Introduction
Osteopontin (OPN) is a secreted
arginine-glycine-aspartic acid-containing phosphoprotein which
exists mainly as a soluble cytokine and can bind to certain
integrins or CD44 variants, which further mediate diverse
biological functions (1–3). OPN is involved in the pathogenesis of
numerous disease states, including cancer and chronic inflammatory
diseases (4,5). OPN levels have been reported to be
markedly increased in both numerous types of human cancers and in
the plasma of patients with cancer (6–8).
Previous studies have reported that OPN is associated with tumor
metastasis and progression (9,10), and
OPN has been reported to promote cell survival through inhibition
of apoptosis (11). It has been
reported that OPN has important roles in mediating the growth,
metastasis and immune response of hepatocellular carcinoma (HCC)
(12,13). OPN has also been shown to be a
promising serum biomarker of HCC (14). Activation of the mitogen activated
protein kinase (MAPK), NF-κB and PI3K/Akt signaling pathways in HCC
cells may be involved in mediating the effects of OPN on liver
cancer cells (15–17). Furthermore, OPN has been reported to
promote the expression of metalloproteinases (MMPs), including
inducing the expression of long non-coding RNAs, such as HOTAIR,
during the invasion of liver cancer cells (18,19).
However, other potential mechanisms that may be involved in the
effects of OPN on liver cancer cells require further
elucidation.
Denticleless E3 ubiquitin protein ligase homolog
[DTL, also known as CDT2, CUL4-DDB1-associated factor (DCAF2) or
RAMP] belongs to the DCAF protein family, contains WD40 repeats,
and exerts a crucial role in the regulation of the degradation of
CDT1 in the DNA damage response. DTL has been reported to be
involved in DNA damage repair, the cell cycle and DNA replication,
processes intimately involved with chromosomal separation and cell
division, and DTL is a key regulator of cell cycle progression and
genome stability (20–22). A previous study reported that DTL
might affect genome stability by modulating the non-homologous end
joining repair pathway. The role of DTL in genome stability
suggests that DTL may be associated with tumorigenesis and a
previous study reported that the expression level of DTL is
increased in numerous types of cancer (23).
Although it is well established that DTL fulfills
important roles in numerous biological processes, to the best of
our knowledge the mechanism underlying the regulation of its
expression in cancer has yet to be fully elucidated. Previous
reports have suggested that SB743921, a selective inhibitor of
kinesin spindle protein (KSP), and the microRNAs (miRs) miR-490-5p
and miR-30a-5p may be associated with the expression of DTL in
cancer cells (24–26). In the present study, it was
demonstrated that OPN was able to induce the expression of DTL in a
dose dependent manner. Moreover, DTL expression was found to be
regulated by OPN siRNAs and a vector expressing OPN. In addition,
the results of the present further demonstrated that the AKT
signaling pathway, which is activated by OPN, may be involved in
mediating the effects of OPN on the expression of DTL in liver
cancer cells. Finally, using luciferase activity assays, it was
demonstrated that both OPN and the AKT signaling pathway were able
to transcriptionally affect the expression of DTL in liver cancer
cells.
Materials and methods
Cell culture and transfection
The Huh7 liver cancer cell line was purchased from
the American Type Culture Collection and the HepG2 liver cancer
cell line was purchased from Guangzhou Saiku Biotechnology Co.,
Ltd. STR profiling was performed to confirm the authenticity of the
HepG2 cell line. The cells were cultured in DMEM (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with heat-inactivated fetal
calf serum (Gibco; Thermo Fisher Scientific, Inc.) and penicillin
(100 U/ml)/streptomycin (100 µg/ml) (Gibco; Thermo Fisher
Scientific, Inc.) in a tissue-culture incubator containing 5%
CO2 at 37°C. The transfection of the plasmids and siRNAs
for the purposes of altering the expression of DTL was performed
using Invitrogen® Lipofectamine 3000 (Thermo Fisher
Scientific, Inc.), according to the manufacturer's
instructions.
Plasmid construction and siRNA
sequences
Total RNA was extracted from Huh7 cells by
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.),
and then 1 µg total RNA was subjected to reverse transcription (RT)
to generate cDNA using the reagent for RT (GoScript™ Reverse
Transcription Mix; cat. no. A2790; Promega Corporation). This cDNA
was used as a template to amplify the coding sequence of OPN by PCR
using PrimeSTAR® HS (cat. no. R040A; Takara Bio, Inc.).
The primers used to amplify the full length of OPN were as follows:
5′-GTAGGTACCATGAGAATTGCAGTGATTTG-3′ and
5′-GTACTCGAGTTAATTGACCTCAGAAGATG-3′. The product of the PCR was
purified by the Kit for DNA purification (cat. no. B110092, Sangon
Biotech, China), and digested by KpnI and XhoI (cat.
nos. 1068A and 1094A; Takara Bio, Inc.). The full length OPN was
further inserted into pcDNA3.1 vector by using a DNA Ligation Kit
(cat. no. 6022Q; Takara Bio, Inc.). The recombinant vector was
verified by sequencing (Invitrogen; Thermo Fisher Scientific,
Inc.). For western blotting and Transwell assay, 3 µg plasmids (OPN
expressing vector or control vector) were transfected into each
well of a 6-well plate containing Huh7 cells or HepG2 cells
(2×105 cells/well) for 24 h using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.). The mixture containing the plasmid and
Lipofectamine 3000 was incubated at room temperature for 20 min
before transfection. A total of 24 h post-transfection, cells were
harvested or seeded into the Transwell chamber. For the cell
viability assay, 0.2 µg corresponding plasmids were transfected
into cells in each well of a 96-well plate at a density of 2,000
cells per well for the indicated time (0, 24, 48 and 72 h), the
mixture containing the plasmid and Lipofectamine 3000 was incubated
at room temperature for 20 min, and then the mixture was added to
the wells of 96-well plate. The 96-well plate was incubated at 37°C
in an atmosphere containing 5% CO2 for the indicated
time (0, 24, 48 and 72 h) before viability assay. The working
concentration of siRNA was 20 nM. Similar to the plasmid
transfection protocol, the mixture containing the siRNA and
Lipofectamine 3000 was incubated at room temperature for 20 min
before transfection. GFP was separately inserted into pcDNA3.1 and
this GFP-expressing vector was used as a control for OPN
overexpression. siRNAs were synthesized by GenePharma Inc,
Shanghai, China. For siRNA transfection, two OPN siRNAs or two DTL
siRNAs were used; the two siRNAs were mixed together for
transfection. The sequences of the siRNAs used for knockdown were
as follows: OPN sense (S), 5′-GUGGGUUGGUCAGUUAUGATT-3′ and
antisense (AS), 5′-UCAUAACUGUCCUUCCCACTT-3′; and S,
5′-GUCUCACCAUUCUGAUGAATT-3′ and AS, 5′-UUCAUCAGAAUGGUGAGACTT-3′;
DTL, S, 5′-CUUCUUAUGGAGAAACAGGTT-3′ and AS,
5′-CCUGUUUCUCCAUAAGAAGTT-3′; and S, 5′-AAUAUGGAACAUGUACUAGTT-3′ and
AS, 5′-CUAGUACAUGUUCCAUAUUTT-3′; and control S,
5′-ACGCAUGCAUGCUUGCUUUTT-3′ and AS, 5′-AAAGCAAGCAUGCAUGCGUTT-3′.
The putative 2,000 bp promoter region of human DTL was determined
by UCSC genome browser (http://genome.ucsc.edu/). The genomic DNA of Huh7
cells was extracted using a kit from Beyotime Institute of
Biotechnology (cat. no. D0061). The genomic DNA was used as a
template to amplify the promoter region of DTL by PCR using
PrimeSTAR® HS (cat. no. R040A; Takara Bio, Inc.). The
forward and reverse primers used were as follows:
5′-GGAGAACCGTTTGAACTCGGG-3′ and 5′-GGGAGAACTCAGAAGCTGAG-3′.
Thermocycling conditions were as follows: Initial denaturation at
98°C for 3 min, followed by 29 cycles of denaturation at 98°C for
10 sec, annealing at 55°C for 20 sec and elongation at 72°C for 2
min; and a final extension step at 72°C for 5 min. The PCR product
was inserted into the pGL3-Basic luciferase-reporter vector
(Promega Corporation).
Cell viability assay
The liver cancer cells were seeded in 96-well plates
at a density of 2,000 cells per well in 150 µl of culture medium.
After 24 h, the siRNAs or OPN overexpression vector were
transfected into the corresponding wells in triplicate. The plates
were then incubated at 37°C in an atmosphere containing 5%
CO2 for the indicated time (0, 24, 48 and 72 h). The
medium was subsequently removed and the cells were washed twice
with PBS. DMEM (90 µl) containing 10 µl CCK8 solution (Beyotime
Institute of Biotechnology) was then added to each well, and the
plates were incubated at 37°C for an additional 2 h. The absorbance
values at 450 nm were assessed using a microplate reader
spectrophotometer (Tecan Group, Ltd.). All experiments were
repeated at least 3 times.
Chemicals and antibodies
The primary antibodies used in the present study
were as follows: Anti-OPN (1:1,000; cat. no. SAB5700738,
Sigma-Aldrich; Merck KGaA), anti-DTL (1:1,000; cat. no. ab174385,
Abcam), phosphorylated (p)-AKT (Ser473; 1:500; cat. no. AA329;
Beyotime Institute of Biotechnology), AKT (1:500; cat. no. AA326;
Beyotime Institute of Biotechnology) and actin (1:500; cat. no.
AA128; Beyotime Institute of Biotechnology). HRP conjugated goat
anti-rabbit IgG (1:2,000; cat. no. A0208) and goat anti-mouse IgG
(1:2,000; cat. no. A0216) secondary antibodies were purchased from
Beyotime Institute of Biotechnology. The PI3K/AKT inhibitors
LY294002 and wortmannin were purchased from Beyotime Institute of
Biotechnology, and recombinant human OPN (rhOPN) was purchased from
PeproTech, Inc.
Luciferase reporter assay
Huh7 and HepG2 cells were seeded at a density of
2×103 cells per well in 96-well plates on the day before
transfection. The cells were co-transfected with 0.1 µg firefly
luciferase reporter construct containing the DTL promoter region
(Promega Corporation), 0.01 µg pRL-TK Renilla luciferase reporter
plasmid (Promega Corporation) and the pcDNA3.1-OPN vector (0.2 µg)
using Lipofectamine™ 3000 (Invitrogen; Thermo Fisher Scientific,
Inc.). After 24 h of transfection, the luciferase activity was
assessed using a dual-luciferase reporter assay system (cat. no.
E1910; Promega Corporation) according to the manufacturer's
instructions, and the signal was normalized to that of the internal
Renilla control in order to assess the transfection
efficiency.
Transwell assay
Cell invasion assays were performed using the
Transwell (Corning, Inc.) system, which allows cells to invade
through a Matrigel™-coated polycarbonate membrane with a pore size
of 8 µm. The aforementioned Huh7 and HepG2 cells transfected with
DTL siRNAs were trypsinized using 0.25% trypsin at 37°C for 5 min.
The cells were seeded into the upper chambers in serum-free DMEM
with or without rhOPN, and were incubated at 37°C for 24 h. DMEM
supplemented with 10% FBS was added to the lower chambers. The
membranes were then washed with PBS, cells above the membrane were
gently removed using a cotton swab, and cells that had migrated
across the membrane were fixed with cold methanol for 15 min at
room temperature. Finally, the cells were stained using crystal
violet for 10 min at room temperature. Cells beneath the membrane
were counted in five fields of view using an inverted light
microscope. Each experiment was repeated three times.
Western blotting
Huh7 and HepG2 cells were exposed to various
experimental conditions, such as transfection and treatment with
rhOPN, prior to being harvested and lysed for protein extraction
using RIPA buffer (cat. no. P0013B; Beyotime Institute of
Biotechnology). Protein concentration was determined using a
Bio-Rad protein assay kit (Bio-Rad Laboratories, Inc.). The samples
were then subjected to 12% SDS-PAGE and transferred to PVDF
membranes. Membranes were blocked using 5% milk in TBS-0.1% Tween
(TBST) buffer at room temperature for 30 min, and then probing with
the aforementioned primary antibodies at 4°C overnight. After
washing with TBST three times, each for ten min, membranes were
further incubated with HRP-conjugated secondary antibodies for 2 h
at room temperature. The western blots were visualized using an
enhanced chemiluminescence detection system (Tiangen Biotech Co.,
Ltd.). β-actin was used as the sample loading control.
Semi-quantification was performed using ImageJ (V1.8.0; National
Institute of Health).
RT-quantitative (q)PCR
Total RNA was extracted using TRIzol reagent and RT
was performed using oligo(dT) 20 (Takara Bio, Inc.) as primer and
M-MLV reverse transcriptase (Promega Corporation) at 42°C for 30
min. The primer sequences for qPCR amplification were as follows:
DTL forward (F), 5′-CCAGTATCTCAGAGCCTCCG-3′ and reverse (R),
5′-TGGATTCTCAGCCTTCCGTT-3′; and β-actin F,
5′-CCCACACTGTGCCCATCTAC-3′ and R, 5′-GGAACCGCTCATTGCCAATG-3′.
β-actin was used as the loading control. The qPCR reactions were
performed using 20 µl 1:1 diluted iTaq™ Universal SYBR®
Green Supermix (Bio-Rad Laboratories, Inc.) with three replicates.
The thermocycling conditions were as follows: Initial heat
activation at 95°C for 3 min, followed by 40 cycles of 95°C for 10
sec and 55°C for 20 sec for. The transcript level of the DTL mRNA
was further analyzed by RT-qPCR using an ABI-7500 Sequence Detector
System (Applied Biosystems; Thermo Fisher Scientific, Inc.). The
relative expression levels of the eighteen selected genes were
calculated using the 2−ΔΔCq method (27).
Statistical analysis
GraphPad Prism 5 (Dotmatics) was used to analyze the
data. Statistical analyses were performed using the unpaired
Student's t-test for comparisons of 2 groups and one-way ANOVA
followed by Tukey's post hoc test for comparisons of ≥3 groups. All
experiments were performed at least 3 times. P<0.05 was
considered to indicate a statistically significant difference.
Results
DTL mediates OPN-induced proliferation
and invasion by liver cancer cells
OPN and DTL have previously been reported to be
associated with both the proliferation and invasion of liver cancer
cells (13,28). Therefore, in the present study, it
was hypothesized that OPN may be able to stimulate both the
proliferation and invasion of liver cancer cells, at least in part,
via DTL. First, the constructed vector expressing OPN, and the
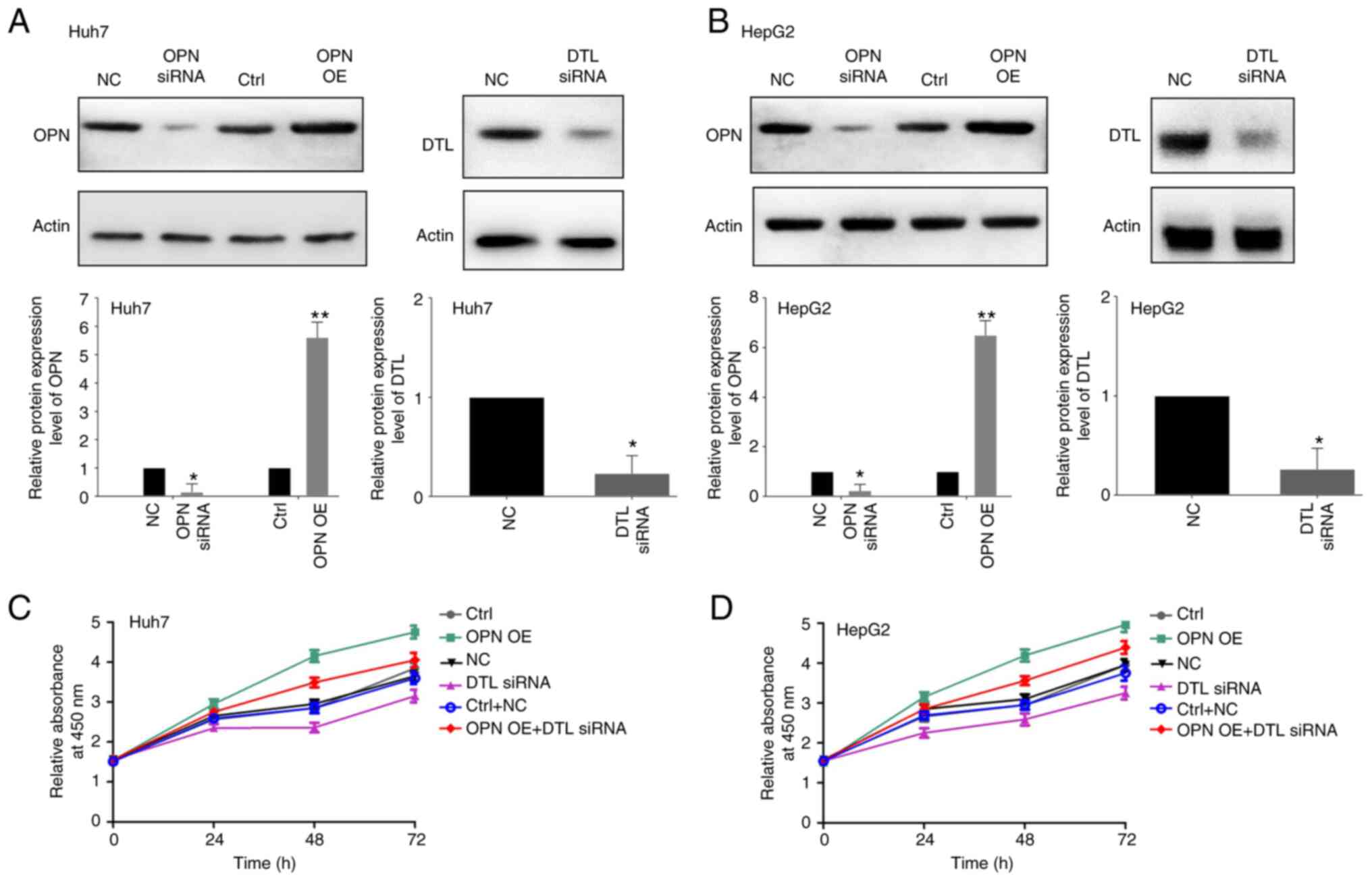
efficacy of the siRNAs against OPN and DTL, were validated. OPN was
significantly overexpressed in both Huh7 and HepG2 cells
transfected with the vector expressing OPN compared with the
control and treating the cells with the siRNAs led to significantly
decreased protein expression levels of both OPN and DTL in both of
the liver cancer cell lines compared with the control (Fig. 1A and B). The viability of the two
cell lines was subsequently assessed using a CCK8 assay following
transfection with OPN expressing vector and/or DTL siRNA for 24, 48
or 72 h. The OPN-induced growth of the Huh7 and HepG2 cells was
demonstrated to be inhibited notably by treatment with DTL siRNA
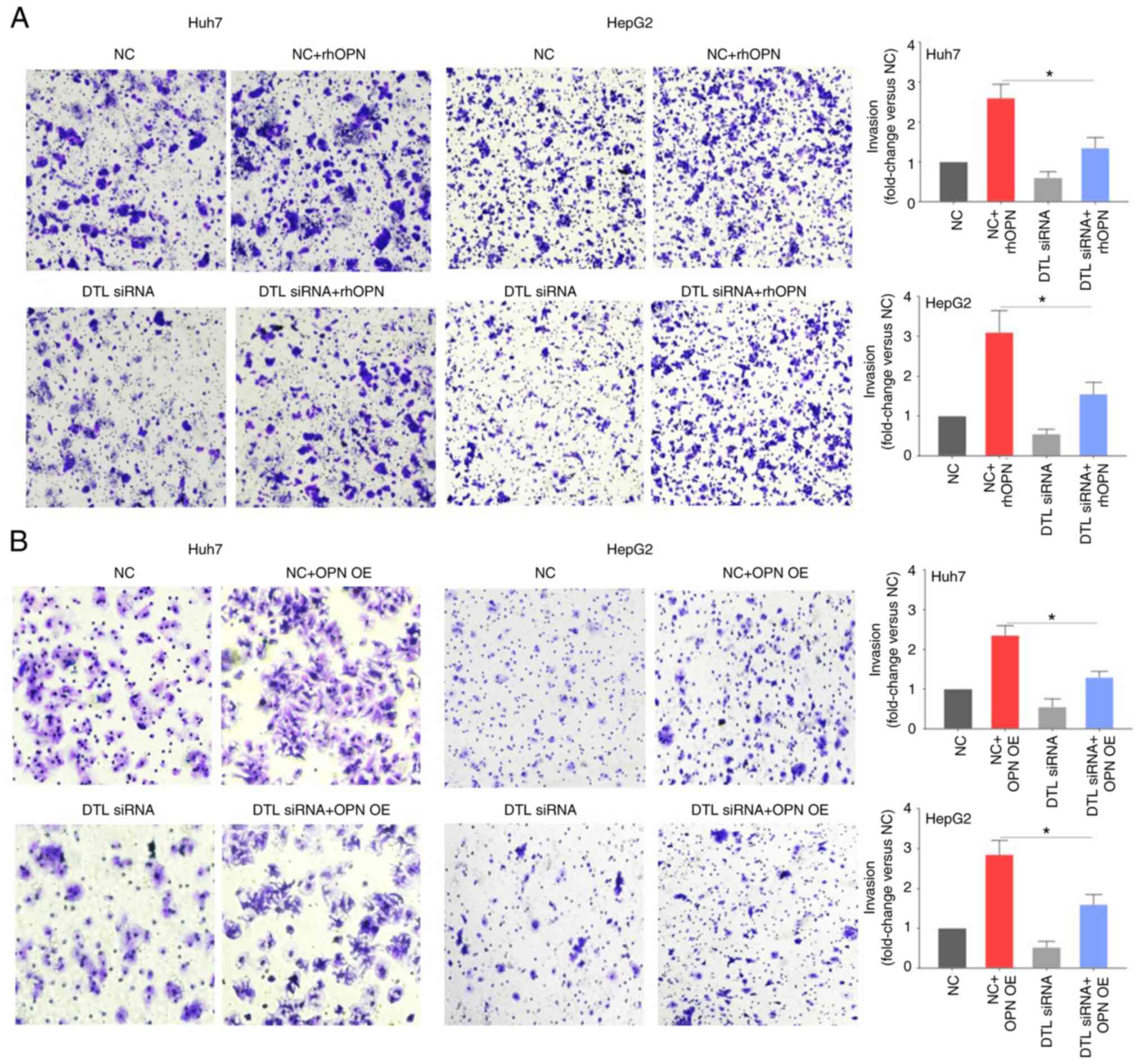
compared with the negative control (Fig. 1C and D). The Transwell assays
(Fig. 2A) demonstrated that though
DTL knockdown by siRNA itself only slightly affected invasion by
liver cancer cells compared with the negative control, the reduced
expression level of DTL caused a significant inhibition of the
rhOPN-induced invasion of Huh7 cells (fold change, 2.60±0.33 vs.
1.38±0.25) compared with the negative control + rhOPN group. This
indicated that DTL may participate in rhOPN-induced invasion by
Huh7 cells. Knockdown of DTL likewise led to a significant decrease
in OPN-induced invasion by HepG2 cells (fold change, 3.15±0.53 vs.
1.56±0.31) compared with the negative control + rhOPN group. To
further evaluate the role of DTL in OPN-induced invasion of liver
cancer cells, a vector expressing OPN was used to assess the effect
of DTL on OPN-induced invasion. Knockdown of DTL significantly
decreased OPN-induced invasion by both Huh7 cells (fold change,
2.36±0.28 vs. 1.32±0.16) and HepG2 cells (fold change, 2.83±0.36
vs. 1.62±0.28) compared with the negative control + OPN
overexpression group (Fig. 2B).
OPN regulates DTL expression
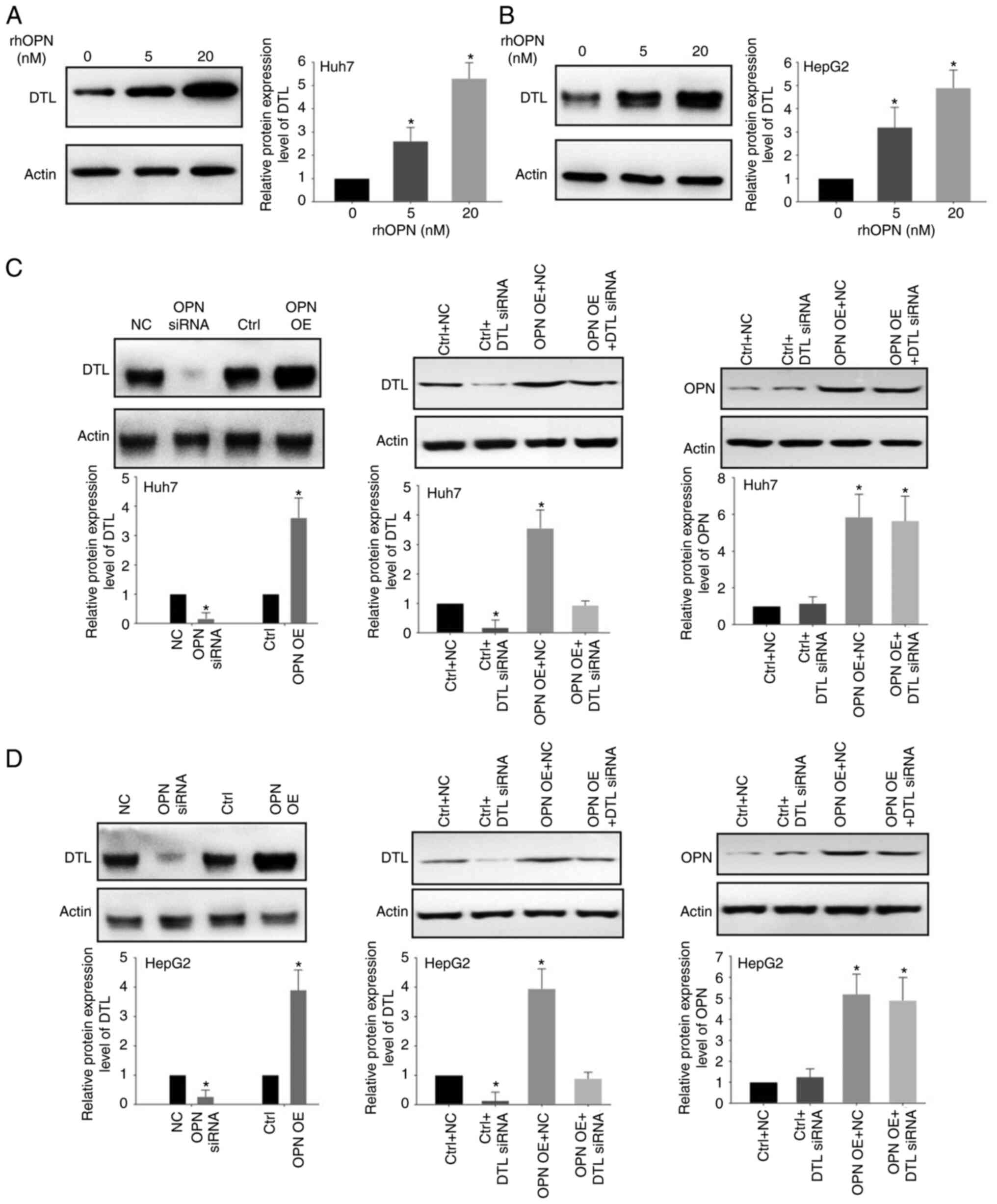
To assess the effect of OPN on DTL protein
expression levels, western blotting was performed using the Huh7
and HepG2 cells. Treatment with rhOPN led to a marked increase in
the expression of DTL in a dose-dependent manner (Fig. 3A and B). Apparent increases in the
protein expression levels of DTL in Huh7 and HepG2 cells were
observed following treatment with 5 or 20 nM rhOPN for 6 h compared
with those in the untreated group (Fig.
3A and B). Knockdown of OPN using siRNAs led to significantly
decreased protein expression levels of DTL in HepG2 and Huh7 cells
compared with the control (Fig. 3C and
D). Moreover, it was demonstrated that OPN overexpression could
significantly increase the expression of DTL compared with the
negative control. It was also demonstrated that knockdown of DTL by
siRNA could markedly reduce the OPN-induced expression of DTL
compared with the OPN overexpression group in both Huh7 cells and
HepG2 cells. However, knockdown of DTL demonstrated little effect
on OPN expression in both Huh7 cells and HepG2 cells. Taken
together, the above data suggested that OPN was able to regulate
the expression of DTL in liver cancer cells.
The PI3K/AKT signaling pathway is
involved in the regulation of DTL expression by OPN
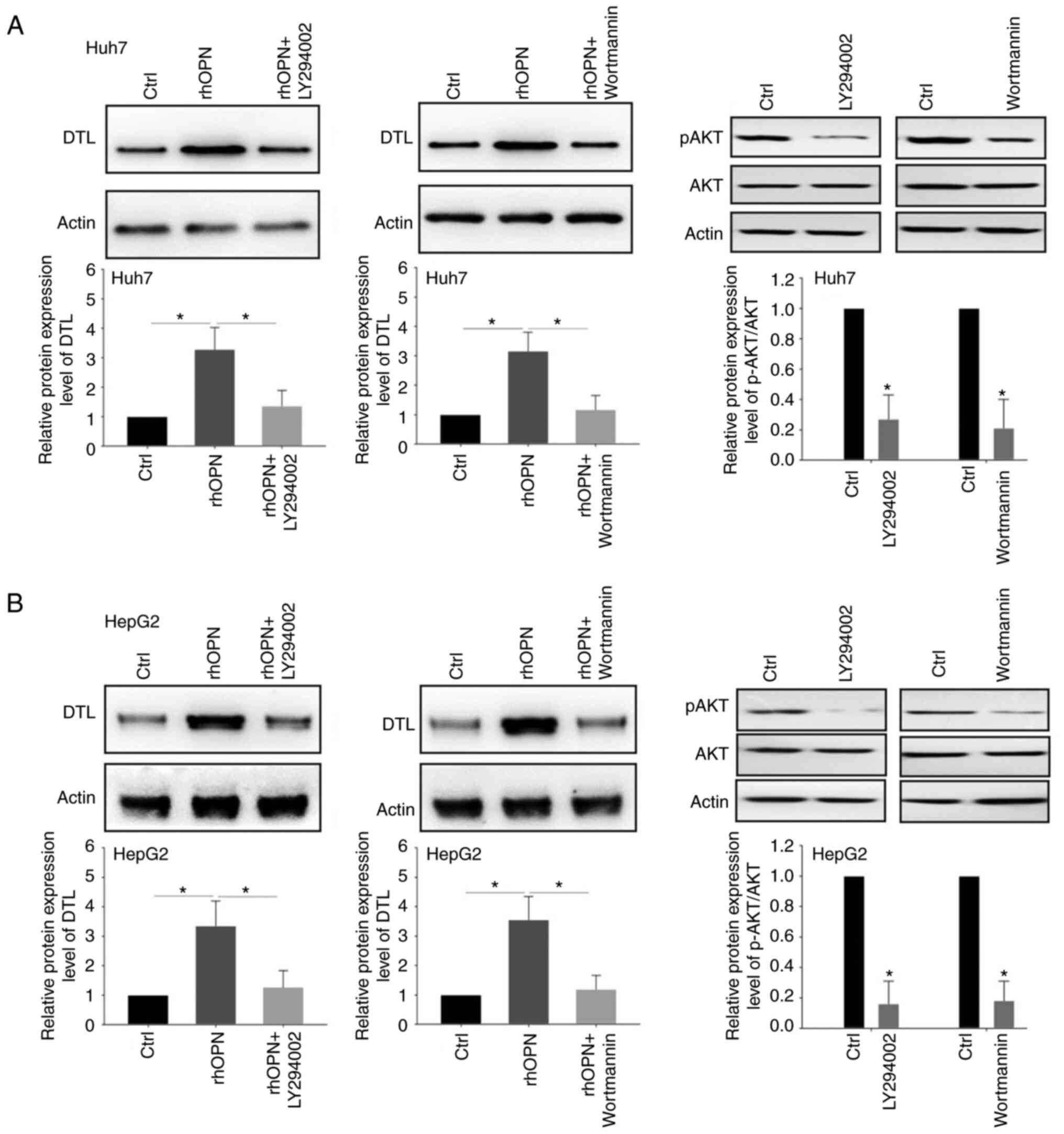
It has been previously reported that OPN is able to
affect the PI3K/AKT pathway in cancer cells (29,30).
Therefore, it was hypothesized that the PI3K/AKT signaling pathway
may participate in the regulation of DTL expression that is
mediated by OPN. The upregulation of DTL protein expression induced
by rhOPN was significantly attenuated when Huh7 cells were
pretreated with the PI3K/AKT inhibitors LY294002 (5 µM) or
wortmannin (5 µM) for 3 h (Fig.
4A). Similar effects were also demonstrated in the HepG2 cells
(Fig. 4B). Inhibition of the AKT
signaling pathway in liver cancer cells by LY294002 and wortmannin
was assessed using p-AKT expression levels. the protein expression
level of p-AKT was significantly downregulated after treatment with
LY294002 or wortmannin in both Huh7 cells and HepG2 cells.
Collectively, these results suggested that the PI3K/AKT signaling
pathway may be involved in the OPN-induced upregulation of DTL
expression in liver cancer cells.
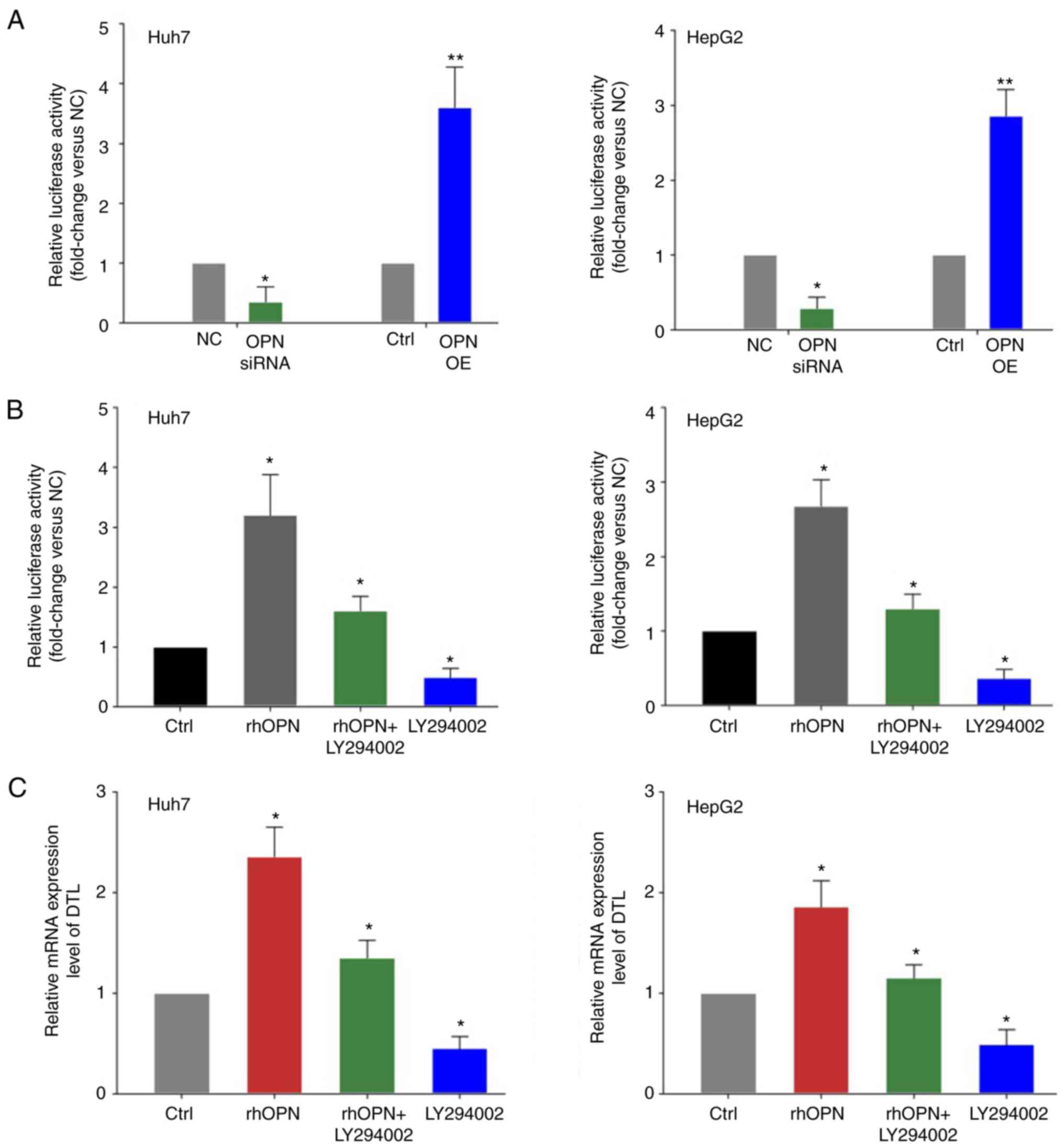
OPN transcriptionally regulates DTL
expression in liver cancer cells via the PI3K/AKT signaling
pathway
It was hypothesized that OPN may transcriptionally
influence the expression of DTL in liver cancer cells. To evaluate
whether DTL may be transcriptionally inhibited by OPN, luciferase
activity assays were performed on extracts from Huh7 or HepG2 cells
that were co-transfected with the luciferase-reporter plasmid in
combination with vectors expressing either OPN or OPN siRNAs and
the respective control groups. OPN knockdown led to a significant
decrease in the DTL promoter activity compared with the control
groups (Fig. 5A). Moreover, the
promoter activity of DTL was significantly increased by OPN
overexpression in liver cancer cells compared with the control.
rhOPN was also used in subsequent experiments to further assess the
aforementioned effects. Treatment with rhOPN led to a marked
increase in the promoter activity of DTL compared with the control
and the PI3K/AKT inhibitor LY294002 strongly attenuated this effect
in liver cancer cells (Fig. 5B).
Furthermore, the mRNA expression level of DTL in liver cancer cells
treated with rhOPN was quantified. rhOPN induced a significant
increase in the expression of DTL mRNA compared with the control
and the PI3K/AKT inhibitor LY294002 was able to markedly reduce
this effect of rhOPN in liver cancer cells (Fig. 5C).
Discussion
It has been reported previously that elevated levels
of OPN in the plasma of patients with certain types of cancer are
closely associated with cancer relapse or that the elevated level
of OPN might decrease the efficacy of treatment (31). Previous studies have reported that
the level of OPN is upregulated in a liver cancer model (32,33).
Furthermore, OPN has been reported to be involved in the regulation
of certain signal transduction pathways, including the PI3K/AKT
signaling pathway, which mainly function in stimulating the
migration, invasion and metastasis of cancer cells (15–17).
The AKT signaling pathway has been reported to be induced by OPN
(29,30,34).
Previous studies reported that OPN induction of Collagen-I occurred
via integrin α(v)β(3) engagement
and activation of the PI3K/pAkt/NFκB signaling pathway. OPN could
also induce activation of phosphatidylinositol 3-kinase and Akt by
binding to the CD44 receptor, an effect which can even affect the
chemoresistance of certain cancer cells (29,30,34).
Therefore, in the present study, whether the AKT signaling pathway
was involved in the relationship between OPN and DTL was evaluated.
As an extracellular cytokine, OPN-mediated signaling has been
previously reported to lead to resistance to apoptosis in cancer
cells. Therefore, the identification of new factors associated with
OPN-mediated signaling could be beneficial in terms of increasing
understanding of OPN function.
DTL is one member of the DCAF family that fulfills
critical roles in the cell cycle and DNA repair. The dysregulation
of DTL expression has previously been reported in different types
of cancer (23,35). Previous studies have also reported
that DTL was involved in proliferation and invasion by cancer cells
(28). An increase in the protein
expression level of DTL was demonstrated to both accelerate the
growth of liver cancer cells and increase their invasive
capabilities. Although the function of DTL in biological processes
has already been reasonably well defined, many other important
aspects still need to be investigated, including its regulation and
identifying its protein interactions with DTL. It has been reported
that KSP inhibitor SB743921, miR-490-5p and miR-30a-5p may affect
the expression of DTL (24–26). However, the manner in which DTL
expression is regulated by extracellular proteins, or their
associated signal transduction pathways, in cancer cells has yet to
be fully elucidated.
Little is known about the associations between OPN,
the PI3K/AKT signaling pathway and DTL in cancer cells. Therefore,
the present study evaluated whether OPN acted as a regulator of the
AKT signaling pathway and DTL in liver cancer cells. The effects of
OPN on the AKT signaling pathway and DTL were first assessed using
an OPN-overexpressing vector and siRNAs against OPN. The results
demonstrated that both rhOPN and overexpression of OPN could
increase the protein expression level of DTL in liver cancer cells,
whereas knockdown of OPN by siRNA treatment led to a decrease in
the protein expression level of DTL. As OPN mainly functions as an
extracellular protein associated with certain signaling processes,
the data obtained suggested that OPN may serve to maintain the DTL
level in liver cancer cells. To further assess the association
between the AKT signaling pathway and the DTL level, liver cancer
cells were treated with the PI3K/AKT pathway inhibitors LY294002
and wortmannin, which demonstrated that both LY294002 and
wortmannin were capable of reducing the protein expression level of
DTL. Furthermore, using a luciferase reporter assay, it was
demonstrated that OPN could transcriptionally induce the expression
of DTL via the AKT signaling pathway. These results indicated that
OPN could regulate expression of DTL, but DTL couldn't affect the
OPN level in liver cancer cells.
In conclusion, the results of the present study have
established a link between OPN and DTL in liver cancer cells, which
indicated that OPN was able to transcriptionally increase the level
of DTL. To the best of our knowledge, this is the first study to
have reported such an effect. Furthermore, the results suggested
that the AKT signaling pathway was involved in mediating the
effects of OPN on the expression of DTL. However, the current data
were mainly derived from liver cancer cells; therefore, the
relationship between OPN and DTL in an animal model of cancer
requires further investigation.
Acknowledgments
The authors would like to thank Dr Zixiong Chen
(Cancer Center, Sun Yat-sen University) for sharing cell lines,
constructs and reagents..
Funding
The present study was funded by the Medical Science and
Technology Foundation of Guangdong Province (grant no.
A2019122).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZL and LY designed the study. ZL, GY, XY and SZ
performed the experiments. ZF, FC and XC performed the data
analysis. ZL and FC confirm the authenticity of all the raw data,
and ZL and LY drafted the manuscript. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chabas D, Baranzini SE, Mitchell D,
Bernard CC, Rittling SR, Denhardt DT, Sobel RA, Lock C, Karpuj M,
Pedotti R, et al: The influence of the proinflammatory cytokine,
osteopontin, on autoimmune demyelinating disease. Science.
294:1731–1735. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Icer MA and Gezmen-Karadag M: The multiple
functions and mechanisms of osteopontin. Clin Biochem. 59:17–24.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yim A, Smith C and Brown AM:
Osteopontin/secreted phosphoprotein-1 harnesses glial-, immune-,
and neuronal cell ligand-receptor interactions to sense and
regulate acute and chronic neuroinflammation. Immunol Rev.
311:224–233. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Klement JD, Paschall AV, Redd PS, Ibrahim
ML, Lu C, Yang D, Celis E, Abrams SI, Ozato K and Liu K: An
osteopontin/CD44 immune checkpoint controls CD8+ T cell activation
and tumor immune evasion. J Clin Invest. 128:5549–5560. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lu C, Liu Z, Klement JD, Yang D, Merting
AD, Poschel D, Albers T, Waller JL, Shi H and Liu K: WDR5-H3K4me3
epigenetic axis regulates OPN expression to compensate PD-L1
function to promote pancreatic cancer immune escape. J Immunother
Cancer. 9:e0026242021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hui EP, Sung FL, Yu BK, Wong CS, Ma BB,
Lin X, Chan A, Wong WL and Chan AT: Plasma osteopontin, hypoxia,
and response to radiotherapy in nasopharyngeal cancer. Clin Cancer
Res. 14:7080–7087. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu CY, Wu MS, Chiang EP, Wu CC, Chen YJ,
Chen CJ, Chi NH, Chen GH and Lin JT: Elevated plasma osteopontin
associated with gastric cancer development, invasion and survival.
Gut. 56:782–789. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Blasberg JD, Pass HI, Goparaju CM, Flores
RM, Lee S and Donington JS: Reduction of elevated plasma
osteopontin levels with resection of non-small-cell lung cancer. J
Clin Oncol. 28:936–941. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qin X, Yan M, Wang X, Xu Q, Wang X, Zhu X,
Shi J, Li Z, Zhang J and Chen W: Cancer-associated
Fibroblast-derived IL-6 promotes head and neck cancer progression
via the osteopontin-NF-kappa B signaling pathway. Theranostics.
8:921–940. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Briones-Orta MA, Avendaño-Vázquez SE,
Aparicio-Bautista DI, Coombes JD, Weber GF and Syn WK: Osteopontin
splice variants and polymorphisms in cancer progression and
prognosis. Biochim Biophys Acta Rev Cancer. 1868:93–108.A. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang RH, Quan YJ, Chen JH, Wang TF, Xu M,
Ye M, Yuan H, Zhang CJ, Liu XJ and Min ZJ: Osteopontin promotes
cell migration and invasion, and inhibits apoptosis and autophagy
in colorectal cancer by activating the p38 MAPK signaling pathway.
Cell Physiol Biochem. 41:1851–1864. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu Q, Li L, Miao C, Hasnat M, Sun L, Jiang
Z and Zhang L: Osteopontin promotes hepatocellular carcinoma
progression through inducing JAK2/STAT3/NOX1-mediated ROS
production. Cell Death Dis. 13:3412022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao J, Dong L, Lu B, Wu G, Xu D, Chen J,
Li K, Tong X, Dai J, Yao S, et al: Down-regulation of osteopontin
suppresses growth and metastasis of hepatocellular carcinoma via
induction of apoptosis. Gastroenterology. 135:956–968. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shang S, Plymoth A, Ge S, Feng Z, Rosen
HR, Sangrajrang S, Hainaut P, Marrero JA and Beretta L:
Identification of osteopontin as a novel marker for early
hepatocellular carcinoma. Hepatology. 55:483–490. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Haga Y, Kanda T, Nakamura M, Nakamoto S,
Sasaki R, Takahashi K, Wu S and Yokosuka O: Overexpression of c-Jun
contributes to sorafenib resistance in human hepatoma cell lines.
PLoS One. 12:e01741532017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Manna D, Reghupaty SC, Camarena MDC,
Mendoza RG, Subler MA, Koblinski JE, Martin R, Dozmorov MG,
Mukhopadhyay ND, Liu J, et al: Melanoma differentiation associated
gene-9/syndecan binding protein promotes hepatocellular carcinoma.
Hepatology. Sep 19–2022.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu X, Zheng Y, Zhu X, Gao X, Wang C, Sheng
Y, Cheng W, Qin L, Ren N, Jia H and Dong Q: Osteopontin promotes
hepatocellular carcinoma progression via the PI3K/AKT/Twist
signaling pathway. Oncol Lett. 16:5299–5308. 2018.PubMed/NCBI
|
|
18
|
Zhang R, Pan X, Huang Z, Weber GF and
Zhang G: Osteopontin enhances the expression and activity of MMP-2
via the SDF-1/CXCR4 axis in hepatocellular carcinoma cell lines.
PLoS One. 6:e238312011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang G, Zhang S, Gao F, Liu Z, Lu M, Peng
S, Zhang T and Zhang F: Osteopontin enhances the expression of
HOTAIR in cancer cells via IRF1. Biochim Biophys Acta.
1839:837–848. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sansam CL, Shepard JL, Lai K, Ianari A,
Danielian PS, Amsterdam A, Hopkins N and Lees JA: DTL/CDT2 is
essential for both CDT1 regulation and the early G2/M checkpoint.
Genes Dev. 20:3117–3129. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Feng M, Wang Y, Bi L, Zhang P, Wang H,
Zhao Z, Mao JH and Wei G: CRL4A(DTL) degrades DNA-PKcs to modulate
NHEJ repair and induce genomic instability and subsequent malignant
transformation. Oncogene. 40:2096–2111. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu YW, Cao LR, Wang M, Xu Y, Wu X, Liu J,
Tong C and Fan HY: Maternal DCAF2 is crucial for maintenance of
genome stability during the first cell cycle in mice. J Cell Sci.
130:3297–3307. 2017.PubMed/NCBI
|
|
23
|
Cui H, Wang Q, Lei Z, Feng M, Zhao Z, Wang
Y and Wei G: DTL promotes cancer progression by PDCD4
ubiquitin-dependent degradation. J Exp Clin Cancer Res. 38:3502019.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu L, Xiao F, Yu Y, Wang H, Fang M, Yang
Y, Sun H, Wang L and Sheng Y: KSP inhibitor SB743921 inhibits
growth and induces apoptosis of breast cancer cells by regulating
p53, Bcl-2, and DTL. Anticancer Drugs. 27:863–872. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li J, Xu X, Liu C, Xi X, Wang Y, Wu X and
Li H: MiR-490-5p restrains progression of Gastric cancer through
DTL repression. Gastroenterol Res Pract. 2021:28941172021.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Baraniskin A, Birkenkamp-Demtroder K,
Maghnouj A, Zöllner H, Munding J, Klein-Scory S, Reinacher-Schick
A, Schwarte-Waldhoff I, Schmiegel W and Hahn SA: MiR-30a-5p
suppresses tumor growth in colon carcinoma by targeting DTL.
Carcinogenesis. 33:732–739. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative pcr and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen YC, Chen IS, Huang GJ, Kang CH, Wang
KC, Tsao MJ and Pan HW: Targeting DTL induces cell cycle arrest and
senescence and suppresses cell growth and colony formation through
TPX2 inhibition in human hepatocellular carcinoma cells. Onco
Targets Ther. 11:1601–1616. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin YH and Yang-Yen HF: The
osteopontin-CD44 survival signal involves activation of the
phosphatidylinositol 3-kinase/Akt signaling pathway. J Biol Chem.
276:46024–46030. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Urtasun R, Lopategi A, George J, Leung TM,
Lu Y, Wang X, Ge X, Fiel MI and Nieto N: Osteopontin, an oxidant
stress sensitive cytokine, up-regulates collagen-I via integrin
α(V)β(3) engagement and PI3K/pAkt/NFκB signaling. Hepatology.
55:594–608. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Le QT, Sutphin PD, Raychaudhuri S, Yu SC,
Terris DJ, Lin HS, Lum B, Pinto HA, Koong AC and Giaccia AJ:
Identification of osteopontin as a prognostic plasma marker for
head and neck squamous cell carcinomas. Clin Cancer Res. 9:59–67.
2003.PubMed/NCBI
|
|
32
|
Cao DX, Li ZJ, Jiang XO, Lum YL, Khin E,
Lee NP, Wu GH and Luk JM: Osteopontin as potential biomarker and
therapeutic target in gastric and liver cancers. World J
Gastroenterol. 18:3923–3930. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhu M, Zheng J, Wu F, Kang B, Liang J,
Heskia F, Zhang X and Shan Y: OPN is a promising serological
biomarker for hepatocellular carcinoma diagnosis. J Med Virol.
92:3596–3603. 2020. View Article : Google Scholar
|
|
34
|
Qian J, LeSavage BL, Hubka KM, Ma C,
Natarajan S, Eggold JT, Xiao Y, Fuh KC, Krishnan V, Enejder A, et
al: Cancer-associated mesothelial cells promote ovarian cancer
chemoresistance through paracrine osteopontin signaling. J Clin
Invest. 131:e1461862021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bao Y, Wang L, Shi L, Yun F, Liu X, Chen
Y, Chen C, Ren Y and Jia Y: Transcriptome profiling revealed
multiple genes and ECM-receptor interaction pathways that may be
associated with breast cancer. Cell Mol Biol Lett. 24:382019.
View Article : Google Scholar : PubMed/NCBI
|