Introduction
Tumor vascularization, as an important influencing
factor in the development of tumorigenesis, metastasis and
treatment resistance, is a process that includes
neovascularization, vascular selection and angiogenic mimicry.
Among them, the formation of angiogenic mimicry increases blood
perfusion; through pathological specimen analysis and in
vitro experiments, angiogenic mimicry role in clinical tumor
occurrence, metastasis, and prognosis was determined. Moreover,
angiogenic mimicry has been confirmed in various diseases,
including glioma, melanoma and lung cancer (1). However, to date, angiogenic mimicry in
ovarian cancer (OC) has been less well, and more superficially,
studied. Xu et al (2)
administered the anti-angiogenic drug bevacizumab in the treatment
of OC in mice and demonstrated through a series of in vitro
experiments that it could promote tumor metastasis, aggravate tumor
hypoxia and lead to the formation of angiogenic mimetic structures
during the process of anti-tumorigenesis. Therefore, angiogenic
mimicry may be an important target for the treatment of OC. In the
present review, the signaling pathways associated with angiogenic
mimicry in OC were investigated, including the vascular endothelial
(VE)-cadherin/cadherin/ephrin-A2 (EphA2)/matrix metalloproteinase
(MMP)-2/laminin 5γ2 (Ln5γ2) signaling pathway and the AKT/mammalian
target of rapamycin (mTOR)/MMP-2/Ln5γ2 signaling pathway. Moreover,
associated influencing factors are described, including the tumor
microenvironment, epithelial-mesenchymal transition (EMT), tumor
stem cells and so on, with an emphasis placed on relevant
inhibitors targeting the angiogenic mimetic state of OC, and the
current clinical application status of these factors.
OC
Concept and prevalence status
OC ranks eighth among malignant tumors worldwide,
and as a highly prevalent malignant tumor in women, its mortality
rate is second only to cervical and uterine cancer (3); however, due to lack of specific
identification targets during the early stage of disease
progression and inconspicuous pathological manifestations (4), the majority of patients are not
identified until at an advanced stage, resulting in poor treatment
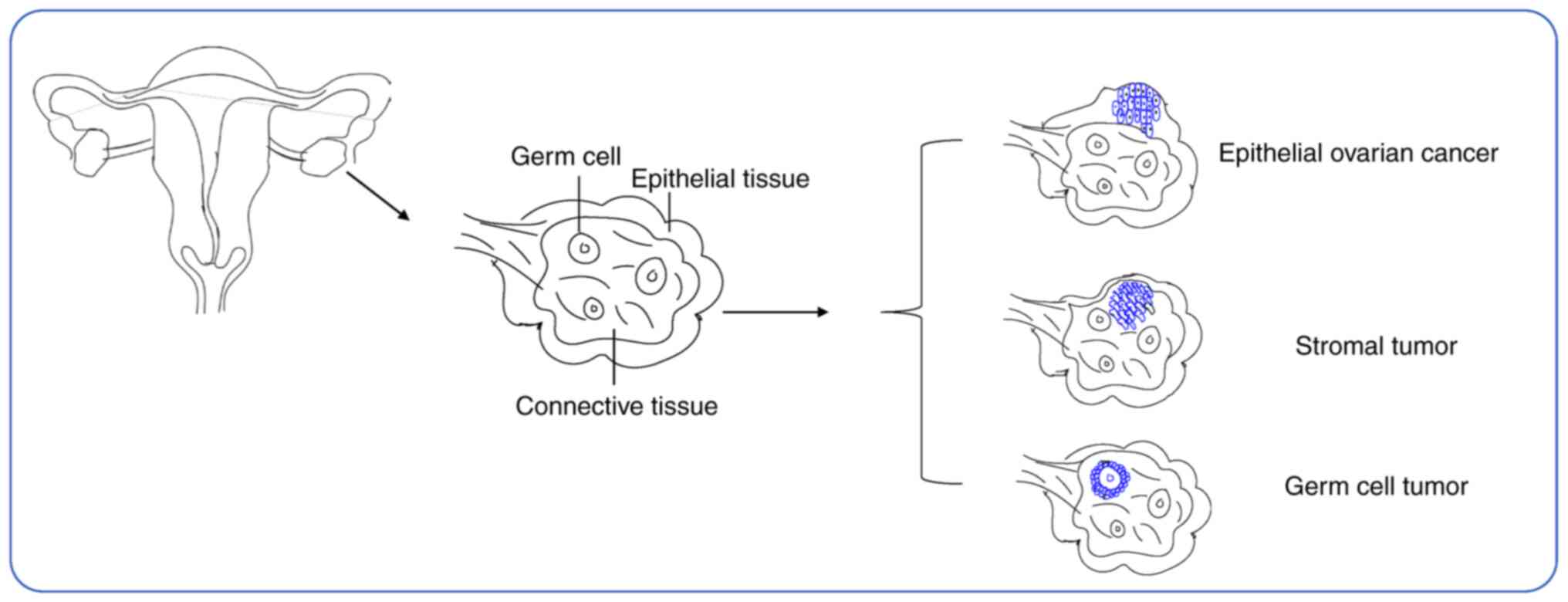
outcomes and low patient survival (5). ~90% of its staging is epithelial OC
(EOC) with high recurrence and metastasis, and the remaining 10% is
ovarian clear carcinoma and ovarian plasma carcinoma (6). Moreover, the heterogeneity of
different staging tumors is very high, which also makes the
treatment of OC more difficult to accomplish (Fig. 1). In addition, previous studies have
shown that the development of OC can be influenced by various
factors, including geographical location, race (7), lifestyle and dietary habits (8).
Difficulties in treatment
The current treatment modality of OC mainly
comprises surgical resection plus post-operative cisplatin (DDP)
and paclitaxel combination chemotherapy, which is highly sensitive
to DDP in the initial stages of chemotherapy for the majority of
patients, although long-term use leads to drug resistance, which
greatly reduces the therapeutic efficacy (3). With the rise of targeted therapies and
immunotherapy, poly (ADP-ribose) polymerase inhibitors, epidermal
growth factor receptor and anti-angiogenesis inhibitors have been
widely used in terms of maintenance therapy (9). Furthermore, an increasing number of
researchers have shifted their attention to herbal medicines and
natural compound extracts; for example, a recently published study
by Wang et al (10) explored
the anti-OC mechanism of the classic Chinese herbal formula ‘Guizhi
Fuling Wan’, which was found to achieve antitumor effects through
inducing apoptosis, inhibiting cancer cell proliferation and
enhancing immunotherapy sensitivity.
Vasculogenic mimicry (VM)
Original concept and research
progress
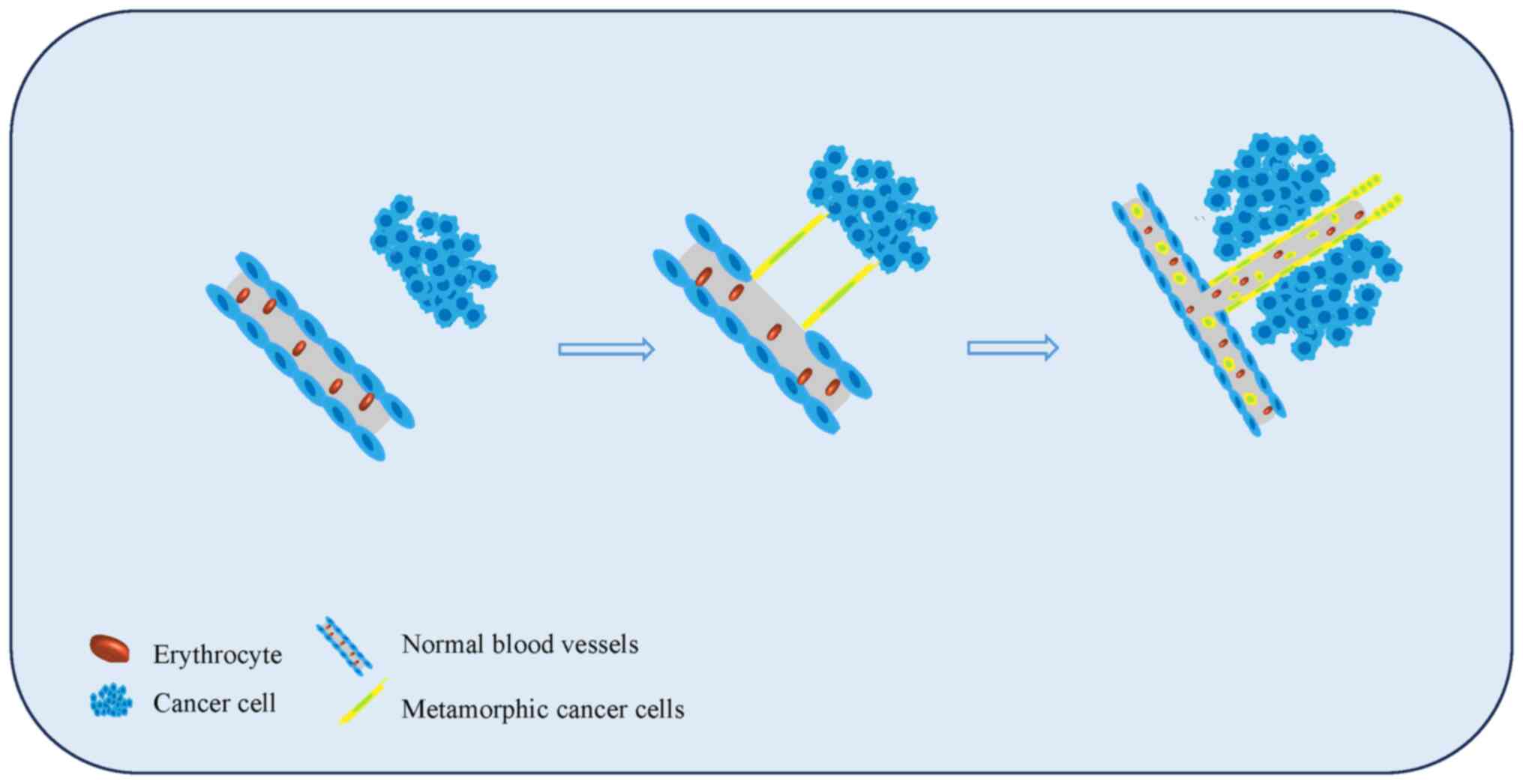
Malignant tumor proliferation is dependent upon
neovascularization to provide the necessary oxygen and nutrients.
When the diameter of a solid tumor grows to 2 mm, the induction of
neovascularization is necessary to meet the supply of oxygen and
nutrients. Tumor cells undergo deformation to induce tumor cells
and extracellular matrix (ECM) remodeling to form structures
similar to blood vessels (Fig. 2).
This angiogenetic process does not rely on endothelial cells [it is
endothelial cell (for example, CD31, CD34) -negative], and cancer
cells are able to be shed and metastasize within the blood at any
time. Furthermore, neither necrosis nor inflammatory cell
infiltration is associated with this process. The special
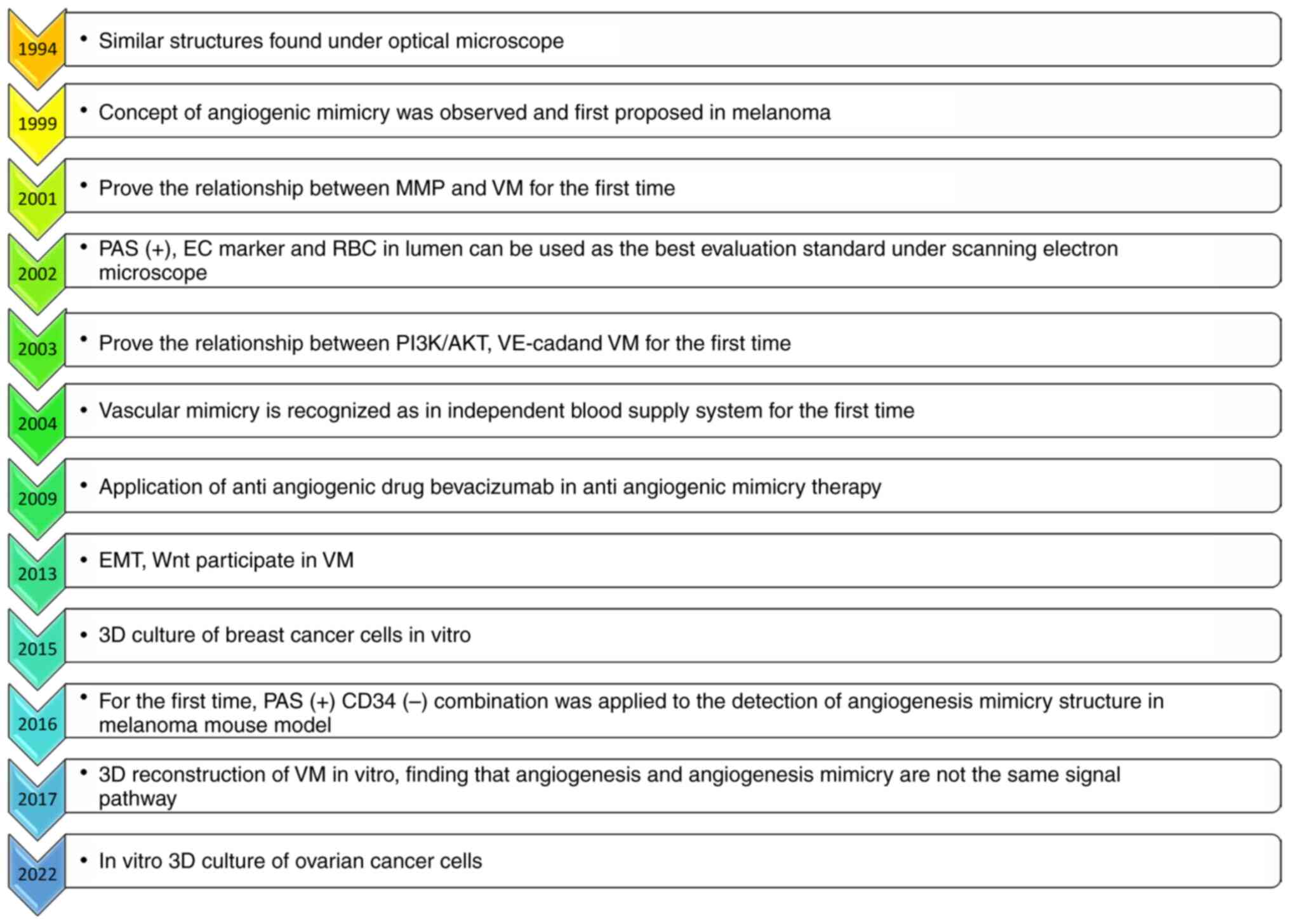
neovascular structure was first discovered under an optical
microscope in 1941 and was observed through techniques such as
Periodic acid-Schiff staining in 1999, when the concept of VM was
first proposed (11). VM
predominantly exists in malignant tumors that have the
characteristics of high recurrence, high metastasis, poor prognosis
for the patient and low survival rate (12). Over a continuous period of
development, the status of research has gradually advanced towards
the identification of pathological structures, the establishment of
in vivo animal models, identification of the associated
signaling pathways and prospective treatments with targeted drugs
(Fig. 3). However, significant
obstacles remain that need to be overcome, including the lack of
reliable VM formation markers and mature methods for VM
identification in vivo (13–15).
An outline of the research progress that has been
made in terms of angiogenesis mimicry, from the first discovery of
angiogenic mimicry in 1941 to the three-dimensional (3D)
observation of angiogenic mimicry structure in angiogenic OC in
2022, moving on towards discovering more about the processes
associated with angiogenic mimicry and their development, is
provided in Fig. 3.
Angiogenic mimicry and tumors
Currently, the presence of angiogenic-mimetic
structures has been identified in the majority of highly aggressive
tumors, including triple-negative breast cancer, glioblastoma,
melanoma and renal cell carcinoma. However, the presence of
angiogenic mimicry in all highly aggressive tumors has yet to be
confirmed, and the means to target angiogenic mimicry to treat
tumors have yet to reach a mature stage of development (16,17).
Angiogenic mimicry and tumor types are listed in Table I (12,18–25).
 | Table I.Angiogenic mimicry and tumor
types. |
Table I.
Angiogenic mimicry and tumor
types.
| Tumor type | Mechanism | Impact | Clinical stage | (Refs.) |
|---|
| Breast cancer | lncRNA, CSC,
multiple signaling pathways | Poor prognosis | Not in the
clinic | (12) |
| Melanoma | ECM matrix
remodeling | Tumor
metastasis | Clinical trial of
immunotherapy in connection with anti-VM therapy | (18) |
| Glioblastoma | Histone deacetylase
inhibitors inhibit VM production | Tumor migration,
Invasion | Not in the
clinic | (19) |
| Hepatocellular
carcinoma | CSC, EMT, hypoxia
signaling factors | Tumor metastasis
invasion, poor prognosis | More clinical
studies are needed | (20) |
| Non-small cell lung
cancer | AGGF1, UBE2C | Tumor metastasis,
invasion and poor prognosis | Clinical sample
staining and correlation study of pathological features | (21) |
| Colon cancer | Wnt/β-catenin
signaling pathway antagonists inhibit VM | Tumor
development | Clinical studies
are being conducted | (22) |
| Prostate
cancer | via hypoxia
signaling factors and hyaluronic acid | New targets for
tumor therapy | In vivo and
in vitro experiments, not yet in clinical trials | (23) |
| Head and neck
squamous cell carcinoma | EMT | Survival and poor
prognosis | Not yet in the
clinical trial stage | (24) |
| Renal cell
carcinoma | Associated with
androgen receptors | Tumor
metastasis | In vivo and
in vitro experiments; not yet in the clinic | (25) |
Signaling pathways associated with
angiogenic mimicry in OC
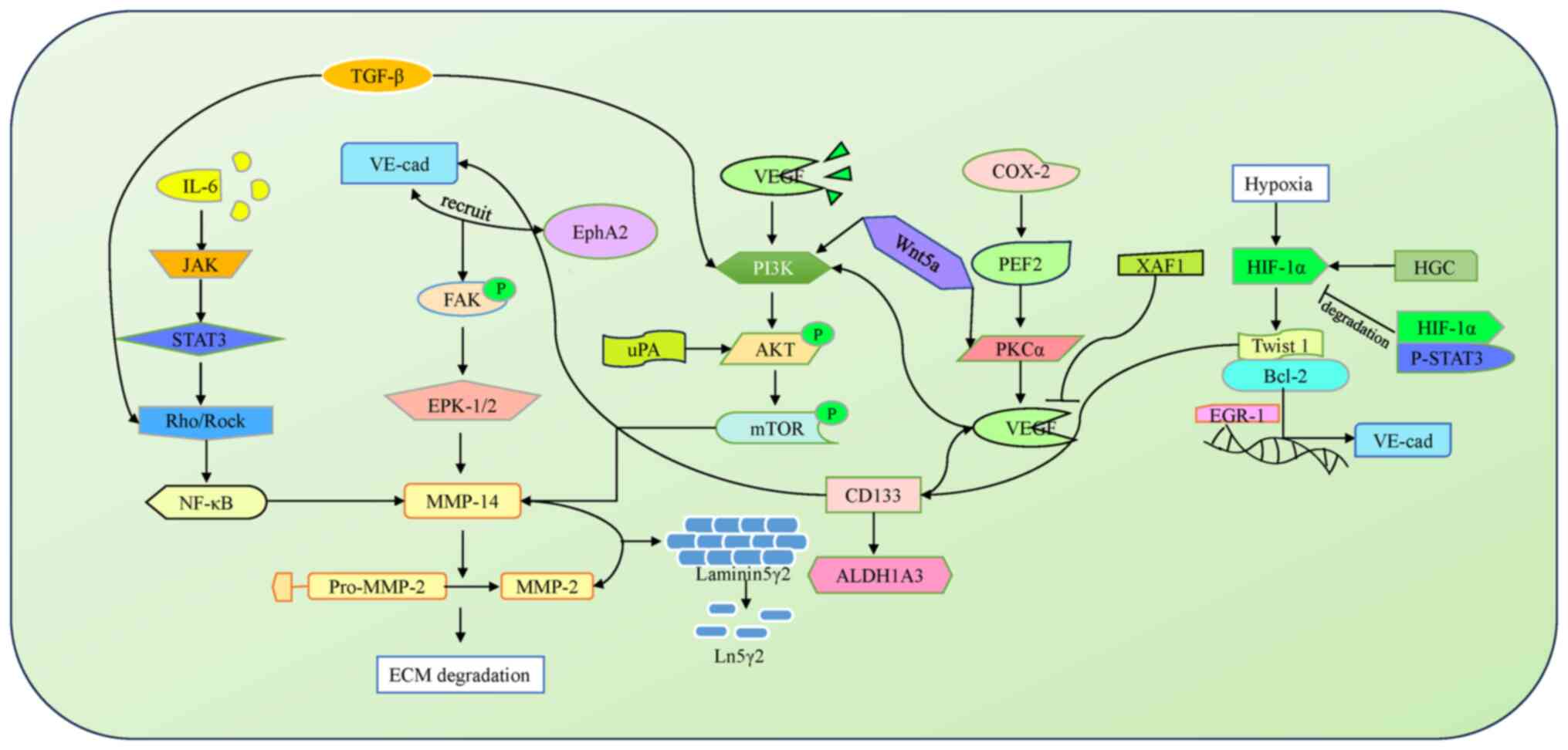
The VM of OC, as a key influencing factor affecting
occurrence, development, invasion and metastasis of OC, has a
complex mechanism of action, which is closely associated with
cancer stem cells (CSCs), the tumor microenvironment, associated
factors. Moreover, various regulatory components and signal
pathways are also interrelated, and can affect each other. For
example, VM, which provides a special means to obtain nutrients and
oxygen during the malignant proliferation of tumors, is closely
associated with the tumor microenvironment. An anoxic
microenvironment can directly regulate vascular endothelial growth
factor (VEGF)-A, VEGF receptor 1 (VEGF-R1), EphA2, Twist and
cyclooxygenase-2 (COX-2) signals, which indirectly regulate
VE-cadherin, tissue factor (TF) and Notch signals (26) and can also induce autophagy and
affect the expression of the CSC markers, CD133 and aldehyde
dehydrogenase 1 (ALDH1). The acidic microenvironment is an
influential factor for cancer cell metastasis and drug resistance.
Recent studies have shown that hypoxia and an acidic
microenvironment can also affect the growth of CSCs (27); inflammatory cytokines, interleukins
and chemokines secreted by the tumor immune microenvironment also
mediate angiogenesis mimicry through activating different signaling
pathways (28). For example, the
inflammatory factor interleukin (IL)-6 activates the JAK/STAT3
signaling pathway to mediate generation of VM, which is associated
with a variety of different mechanisms of tumor chemotherapy
resistance (29). In 2019,
Ayala-Dominguez et al (1)
reported how the VE-cadherin/cadherin /EphA2/MMP-2/Ln5γ2 signaling
axis acted as a key signaling pathway for the formation of
angiogenic mimicry in OC, wherein cancer cell metastasis was
promoted through ECM remodeling. In addition, another study
(30) demonstrated that there are
several other signaling pathways involved in the generation of
angiogenic mimicry in OC, including the AKT/mTOR/MMP-2/laminin5γ2
and the phosphoinositide 3-kinase (PI3K)/AKT/mTOR signaling
pathways, and so on. Furthermore, a recent study identified that
platelets, as coagulants, can function as agents of anti-VM
generation in breast cancer (31),
although whether similar blood cells also have an identical role in
OC requires further research, and may provide a new direction and
further ideas for subsequent research strategies. A detailed
introduction to the important signaling pathways and major
regulatory components that affect the formation of VM in OC is
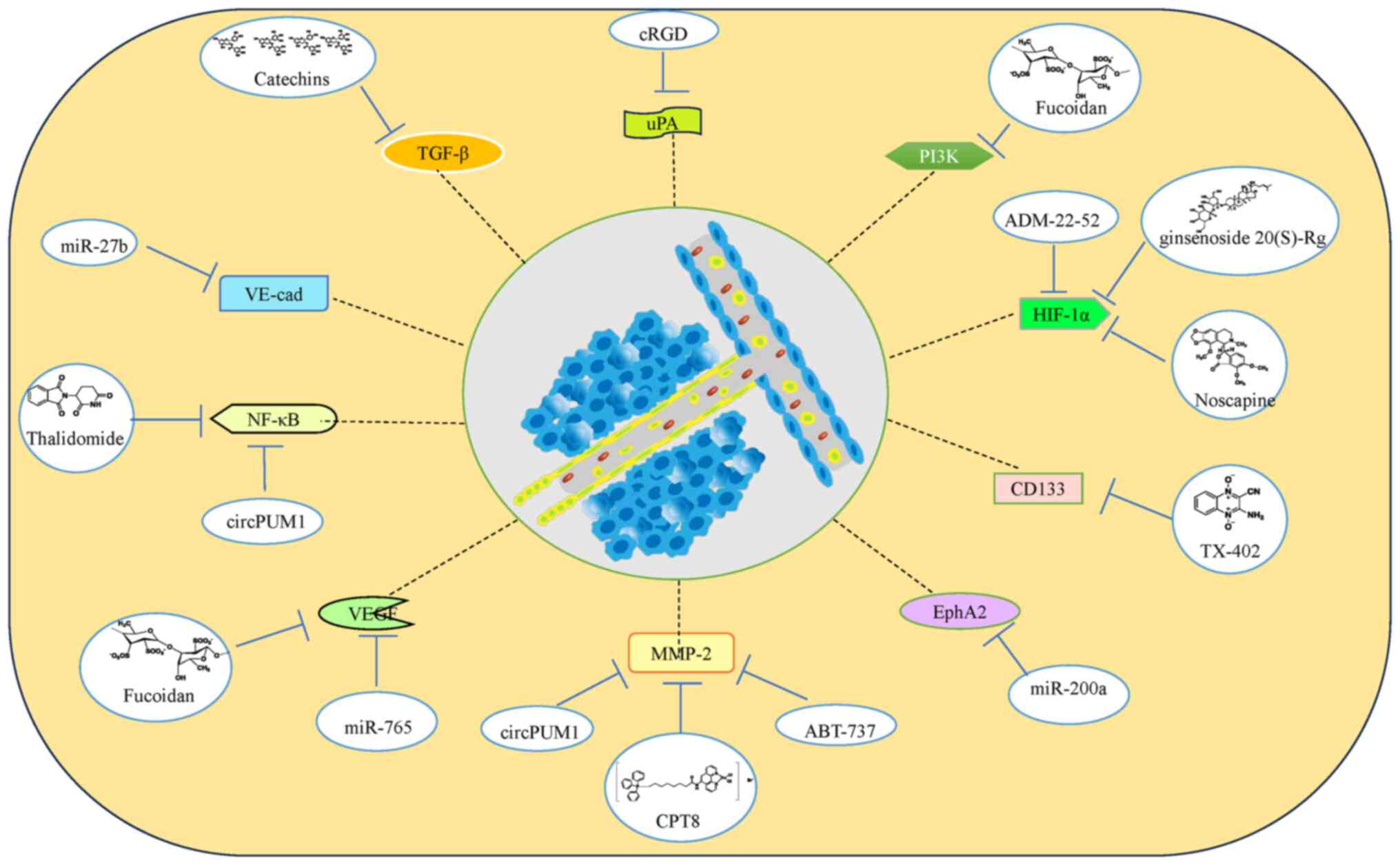
provided in Fig. 4.
Signaling pathways
VE-cadherin/cadherin/EphA2/MMP-2/Ln5γ2
signaling pathway
VE-cadherin is an important protein that adheres to
endothelial cells (32). The
protein is closely associated with the formation of VM in OC.
VE-cadherin promotes activation of the extracellular
signal-regulated kinases 1 and 2 (ERK1/2) and MMP-14 through
recruiting EphA2 receptors. MMP-degradable cell matrix components
promote OC transfer and the generation of VM. In addition, MMP-2
and MMP-14 have been shown to induce Ln5γ2, and remodeling of the
ECM further promotes the production of VM (33,34).
AKT/mTOR/MMP-2/Ln5γ2 signaling
pathway
Urokinase-type plasminogen activator (uPA) is a
serine protein that is able to activate the AKT/mTOR/MMP-2/Ln5γ2
signaling pathway to promote VM generation in OC. The specific
mechanism involves AKT phosphorylation mediated by uPA, which
thereby promotes mTOR phosphorylation. Phosphorylated mTOR promote
the conversion of pro-MMP2 into MMP2, thereby enhancing the
degradation of Ln5γ2 and promoting ECM reconstruction, leading to
the further generation of VM (35).
PI3K/AKT/mTOR signaling pathway
Ediriweera et al (36) revealed that the PI3K/AKT/mTOR
pathway is activated in OC, accompanied by changes in the pathway
structure and gene expression of its various components (including
PTEN and PI3K). At present, numerous inhibitors associated with
this pathway have been applied during preclinical trials of OC,
although further research is needed to improve their targeting
efficiency, since this pathway not only regulates cancer cell
growth and the generation of VM, but it also participates in normal
cell growth.
Wnt signaling pathway
The abnormal activation of the Wnt signaling pathway
in OC is closely related to the malignant proliferation and
metastasis of OC. The family member Wnt5a regulates the expression
of protein kinase Cα (PKCa) to promote generation of VM in OC. In
addition, an increase in Wnt5a has also been shown to lead to an
upregulation of the expression of PI3K, although the specific
regulatory mechanism underlying this process still requires further
research (35,37).
Regulatory factors
CSCs
CSCs have unlimited proliferative potential compared
with mature stem cells. In 2005, Bapat et al (38) discovered CSCs in the ascites of
patients with OC, suggesting that the high invasiveness of OC is
associated with CSCs. CD133, as a marker of CSCs, is positive in
patients with OC, inducing both an increase in VM and an
upregulation of biomarkers associated with VM formation, including
VE-cadherin and VEGF. In addition, CD133 was also found to be
associated with a high expression of ALDH1, and previous studies
have shown that high expression of ALDH1 is also positively
correlated with the generation of VM (39,40).
Hypoxic microenvironment
Hypoxia is able to regulate various pathways of
cancer generation, including angiogenic mimicry. A study in
vitro showed that hypoxia is positively correlated with the
generation of VM in melanoma, hepatocellular carcinoma, breast
cancer, and other types of cancer (38). Hypoxia has been shown to upregulate
the expression of VE-cadherin and p-STAT3, thereby promoting VM
generation. Gest et al (41)
found that inhibiting p-STAT3 could effectively reduce the number
of VM structures in OC. Therefore, developing targeted inhibitors
for p-STAT3 should provide a new source of potential inhibitors for
the generation of VM.
EMT and ECM reconstruction
EMT is a necessary process contributing towards
cancer cell metastasis, and numerous EMT-associated molecules have
been shown to fulfill important roles in the formation of VM,
including transforming growth factor-β (TGF-β). Previous studies
also identified that the inhibition of TGF-β induced cell migration
and EMT could further inhibit the formation of VM structures in OC
(42,43). The role of ECM in tumor metastasis
is essentially twofold: ECM initially serves as a regional barrier
against cancer cell metastasis, although when it is reshaped, it
becomes an important mediator of metastasis (44,45).
Other components
Human chorionic gonadotropin (HGC) has been
demonstrated to promote the production of VM through its fifth
subunit in vitro. When the HGC is introduced externally, the
overexpression of hypoxia-inducible factor-1α (HIF-1α) and
significant upregulation of vascular markers, including CD31 and
VEGF, induce the production of VM through the HGC/VEGF axis
(46,47). In addition, in vivo
inhibition experiments revealed that the overexpression of
apoptosis-associated factors [for example, XIAP-associated factor 1
(XAF1)] and decreased expression of VEGF led to a decrease in the
number of VM structures and inhibition of cell proliferation and
migration. These findings suggested that XAF1 may serve as a useful
inhibitor of VM generation (48).
OC angiogenesis mimetic-associated
inhibitors
At present, the reported inhibitors associated with
angiogenesis mimicry in OC can be essentially divided into the
following categories: Synthetic drugs and drug analogues, natural
extracts, non-coding RNAs, small-molecule inhibitors, protein
peptides and associated targets (Fig.
5). Among these inhibitors, synthetic drugs and drug analogues
refer to drugs that have been widely used in clinical practice for
the treatment of other diseases. Therefore, their safety, efficacy,
side effects and medication methods have been clearly demonstrated,
although as a downside, the multiplicity of their effects does
present a number of obstacles to the rational use of these drugs
(49). As commonly used agents for
cancer treatment, natural extracts have the advantages of low
toxicity and high efficiency. However, due to their complex
components and diverse impact targets, it is necessary to perform a
prerequisite number of preclinical trials (50). Non-coding RNAs, small-molecule
inhibitors and protein peptides all exert targeted inhibitory
effects. Non-coding RNAs affect the process of angiogenic mimicry
through regulating the expression of target genes, which in turn
affects the occurrence, development, treatment and prognosis of OC.
However, their mechanism of action, and overall function, remain
largely unknown (51).
Small-molecule inhibitors can target specific proteins or factors
to affect the inhibition of angiogenic mimicry in OC and have
strong specificity. However, concerning VEGF angiogenic inhibitors
that are associated with angiogenic mimicry, although they can
significantly inhibit angiogenesis, they are prone to drug
resistance and have the ability to compensate for the increased
effects of angiogenesis mimicry, thereby promoting cancer cell
recurrence and metastasis (52).
The mechanisms underlying the action of protein peptide drugs are
relatively clear, although they are easily cleared by the organism,
and therefore their efficient utilization rate continues to remain
an important focus of research (53). Previous studies have also identified
a new modality for angiogenesis mimicry, in that it is jointly
affected by multiple signal pathways and signaling molecules. The
single inhibition of a certain pathway or molecule tends to
activate other signaling pathways in a compensatory manner, which
suggests that multiple types of inhibitors will be required in
order to improve the therapeutic effects during treatment (54–56)
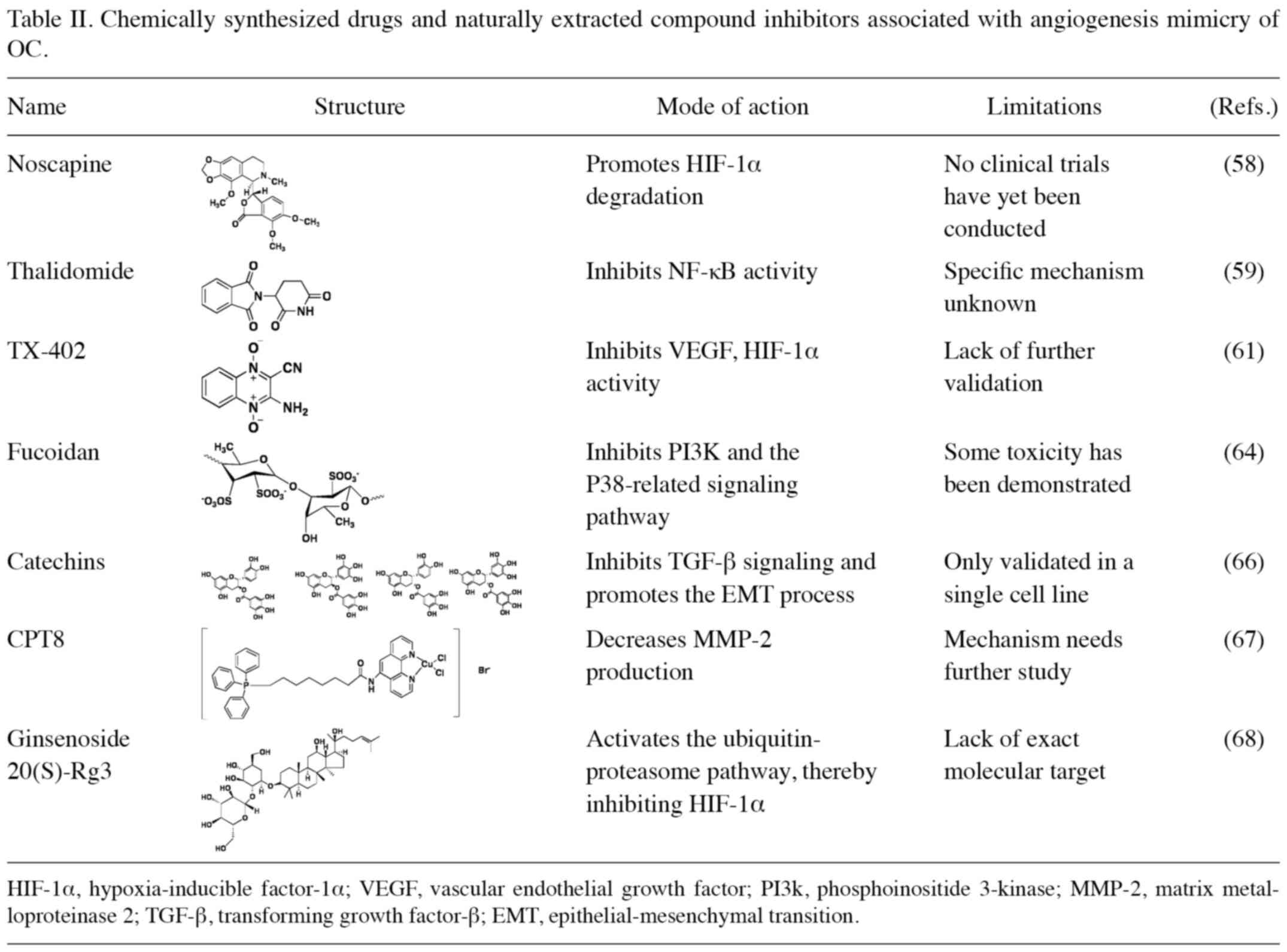
(Table II).
Synthetic drugs and drug analogs
Noscapine is mainly used in the clinic for cough
suppression, and exerts similar effects to codeine, although it is
non-addictive. Previous studies have shown that it acts as a HIF-1α
inhibitor through promoting degradation of HIF-1α by associated
proteases in combination with DDP to treat the proliferation of OC
resistant to paclitaxel (57).
Currently, clinical trials have been conducted for both lymphoma
and chronic lymphatic leukemia, and in vivo and in
vitro experiments have been performed to validate the use of
DDP in OC (58).
Thalidomide, as an immunomodulatory inhibitor, has
been used in the clinical treatment of OC, myeloma, glioblastoma
and other tumors. Previous studies have found that it can be
employed as an anti-angiogenesis inhibitor through exploiting its
ability to effectively inhibit VEGF production, and it has also
been shown to downregulate the NF-κB signaling pathway (59), which has been revealed to be closely
associated with angiogenesis and cancer cell metastasis. Therefore,
it has been hypothesized that the mechanism of action of
thalidomide may involve decreasing the level of NF-κB, which
further inhibits ECM degradation and reduces both the generation of
VM and cancer cell metastasis (60).
TX-402, as a selective prodrug (61), possesses both angiogenic and
HIF-1α-inhibitory effects, and exerts its function through
eliminating tumor stem cell (CSC)-associated growth factors (for
example, CD33 and CD44) in the hypoxic zone at the core of rapid
tumor proliferation. It is anticipated that it may be used as a
treatment for hypoxia-induced tumor cell proliferation and
angiogenesis-resistant tumors.
In addition, lupeol is a triterpene that has a wide
range of applications both in vivo and in vitro, and
previous studies have shown that it possesses favorable antitumor
activity (62). In melanoma, it can
inhibit the production of VM through downregulating the CSC marker,
CD133. However, its anti-angiogenic mimicry effects in OC have yet
to be reported on (63).
Natural extracts
Fucoidans are a class of sulfated polysaccharide
analogs extracted from brown algae that exert a killing effect on a
variety of cancer cells, including ovarian, breast, hepatocellular
and bladder cancer. Bae et al (64) used fucoidan as a means of
therapeutic intervention both in zebrafish in vivo and on
ES-2 and OV-90 cells in vitro, and it was found that this
treatment led to an increased production of reactive oxygen species
(ROS) in cell lines, mitochondrial oxidative damage, the production
of cytochrome c, to promote apoptotic vesicle cleavage. In
addition, fucoidan was found to inhibit OC angiogenic mimicry
through inhibiting angiogenic genes (e.g., VEGFs) and FMS-related
tyrosine kinases (Fits) and kinase insertion domain receptors
(KDRs), exerting synergistic effects in combination with natural
antitumor agents such as DDP and paclitaxel. However, it was found
to exert toxic effects on the zebrafish breeding females in the
in vivo animal model, and therefore its application in the
clinic requires further refinement of the dose used, timing of drug
administration and molecular targets.
Catechins is extracted from green tea. Studies have
shown that it can be used with TGF-β receptor binding, suppress
TGF-β activation of pathway related downstream Smad3/P38 signal
inhibits the degradation of MMPS on ECM to reduce VM production and
prevent OC metastasis and chemoresistance (65,66).
Low-copper complexes [for example, the
phenanthroline copper (ii) complex CPT8] are able to destroy
intracellular DNA by producing ROS, thereby exerting antitumor
effects. A previous study has revealed that the copper ligand
compound CPT8 exhibits anti-angiogenic mimicry activity via
inhibiting the production of MMP-2, thereby blocking the
nutritional supply system of tumor cells and reducing the
likelihood of cancer cell metastasis and recurrence (67).
EOC, a phenotype of OC that currently has a high
mortality rate, is closely associated with low patient survival due
to its aggressive and metastatic nature. Liu et al (68) showed for the first time that
ginsenoside 20(S)-Rg3 inhibited EMT, which consequently affected
cell invasion and metastasis both in vitro and in
vivo by reducing HIF-1α expression, which in turn inhibited
downregulation of the epithelial marker, E-cadherin, and
upregulation of the mesenchymal marker, vimentin. Ginsenoside
20(S)-Rg3 has the advantage of being a natural inhibitor of HIF-1α
with low toxicity and high efficiency, although the lack of
relevant molecular targets at the present time hinders its clinical
application.
The weak alkaline indole alkaloid brucine, extracted
from the seeds of Strychnos nux-vomica L.
(Loganiacaee), has been identified to possess antitumor
activity. Xu et al (69)
demonstrated that it can inhibit the generation of VM through
inhibiting expression of EphA2 and MMP in the triple-negative
breast cancer cell line MDA-MB-231 in a dose-dependent manner.
Polyphyllin I (PPI), as the main component of the commonly used
traditional Chinese medicine Rhizoma Paridis, has been demonstrated
to inhibit both the progression of hepatocellular carcinoma and the
generation of VM. It was also found to regulate the expression of
Twist1 and to inhibit the generation of VM through inhibiting the
PI3K/AKT/Twist1/VE-cadherin signaling pathway (70). However, whether Brucine and PPI have
the same efficacy in OC angiogenesis mimicry has yet to be
explored, and further studies are required to address this
question.
The small-molecule drug D-39, extracted from the
evergreen perennial plant Liriope muscari, is an inhibitor
of the cysteine-rich protein, Mig-7. Its encoding gene,
Mig-7, is a migration-inducing gene that is rich in
cysteine, which was first identified in metastatic hepatocellular
carcinoma, and later shown to be expressed in a variety of cancer
cells, but almost not at all in normal tissues (71,72).
Huang et al (73) showed
through phenotypic studies in EOC tissues that included
investigation of Mig-7 protein expression levels and
histopathological status analysis, the introduction of Mig-7 cell
metastasis and invasion in vitro, tumor volume size analysis
in nude mice in vivo, the addition of the Mig-7 inhibitor
D-39 for reverse validation experiments, and statistical analysis
in combination with clinical samples, that D-39 specifically
inhibited expression of VEGFA without affecting other angiogenic
factors. The aformentioned study provided further clues for the
development of specific Mig-7 inhibitors or monoclonal antibodies
to influence VM production, and thereby increase the options
available for the therapeutic treatment of OC.
Non-coding RNAs
MicroRNAs (miRNAs)
Sun et al (74) observed the phenotype of clinical
samples of OC, performed in vitro cellular experiments and
combined with previous findings, concluded that VM is associated
with disease clinicopathological typing, metastasis and survival,
fulfilling an important role in OC development and prognosis.
Previous studies have shown that several miRNAs are associated with
development and prognosis of OC and can be used as biological
targets for the diagnosis and prognosis of OC. Among them, miR-200a
inhibits the production of VM by reducing the expression level of
the EphA2 gene, and it has been hypothesized that a reduced level
of miR-200a expression would inhibit E-cadherin expression in tumor
stem cells, thereby affecting the migration and invasion of OC
cells. By contrast, high expression of miR-200a may also be a
predictor of a higher survival rate of patients with OC, and
therefore this can be used as a predictive target for OC diagnosis
and prognosis. However, the number of clinical samples included in
the study by Sun et al (74)
was small, and further studies are required to refine the
conclusions. In an extensive study on the miR-27b regulation of
tumor angiogenesis and cancer metastasis, Liu et al
(75) were the first research group
to have explored its inhibition of VM formation in OC through the
expression of VE-cadherin. This miRNA accomplishes its function
through binding to the 3′-untranslated region of VE-cadherin mRNA,
resulting in reduced expression of its associated proteins,
decreased expression of tight junction proteins and a decrease in
invasion signals, which leads to the inhibition of tumor migration,
invasion, endothelial vascularity and tumor neovascularization, and
therefore, of the generation of VM. Hypoxia-regulated miRNAs serve
an important role in the early regulation of VM formation, and it
was shown that miR-765 regulates the VEGFA/AKT1/SRC-α signaling
axis to coordinate the formation of 3D channel-like structures
(76). That study, in its
investigation of miR-765′s critical role in early VM formation,
employed a combination of mechanistic and in vitro cellular
experiments to demonstrate the effects exerted by miR-765 on the
number of 3D channel-like structural branches and patterned tubular
structures; however, a limitation to the study was that only a
single cellular model was employed to study early VM formation at
48 h, and no in vivo experiments were performed to validate
the findings. Therefore, that study lacked theoretical support for
the mechanism of late VM formation, and the application of miR-765
in the clinical setting needs to be further investigated, in depth
and over a longer time period.
Circular RNAs (circRNAs)
CircRNAs act as non-coding RNAs, which can serve
important roles via linking to the tumor microenvironment through
neovascularization and tumor cell metastasis (76). A previous study revealed that
circPUM1 is more highly expressed in cancerous OC tissues, and it
has been surmised that it regulates the expression of NF-κB and
MMP2 through targeting the miRNAs miR-615-5p and miR-6753-5p;
moreover, its exosomes are able to reach the peritoneum, thereby
promoting tumor cell metastasis (77). Furthermore, Shao and Lu (78) demonstrated that circPUM1 could be an
important target for the generation of VM in OC in the future.
Collectively, the aforementioned studies have shown that VM
production is an important target for OC.
Small-molecule inhibitors
VEGR and MMP-2/-9 are positive regulators of tumor
angiogenesis, and through the use of an in vitro pipeline by
in vitro co-culture, angiogenesis mimetic assay and the
western blotting detection of MMP-2/-9 protein expression branch
formation, a research group (79)
identified that treatment with ABT-737, a small-molecule inhibitor
of the anti-apoptotic protein B-cell lymphoma-2 (Bcl-2), led to
downregulation of VEGR and MMP-2/-9 protein expression, therefore
implying that ABT-737 could inhibit VM production by affecting
MMP-2/-9.
Other small-molecule target inhibitors serve roles
in generation of VM. For example, histone deacetylase (HDAC) has
been found to inhibit the generation of VM structure in
triple-negative breast cancer and glioma, and the protein was found
to exert its role through downregulating the expression of VEGF-A
and EMT-associated genes, although all the studies that have been
performed to date have been in vitro cellular experiments,
and no clinical studies have yet been conducted (80); therefore, further experiments are
needed to verify whether HDAC can be used as an effective inhibitor
of VM and applied in the treatment of OC (81).
VEGF serves as a common influential target for
angiogenesis and angiogenic mimicry, and a range of anti-angiogenic
VEGF inhibitors (for example, cediranib, nintedanib, bevacizumab
and humv833) have been used to reduce the production of VM
(82). However, numerous studies
have reported that pro-angiogenic mimicry may occur during
inhibition of angiogenesis, and therefore an in-depth study of the
underlying mechanisms accounting for how they are able to reduce VM
production will not only lead to an improved understanding of the
difference between angiogenic mimicry and angiogenesis, but it
should also contribute towards future studies of specific
anti-angiogenic mimicry therapy (83,84).
Similarly, it remains to be identified whether further studies are
necessary to investigate more deeply markers of angiogenesis, such
as TEM8 (85), which, to date, has
not been applied in the VM generation in OC.
A previous study (86) reported on the use of galunisertib as
a TGF-β inhibitor that can inhibit autophagy and improve the
survival rates of patients with breast cancer. Moreover, a close
association exists between cell autophagy and VM, and thus
galunisertib may inhibit angiogenesis mimicry in breast cancer via
inhibiting TGF-β and autophagy. However, whether it can be applied
to angiogenesis mimicry in OC needs to be validated by further
research.
Protein peptides
Adrenomedullin (ADM), as a peptide substance, is
expressed in various types of tumor tissue and fulfills an
important role in tumor cell growth, inducing apoptosis and tumor
angiogenesis. A previous study (87) observed the mimicry effect resulting
from the overexpression of ADM, and by observing the effects of
adding an ADM-22-52 inhibitor on OC angiogenesis through a series
of in vitro cell experiments, it was found that ADM could
upregulate HIF-1α and promote the role of VEGF in angiogenesis
in vitro. This suggested that the inhibition of ADM may be
used as a target for treating VM in the future.
As a component of a proteoglycan complex, syndecan-1
(SDC1) is mainly expressed in the epithelium, and its expression is
associated with the poor prognosis of OC. As a marginal substance
of benign and malignant stroma, SDC1 may be used as a predictive
target. Through combining anti-SDC1 peptide with L19-IL2 factor
(B-Fn-specific factor) to intervene in an EOC mouse model, it was
found that it regulate EMT, alleviate hypoxia in the tumor center
and promote the loss of the stemness characteristics of CSCs,
thereby inhibiting the progression of OC tumors. However, further
research is needed to determine whether the inhibition of
angiogenesis mimicry is included as a part of the process of
inhibiting the progression of OC. In addition, SDC1 has been shown
to serve as a reverse target gene for miR-302a, suggesting that
miR-302a may serve as a targeted inhibitor for the treatment of OC
progression (88).
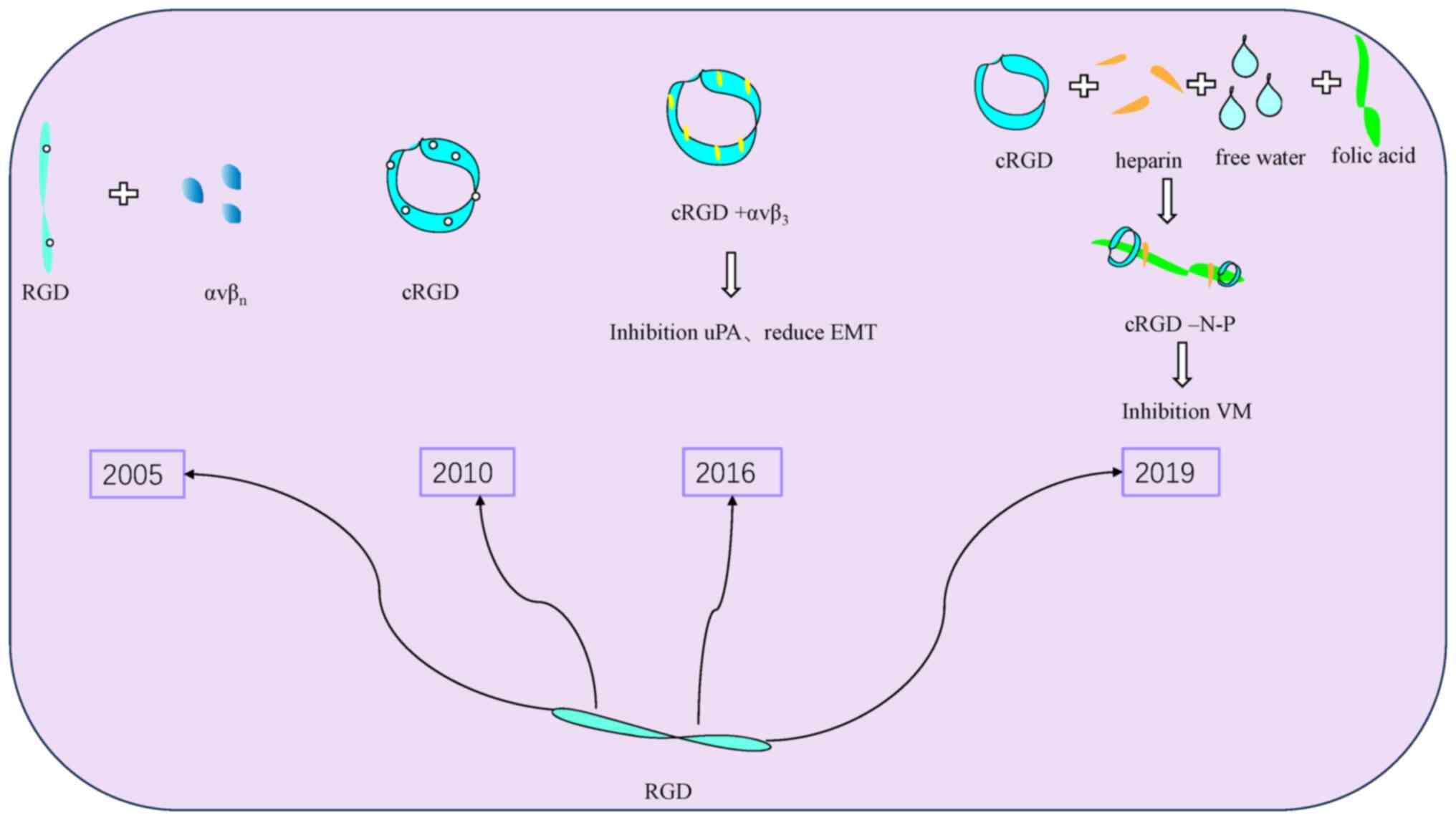
Cyclic Arg-Gly-Asp (cRGD) is an endogenous peptide,
and in 2016 Tang et al (35), in their study involving tissue
immunohistochemical analysis and in vitro culture of various
OC cell lines, including SKOV-3, OVCAR and A2780 cells, found that
uPA exerted a positive regulatory role in VM generation via
activating the AKT/mTOR/MMP-2/laminin5γ2 signaling pathway. cRGD, a
uPA inhibitor, is able to reduce VM production through
downregulating its expression, and it can also inhibit the
angiogenic mimetic state of OC by reversing EMT. In 2019, Wang
et al (89) made it into a
functional nanoparticle and demonstrated through performing a
series of in vivo and in vitro experimental studies
that it could achieve both anti-EDV and anti-VM effects by
affecting MMPs and through reducing EMT production. This study
provided a new direction for the use of functional nanomaterials as
a novel drug delivery method at a later stage (Fig. 6).
Other associated targets
Combined with incidence and therapeutic difficulties
of OC, the discovery of more specific predictive targets is
indispensable for early disease screening, disease diagnosis and
prognosis prediction. Currently, a number of specific targets have
been identified through the analysis of clinical samples or by
validation through the employment of in vivo and in
vitro experiments, as revealed in Table III (90–95).
 | Table III.Angiogenesis mimicry-related targets
in OC. |
Table III.
Angiogenesis mimicry-related targets
in OC.
| Target | Definition | Study
methodology | VM | Limitations | Role | (Refs.) |
|---|
| CGB5 | Chorionic
gonadotropin subunit V | In vitro
plasmid transfection, in vivo tissue staining | As an important
subunit of HCG secretion by trophoblast cells, it promotes vascular
endothelial cell proliferation and VEGF expression | Lack of clinical
sample analysis and in-depth study of specific mechanisms | Future use as
therapeutic target | (90) |
| CD177 | Tumor stem cell
surface markers |
Immunohistochemistry of clinical
samples | Predicting patient
survival | Small sample size
and lack of long-term prognostic studies | Assessment of EOC
malignancy and chemotherapy sensitivity | (91) |
| CD133 | Tumor stem cell
surface markers | Clinical sample
analysis | Determine patient
survival | Small clinical
samples and lack of quantification of CD133 expression | Diagnosis,
prognosis | (92) |
| ALDH1 | Stem cell
markers |
Immunohistochemistry of clinical tissue
samples | Associated with EOC
development, invasion, metastasis and poor prognosis | Lack of in
vivo and in vitro experimental validation | EOC prognosis | (93) |
| FOXC2 | Embryonic
transcription factors | In vivo and
in vitro experimental validation | Mainly involved in
EMT process, decreased VE-cadherin protein expression, and
decreased tube-forming ability of cells | Few clinical
samples | Metastasis,
survival | (94) |
| PRRX1 | Homologous frame
transcription factors | Immunohistochemical
analysis of clinical tissue samples | Acts as an EMT
inducer, presumably activating the Wnt pathway for VM by promoting
-catenin entry into the nucleus | Lack of in
vivo and in vitro experimental validation | Prognosis | (95) |
Clinical applications
As aforementioned, old and new chemical drugs,
natural extracts, non-coding RNAs and small-molecule inhibitors can
inhibit angiogenesis mimicry in OC, although the majority of the
studies published to date are at stages prior to these compounds
entering clinical trials. Monoclonal antibodies that work against
the establishment of VM have been developed which target the
VE-cadherin protease receptor, which inhibit its binding to achieve
anti-angiogenic mimicry, and this has been shown to have a clear
role in the treatment of lung cancer (96), although this has yet to be applied
in treatment of OC. Due to monoclonal antibodies strong
specificity, the rational and efficient application of these
antibodies will be expected to achieve a multiplier effect
(1). In addition, the study of
biomimetic mechanisms and associated inhibitors in OC remains a
difficult research area. Certain anti-angiogenic mimetic drugs that
have been applied to other tumors, including flavonoids (97), curcumin (98), doxycycline (20), thalidomide (60), and so on, need to be tested for
their pharmaco-toxicological and pharmacokinetic properties before
they can be formally applied in a clinical setting; therefore,
there remains a long way to go before they can be applied for the
treatment of OC angiogenic mimicry (99). Thus, there remains a long way to go
before these agents can be used in therapeutic strategies for the
treatment of OC angiogenic mimicry. Currently, studies have shown
that the development of anti-angiogenic mimetic drugs and
inhibitors is beneficial for the treatment of anti-angiogenic
resistant tumors, although whether angiogenic mimetics and
angiogenesis are interlinked or processes that are independent of
each other, or whether there is a common signaling pathway, will
impact on the therapeutic efficacy of the associated inhibitors
(100).
Summary and outlook
As a unique way of supplying oxygen and nutrients to
malignant tumors, the special mode of generation, complex signaling
pathways and variety of influencing factors all increase the
challenge of targeting the specificity of angiogenic mimicry, and
moreover, these factors all serve to increase the complexity of the
desired targeted therapies. The compounds and drugs under
consideration have been applied in the treatment of numerous types
of commonly occurring tumors; however, research on the treatment of
OC remains in its infancy stages. In the present review, the
underlying mechanisms of OC angiogenesis mimicry and its associated
inhibitors have been examined and how certain specific targets can
elicit effects on the occurrence were discussed, development,
treatment, metastasis and prognosis of OC, although the majority of
the targets require further clarification in terms of their
identification criteria and the research mainly remains at an early
stage (Fig. 7). However, whether a
single inhibitory target or an entire signaling pathway can exert a
role, or whether other targets or signaling pathways, in turn, will
be activated, requires further study. Therefore, a complete
understanding of the overall signaling network of OC angiogenesis
mimicry and the integration of multiple pathways and targets, or
even of other therapeutic modalities such as chemotherapy, should
provide novel directions for future research. In conclusion, the
rational use of targeted inhibition of OC angiogenesis mimicry is
expected to resolve problems, namely, late detection, difficult
treatment, high recurrence rates and low survival rates of patients
with OC, and provide new research directions working towards
improving the survival rate of patients with OC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the General program of
Natural Science Foundation of Inner Mongolia Autonomous Region
(grant no. 2022MS08060), the General Project of Inner Mongolia
Medical University (grant no. YKD2022MS045), the Inner Mongolia
Autonomous Region Health Science and Technology Plan Project
Assignment (grant nos. 202202156, 202201337 and 202202378), the
Inner Mongolia Medical University Zhiyuan Talent Program (Good
Learning Talent Program) (grant no. ZY0202031), Program for Young
Talents of Science and Technology in Universities of Inner Mongolia
Autonomous Region (grant no. NJYT23050) and the Inner Mongolia
Autonomous Region ‘Grassland Talent’ project youth innovation and
entrepreneurship talent project (grant no. 2022073).
Availability of data and materials
Not applicable.
Authors' contributions
HC and HG conceived and designed the present review.
XT, QS and ML wrote the first draft of the manuscript. JS, RZ, YX
and LY participated in writing of the manuscript. All authors read
and approved the final version of the manuscript. Data
authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ayala-Dominguez L, Olmedo-Nieva L,
Munoz-Bello JO, Contreras-Paredes A, Manzo-Merino J,
Martinez-Ramirez I and Lizano M: Mechanisms of vasculogenic mimicry
in ovarian cancer. Front Oncol. 9:9982019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xu Y, Li Q, Li XY, Yang QY, Xu WW and Liu
GL: Short-term anti-vascular endothelial growth factor treatment
elicits vasculogenic mimicry formation of tumors to accelerate
metastasis. J Exp Clin Cancer Res. 31:162012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yin M, Li C, Li X, Lou G, Miao B, Liu X,
Meng F, Zhang H, Chen X, Sun M, et al: Over-expression of LAPTM4B
is associated with poor prognosis and chemotherapy resistance in
stages III and IV epithelial ovarian cancer. J Surg Oncol.
104:29–36. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Williams TI, Toups KL, Saggese DA, Kalli
KR, Cliby WA and Muddiman DC: Epithelial ovarian cancer: Disease
etiology, treatment, detection, and investigational gene,
metabolite, and protein biomarkers. J Proteome Res. 6:2936–2962.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lino-Silva LS: Ovarian carcinoma:
Pathology review with an emphasis in their molecular
characteristics. Chin Clin Oncol. 9:452020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McCluggage WG, Judge MJ, Clarke BA,
Davidson B, Gilks CB, Hollema H, Ledermann JA, Matias-Guiu X,
Mikami Y, Stewart CJ, et al: Data set for reporting of ovary,
fallopian tube and primary peritoneal carcinoma: Recommendations
from the international collaboration on cancer reporting (ICCR).
Mod Pathol. 28:1101–1122. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Momenimovahed Z, Tiznobaik A, Taheri S and
Salehiniya H: Ovarian cancer in the world: Epidemiology and risk
factors. Int J Womens Health. 11:287–299. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
El-Sherif A, El-Sherif S, Taylor AH and
Ayakannu T: Ovarian cancer: Lifestyle, diet and nutrition. Nutr
Cancer. 73:1092–1107. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fan YZ and Sun M: Molecular regulation of
vasculogenic mimicry in tumors and potential tumor-target therapy.
World J Gastrointest Surg. 2:117–127. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang X, Su P, Hao Q, Zhang X, Xia L and
Zhang Y: A Chinese classical prescription Guizhi-Fuling Wan in
treatment of ovarian cancer: An overview. Biomed Pharmacother.
153:1134012022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Maniotis AJ, Folberg R, Hess A, Seftor EA,
Gardner LM, Pe'er J, Trent JM, Meltzer PS and Hendrix MJ: Vascular
channel formation by human melanoma cells in vivo and in vitro:
Vasculogenic mimicry. Am J Pathol. 155:739–752. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chavoshi H, Poormolaie N, Vahedian V,
Kazemzadeh H, Mir A, Nejabati HR, Behroozi J, Isazadeh A,
Hajezimian S, Nouri M and Maroufi NF: Vascular mimicry: A potential
therapeutic target in breast cancer. Pathol Res Pract.
234:1539222022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Marques dos Reis E and Berti FV:
Vasculogenic mimicry-an overview. Methods Mol Biol. 2514:3–13.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Salinas-Vera YM, Gallardo-Rincón D,
Ruíz-García E, Marchat LA, Valdés J, Vázquez-Calzada C and
López-Camarillo C: A three-dimensional culture-based assay to
detect early stages of vasculogenic mimicry in ovarian cancer
cells. Methods Mol Biol. 2514:53–60. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Folberg R and Maniotis AJ: Vasculogenic
mimicry. APMIS. 112:508–525. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang SY, Ke YQ, Lu GH, Song ZH, Yu L, Xiao
S, Sun XL, Jiang XD, Yang ZL and Hu CC: Vasculogenic mimicry is a
prognostic factor for postoperative survival in patients with
glioblastoma. J Neurooncol. 112:339–345. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen L, Lin ZX, Lin GS, Zhou CF, Chen YP,
Wang XF and Zheng ZQ: Classification of microvascular patterns via
cluster analysis reveals their prognostic significance in
glioblastoma. Hum Pathol. 46:120–128. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Quaresmini D and Guida M: Neoangiogenesis
in melanoma: An issue in biology and systemic treatment. Front
Immunol. 11:5849032020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pastorino O, Gentile MT, Mancini A, Del
Gaudio N, Di Costanzo A, Bajetto A, Franco P, Altucci L, Florio T,
Stoppelli MP and Colucci-D'Amato L: Histone deacetylase inhibitors
impair vasculogenic mimicry from glioblastoma cells. Cancers
(Basel). 11:7472019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zheng N, Zhang S, Wu W, Zhang N and Wang
J: Regulatory mechanisms and therapeutic targeting of vasculogenic
mimicry in hepatocellular carcinoma. Pharmacol Res. 166:1055072021.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Y, Shi F, Tao R, Wu J, Gu J, Yang R
and Wu S: The relationship Between UBE2C and AGGF1 overexpression
and tumor angiogenesis in non-small cell lung cancer. Cancer Manag
Res. 13:5919–5930. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qi L, Song W, Liu Z, Zhao X, Cao W and Sun
B: Wnt3a promotes the vasculogenic mimicry formation of colon
cancer via Wnt/β-catenin signaling. Int J Mol Sci. 16:18564–18579.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Luo Y, Yang Z, Yu Y and Zhang P: HIF1α
lactylation enhances KIAA1199 transcription to promote angiogenesis
and vasculogenic mimicry in prostate cancer. Int J Biol Macromol.
222:2225–2243. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Salem A and Salo T: Vasculogenic mimicry
in head and neck squamous cell carcinoma-time to take notice. Front
Oral Health. 2:6668952021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
You B, Sun Y, Luo J, Wang K, Liu Q, Fang
R, Liu B, Chou F, Wang R, Meng J, et al: Androgen receptor promotes
renal cell carcinoma (RCC) vasculogenic mimicry (VM) via altering
TWIST1 nonsense-mediated decay through lncRNA-TANAR. Oncogene.
40:1674–1689. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Seftor RE, Hess AR, Seftor EA, Kirschmann
DA, Hardy KM, Margaryan NV and Hendrix MJ: Tumor cell vasculogenic
mimicry: From controversy to therapeutic promise. Am J Pathol.
181:1115–1125. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Andreucci E, Peppicelli S, Ruzzolini J,
Bianchini F, Biagioni A, Papucci L, Magnelli L, Mazzanti B, Stecca
B and Calorini L: The acidic tumor microenvironment drives a
stem-like phenotype in melanoma cells. J Mol Med (Berl).
98:1431–1446. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hu H, Ma T, Liu N, Hong H, Yu L, Lyu D,
Meng X, Wang B and Jiang X: Immunotherapy checkpoints in ovarian
cancer vasculogenic mimicry: Tumor immune microenvironments, and
drugs. Int Immunopharmacol. 111:1091162022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Annett S, Moore G, Short A, Marshall A,
McCrudden C, Yakkundi A, Das S, McCluggage WG, Nelson L, Harley I,
et al: FKBPL-based peptide, ALM201, targets angiogenesis and cancer
stem cells in ovarian cancer. Br J Cancer. 122:361–371. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Graupera M and Potente M: Regulation of
angiogenesis by PI3K signaling networks. Exp Cell Res.
319:1348–1355. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Morales-Guadarrama G, García-Becerra R,
Méndez-Pérez EA, García-Quiroz J, Avila E and Díaz L: Vasculogenic
mimicry in breast cancer: Clinical relevance and drivers. Cells.
10:17582021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Giannotta M, Trani M and Dejana E:
VE-cadherin and endothelial adherens junctions: Active guardians of
vascular integrity. Dev Cell. 26:441–454. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Giannelli G, Falk-Marzillier J, Schiraldi
O, Stetler-Stevenson WG and Quaranta V: Induction of cell migration
by matrix metalloprotease-2 cleavage of laminin-5. Science.
277:225–258. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Koshikawa N, Giannelli G, Cirulli V,
Miyazaki K and Quaranta V: Role of cell surface metalloprotease
MT1-MMP in epithelial cell migration over laminin-5. J Cell Biol.
148:615–624. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tang J, Wang J, Fan L, Li X, Liu N, Luo W,
Wang J and Wang Y and Wang Y: cRGD inhibits vasculogenic mimicry
formation by down-regulating uPA expression and reducing EMT in
ovarian cancer. Oncotarget. 7:24050–24062. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ediriweera MK, Tennekoon KH and Samarakoon
SR: Role of the PI3K/AKT/mTOR signaling pathway in ovarian cancer:
Biological and therapeutic significance. Semin Cancer Biol.
59:147–160. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Qi H, Sun B, Zhao X, Du J, Gu Q, Liu Y,
Cheng R and Dong X: Wnt5a promotes vasculogenic mimicry and
epithelial-mesenchymal transition via protein kinase Cα in
epithelial ovarian cancer. Oncol Rep. 32:771–779. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bapat SA, Mali AM, Koppikar CB and Kurrey
NK: Stem and progenitor-like cells contribute to the aggressive
behavior of human epithelial ovarian cancer. Cancer Res.
65:3025–3029. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Taniguchi H, Suzuki Y and Natori Y: The
evolving landscape of cancer stem cells and ways to overcome cancer
heterogeneity. Cancers (Basel). 11:5322019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang HF, Wang SS, Zheng M, Dai LL, Wang K,
Gao XL, Cao MX, Yu XH, Pang X, Zhang M, et al: Hypoxia promotes
vasculogenic mimicry formation by vascular endothelial growth
factor A mediating epithelial-mesenchymal transition in salivary
adenoid cystic carcinoma. Cell Prolif. 52:e126002019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gest C, Mirshahi P, Li H, Pritchard LL,
Joimel U, Blot E, Chidiac J, Poletto B, Vannier JP, Varin R, et al:
Ovarian cancer: Stat3, RhoA and IGF-IR as therapeutic targets.
Cancer Lett. 317:207–217. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dongre A and Weinberg RA: New insights
into the mechanisms of epithelial-mesenchymal transition and
implications for cancer. Nat Rev Mol Cell Biol. 20:69–84. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sicard AA, Dao T, Suarez NG and Annabi B:
Diet-derived gallated catechins prevent TGF-β-mediated
epithelial-mesenchymal transition, cell migration and vasculogenic
mimicry in chemosensitive ES-2 ovarian cancer cells. Nutr Cancer.
73:169–180. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sun B, Zhang D, Zhao N and Zhao X:
Epithelial-to-endothelial transition and cancer stem cells: Two
cornerstones of vasculogenic mimicry in malignant tumors.
Oncotarget. 8:30502–30510. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhao X, Sun B, Li Y, Liu Y, Zhang D, Wang
X, Gu Q, Zhao J, Dong X, Liu Z and Che N: Dual effects of
collagenase-3 on melanoma: Metastasis promotion and disruption of
vasculogenic mimicry. Oncotarget. 6:8890–8899. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Su M, Wei W, Xu X, Wang X, Chen C, Su L
and Zhang Y: Role of hCG in vasculogenic mimicry in OVCAR-3 ovarian
cancer cell line. Int J Gynecol Cancer. 21:1366–1374. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gao S, Fan C, Huang H, Zhu C, Su M and
Zhang Y: Effects of HCG on human epithelial ovarian cancer
vasculogenic mimicry formation in vivo. Oncol Lett.
12:459–466. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang Y, Liu P, Wang X and Mao H: Role of
X-linked inhibitor of apoptosis-associated factor-1 in vasculogenic
mimicry in ovarian cancer. Mol Med Rep. 16:325–330. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ashburn TT and Thor KB: Drug
repositioning: Identifying and developing new uses for existing
drugs. Nat Rev Drug Discov. 3:673–683. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang S, Long S, Deng Z and Wu W: Positive
role of Chinese herbal medicine in cancer immune regulation. Am J
Chin Med. 48:1577–1592. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hernández de la Cruz ON, López-González
JS, García-Vázquez R, Salinas-Vera YM, Muñiz-Lino MA,
Aguilar-Cazares D, López-Camarillo C and Carlos-Reyes Á: Regulation
networks driving vasculogenic mimicry in solid tumors. Front Oncol.
9:14192019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Mahfouz N, Tahtouh R, Alaaeddine N, El
Hajj J, Sarkis R, Hachem R, Raad I and Hilal G: Gastrointestinal
cancer cells treatment with bevacizumab activates a VEGF
autoregulatory mechanism involving telomerase catalytic subunit
hTERT via PI3K-AKT, HIF-1α and VEGF receptors. PLoS One.
12:e01792022017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wu H and Huang J: Optimization of protein
and peptide drugs based on the mechanisms of kidney clearance.
Protein Pept Lett. 25:514–521. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Pinto MP, Sotomayor P, Carrasco-Avino G,
Corvalan AH and Owen GI: Escaping antiangiogenic therapy:
Strategies employed by cancer cells. Int J Mol Sci. 17:14892016.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Vasudev NS and Reynolds AR:
Anti-angiogenic therapy for cancer: Current progress, unresolved
questions and future directions. Angiogenesis. 17:471–494. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lu XS, Sun W, Ge CY, Zhang WZ and Fan YZ:
Contribution of the PI3K/MMPs/Ln-5γ2 and EphA2/FAK/Paxillin
signaling pathways to tumor growth and vasculogenic mimicry of
gallbladder carcinomas. Int J Oncol. 42:2103–2115. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Altinoz MA, Topcu G, Hacimuftuoglu A,
Ozpinar A, Ozpinar A, Hacker E and Elmaci I: Noscapine, a
non-addictive opioid and microtubule-inhibitor in potential
treatment of glioblastoma. Neurochem Res. 44:1796–1806. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Su W, Huang L, Ao Q, Zhang Q, Tian X, Fang
Y and Lu Y: Noscapine sensitizes chemoresistant ovarian cancer
cells to cisplatin through inhibition of HIF-1α. Cancer Lett.
305:94–99. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Peach ML, Beedie SL, Chau CH, Collins MK,
Markolovic S, Luo W, Tweedie D, Steinebach C, Greig NH, Gütschow M,
et al: Antiangiogenic activity and in silico cereblon binding
analysis of novel thalidomide analogs. Molecules. 25:56832020.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhang S, Li M, Gu Y, Liu Z, Xu S, Cui Y
and Sun B: Thalidomide influences growth and vasculogenic mimicry
channel formation in melanoma. J Exp Clin Cancer Res. 27:602008.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Nozawa-Suzuki N, Nagasawa H, Ohnishi K and
Morishige K: The inhibitory effect of hypoxic cytotoxin on the
expansion of cancer stem cells in ovarian cancer. Biochem Biophys
Res Commun. 457:706–711. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Liu K, Zhang X, Xie L, Deng M, Chen H,
Song J, Long J, Li X and Luo J: Lupeol and its derivatives as
anticancer and anti-inflammatory agents: Molecular mechanisms and
therapeutic efficacy. Pharmacol Res. 164:1053732021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Bhattacharyya S, Mitra D, Ray S, Biswas N,
Banerjee S, Majumder B, Mustafi SM and Murmu N: Reversing effect of
Lupeol on vasculogenic mimicry in murine melanoma progression.
Microvasc Res. 121:52–62. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Bae H, Lee JY, Yang C, Song G and Lim W:
Fucoidan derived from fucus vesiculosus inhibits the development of
human ovarian cancer via the disturbance of calcium homeostasis,
endoplasmic reticulum stress, and angiogenesis. Mar Drugs.
18:452020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Sicard AA, Dao T, Suarez NG and Annabi B:
Diet-derived gallated catechins prevent TGF-beta-mediated
epithelial-mesenchymal transition, cell migration and vasculogenic
mimicry in chemosensitive ES-2 ovarian cancer cells. Nutr Cancer.
73:169–180. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Shi X, Chen Z, Wang Y, Guo Z and Wang X:
Hypotoxic copper complexes with potent anti-metastatic and
anti-angiogenic activities against cancer cells. Dalton Trans.
47:5049–5054. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Liu T, Zhao L, Zhang Y, Chen W, Liu D, Hou
H, Ding L and Li X: Ginsenoside 20(S)-Rg3 targets HIF-1alpha to
block hypoxia-induced epithelial-mesenchymal transition in ovarian
cancer cells. PLoS One. 9:e1038872014. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Xu MR, Wei PF, Suo MZ, Hu Y, Ding W, Su L,
Zhu YD, Song WJ, Tang GH, Zhang M and Li P: Brucine suppresses
vasculogenic mimicry in human triple-negative breast cancer cell
line MDA-MB-231. Biomed Res Int. 2019:65432302019.PubMed/NCBI
|
|
70
|
Xiao T, Zhong W, Zhao J, Qian B, Liu H,
Chen S, Qiao K, Lei Y, Zong S, Wang H, et al: Polyphyllin I
suppresses the formation of vasculogenic mimicry via
Twist1/VE-cadherin pathway. Cell Death Dis. 9:9062018. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Phillips TM and Lindsey JS: Carcinoma
cell-specific Mig-7: A new potential marker for circulating and
migrating cancer cells. Oncol Rep. 13:37–44. 2005.PubMed/NCBI
|
|
72
|
Crouch S, Spidel CS and Lindsey JS: HGF
and ligation of alphavbeta5 integrin induce a novel, cancer
cell-specific gene expression required for cell scattering. Exp
Cell Res. 292:274–287. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Huang B, Yin M, Li X, Cao G, Qi J, Lou G,
Sheng S, Kou J, Chen K and Yu B: Migration-inducing gene 7 promotes
tumorigenesis and angiogenesis and independently predicts poor
prognosis of epithelial ovarian cancer. Oncotarget. 7:27552–27566.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Sun Q, Zou X, Zhang T, Shen J, Yin Y and
Xiang J: The role of miR-200a in vasculogenic mimicry and its
clinical significance in ovarian cancer. Gynecol Oncol.
132:730–738. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Liu W, Lv C, Zhang B, Zhou Q and Cao Z:
MicroRNA-27b functions as a new inhibitor of ovarian
cancer-mediated vasculogenic mimicry through suppression of
VE-cadherin expression. RNA. 23:1019–1027. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Kristensen LS, Andersen MS, Stagsted LVW,
Ebbesen KK, Hansen TB and Kjems J: The biogenesis, biology and
characterization of circular RNAs. Nat Rev Genet. 20:675–691. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Guan X, Zong ZH, Liu Y, Chen S, Wang LL
and Zhao Y: circPUM1 promotes tumorigenesis and progression of
ovarian cancer by sponging miR-615-5p and miR-6753-5p. Mol Ther
Nucleic Acids. 18:882–892. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Shao Y and Lu B: The emerging roles of
circular RNAs in vessel co-option and vasculogenic mimicry:
Clinical insights for anti-angiogenic therapy in cancers. Cancer
Metastasis Rev. 41:173–191. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Li J, Ke Y, Huang M, Huang S and Liang Y:
Inhibitory effects of B-cell lymphoma 2 on the vasculogenic mimicry
of hypoxic human glioma cells. Exp Ther Med. 9:977–981. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Li T, Zhang C, Hassan S, Liu X, Song F,
Chen K, Zhang W and Yang J: Histone deacetylase 6 in cancer. J
Hematol Oncol. 11:1112018. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Treps L, Faure S and Clere N: Vasculogenic
mimicry, a complex and devious process favoring
tumorigenesis-interest in making it a therapeutic target. Pharmacol
Ther. 223:1078052021. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Ledermann JA, Embleton AC, Raja F, Perren
TJ, Jayson GC, Rustin GJS, Kaye SB, Hirte H, Eisenhauer E, Vaughan
M, et al: Cediranib in patients with relapsed platinum-sensitive
ovarian cancer (ICON6): A randomised, double-blind,
placebo-controlled phase 3 trial. Lancet. 387:1066–1074. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Lim D, Do Y, Kwon BS, Chang W, Lee MS, Kim
J and Cho JG: Angiogenesis and vasculogenic mimicry as therapeutic
targets in ovarian cancer. BMB Rep. 53:291–298. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Sun H, Zhang D, Yao Z, Lin X, Liu J, Gu Q,
Dong X, Liu F, Wang Y, Yao N, et al: Anti-angiogenic treatment
promotes triple-negative breast cancer invasion via vasculogenic
mimicry. Cancer Biol Ther. 18:205–213. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Sun M, Li H, Liu J, Ning L, Zhao D and Liu
S: The relationship between TEM8 and early diagnosis and prognosis
of lung cancer. Minerva Med. 112:359–364. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Zhang C, Chen W, Zhang X, Huang B, Chen A,
He Y, Wang J and Li X: Galunisertib inhibits glioma vasculogenic
mimicry formation induced by astrocytes. Sci Rep. 6:230562016.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Zhang Y, Xu Y, Ma J, Pang X and Dong M:
Adrenomedullin promotes angiogenesis in epithelial ovarian cancer
through upregulating hypoxia-inducible factor-1α and vascular
endothelial growth factor. Sci Rep. 7:405242017. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Guo T, Yu W, Lv S, Zhang C and Tian Y:
MiR-302a inhibits the tumorigenicity of ovarian cancer cells by
suppression of SDC1. Int J Clin Exp Pathol. 8:4869–4880.
2015.PubMed/NCBI
|
|
89
|
Wang Y, Tong L, Wang J, Luo J, Tang J,
Zhong L, Xiao Q, Niu W, Li J, Zhu J, et al: cRGD-functionalized
nanoparticles for combination therapy of anti-endothelium dependent
vessels and anti-vasculogenic mimicry to inhibit the proliferation
of ovarian cancer. Acta Biomater. 94:495–504. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Dueñas-Garcia OF, Diaz-Sotomayor M and
Rico-Olvera H: Utility of the pulsatility index of the uterine
arteries and human chorionic gonadotropin in a series of cases of
placenta accreta. J Obstet Gynaecol Res. 37:1112–1116. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Jiang J, Chen Y, Zhang M, Zhou H and Wu H:
Relationship between CD177 and the vasculogenic mimicry,
clinicopathological parameters, and prognosis of epithelial ovarian
cancer. Ann Palliat Med. 9:3985–3992. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Liang J, Yang B, Cao Q and Wu X:
Association of vasculogenic mimicry formation and CD133 expression
with poor prognosis in ovarian cancer. Gynecol Obstet Invest.
81:529–536. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Yu L, Zhu B, Wu S, Zhou L, Song W, Gong X
and Wang D: Evaluation of the correlation of vasculogenic mimicry,
ALDH1, KiSS-1, and MACC1 in the prediction of metastasis and
prognosis in ovarian carcinoma. Diagn Pathol. 12:232017. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Recouvreux MS, Miao J, Gozo MC, Wu J,
Walts AE, Karlan BY and Orsulic S: FOXC2 promotes vasculogenic
mimicry in ovarian cancer. Cancers (Basel). 14:48512022. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Ocaña OH, Córcoles R, Fabra A,
Moreno-Bueno G, Acloque H, Vega S, Barrallo-Gimeno A, Cano A and
Nieto MA: Metastatic colonization requires the repression of the
epithelial-mesenchymal transition inducer Prrx1. Cancer Cell.
22:709–724. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Ding J, Jia X, Zuo B, He J, Yang J and He
Y: A novel monoclonal antibody targeting a novel epitope of
VE-cadherin inhibits vasculogenic mimicry of lung cancer cells.
Oncol Rep. 39:2837–2844. 2018.PubMed/NCBI
|
|
97
|
Liu LZ, Jing Y, Jiang LL, Jiang XE, Jiang
Y, Rojanasakul Y and Jiang BH: Acacetin inhibits VEGF expression,
tumor angiogenesis and growth through AKT/HIF-1α pathway. Biochem
Biophys Res Commun. 413:299–305. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Morales-Guadarrama G, Mendez-Perez EA,
Garcia-Quiroz J, Avila E, Garcia-Becerra R, Zentella-Dehesa A,
Larrea F and Díaz L: Endothelium-dependent induction of
vasculogenic mimicry in human triple-negative breast cancer cells
is inhibited by calcitriol and curcumin. Int J Mol Sci.
23:76592022. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Selick HE, Beresford AP and Tarbit MH: The
emerging importance of predictive ADME simulation in drug
discovery. Drug Discov Today. 7:109–116. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Tang HS, Feng YJ and Yao LQ: Angiogenesis,
vasculogenesis, and vasculogenic mimicry in ovarian cancer. Int J
Gynecol Cancer. 19:605–610. 2009. View Article : Google Scholar : PubMed/NCBI
|