistance, as the present study only investigated the prognostic
value of OCT4+ CTC in patients with mCRPC treated with
abiraterone + prednisone. The present study aimed to investigate
the prognostic value of OCT4 expression in CTCs and did not enroll
non-cancer patients (who have no CTCs) as controls; the lack of
control group was a limitation. As a result, the OCT4
tumor-specificity in mCRPC needs validations in further study with
a health control group.
In conclusion, OCT4+ CTCs were highly
prevalent and associated with visceral metastasis and increased LDH
levels. Thus, OCT4+ CTCs may exhibit potential in
predicting prognosis of patients with mCRPC treated with
abiraterone + prednisone.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
The author would like to thank Dr Qiongying Ma and
Dr Ziyi Sheng (Department of Nursing, Shanghai Songjiang District
Sijing Hospital) for their assistance in data entry and peripheral
blood collection.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YM was conceived the study, analyzed and interpreted
data, constructed figures and wrote and reviewed the manuscript. YM
confirms the authenticity of all the raw data. The author has read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by The Ethics
Committee of Shanghai Songjiang District Sijing Hospital, Shanghai,
China (approval no. 20180314sjyy01). All patients provided written
informed consent.
Patient consent for publication
Not applicable.
Competing interests
The author declares that they have no competing
interests.
References
|
1
|
Rebello RJ, Oing C, Knudsen KE, Loeb S,
Johnson DC, Reiter RE, Gillessen S, Van der Kwast T and Bristow RG:
Prostate cancer. Nat Rev Dis Primers. 7:92021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Qiu H, Cao S and Xu R: Cancer incidence,
mortality, and burden in China: A time-trend analysis and
comparison with the United States and United Kingdom based on the
global epidemiological data released in 2020. Cancer Commun (Lond).
41:1037–1048. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu X, Yu C, Bi Y and Zhang ZJ: Trends and
age-period-cohort effect on incidence and mortality of prostate
cancer from 1990 to 2017 in China. Public Health. 172:70–80. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mansinho A, Macedo D, Fernandes I and
Costa L: Castration-resistant prostate cancer: Mechanisms, targets
and treatment. Adv Exp Med Biol. 1096:117–133. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tucci M, Scagliotti GV and Vignani F:
Metastatic castration-resistant prostate cancer: Time for
innovation. Future Oncol. 11:91–106. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Henriquez I, Roach M III, Morgan TM, Bossi
A, Gómez JA, Abuchaibe O and Couñago F: Current and emerging
therapies for metastatic castration-resistant prostate cancer
(mCRPC). Biomedicines. 9:12472021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Desai K, McManus JM and Sharifi N:
Hormonal therapy for prostate cancer. Endocr Rev. 42:354–373. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
de Bono JS, Logothetis CJ, Molina A,
Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB Jr, Saad F,
et al: Abiraterone and increased survival in metastatic prostate
cancer. N Engl J Med. 364:1995–2005. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pan J, Zhao J, Ni X, Gan H, Wei Y, Wu J,
Zhang T, Wang Q, Freedland SJ, Wang B, et al: The prevalence and
prognosis of next-generation therapeutic targets in metastatic
castration-resistant prostate cancer. Mol Oncol. 16:4011–4022.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin D, Shen L, Luo M, Zhang K, Li J, Yang
Q, Zhu F, Zhou D, Zheng S, Chen Y and Zhou J: Circulating tumor
cells: Biology and clinical significance. Signal Transduct Target
Ther. 6:4042021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Castro-Giner F and Aceto N: Tracking
cancer progression: From circulating tumor cells to metastasis.
Genome Med. 12:312020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Barnett ES, Schultz N, Stopsack KH, Lam
ET, Arfe A, Lee J, Zhao JL, Schonhoft JD, Carbone EA, Keegan NM, et
al: Analysis of BRCA2 copy number loss and genomic instability in
circulating tumor cells from patients with metastatic
castration-resistant prostate cancer. Eur Urol. 83:112–120. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen Z, Wang J, Lu Y, Lai C, Qu L and Zhuo
Y: Ezrin expression in circulating tumor cells is a predictor of
prostate cancer metastasis. Bioengineered. 13:4076–4084. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Scher HI, Heller G, Molina A, Attard G,
Danila DC, Jia X, Peng W, Sandhu SK, Olmos D, Riisnaes R, et al:
Circulating tumor cell biomarker panel as an individual-level
surrogate for survival in metastatic castration-resistant prostate
cancer. J Clin Oncol. 33:1348–1355. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Werner S, Stenzl A, Pantel K and
Todenhöfer T: Expression of epithelial mesenchymal transition and
cancer stem cell markers in circulating tumor cells. Adv Exp Med
Biol. 994:205–228. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Deng Z, Wu S, Wang Y and Shi D:
Circulating tumor cell isolation for cancer diagnosis and
prognosis. EBioMedicine. 83:1042372022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mohiuddin IS, Wei SJ and Kang MH: Role of
OCT4 in cancer stem-like cells and chemotherapy resistance. Biochim
Biophys Acta Mol Basis Dis. 1866:1654322020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Iki K and Pour PM: Expression of Oct4, a
stem cell marker, in the hamster pancreatic cancer model.
Pancreatology. 6:406–413. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Villodre ES, Kipper FC, Pereira MB and
Lenz G: Roles of OCT4 in tumorigenesis, cancer therapy resistance
and prognosis. Cancer Treat Rev. 51:1–9. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xie W, Yu J, Yin Y, Zhang X, Zheng X and
Wang X: OCT4 induces EMT and promotes ovarian cancer progression by
regulating the PI3K/AKT/mTOR pathway. Front Oncol. 12:8762572022.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li S, Chen Q, Li H, Wu Y, Feng J and Yan
Y: Mesenchymal circulating tumor cells (CTCs) and OCT4 mRNA
expression in CTCs for prognosis prediction in patients with
non-small-cell lung cancer. Clin Transl Oncol. 19:1147–1153. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang R, Xia J, Wang Y, Cao M, Jin D, Xue
W, Huang Y and Chen H: Co-expression of stem cell and epithelial
mesenchymal transition markers in circulating tumor cells of
bladder cancer patients. Onco Targets Ther. 13:10739–10748. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Q, Zhang JG and Wang W: Expression
and significance of S100P, CD147, and OCT4 in different prostate
cancer tissue TNM stages. Genet Mol Res. 14:6844–6851. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang H, Wang C, Liu F, Li HZ, Peng G, Gao
X, Dong KQ, Wang HR, Kong DP, Qu M, et al: Reciprocal network
between cancer stem-like cells and macrophages facilitates the
progression and androgen deprivation therapy resistance of prostate
cancer. Clin Cancer Res. 24:4612–4626. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vaddi PK, Stamnes MA, Cao H and Chen S:
Elimination of SOX2/OCT4-associated prostate cancer stem cells
blocks tumor development and enhances therapeutic response. Cancers
(Basel). 11:13312019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu Y, Ye D and Expert G: Chinese expert
consensus on the diagnosis and treatment of castration-resistant
prostate cancer (2019 update). Cancer Manag Res. 12:2127–2140.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Epstein JI: An update of the Gleason
grading system. J Urol. 183:433–440. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Epstein JI, Egevad L, Amin MB, Delahunt B,
Srigley JR and Humphrey PA; Grading Committee, : The 2014
international society of urological pathology (ISUP) consensus
conference on Gleason grading of prostatic carcinoma: Definition of
grading patterns and proposal for a new grading system. Am J Surg
Pathol. 40:244–252. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu S, Liu S, Liu Z, Huang J, Pu X, Li J,
Yang D, Deng H, Yang N and Xu J: Classification of circulating
tumor cells by epithelial-mesenchymal transition markers. PLoS One.
10:e01239762015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lindsay CR, Le Moulec S, Billiot F, Loriot
Y, Ngo-Camus M, Vielh P, Fizazi K, Massard C and Farace F: Vimentin
and Ki67 expression in circulating tumour cells derived from
castrate-resistant prostate cancer. BMC Cancer. 16:1682016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lowes LE, Lock M, Rodrigues G, D'Souza D,
Bauman G, Ahmad B, Venkatesan V, Allan AL and Sexton T: The
significance of circulating tumor cells in prostate cancer patients
undergoing adjuvant or salvage radiation therapy. Prostate Cancer
Prostatic Dis. 18:358–364. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Saad F, Efstathiou E, Attard G, Flaig TW,
Franke F, Goodman OB Jr, Oudard S, Steuber T, Suzuki H, Wu D, et
al: Apalutamide plus abiraterone acetate and prednisone versus
placebo plus abiraterone and prednisone in metastatic,
castration-resistant prostate cancer (ACIS): A randomised,
placebo-controlled, double-blind, multinational, phase 3 study.
Lancet Oncol. 22:1541–1559. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chi KN, Rathkopf D, Smith MR, Efstathiou
E, Attard G, Olmos D, Lee JY, Small EJ, Pereira de Santana Gomes
AJ, Roubaud G, et al: Niraparib and abiraterone acetate for
metastatic castration-resistant prostate cancer. J Clin Oncol.
41:3339–3351. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lorente D, Olmos D, Mateo J, Dolling D,
Bianchini D, Seed G, Flohr P, Crespo M, Figueiredo I, Miranda S, et
al: Circulating tumour cell increase as a biomarker of disease
progression in metastatic castration-resistant prostate cancer
patients with low baseline CTC counts. Ann Oncol. 29:1554–1560.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
de Bono JS, Scher HI, Montgomery RB,
Parker C, Miller MC, Tissing H, Doyle GV, Terstappen LW, Pienta KJ
and Raghavan D: Circulating tumor cells predict survival benefit
from treatment in metastatic castration-resistant prostate cancer.
Clin Cancer Res. 14:6302–6309. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
de Kruijff IE, Sieuwerts AM, Onstenk W,
Kraan J, Smid M, Van MN, van der Vlugt-Daane M, Hoop EO, Mathijssen
RHJ, Lolkema MP, et al: Circulating tumor cell enumeration and
characterization in metastatic castration-resistant prostate cancer
patients treated with cabazitaxel. Cancers (Basel). 11:12122019.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Heller G, McCormack R, Kheoh T, Molina A,
Smith MR, Dreicer R, Saad F, de Wit R, Aftab DT, Hirmand M, et al:
Circulating tumor cell number as a response measure of prolonged
survival for metastatic castration-resistant prostate cancer: A
comparison with prostate-specific antigen across five randomized
phase III clinical trials. J Clin Oncol. 36:572–580. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Scher HI, Armstrong AJ, Schonhoft JD, Gill
A, Zhao JL, Barnett E, Carbone E, Lu J, Antonarakis ES, Luo J, et
al: Development and validation of circulating tumour cell
enumeration (Epic Sciences) as a prognostic biomarker in men with
metastatic castration-resistant prostate cancer. Eur J Cancer.
150:83–94. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kosaka T, Mikami S, Yoshimine S, Miyazaki
Y, Daimon T, Kikuchi E, Miyajima A and Oya M: The prognostic
significance of OCT4 expression in patients with prostate cancer.
Hum Pathol. 51:1–8. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Vasseur A, Kiavue N, Bidard FC, Pierga JY
and Cabel L: Clinical utility of circulating tumor cells: An
update. Mol Oncol. 15:1647–1666. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lu Y, Zhu H, Shan H, Lu J, Chang X, Li X,
Lu J, Fan X, Zhu S, Wang Y, et al: Knockdown of Oct4 and Nanog
expression inhibits the stemness of pancreatic cancer cells. Cancer
Lett. 340:113–123. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Patra SK: Roles of OCT4 in pathways of
embryonic development and cancer progression. Mech Ageing Dev.
189:1112862020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Komura K, Fujiwara Y, Uchimoto T, Saito K,
Tanda N, Matsunaga T, Ichihashi A, Tsutsumi T, Tsujino T, Yoshikawa
Y, et al: Comparison of radiographic progression-free survival and
PSA response on sequential treatment using abiraterone and
enzalutamide for newly diagnosed castration-resistant prostate
cancer: A propensity score matched analysis from multicenter
cohort. J Clin Med. 8:12512019. View Article : Google Scholar : PubMed/NCBI
|