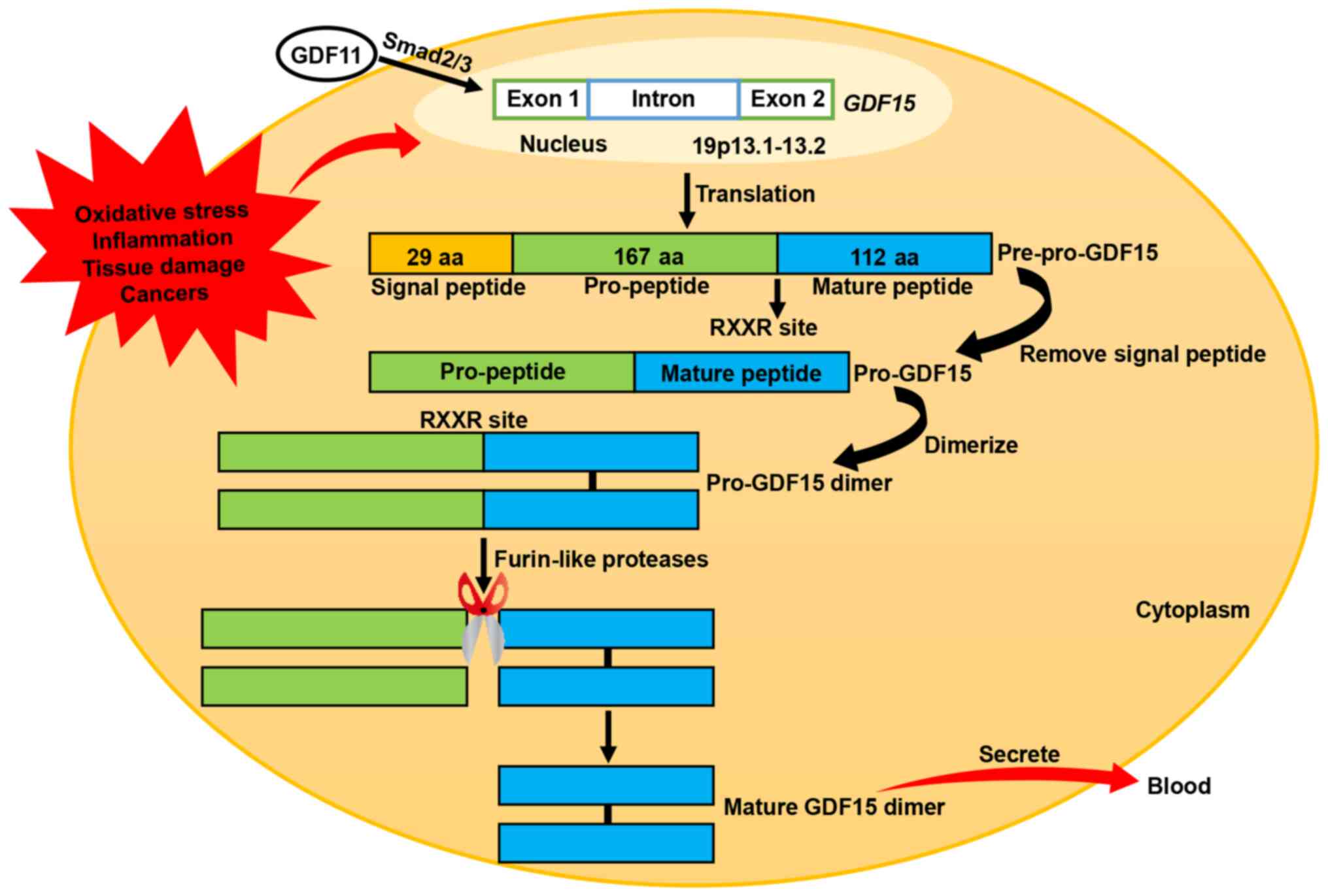
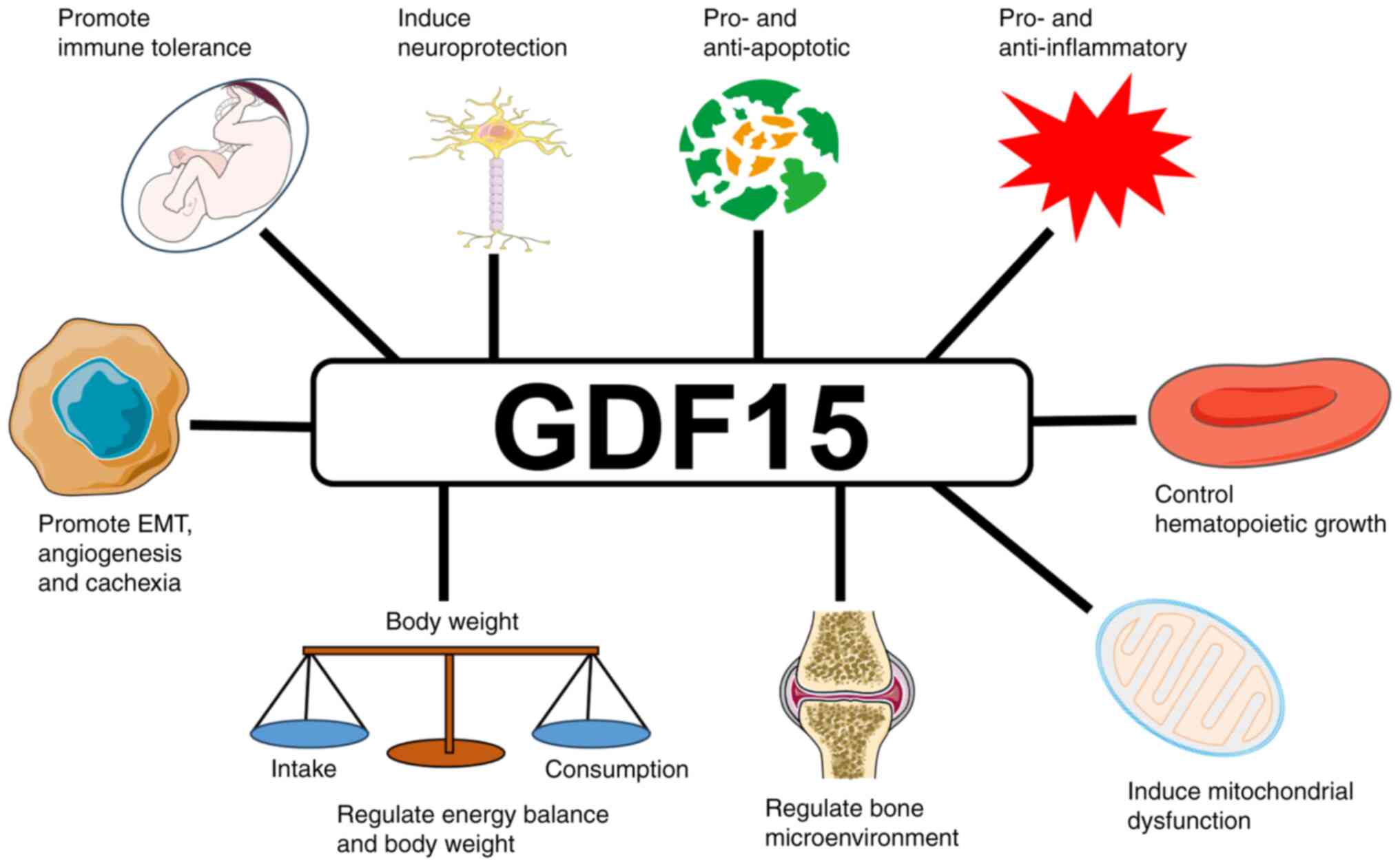
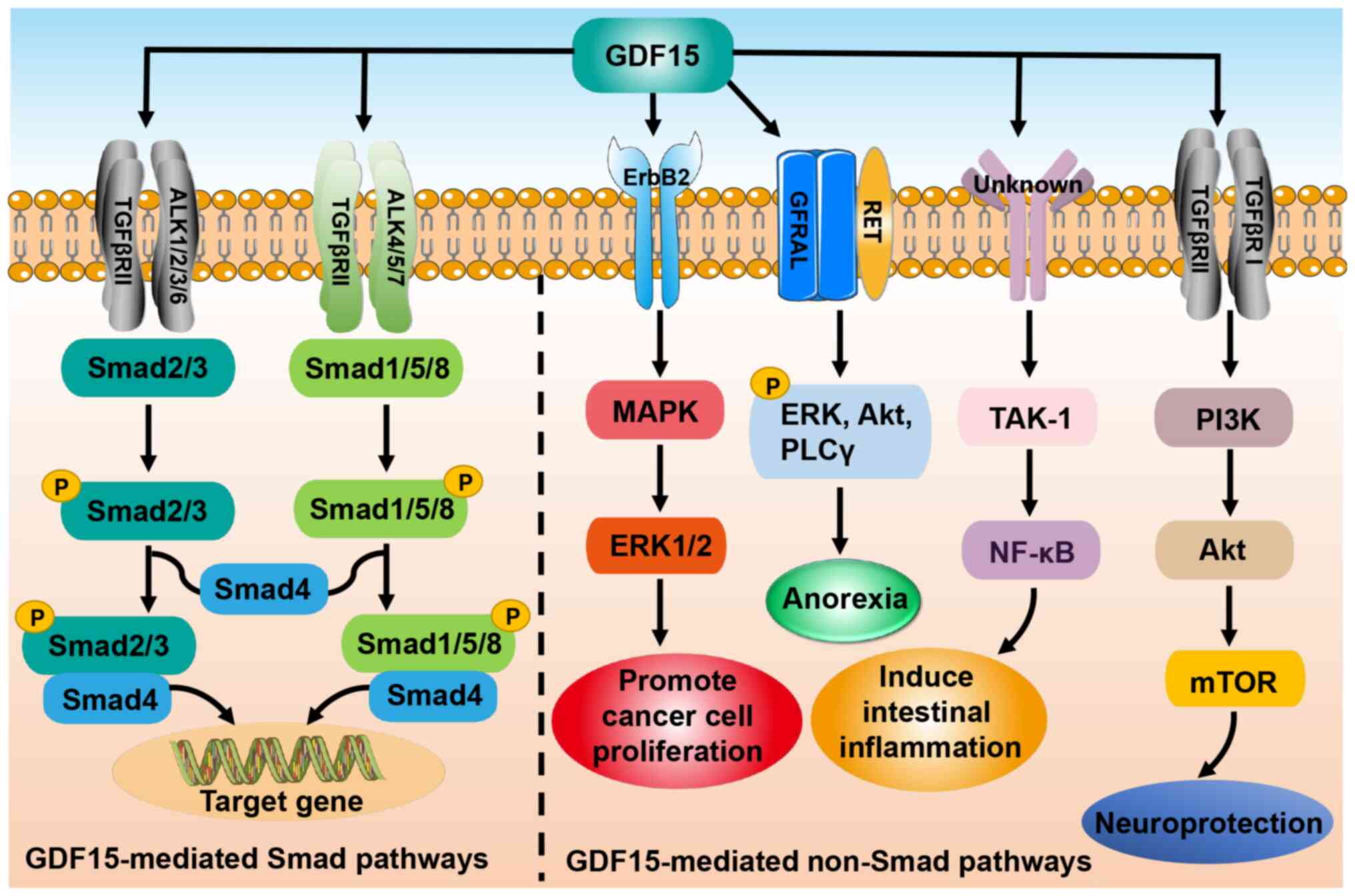
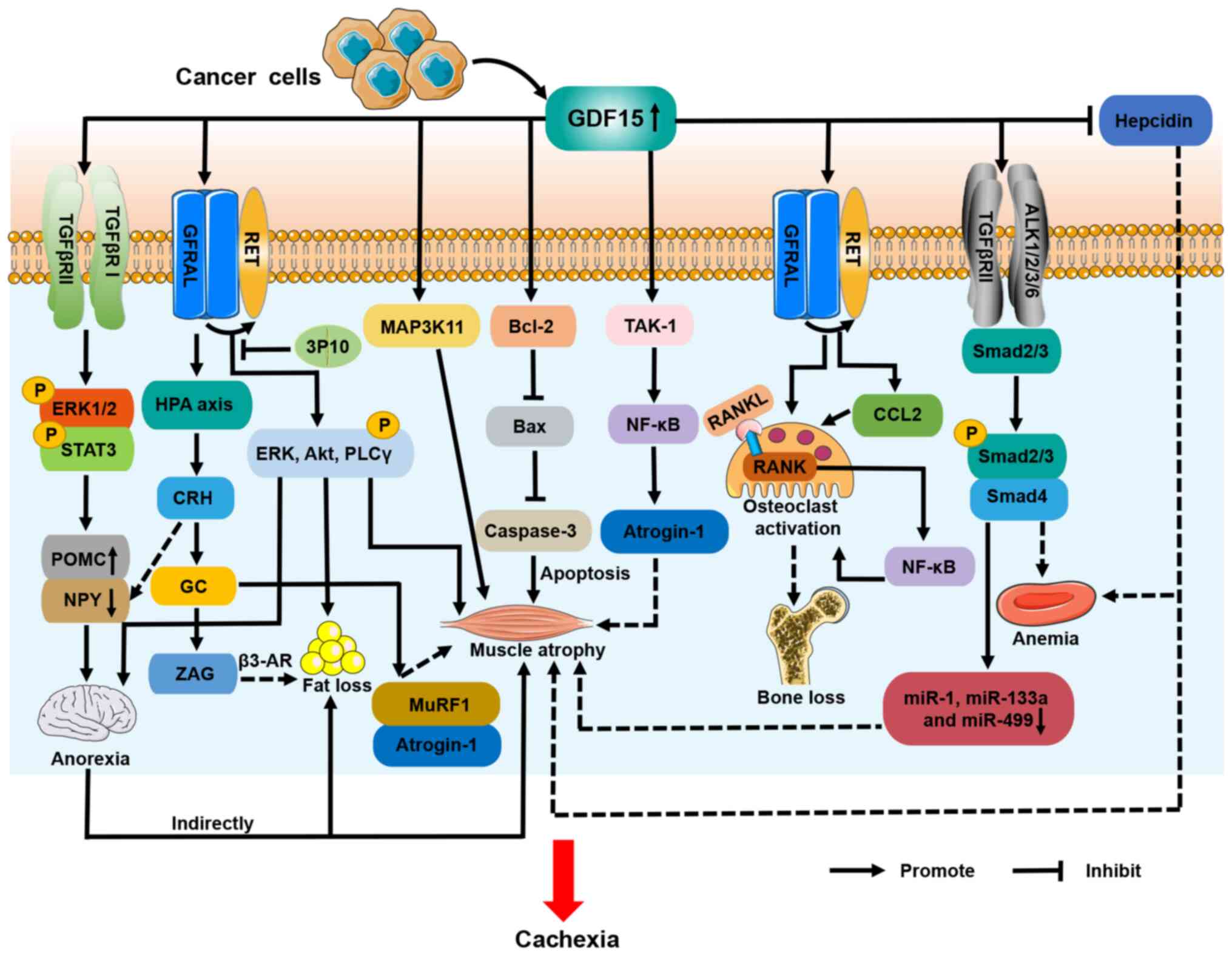
|
1
|
Evans WJ, Morley JE, Argilés J, Bales C,
Baracos V, Guttridge D, Jatoi A, Kalantar-Zadeh K, Lochs H,
Mantovani G, et al: Cachexia: A new definition. Clin Nutr.
27:793–799. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baazim H, Antonio-Herrera L and Bergthaler
A: The interplay of immunology and cachexia in infection and
cancer. Nat Rev Immunol. 22:309–321. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fearon K, Strasser F, Anker SD, Bosaeus I,
Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N,
Mantovani G, et al: Definition and classification of cancer
cachexia: An international consensus. Lancet Oncol. 12:489–495.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Porporato PE: Understanding cachexia as a
cancer metabolism syndrome. Oncogenesis. 5:e2002016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu J, Choi S, Park A, Do J, Nam D, Kim Y,
Noh J, Lee KY, Maeng CH and Park KS: Bone marrow homeostasis is
impaired via JAK/STAT and glucocorticoid signaling in cancer
cachexia model. Cancers (Basel). 13:10592021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Argilés JM, Stemmler B, López-Soriano FJ
and Busquets S: Inter-tissue communication in cancer cachexia. Nat
Rev Endocrinol. 15:9–20. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Baracos VE, Martin L, Korc M, Guttridge DC
and Fearon KCH: Cancer-associated cachexia. Nat Rev Dis Primers.
4:171052018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gupta A, Nshuti L, Grewal US, Sedhom R,
Check DK, Parsons HM, Blaes AH, Virnig BA, Lustberg MB, Subbiah IM,
et al: Financial burden of drugs prescribed for cancer-associated
symptoms. JCO Oncol Pract. 18:140–147. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu CA, Zhang Q, Ruan GT, Shen LY, Xie HL,
Liu T, Tang M, Zhang X, Yang M, Hu CL, et al: Novel diagnostic and
prognostic tools for lung cancer cachexia: Based on nutritional and
inflammatory status. Front Oncol. 12:8907452022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Malla J, Zahra A, Venugopal S, Selvamani
TY, Shoukrie SI, Selvaraj R, Dhanoa RK, Hamouda RK and Mostafa J:
What role do inflammatory cytokines play in cancer cachexia?
Cureus. 14:e267982022.PubMed/NCBI
|
|
11
|
Zeng R, Tong C and Xiong X: The molecular
basis and therapeutic potential of leukemia inhibitory factor in
cancer cachexia. Cancers (Basel). 14:29552022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu SW, Pan HC, Hsu YH, Chang KC, Wu LW,
Chen WY and Chang MS: IL-20 antagonist suppresses PD-L1 expression
and prolongs survival in pancreatic cancer models. Nat Commun.
11:46112020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Di Girolamo D and Tajbakhsh S:
Pathological features of tissues and cell populations during cancer
cachexia. Cell Regen. 11:152022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Callaway CS, Delitto AE, Patel R, Nosacka
RL, D'Lugos AC, Delitto D, Deyhle MR, Trevino JG, Judge SM and
Judge AR: IL-8 released from human pancreatic cancer and
tumor-associated stromal cells signals through a CXCR2-ERK1/2 axis
to induce muscle atrophy. Cancers (Basel). 11:18632019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xiong H, Ye J, Xie K, Hu W, Xu N and Yang
H: Exosomal IL-8 derived from Lung Cancer and Colon Cancer cells
induced adipocyte atrophy via NF-κB signaling pathway. Lipids
Health Dis. 21:1472022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Suriben R, Chen M, Higbee J, Oeffinger J,
Ventura R, Li B, Mondal K, Gao Z, Ayupova D, Taskar P, et al:
Antibody-mediated inhibition of GDF15-GFRAL activity reverses
cancer cachexia in mice. Nat Med. 26:1264–1270. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Belloum Y, Rannou-Bekono F and Favier FB:
Cancer-induced cardiac cachexia: Pathogenesis and impact of
physical activity (Review). Oncol Rep. 37:2543–2552. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nissinen TA, Hentilä J, Penna F, Lampinen
A, Lautaoja JH, Fachada V, Holopainen T, Ritvos O, Kivelä R and
Hulmi JJ: Treating cachexia using soluble ACVR2B improves survival,
alters mTOR localization, and attenuates liver and spleen
responses. J Cachexia Sarcopenia Muscle. 9:514–529. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thibaut MM, Sboarina M, Roumain M, Pötgens
SA, Neyrinck AM, Destrée F, Gillard J, Leclercq IA, Dachy G,
Demoulin JB, et al: Inflammation-induced cholestasis in cancer
cachexia. J Cachexia Sarcopenia Muscle. 12:70–90. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Peyta L, Jarnouen K, Pinault M, Coulouarn
C, Guimaraes C, Goupille C, de Barros JP, Chevalier S, Dumas JF,
Maillot F, et al: Regulation of hepatic cardiolipin metabolism by
TNFα: Implication in cancer cachexia. Biochim Biophys Acta.
1851:1490–1500. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Patel HJ and Patel BM: TNF-α and cancer
cachexia: Molecular insights and clinical implications. Life Sci.
170:56–63. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Black K, Garrett IR and Mundy GR: Chinese
hamster ovarian cells transfected with the murine interleukin-6
gene cause hypercalcemia as well as cachexia, leukocytosis and
thrombocytosis in tumor-bearing nude mice. Endocrinology.
128:2657–2659. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bindels LB, Neyrinck AM, Loumaye A, Catry
E, Walgrave H, Cherbuy C, Leclercq S, Van Hul M, Plovier H,
Pachikian B, et al: Increased gut permeability in cancer cachexia:
mechanisms and clinical relevance. Oncotarget. 9:18224–18238. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
White JP, Puppa MJ, Narsale A and Carson
JA: Characterization of the male ApcMin/+ mouse as a hypogonadism
model related to cancer cachexia. Biol Open. 2:1346–1353. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tracey KJ, Wei H, Manogue KR, Fong Y,
Hesse DG, Nguyen HT, Kuo GC, Beutler B, Cotran RS, Cerami A, et al:
Cachectin/tumor necrosis factor induces cachexia, anemia, and
inflammation. J Exp Med. 167:1211–1227. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Johns N, Stretch C, Tan BH, Solheim TS,
Sørhaug S, Stephens NA, Gioulbasanis I, Skipworth RJ, Deans DA,
Vigano A, et al: New genetic signatures associated with cancer
cachexia as defined by low skeletal muscle index and weight loss. J
Cachexia Sarcopenia Muscle. 8:122–130. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhong X, Narasimhan A, Silverman LM, Young
AR, Shahda S, Liu S, Wan J, Liu Y, Koniaris LG and Zimmers TA: Sex
specificity of pancreatic cancer cachexia phenotypes, mechanisms,
and treatment in mice and humans: Role of Activin. J Cachexia
Sarcopenia Muscle. 13:2146–2161. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Avancini A, Trestini I, Tregnago D, Lanza
M, Menis J, Belluomini L, Milella M and Pilotto S: A multimodal
approach to cancer-related cachexia: from theory to practice.
Expert Rev Anticancer Ther. 21:819–826. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lockhart SM, Saudek V and O'Rahilly S:
GDF15: A hormone conveying somatic distress to the brain. Endocr
Rev. 41:bnaa0072020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lerner L, Tao J, Liu Q, Nicoletti R, Feng
B, Krieger B, Mazsa E, Siddiquee Z, Wang R, Huang L, et al:
MAP3K11/GDF15 axis is a critical driver of cancer cachexia. J
Cachexia Sarcopenia Muscle. 7:467–482. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Suzuki H, Mitsunaga S, Ikeda M, Aoyama T,
Yoshizawa K, Yoshimatsu H, Kawai N, Masuda M, Miura T and Ochiai A:
Clinical and tumor characteristics of patients with high serum
levels of growth differentiation factor 15 in advanced pancreatic
cancer. Cancers (Basel). 13:48422021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lerner L, Hayes TG, Tao N, Krieger B, Feng
B, Wu Z, Nicoletti R, Chiu MI, Gyuris J and Garcia JM: Plasma
growth differentiation factor 15 is associated with weight loss and
mortality in cancer patients. J Cachexia Sarcopenia Muscle.
6:317–324. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Johnen H, Lin S, Kuffner T, Brown DA, Tsai
VW, Bauskin AR, Wu L, Pankhurst G, Jiang L, Junankar S, et al:
Tumor-induced anorexia and weight loss are mediated by the TGF-beta
superfamily cytokine MIC-1. Nat Med. 13:1333–1340. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li P, Lv H, Zhang B, Duan R, Zhang X, Lin
P, Song C and Liu Y: Growth differentiation factor 15 protects
SH-SY5Y cells from rotenone-induced toxicity by suppressing
mitochondrial apoptosis. Front Aging Neurosci. 14:8695582022.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Asrih M, Wei S, Nguyen TT, Yi HS, Ryu D
and Gariani K: Overview of growth differentiation factor 15 in
metabolic syndrome. J Cell Mol Med. 27:1157–1167. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wan Y and Fu J: GDF15 as a key disease
target and biomarker: Linking chronic lung diseases and ageing. Mol
Cell Biochem. Apr 24–2023.(Epub ahead of print). View Article : Google Scholar
|
|
37
|
Assadi A, Zahabi A and Hart RA: GDF15, an
update of the physiological and pathological roles it plays: A
review. Pflugers Arch. 472:1535–1546. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Johann K, Kleinert M and Klaus S: The Role
of GDF15 as a Myomitokine. Cells. 10:29902021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wischhusen J, Melero I and Fridman WH:
Growth/Differentiation Factor-15 (GDF-15): From biomarker to novel
targetable immune checkpoint. Front Immunol. 11:9512020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tsai VW, Brown DA and Breit SN: Targeting
the divergent TGFβ superfamily cytokine MIC-1/GDF15 for therapy of
anorexia/cachexia syndromes. Curr Opin Support Palliat Care.
12:404–409. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Siddiqui JA, Pothuraju R, Khan P, Sharma
G, Muniyan S, Seshacharyulu P, Jain M, Nasser MW and Batra SK:
Pathophysiological role of growth differentiation factor 15 (GDF15)
in obesity, cancer, and cachexia. Cytokine Growth Factor Rev.
64:71–83. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Niu Y, Zhang W, Shi J, Liu Y, Zhang H, Lin
N, Li X, Qin L, Yang Z and Su Q: The relationship between
circulating growth differentiation factor 15 levels and diabetic
retinopathy in patients with type 2 diabetes. Front Endocrinol
(Lausanne). 12:6273952021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tsui KH, Hsu SY, Chung LC, Lin YH, Feng
TH, Lee TY, Chang PL and Juang HH: Growth differentiation
factor-15: A p53- and demethylation-upregulating gene represses
cell proliferation, invasion, and tumorigenesis in bladder
carcinoma cells. Sci Rep. 5:128702015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Joo M, Kim D, Lee MW, Lee HJ and Kim JM:
GDF15 promotes cell growth, migration, and invasion in gastric
cancer by inducing STAT3 activation. Int J Mol Sci. 24:29252023.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li S, Ma YM, Zheng PS and Zhang P: GDF15
promotes the proliferation of cervical cancer cells by
phosphorylating AKT1 and Erk1/2 through the receptor ErbB2. J Exp
Clin Cancer Res. 37:802018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Spanopoulou A and Gkretsi V: Growth
differentiation factor 15 (GDF15) in cancer cell metastasis: From
the cells to the patients. Clin Exp Metastasis. 37:451–464. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Rochette L, Dogon G, Zeller M, Cottin Y
and Vergely C: GDF15 and cardiac cells: Current concepts and new
insights. Int J Mol Sci. 22:88892021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li L, Zhang R, Yang H, Zhang D, Liu J, Li
J and Guo B: GDF15 knockdown suppresses cervical cancer cell
migration in vitro through the TGF-β/Smad2/3/Snail1 pathway. FEBS
Open Bio. 10:2750–2760. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang CY, Huang AQ, Zhou MH and Mei YA:
GDF15 regulates Kv2.1-mediated outward K+ current through the
Akt/mTOR signalling pathway in rat cerebellar granule cells.
Biochem J. 460:35–47. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Park SH, Yu M, Kim J and Moon Y: C/EBP
homologous protein promotes NSAID-activated gene 1-linked
pro-inflammatory signals and enterocyte invasion by
enteropathogenic Escherichia coli. Microbes Infect. 19:110–121.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Molfino A, Amabile MI, Imbimbo G, Rizzo V,
Pediconi F, Catalano C, Emiliani A, Belli R, Ramaccini C, Parisi C,
et al: Association between growth differentiation factor-15
(GDF-15) serum levels, anorexia and low muscle mass among cancer
patients. Cancers (Basel). 13:992020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sabatini PV, Frikke-Schmidt H, Arthurs J,
Gordian D, Patel A, Rupp AC, Adams JM, Wang J, Beck Jørgensen S,
Olson DP, et al: GFRAL-expressing neurons suppress food intake via
aversive pathways. Proc Natl Acad Sci USA. 118:e20213571182021.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Cimino I, Kim H, Tung YCL, Pedersen K,
Rimmington D, Tadross JA, Kohnke SN, Neves-Costa A, Barros A,
Joaquim S, et al: Activation of the hypothalamic-pituitary-adrenal
axis by exogenous and endogenous GDF15. Proc Natl Acad Sci USA.
118:e21068681182021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Setiawan T, Sari IN, Wijaya YT, Julianto
NM, Muhammad JA, Lee H, Chae JH and Kwon HY: Cancer cachexia:
Molecular mechanisms and treatment strategies. J Hematol Oncol.
16:542023. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Bloch SA, Lee JY, Syburra T, Rosendahl U,
Griffiths MJ, Kemp PR and Polkey MI: Increased expression of GDF-15
may mediate ICU-acquired weakness by down-regulating muscle
microRNAs. Thorax. 70:219–228. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yamamoto H, Takeshima F, Haraguchi M,
Akazawa Y, Matsushima K, Kitayama M, Ogihara K, Tabuchi M,
Hashiguchi K, Yamaguchi N, et al: High serum concentrations of
growth differentiation factor-15 and their association with Crohn's
disease and a low skeletal muscle index. Sci Rep. 12:65912022.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Garfield BE, Crosby A, Shao D, Yang P,
Read C, Sawiak S, Moore S, Parfitt L, Harries C, Rice M, et al:
Growth/differentiation factor 15 causes TGFβ-activated kinase
1-dependent muscle atrophy in pulmonary arterial hypertension.
Thorax. 74:164–176. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Deng M, Bian Y, Zhang Q, Zhou X and Hou G:
Growth differentiation factor-15 as a biomarker for sarcopenia in
patients with chronic obstructive pulmonary disease. Front Nutr.
9:8970972022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Song M, Zhang Q, Tang M, Zhang X, Ruan G,
Zhang X, Zhang K, Ge Y, Yang M, Li Q, et al: Associations of low
hand grip strength with 1 year mortality of cancer cachexia: A
multicentre observational study. J Cachexia Sarcopenia Muscle.
12:1489–1500. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhang W, Sun W, Gu X, Miao C, Feng L, Shen
Q, Liu X and Zhang X: GDF-15 in tumor-derived exosomes promotes
muscle atrophy via Bcl-2/caspase-3 pathway. Cell Death Discov.
8:1622022. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Tanno T, Bhanu NV, Oneal PA, Goh SH,
Staker P, Lee YT, Moroney JW, Reed CH, Luban NL, Wang RH, et al:
High levels of GDF15 in thalassemia suppress expression of the iron
regulatory protein hepcidin. Nat Med. 13:1096–1101. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhou D, Zhang Y, Mamtawla G, Wan S, Gao X,
Zhang L, Li G and Wang X: Iron overload is related to muscle
wasting in patients with cachexia of gastric cancer: using
quantitative proteome analysis. Med Oncol. 37:1132020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Martin A, Castells J, Allibert V, Emerit
A, Zolotoff C, Cardot-Ruffino V, Gallot YS, Vernus B, Chauvet V,
Bartholin L, et al: Hypothalamic-pituitary-adrenal axis activation
and glucocorticoid-responsive gene expression in skeletal muscle
and liver of Apc mice. J Cachexia Sarcopenia Muscle. 13:1686–1703.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Laurens C, Parmar A, Murphy E, Carper D,
Lair B, Maes P, Vion J, Boulet N, Fontaine C, Marquès M, et al:
Growth and differentiation factor 15 is secreted by skeletal muscle
during exercise and promotes lipolysis in humans. JCI Insight.
5:e1318702020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Fouladiun M, Körner U, Bosaeus I, Daneryd
P, Hyltander A and Lundholm KG: Body composition and time course
changes in regional distribution of fat and lean tissue in
unselected cancer patients on palliative care-correlations with
food intake, metabolism, exercise capacity, and hormones. Cancer.
103:2189–2198. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Elattar S, Dimri M and Satyanarayana A:
The tumor secretory factor ZAG promotes white adipose tissue
browning and energy wasting. FASEB J. 32:4727–4743. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Weber BZC, Arabaci DH and Kir S: Metabolic
reprogramming in adipose tissue during cancer cachexia. Front
Oncol. 12:8483942022. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Plotkin LI, Sanz N and Brun LR: Messages
from the Mineral: How bone cells communicate with other tissues.
Calcif Tissue Int. 113:39–47. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zhang Y, Wang H, Zhu G, Qian A and Chen W:
F2r negatively regulates osteoclastogenesis through inhibiting the
Akt and NFκB signaling pathways. Int J Biol Sci. 16:1629–1639.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Zwickl H, Zwickl-Traxler E, Haushofer A,
Seier J, Podar K, Weber M, Hackner K, Jacobi N, Pecherstorfer M and
Vallet S: Effect of cachexia on bone turnover in cancer patients: A
case-control study. BMC Cancer. 21:7442021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Bonetto A, Kays JK, Parker VA, Matthews
RR, Barreto R, Puppa MJ, Kang KS, Carson JA, Guise TA, Mohammad KS,
et al: Differential bone loss in mouse models of colon cancer
cachexia. Front Physiol. 7:6792016.PubMed/NCBI
|
|
72
|
Pin F, Jones AJ, Huot JR, Narasimhan A,
Zimmers TA, Bonewald LF and Bonetto A: RANKL blockade reduces
cachexia and bone loss induced by non-metastatic ovarian cancer in
mice. J Bone Miner Res. 37:381–396. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Adesina OO, Jenkins IC, Wu QV, Fung EB,
Narla RR, Lipkin EW, Mahajan K, Konkle BA and Kruse-Jarres R:
Urinary cross-linked carboxyterminal telopeptide, a bone resorption
marker, decreases after vaso-occlusive crises in adults with sickle
cell disease. Blood Cells Mol Dis. 80:1023692020. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Cameron ME, Underwood PW, Williams IE,
George TJ, Judge SM, Yarrow JF, Trevino JG and Judge AR: Osteopenia
is associated with wasting in pancreatic adenocarcinoma and
predicts survival after surgery. Cancer Med. 11:50–60. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Westhrin M, Moen SH, Holien T, Mylin AK,
Heickendorff L, Olsen OE, Sundan A, Turesson I, Gimsing P, Waage A
and Standal T: Growth differentiation factor 15 (GDF15) promotes
osteoclast differentiation and inhibits osteoblast differentiation
and high serum GDF15 levels are associated with multiple myeloma
bone disease. Haematologica. 100:e511–e514. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Hinoi E, Ochi H, Takarada T, Nakatani E,
Iezaki T, Nakajima H, Fujita H, Takahata Y, Hidano S, Kobayashi T,
et al: Positive regulation of osteoclastic differentiation by
growth differentiation factor 15 upregulated in osteocytic cells
under hypoxia. J Bone Miner Res. 27:938–949. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Wakchoure S, Swain TM, Hentunen TA,
Bauskin AR, Brown DA, Breit SN, Vuopala KS, Harris KW and Selander
KS: Expression of macrophage inhibitory cytokine-1 in prostate
cancer bone metastases induces osteoclast activation and weight
loss. Prostate. 69:652–661. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Siddiqui JA, Seshacharyulu P, Muniyan S,
Pothuraju R, Khan P, Vengoji R, Chaudhary S, Maurya SK, Lele SM,
Jain M, et al: GDF15 promotes prostate cancer bone metastasis and
colonization through osteoblastic CCL2 and RANKL activation. Bone
Res. 10:62022. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Ahmed DS, Isnard S, Lin J, Routy B and
Routy JP: GDF15/GFRAL pathway as a metabolic signature for cachexia
in patients with cancer. J Cancer. 12:1125–1132. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Zhang XW, Zhang Q, Song MM, Zhang KP,
Zhang X, Ruan GT, Yang M, Ge YZ, Tang M, Li XR, et al: The
prognostic effect of hemoglobin on patients with cancer cachexia: A
multicenter retrospective cohort study. Support Care Cancer.
30:875–885. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Wang B, Wang Y, Chen H, Yao S, Lai X, Qiu
Y, Cai J, Huang Y, Wei X, Guan Y, et al: Inhibition of TGFβ
improves hematopoietic stem cell niche and ameliorates
cancer-related anemia. Stem Cell Res Ther. 12:652021. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Jiang F, Yu WJ, Wang XH, Tang YT, Guo L
and Jiao XY: Regulation of hepcidin through GDF-15 in
cancer-related anemia. Clin Chim Acta. 428:14–19. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Balsano R, Kruize Z, Lunardi M,
Comandatore A, Barone M, Cavazzoni A, Re Cecconi AD, Morelli L,
Wilmink H, Tiseo M, et al: Transforming growth factor-beta
signaling in cancer-induced cachexia: From molecular pathways to
the clinics. Cells. 11:26712022. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Liu S, Ren J and Ten Dijke P: Targeting
TGFβ signal transduction for cancer therapy. Signal Transduct
Target Ther. 6:82021. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Greco SH, Tomkötter L, Vahle AK, Rokosh R,
Avanzi A, Mahmood SK, Deutsch M, Alothman S, Alqunaibit D, Ochi A,
et al: TGF-β blockade reduces mortality and metabolic changes in a
validated murine model of pancreatic cancer cachexia. PLoS One.
10:e01327862015. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Wang Z, Jiang P, Liu F, Du X, Ma L, Ye S,
Cao H, Sun P, Su N, Lin F, et al: GDF11 Regulates PC12 neural stem
cells via ALK5-Dependent PI3K-Akt signaling pathway. Int J Mol Sci.
23:122792022. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Jones JE, Cadena SM, Gong C, Wang X, Chen
Z, Wang SX, Vickers C, Chen H, Lach-Trifilieff E, Hadcock JR and
Glass DJ: Supraphysiologic administration of GDF11 induces cachexia
in part by upregulating GDF15. Cell Rep. 22:1522–1530. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Zimmers TA, Jiang Y, Wang M, Liang TW,
Rupert JE, Au ED, Marino FE, Couch ME and Koniaris LG: Exogenous
GDF11 induces cardiac and skeletal muscle dysfunction and wasting.
Basic Res Cardiol. 112:482017. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Ebner N, Anker SD and von Haehling S:
Recent developments in the field of cachexia, sarcopenia, and
muscle wasting: highlights from the 11th Cachexia Conference. J
Cachexia Sarcopenia Muscle. 10:218–225. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Ebner N, Anker SD and von Haehling S:
Recent developments in the field of cachexia, sarcopenia, and
muscle wasting: Highlights from the 12th Cachexia Conference. J
Cachexia Sarcopenia Muscle. 11:274–285. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
da Fonseca GWP, Sato R, de Nazaré Nunes
Alves MJ and von Haehling S: Current advancements in
pharmacotherapy for cancer cachexia. Expert Opin Pharmacother.
24:629–639. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Crawford J, Calle RA, Collins SM, Weng Y,
Lubaczewski SL, Buckeridge C, Wang EQ, Harrington MA, Tarachandani
A, Rossulek MI, et al: CT108/16-First-in-patient study of the
GDF-15 inhibitor ponsegromab in patients with cancer and cachexia:
Safety, tolerability, and exploratory measures of efficacy.
Proceedings of the 114th Annual Meeting of the American Association
for Cancer Research. 14–19. 2023.https://www.abstractsonline.com/pp8/#!/10828/presentation/10304
|
|
93
|
Kim-Muller JY, Song L, LaCarubba Paulhus
B, Pashos E, Li X, Rinaldi A, Joaquim S, Stansfield JC, Zhang J,
Robertson A, et al: GDF15 neutralization restores muscle function
and physical performance in a mouse model of cancer cachexia. Cell
Rep. 42:1119472023. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Vignjević Petrinović S, Jauković A,
Milošević M, Bugarski D and Budeč M: Targeting stress
erythropoiesis pathways in cancer. Front Physiol. 13:8440422022.
View Article : Google Scholar : PubMed/NCBI
|