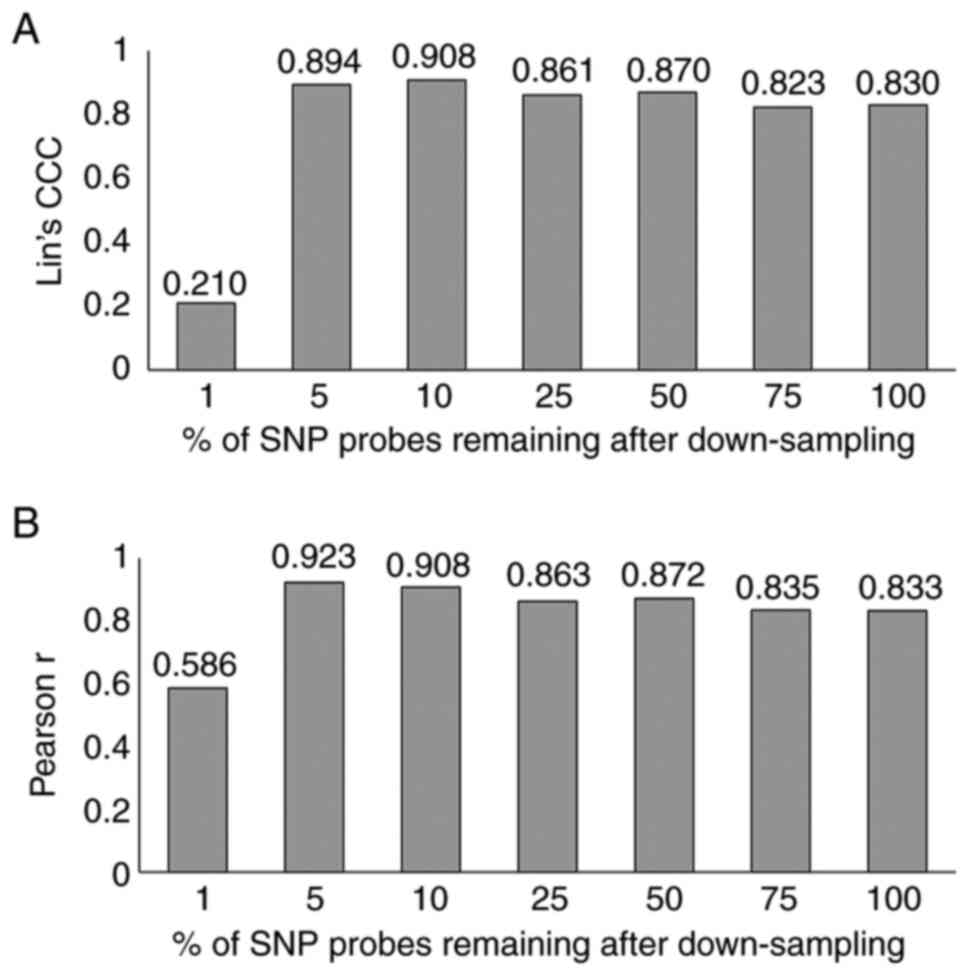
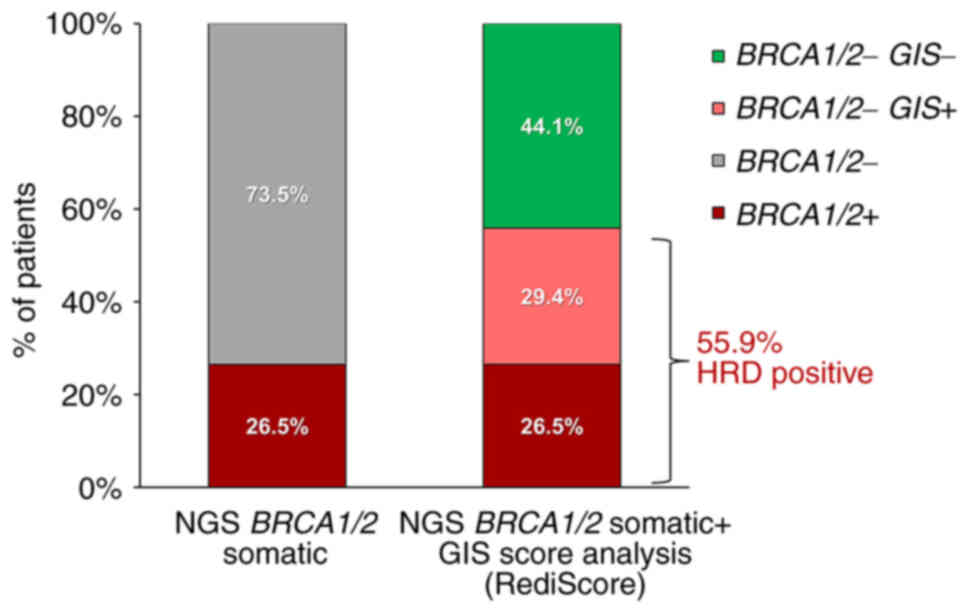
|
1
|
Topatana W, Juengpanich S, Li S, Cao J, Hu
J, Lee J, Suliyanto K, Ma D, Zhang B, Chen M and Cai X: Advances in
synthetic lethality for cancer therapy: Cellular mechanism and
clinical translation. J Hematol Oncol. 13:1182020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Swisher EM, Kwan TT, Oza AM, Tinker AV,
Ray-Coquard I, Oaknin A, Coleman RL, Aghajanian C, Konecny GE,
O'Malley DM, et al: Molecular and clinical determinants of response
and resistance to rucaparib for recurrent ovarian cancer treatment
in ARIEL2 (Parts 1 and 2). Nat Commun. 12:24872021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Konstantinopoulos PA, Ceccaldi R, Shapiro
GI and D'Andrea AD: Homologous recombination deficiency: Exploiting
the fundamental vulnerability of ovarian cancer. Cancer Discov.
5:1137–1154. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pujade-Lauraine E, Brown J, Barnicle A,
Wessen J, Lao-Sirieix P, Criscione SW, du Bois A, Lorusso D, Romero
I, Petru E, et al: Homologous recombination repair gene mutations
to predict olaparib plus bevacizumab efficacy in the first-line
ovarian cancer PAOLA-1/ENGOT-ov25 trial. JCO Precis Oncol.
7:e22002582023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miller RE, Leary A, Scott CL, Serra V,
Lord CJ, Bowtell D, Chang DK, Garsed DW, Jonkers J, Ledermann JA,
et al: ESMO recommendations on predictive biomarker testing for
homologous recombination deficiency and PARP inhibitor benefit in
ovarian cancer. Ann Oncol. 31:1606–1622. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ray-Coquard I, Pautier P, Pignata S, Pérol
D, González-Martín A, Berger R, Fujiwara K, Vergote I, Colombo N,
Mäenpää J, et al: Olaparib plus bevacizumab as first-line
maintenance in ovarian cancer. N Engl J Med. 381:2416–2428. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stewart MD, Merino Vega D, Arend RC, Baden
JF, Barbash O, Beaubier N, Collins G, French T, Ghahramani N,
Hinson P, et al: Homologous recombination deficiency: Concepts,
definitions, and assays. Oncologist. 27:167–174. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
O'Sullivan Coyne G, Karlovich C, Wilsker
D, Voth AR, Parchment RE, Chen AP and Doroshow JH: PARP inhibitor
applicability: Detailed assays for homologous recombination repair
pathway components. Onco Targets Ther. 15:165–180. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cristescu R, Liu XQ, Arreaza G, Chen C,
Albright A, Qiu P and Marton MJ: Concordance between
single-nucleotide polymorphism-based genomic instability assays and
a next-generation sequencing-based homologous recombination
deficiency test. BMC Cancer. 22:13102022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ngoi NYL and Tan DSP: The role of
homologous recombination deficiency testing in ovarian cancer and
its clinical implications: do we need it? ESMO Open. 6:1001442021.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Foster JM, Oumie A, Togneri FS, Vasques
FR, Hau D, Taylor M, Tinkler-Hundal E, Southward K, Medlow P,
McGreeghan-Crosby K, et al: Cross-laboratory validation of the
OncoScan® FFPE assay, a multiplex tool for whole genome
tumour profiling. BMC Med Genomics. 8:52015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rowe LR, Thaker HM, Opitz JM, Schiffman
JD, Haddadin ZM, Erickson LK and South ST: Molecular inversion
probe array for the genetic evaluation of stillbirth using
formalin-fixed, paraffin-embedded tissue. J Mol Diagn. 15:466–472.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Y, Moorhead M, Karlin-Neumann G,
Falkowski M, Chen C, Siddiqui F, Davis RW, Willis TD and Faham M:
Allele quantification using molecular inversion probes (MIP).
Nucleic Acids Res. 33:e1832005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ross EM, Haase K, Van Loo P and Markowetz
F: Allele-specific multi-sample copy number segmentation in ASCAT.
Bioinformatics. 37:1909–1911. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Van Loo P, Nordgard SH, Lingjærde OC,
Russnes HG, Rye IH, Sun W, Weigman VJ, Marynen P, Zetterberg A,
Naume B, et al: Allele-specific copy number analysis of tumors.
Proc Natl Acad Sci USA. 107:16910–16915. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Imanishi S, Naoi Y, Shimazu K, Shimoda M,
Kagara N, Tanei T, Miyake T, Kim SJ and Noguchi S:
Clinicopathological analysis of homologous recombination-deficient
breast cancers with special reference to response to neoadjuvant
paclitaxel followed by FEC. Breast Cancer Res Trea. 174:627–637.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Abkevich V, Timms KM, Hennessy BT, Potter
J, Carey MS, Meyer LA, Smith-McCune K, Broaddus R, Lu KH, Chen J,
et al: Patterns of genomic loss of heterozygosity predict
homologous recombination repair defects in epithelial ovarian
cancer. Br J Cancer. 107:1776–1782. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Birkbak NJ, Wang ZC, Kim JY, Eklund AC, Li
Q, Tian R, Bowman-Colin C, Li Y, Greene-Colozzi A, Iglehart JD, et
al: Telomeric allelic imbalance indicates defective DNA repair and
sensitivity to DNA-damaging agents. Cancer Discov. 2:366–375. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Popova T, Manié E, Rieunier G,
Caux-Moncoutier V, Tirapo C, Dubois T, Delattre O, Sigal-Zafrani B,
Bollet M, Longy M, et al: Ploidy and large-scale genomic
instability consistently identify basal-like breast carcinomas with
BRCA1/2 inactivation. Cancer Res. 72:5454–5462. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Timms KM, Abkevich V, Hughes E, Neff C,
Reid J, Morris B, Kalva S, Potter J, Tran TV, Chen J, et al:
Association of BRCA1/2 defects with genomic scores predictive of
DNA damage repair deficiency among breast cancer subtypes. Breast
Cancer Res. 16:4752014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Popova T, Manié E and Stern MH: Genomic
signature of homologous recombination deficiency in breast and
ovarian cancers. Bio-Protocol. 3:2013. View Article : Google Scholar
|
|
22
|
Christinat Y, Ho L, Clément S, Genestie C,
Sehouli J, Gonzalez Martin A, Denison U, Fujiwara K, Vergote I,
Tognon G, et al: Normalized LST is an efficient biomarker for
homologous recombination deficiency and Olaparib response in
ovarian carcinoma. medRxiv. 2022.08.22.22278669. 2022.
|
|
23
|
Wiggans AJ, Cass GKS, Bryant A, Lawrie TA
and Morrison J: Poly(ADP-ribose) polymerase (PARP) inhibitors for
the treatment of ovarian cancer. Cochrane Database Syst Rev.
2015:CD0079292015.PubMed/NCBI
|
|
24
|
Konecny GE and Kristeleit RS: PARP
inhibitors for BRCA1/2-mutated and sporadic ovarian cancer: Current
practice and future directions. Br J Cancer. 115:1157–1173. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sabatier R, Rousseau F, Joly F, Cropet C,
Montégut C, Frindte J, Cinieri S, Guerra Alía EM, Polterauer S,
Yoshida H, et al: Efficacy and safety of maintenance olaparib and
bevacizumab in ovarian cancer patients aged ≥65 years from the
PAOLA-1/ENGOT-ov25 trial. Eur J Cancer. 181:42–52. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Labidi-Galy SI, Rodrigues M, Sandoval JL,
Kurtz JE, Heitz F, Mosconi AM, Romero I, Denison U, Nagao S,
Vergote I, et al: Association of location of BRCA1 and BRCA2
mutations with benefit from olaparib and bevacizumab maintenance in
high-grade ovarian cancer: Phase III PAOLA-1/ENGOT-ov25 trial
subgroup exploratory analysis. Ann Oncol. 34:152–162. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Telli ML, Timms KM, Reid J, Hennessy B,
Mills GB, Jensen KC, Szallasi Z, Barry WT, Winer EP, Tung NM, et
al: Homologous recombination deficiency (HRD) score predicts
response to platinum-containing neoadjuvant chemotherapy in
patients with triple-negative breast cancer. Clin Cancer Re.
22:3764–3773. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Doig KD, Fellowes AP and Fox SB:
Homologous recombination repair deficiency: An overview for
pathologists. Mod Pathol. 36:1000492023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fumagalli C, Betella I, Ranghiero A,
Guerini-Rocco E, Bonaldo G, Rappa A, Vacirca D, Colombo N and
Barberis M: In-house testing for homologous recombination repair
deficiency (HRD) testing in ovarian carcinoma: A feasibility study
comparing AmoyDx HRD focus panel with myriad myChoiceCDx assay.
Pathologica. 114:288–294. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stronach EA, Paul J, Timms KM, Hughes E,
Brown K, Neff C, Perry M, Gutin A, El-Bahrawy M, Steel JH, et al:
Biomarker assessment of HR deficiency, tumor BRCA1/2 mutations, and
CCNE1 copy number in ovarian cancer: Associations with clinical
outcome following platinum monotherapy. Mol Cancer Res.
16:1103–1111. 2018. View Article : Google Scholar : PubMed/NCBI
|