Introduction
Cervical cancer (CC) is one of the prevalent
malignant tumors affecting the reproductive system in female
patients and ranks as the fourth most common malignant tumor
globally (1). According to the
International Federation of Gynecology and Obstetrics (FIGO)
clinical staging system, radical hysterectomy with pelvic
lymphadenectomy (RHPL) with or without para-aortic lymphadenectomy
is the standard surgical treatment for patients with stage IB1-IIA2
CC. Patients with local advanced CC are usually given concurrent
chemoradiotherapy (2). The Sedlis
criteria classifies lymph node metastasis (LNM), surgical margin
and parametrial involvement as high risk factors and stromal
invasion, lymphatic space involvement and primary tumor size as
intermediate risk factors related to the diagnosis, prognosis and
treatment of CC (3). Moreover,
hematological indices, including hemoglobin, lymphocyte, cancer
antigen 125 (Ca125) and squamous cell carcinoma antigen (SCC-Ag),
are particularly valuable for predicting LNM and prognosis
(4–6).
Serum lactate dehydrogenase (LDH), a rate-limiting
enzyme, contributes to the conversion of pyruvate to lactic acid
under hypoxic conditions (7),
serving an important role in tumor cell proliferation and
metastasis (8). Hypoxia can promote
cancer development, contributing to treatment resistance through
new blood vessel formation (9).
Serum LDH has been associated with the prognosis of several cancer
types, including non-Hodgkin lymphoma, colon and lung cancer
(8,10,11),
and elevated LDH levels have been reported to be associated with
poor prognosis in CC (12–14). Research by Ye et al (14) provided evidence for the association
between elevated LDH and poor prognosis of CC using RNA-seq and
microarray datasets. Wang et al (13) reported the prognostic role of the
combination of C-reactive protein and LDH in patients with locally
advanced CC. However, these studies failed to demonstrate the
relationship between LDH and LNM in CC, as well as the lack of
adjustment for relevant risk factors. Therefore, the aim of the
present retrospective study was to investigate the relationship
between LDH levels and LNM in patients who have undergone RHPL
treatment, adjusting for other risk factors (Sedlis criteria) and
hematological indices.
Materials and methods
Study design and population
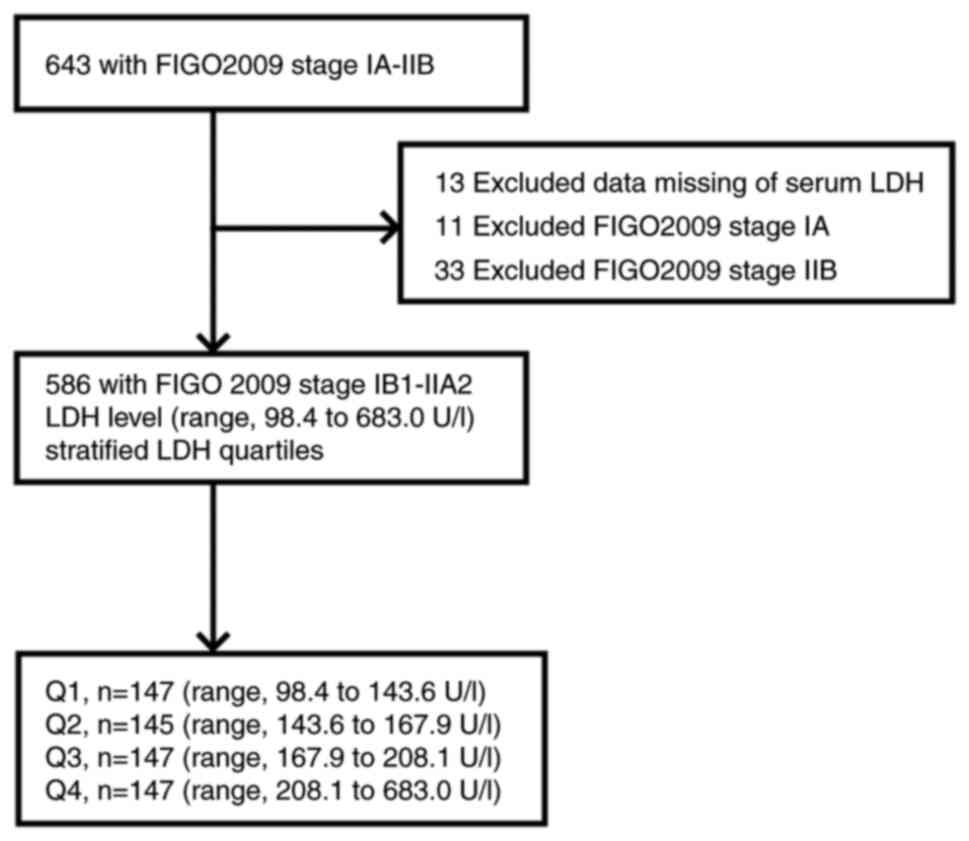
A total of 586 patients with CC who underwent a
radical hysterectomy, pelvic lymphadenectomy with or without
para-aortic lymphadenectomy, were admitted to Fujian Provincial
Maternity and Children's Hospital (Fuzhou, China) between January
2012 and January 2022 and used in the present retrospective study.
The following inclusion criteria were applied: i) First treatment
was administered and completed in the hospital, ii) the case was
assessed preoperatively by >2 gynecological oncologists with
senior professional titles in the hospital and was determined to
fall within stages IB1-IIA2 according to the staging standards of
FIGO (2009), iii) pathological diagnosis of CC, iv) complete
information, including lymph node dissection and hematological
data. Exclusion criteria were as follows: i) Patients staged as Ia
or IIB, ii) Missing LDH data, iii) patients with a history of other
malignant tumors, myocardial infarction, or liver disease. The
hematological data of patients were tested routinely within two
weeks prior to surgery. The other detailed inclusion and exclusion
criteria are listed in Fig. 1.
Patient age range was 24–73 years. The tumor size was divided into
three groups: <2, ≥2 and <4, ≥4 cm; and DSI was divided into
three groups: <1/3, ≥1/3 and <2/3, ≥2/3 of cervical stroma
thickness.
Data collection
Clinical information, pathological results and
hematological data were collected from each patient. Clinical
information included age, FIGO stage, tumor size and neoadjuvant
chemotherapy (NACT). Pathological results included LNM,
pathological type, deep stromal invasion (DSI), lymph-vascular
space invasion (LVSI), surgical margin and parametrial involvement.
DSI definition was primarily based on the ratio of the tumor
invading the cervical stroma. Hematological data, including white
blood cell (WBC) count, neutrophil (NE) count, lymphocyte (LY)
count, platelet count (PLT), cancer antigen 199 (Ca199), Ca125,
α-fetoprotein (AFP), LDH and SCC-Ag, were collected one week before
treatment.
LDH was detected using the lactate to ketone acid
method. The detection range of LDH is 30–4,500 U/l, the normal
reference range is 125–250 U/l and the maximum detection values for
the blank limit, detection limit and quantification limit were 9,
15 and 25 U/l, respectively (15,16).
Statistical analysis
All analyses were performed using the statistical
software packages R 3.3.2 (R-project.org; The R Foundation) and
Free Statistics software v1.3
(clinicalscientists.cn/freestatistics/). Patient characteristics
were calculated according to stratified LDH quartiles. LDH was
entered as a categorical variable (quartiles) and a continuous
variable [with odds ratio (OR)/hazard ratio (HR) calculated per 10
U/l LDH increase]. Data were expressed as the mean ± SD if normally
distributed or as median and interquartile range if skewed. The
χ2 test or Fisher's exact probability method was used to
compare the differences in the rate/composition ratio between
groups for the count data. Uni-/multi-variate analysis was used to
identify the influencing factors. Further analyses were adjusted
cumulatively for logistic stepwise regression analysis and
professional knowledge. Additional subgroup analyses were performed
when effect modification was observed or differences in LDH were
expected in patient subgroups. OR and 95% CI were calculated to
assess the association between LDH and LNM using logistic
regression models. Statistical significance was set at P<0.05.
Missing data were imputed by multiple imputations (17). Splines were fitted using a logistic
regression model based on restricted cubic splines and model
adjustments used (18,19).
Results
Patient characteristics
The present study included 586 female patients with
confirmed pathological diagnoses; among them, 91 patients were
diagnosed with LNM. The first, second and third quartiles of LDH
level (range, 98.4-683.0 U/l) were 143.6, 167.9 and 208.1 U/l,
divided into Q1, Q2, Q3 and Q4 groups. According to this grouping
of LDH levels, the LNM rates of patients were 8.8, 13.8, 15.6 and
23.8%, for Q1, Q2, Q3 and Q4, respectively (P=0.005). Table I displays the baseline patient
characteristics for age, pathology, SCC-Ag, Ca199, Ca125, AFP, NE,
LY, PLT, LNM, NACT, tumor size, DSI, LVSI, parametrial involvement,
surgical margin, WBC count and FIGO stage. The groups with an
higher LDH level were more likely to have LVSI, DSI, LNM and be of
an older age.
 | Table I.Comparison of clinicopathological
characteristics between patients in the different LDH level
groups. |
Table I.
Comparison of clinicopathological
characteristics between patients in the different LDH level
groups.
|
| Serum lactate
dehydrogenase level quartilesa |
|---|
|
|
|
|---|
| Clinicopathological
characteristic | Total (%),
n=586 | Q1 (%), n=147 | Q2 (%), n=145 | Q3 (%), n=147 | Q4 (%), n=147 | P-value | χ2 |
|---|
| FIGO stage |
|
|
|
|
| 0.489 | 8.454 |
|
IB1 | 317 (54.1) | 88 (59.9) | 80 (55.2) | 77 (52.4) | 72 (49.0) |
|
|
|
1B2 | 110 (18.8) | 29 (19.7) | 29 (20.0) | 28 (19.0) | 24 (16.3) |
|
|
|
IIA1 | 80 (13.7) | 15 (10.2) | 18 (12.4) | 22 (15.0) | 25 (17.0) |
|
|
|
IIA2 | 79 (13.5) | 15 (10.2) | 18 (12.4) | 20 (13.6) | 26 (17.7) |
|
|
| Median age, years
(IQR) | 47.0 | 43.0 | 47.0 | 49.0 | 48.0 | <0.001 | 24.582 |
|
| (42.0, 53.0) | (38.0, 51.0) | (42.0, 55.0) | (44.0, 54.0) | (42.0, 54.5) |
|
|
| Tumor size, cm |
|
|
|
|
| 0.791 | 3.143 |
|
<2 | 226 (38.6) | 59 (40.1) | 55 (37.9) | 59 (40.1) | 53 (36.1) |
|
|
|
≥2-<4 | 218 (37.2) | 59 (40.1) | 55 (37.9) | 50 (34.0) | 54 (36.7) |
|
|
| ≥4 | 142 (24.2) | 29 (19.7) | 35 (24.1) | 38 (25.9) | 40 (27.2) |
|
|
| Pathology |
|
|
|
|
| 0.428 | 5.960 |
|
Squamous cell carcinoma | 466 (79.5) | 117 (79.6) | 110 (75.9) | 117 (79.6) | 122 (83.0) |
|
|
|
Adenocarcinoma | 74 (12.6) | 16 (10.9) | 25 (17.2) | 20 (13.6) | 13 (8.8) |
|
|
|
Other | 46 (7.8) | 14 (9.5) | 10 (6.9) | 10 (6.8) | 12 (8.2) |
|
|
| Deep stromal
invasion |
|
|
|
|
| 0.018 | 15.324 |
|
<1/3 | 267 (45.6) | 68 (46.3) | 77 (53.1) | 66 (44.9) | 56 (38.1) |
|
|
|
≥1/3-<2/3 | 202 (34.5) | 48 (32.7) | 47 (32.4) | 42 (28.6) | 65 (44.2) |
|
|
|
≥2/3 | 117 (20.0) | 31 (21.1) | 21 (14.5) | 39 (26.5) | 26 (17.7) |
|
|
| Lymph-vascular
space invasion |
|
|
|
|
| 0.002 | 15.339 |
|
Negative | 388 (66.2) | 99 (67.3) | 110 (75.9) | 99 (67.3) | 80 (54.4) |
|
|
|
Positive | 198 (33.8) | 48 (32.7) | 35 (24.1) | 48 (32.7) | 67 (45.6) |
|
|
| Lymph node
metastasis |
|
|
|
|
| 0.005 | 13.027 |
|
Negative | 495 (84.5) | 134 (91.2) | 125 (86.2) | 124 (84.4) | 112 (76.2) |
|
|
|
Positive | 91 (15.5) | 13 (8.8) | 20 (13.8) | 23 (15.6) | 35 (23.8) |
|
|
| Parametrial
involvement |
|
|
|
|
| 0.905 | Fisher |
|
Negative | 575 (98.1) | 144 (98.0) | 143 (98.6) | 145 (98.6) | 143 (97.3) |
|
|
|
Positive | 11 (1.9) | 3 (2.0) | 2 (1.4) | 2 (1.4) | 4 (2.7) |
|
|
| Surgical
margin |
|
|
|
|
| 0.417 | 2.841 |
|
Negative | 565 (96.4) | 141 (95.9) | 143 (98.6) | 141 (95.9) | 140 (95.2) |
|
|
|
Positive | 21 (3.6) | 6 (4.1) | 2 (1.4) | 6 (4.1) | 7 (4.8) |
|
|
| Neoadjuvant
chemotherapy |
|
|
|
|
| 0.362 | 3.197 |
| No | 392 (66.9) | 97 (66.0) | 99 (68.3) | 105 (71.4) | 91 (61.9) |
|
|
|
Yes | 194 (33.1) | 50 (34.0) | 46 (31.7) | 42 (28.6) | 56 (38.1) |
|
|
| White blood cell
count, ×109/l |
|
|
|
|
| 0.847 | 2.691 |
|
<3.5 | 26 (4.4) | 7 (4.8) | 9 (6.2) | 5 (3.4) | 5 (3.4) |
|
|
|
3.5-9.5 | 529 (90.3) | 133 (90.5) | 129 (89) | 132 (89.8) | 135 (91.8) |
|
|
|
>9.5 | 31 (5.3) | 7 (4.8) | 7 (4.8) | 10 (6.8) | 7 (4.8) |
|
|
| Neutrophil count,
×109/l |
|
|
|
|
| 0.723 | 3.656 |
|
<1.8 | 22 (3.8) | 7 (4.8) | 3 (2.1) | 7 (4.8) | 5 (3.4) |
|
|
|
1.8-6.3 | 542 (92.5) | 137 (93.2) | 136 (93.8) | 134 (91.2) | 135 (91.8) |
|
|
|
>6.3 | 22 (3.8) | 3 (2.0) | 6 (4.1) | 6 (4.1) | 7 (4.8) |
|
|
| Lymphocyte count,
109/l |
|
|
|
|
| 0.971 | Fisher |
|
<1.1 | 47 (8.0) | 11 (7.5) | 12 (8.3) | 12 (8.2) | 12 (8.2) |
|
|
|
1.1-3.2 | 525 (89.6) | 132 (89.8) | 130 (89.7) | 130 (88.4) | 133 (90.5) |
|
|
|
>3.2 | 14 (2.4) | 4 (2.7) | 3 (2.1) | 5 (3.4) | 2 (1.4) |
|
|
| Platelet count,
×109/l |
|
|
|
|
| 0.659 | Fisher |
|
<125 | 8 (1.4) | 0 (0) | 3 (2.1) | 2 (1.4) | 3 (2) |
|
|
|
125-350 | 534 (91.1) | 138 (93.9) | 131 (90.3) | 134 (91.2) | 131 (89.1) |
|
|
|
>350 | 44 (7.5) | 9 (6.1) | 11 (7.6) | 11 (7.5) | 13 (8.8) |
|
|
| Ca125, ng/ml |
|
|
|
|
| 0.848 | 0.806 |
|
<35 | 540 (92.2) | 136 (92.5) | 135 (93.1) | 133 (90.5) | 136 (92.5) |
|
|
|
≥35 | 46 (7.8) | 11 (7.5) | 10 (6.9) | 14 (9.5) | 11 (7.5) |
|
|
| Ca199, ng/ml |
|
|
|
|
| 0.299 | 3.672 |
|
<37 | 563 (96.1) | 140 (95.2) | 143 (98.6) | 141 (95.9) | 139 (94.6) |
|
|
|
≥37 | 23 (3.9) | 7 (4.8) | 2 (1.4) | 6 (4.1) | 8 (5.4) |
|
|
| -fetoprotein,
ng/ml |
|
|
|
|
| 0.633 | Fisher |
|
<8.78 | 584 (99.7) | 147 (100) | 144 (99.3) | 147 (100) | 146 (99.3) |
|
|
|
≥8.78 | 2 (0.3) | 0 (0) | 1 (0.7) | 0 (0) | 1 (0.7) |
|
|
| Squamous cell
carcinoma antigen, ng/ml |
|
|
|
|
| 0.064 | 7.267 |
|
<1.5 | 290 (49.5) | 76 (51.7) | 68 (46.9) | 84 (57.1) | 62 (42.2) |
|
|
|
≥1.5 | 296 (50.5) | 71 (48.3) | 77 (53.1) | 63 (42.9) | 85 (57.8) |
|
|
Univariate and multivariate analyses
for LNM
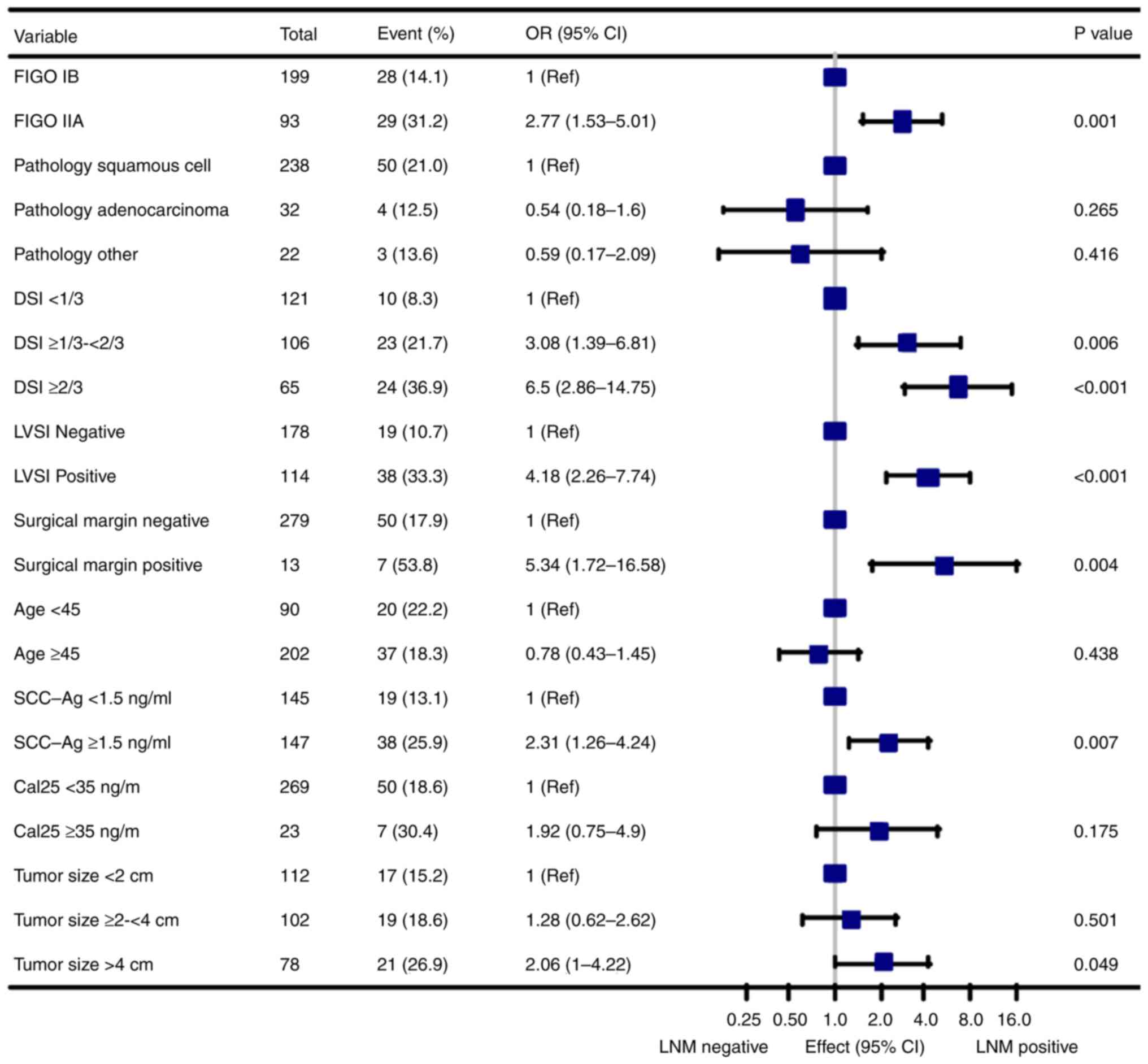
Univariate analysis indicated that LDH of groups
age, FIGO stage, NACT, tumor size, DSI, LVSI, parametrial
involvement, surgical margin, Ca125 and SCC-Ag were linked to LNM
(all P<0.05; Fig. 1; Table II). Multivariate analysis confirmed
LDH, NACT, DSI, age and LVSI as independent factors for LNM (all
P<0.05; Table II). In
multivariable logistic regression analyses, there was a 2.5-fold
increased risk of LNM in Q4 group compared to Q1 group (OR 3.50;
95% CI, 1.57-7.81; P=0.002; Table
II).
 | Table II.Univariate and multivariate logistic
regression for predicting LNM. |
Table II.
Univariate and multivariate logistic
regression for predicting LNM.
|
| Univariate
analysis | Multivariate
analysis |
|---|
| Clinicopathological
characteristic |
|
|
|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value |
|---|
| Trenda | 1.44
(1.17-1.78) | 0.001 | 1.4
(1.11-1.78) | 0.005 |
| Q2 | 1.65
(0.79-3.45) | 0.185 | 2.79
(1.19-6.55) | 0.018 |
| Q3 | 1.91
(0.93-3.94) | 0.079 | 2.52
(1.07-5.9) | 0.034 |
| Q4 | 3.22
(1.63-6.38) | 0.001 | 3.5
(1.57-7.81) | 0.002 |
| Age, years | 0.97 (0.95-1) | 0.045 | 0.95
(0.92-0.98) | 0.001 |
| FIGO stage |
|
|
|
|
|
IB2 | 1.86
(1.01-3.44) | 0.048 | 0.99
(0.47-2.11) | 0.985 |
|
IIA1 | 2.59
(1.36-4.9) | 0.004 | 1.78
(0.83-3.83) | 0.140 |
|
IIA2 | 3.44
(1.86-6.34) | <0.001 | 1.71
(0.76-3.85) | 0.195 |
| Neoadjuvant
chemotherapy | 2.15
(1.37-3.39) | 0.001 | 2.04 (1.1-3.8) | 0.024 |
| Tumor size, cm |
|
|
|
|
|
≥2-<4 | 1.49
(0.85-2.58) | 0.161 | 0.6 (0.3-1.23) | 0.164 |
|
>4 | 2.34
(1.32-4.15) | 0.004 | 0.72
(0.31-1.68) | 0.446 |
| DSI |
|
|
|
|
|
≥1/3-<2/3 | 3.99
(2.17-7.36) | <0.001 | 3.04
(1.52-6.1) | 0.002 |
|
≥2/3 | 6.43
(3.38-12.24) | <0.001 | 4.89
(2.21-10.79) | <0.001 |
| Lymph-vascular
space invasion positive | 3.76
(2.37-5.97) | <0.001 | 2.09
(1.17-3.72) | 0.012 |
| Parametrial
involvement positive | 27.05
(5.74-127.46) | <0.001 | 6.57
(0.97-44.67) | 0.054 |
| Surgical margin
positive | 5.43
(2.23-13.2) | <0.001 | 3.16
(0.92-10.88) | 0.068 |
| Pathology |
|
|
|
|
|
Adenocarcinoma | 0.6
(0.28-1.31) | 0.2 | 0.85
(0.33-2.16) | 0.726 |
|
Other | 0.61
(0.23-1.58) | 0.307 | 0.63
(0.21-1.93) | 0.419 |
| WBC count,
3.5-9.5×109/l | 0.97
(0.32-2.88) | 0.95 | 0.59
(0.15-2.3) | 0.45 |
| WBC count,
>9.5×109/l | 1.91
(0.5-7.27) | 0.341 | 0.99
(0.15-6.34) | 0.988 |
| NE count,
1.8-6.3×109/l | 1.78
(0.41-7.77) | 0.442 | 2.38
(0.32-17.5) | 0.395 |
| NE count,
>6.3×109/l | 4.67
(0.85-25.75) | 0.077 | 4.51
(0.3-67.94) | 0.277 |
| LY count,
1.1-3.2×109/l | 0.67
(0.32-1.39) | 0.279 | 1.46
(0.35-6.1) | 0.603 |
| LY count,
>3.2×109/l | 0.28
(0.03-2.44) | 0.252 | 0.5
(0.03-9.44) | 0.643 |
| Platelet count,
×109/l |
|
|
|
|
|
125-350 | 0.53
(0.1-2.67) | 0.44 | 0.51
(0.06-4.3) | 0.538 |
|
>350×109/l | 0.77
(0.13-4.48) | 0.773 | 0.45
(0.04-4.68) | 0.506 |
| Ca125, ≥35
ng/ml | 2.63
(1.34-5.16) | 0.005 | 2.3
(0.98-5.39) | 0.055 |
| Ca199, ≥37
ng/ml | 2.49 (1–6.25) | 0.051 | 1.37
(0.42-4.47) | 0.606 |
| α-fetoprotein,
≥8.78 ng/ml | 0 (0-Inf) | 0.984 | 0 (0-Inf) | 0.983 |
| Squamous cell
carcinoma antigen, ≥3.75 ng/ml | 2.12
(1.33-3.39) | 0.002 | 1.55
(0.82-2.91) | 0.175 |
Association between LDH and LNM
The following adjustments were made to assess the
robustness of the findings of the present study: i) Model 1 was not
adjusted, ii) Model 2 was adjusted for age, iii) Model 3 was
adjusted for age and FIGO stage, iv) Model 4 was adjusted for age,
FIGO stage and NACT, v) Model 5 was adjusted for age, FIGO stage,
NACT and hematological indicator variables (SCC-Ag and Ca125), vi)
Model 6 was adjusted for age, FIGO stage, NACT, hematological
indicator variables and high risk factors (surgical margin and
parametrial involvement) and vii) Model 7 was adjusted for age,
FIGO stage, NACT, hematological indicator variables, high risk
factors and intermediate risk factors (tumor size, LVSI and
DSI).
In multivariable logistic regression analysis with
LDH quartiles, Q4 group were associated with a 2.37-fold increased
risk of LNM compared to Q1 group, independent of potential
confounders (Model 7; OR 3.37; 95% CI, 1.54-7.36; P=0.055; Table III). The risk of LNM in the Q2
group is 1.65-2.79 times higher than in the Q1 group after
adjusting for confounding factors (Models 1–7; Table III). The risk of LNM in the Q3
group is 1.91-2.66 times higher than in the Q1 group after
adjusting for confounding factors (Models 1–7; Table III). LDH was entered as a
continuous variable per 5 U/l increase, and LDH and LNM remained
significantly associated (OR 1.03; 95% CI, 1.00-1.04).
 | Table III.Multivariable logistic regression to
assess the association of serum LDH with LNM. |
Table III.
Multivariable logistic regression to
assess the association of serum LDH with LNM.
|
| LDH
levelb, U/l | Q1, n=147 | Q2, n=145 | Q3, n=147 | Q4, n=147 | Trend |
|---|
|
|
|
|
|
|
|
|
|---|
| Modela | OR (95% CI) | P-value | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) |
|---|
| 1 | 1.03
(1.01-1.05) | 0.002 | 1 (Ref) | 1.65
(0.79-3.45) | 1.91
(0.93-3.94) | 3.22
(1.63-6.38) | 1.44
(1.17-1.78) |
| 2 | 1.03
(1.01-1.05) | 0.002 | 1 (Ref) | 1.88
(0.89-3.98) | 2.29
(1.09-4.8) | 3.74
(1.86-7.53) | 1.51
(1.22-1.86) |
| 3 | 1.03
(1.01-1.05) | 0.006 | 1 (Ref) | 1.91
(0.89-4.1) | 2.26
(1.06-4.79) | 3.45
(1.69-7.03) | 1.46
(1.18-1.81) |
| 4 | 1.03
(1.01-1.05) | 0.006 | 1 (Ref) | 1.98
(0.92-4.27) | 2.42
(1.13-5.18) | 3.54
(1.72-7.26) | 1.47
(1.18-1.82) |
| 5 | 1.03
(1.01-1.05) | 0.007 | 1 (Ref) | 1.94
(0.9-4.21) | 2.36
(1.09-5.08) | 3.38
(1.64-6.97) | 1.45
(1.16-1.8) |
| 6 | 1.02 (1–1.04) | 0.054 | 1 (Ref) | 2.21
(0.99-4.96) | 2.66
(1.19-5.95) | 3.71
(1.74-7.94) | 1.47
(1.18-1.84) |
| 7 | 1.02 (1–1.04) | 0.055 | 1 (Ref) | 2.79
(1.21-6.4) | 2.55
(1.11-5.86) | 3.37
(1.54-7.36) | 1.39
(1.1-1.75) |
Subgroup analyses
Subgroup analysis was used to address the
association between LDH and LNM. Additional subgroup and
sensitivity analyses concerning the role of confounding factors are
presented in Table III, Fig. 2 and the supplementary material
(Table SI, Table SII, Table SIII, Table SIV, Table SV, Table SVI, Table SVII, Table SVIII and Fig. S1). When LDH was >167.9 ng/ml,
factors that were related to LNM include FIGO stage, DSI, LVSI,
surgical margin, SCC-Ag, tumor size (all P<0.05). Similar
associations were discovered between LDH and LNM in some subgroup
analyses. Special attention should be paid among patients with FIGO
IIA stage (OR 1.83; 95% CI, 1.18-2.84; P=0.007; Table SI), age ≥45 (OR 1.94; 95% CI,
1.35-2.78; P<0.001; Table SII),
SCC-Ag ≥1.5 ng/ml (OR 1.41; 95% CI, 1.06-1.88; P=0.019; Table SV), Ca125 <35 ng/ml (OR 1.48;
95% CI, 1.16-1.88; P<0.001; Table
SIII), LVSI positive (OR 1.39; 95% CI, 1.00-1.93; P=0.047;
Table SVI), DSI ≥2/3 (OR 1.58; 95%
CI, 0.95-2.64; P=0.077; Table
SVII), tumor size <2 cm (OR 1.64; 95% CI, 1.05-2.56;
P=0.029; Table SVIII) and squamous
cell carcinoma (OR 1.40; 95% CI, 1.09-1.79; P=0.009; Table SIV).
Value-dependent effects of LDH on
LNM
Fig. 3 depicts a
multivariable-adjusted restricted cubic spline for the association
between LDH and LNM to quantify the effect of LDH on LNM. The
analysis indicated that the risk of LNM increased sharply when LDH
<167.9 ng/ml. The risk of LNM began to decline in the Q3 range.
The risk of LNM entered a plateau in the Q4 range (Fig. 3A). After adjustment for potential
confounders (Model 7; Fig. 3B),
this trend became more notable regarding the association between
LDH and LNM.
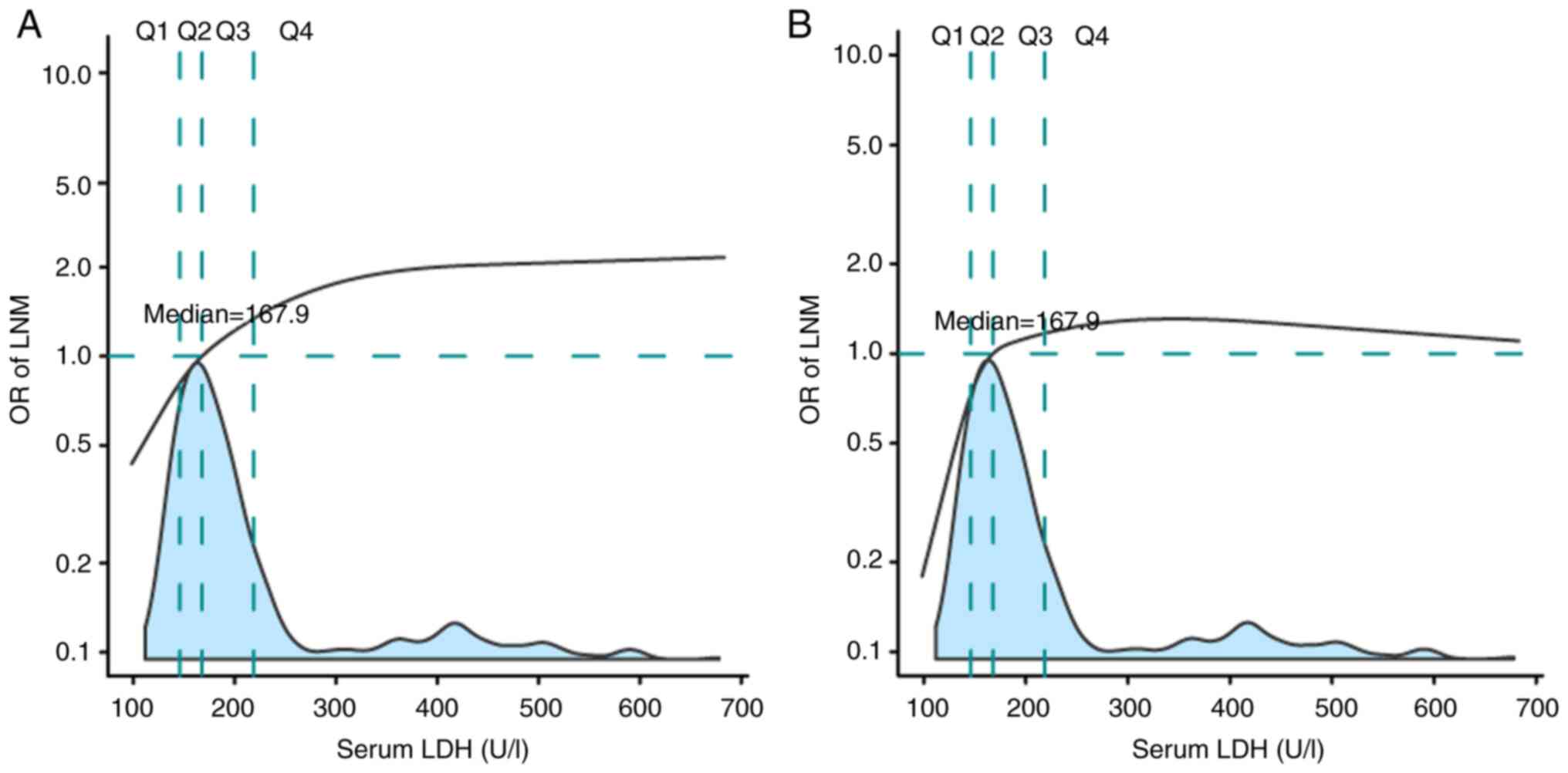 | Figure 3.Effects of LDH level on LNM are
modeled with a P-spline expansion. (A) no adjustment; (B) adjusted
for age, International Federation of Gynecology and Obstetrics
stage, neoadjuvant chemotherapy, squamous cell carcinoma antigen,
cancer antigen 125, surgical margin and parametrial involvement,
tumor size, lymph-vascular space invasion and deep stromal
invasion. The first, second and third quartiles of LDH level
(98.4-683.0 U/l) were 143.6, 167.9 and 208.1 U/l, divided into Q1,
Q2, Q3 and Q4 group. LDH, serum lactate dehydrogenase; LNM, lymph
node metastasis; OR, odds ratio. |
Discussion
The present study aimed to evaluate the association
between serum LDH level and the risk of LNM in patients with CC.
LDH level may serve as a potential tumor maker and
treatment-related indicator. The present study has demonstrated
that patients with elevated LDH levels were more likely to have
LVSI, DSI, LNM and be of an older age. After adjusting for other
factors, patients in the highest LDH quartile had an increased risk
of LNM compared with those in the lowest LDH quartile. A
multivariable-adjusted restricted cubic spline also confirmed the
association between LDH and LNM. If LDH levels are elevated,
further MRI or lymph node biopsy is needed to clarify the lymph
node status to determine whether to perform radical surgery or
concurrent radiotherapy treatment (2).
Unlike previous reports (13,14),
the present study was a comprehensive association analysis that
focused on the detailed relationship between serum LDH and LNM.
Particularly noteworthy was that even after adjusting for other
important prognostic factors, LDH still demonstrated its value. The
present study comprehensively analyzed the association between LDH
and LNM in CC. A previous study reported that LDH was related to a
poor prognosis in CC; however, the small number of cases was
insufficient to analyze relevant influencing factors (13). Unlike previous reports on
non-surgical patients, to the best of our knowledge, this is the
first study on patients undergoing radical surgery in the early
stage and is the largest population-based analysis of LDH in
CC.
Serum LDH is associated with the prognosis of
numerous cancer types, including non-Hodgkin lymphoma, colon, lung,
breast cancer and melanoma (8,10,11,20).
Ovarian and uterine cancer have been associated with elevated LDH
expression and aggressive phenotypes among gynecologic malignancies
(13,20). This accords with a previous study,
which discovered that high LDH levels were more likely to have
LVSI, DSI and LNM in CC (13).
Contrary to earlier findings, patients with elevated LDH levels
were linked to older age and were not likely to have a high level
of SCC-Ag (12). Therefore, the
present study included these factors in subsequent model
adjustments and subgroup analyses.
Univariate and multivariate analyses confirmed that
LDH was an independent factor for LNM. Other factors related to LNM
include age, NACT, SCC-Ag, Ca125, FIGO stage, tumor size, DSI,
LVSI, parametrial involvement and surgical margin. According to the
Sedlis criteria, LNM, surgical margin and parametrial involvement
are high risk factors, whereas stromal invasion, lymphatic space
involvement and primary tumor size are intermediate risk factors,
and a previous study has reported on other factors related to LNM,
such as age, NACT, SCC-Ag, Ca125 (3). Therefore, these confounders were
adjusted in the present study.
In the present study, a positive association between
LDH and LNM was consistently observed, independent of important
covariates and confounders. One possible explanation could be
because LDH is a ubiquitous cellular enzyme and comprises the
rate-limiting step in converting pyruvate to lactic acid under
anaerobic conditions (21). Hypoxia
is a characteristic property of solid tumors owing to rapid cancer
cell proliferation, high metabolic demands and functional
angiogenesis (22). Therefore,
elevated LDH levels indicate an aggressive phenotype, which is more
prone to LNM. Secondly, higher LDH levels cause lactic acid
accumulation due to anaerobic glycolysis, resulting in an acidic
tumor microenvironment and promoting invasion and metastasis
(23). Thirdly, vascular density is
significantly higher in patients with elevated LDH levels,
suggesting aggressive angiogenesis (24). Patients with increased LDH levels
are more likely to have LNM, DSI and LVSI, as angiogenesis is
essential for tumor proliferation and metastasis (12,13).
Additionally, vascular density is significantly associated with
tumor VEGFA and VEGFR expression, and treatment with bevacizumab,
an angiogenesis inhibitor, can significantly improve the prognosis,
particularly in metastatic colorectal cancer with high LDH levels
(25). Vascular density is
significantly higher in patients with elevated LDH levels,
suggesting aggressive angiogenesis (24). In cervical cancer with elevated LDH
levels (25), it is worth noting
that bevacizumab is recommended as a first-line treatment in the
treatment guidelines for advanced cases (26). Hence, future attention can be
directed towards assessing whether there could be more advantages
in patients with high LDH levels.
A subgroup analysis was also conducted in the
present study to investigate the association between LDH and LNM,
which revealed a significant relationship between LDH and LNM in
the FIGO IIA stage, age ≥45 years, SCC-Ag <1.5 ng/ml, Ca125
<35 ng/ml, LVSI positive, DSI ≥2/3, tumor size <2 cm, and
squamous cell carcinoma. The possible explanation could firstly be
due to the more advanced tumor stage, larger tumor size, more
vascular invasion, high tumor burden, increased vascular density
and tumor cell invasion into endothelial lymphatic vessels and/or
blood vessels to form emboli that release tumor cells through
lymphatic system and blood vessels (27). In high tumor burdens, elevated LDH
levels indicate increased tumor glycolysis and hypoxia-induced
tumor necrosis (28). Secondly,
advanced age is a risk factor for different degrees of
angiosclerosis and cardiovascular disease associated with hypoxia
(29). Therefore, this might
strengthen the link between LNM and LDH elevation.
A significant positive association was found between
the two factors when a multivariable-adjusted restricted cubic
spline was used to determine the association between LDH and the
risk of LNM. The present study discovered a rapid rise in the risk
of LNM with low LDH levels (LDH <167.9 U/l). Thereafter, the
risk of LNM growth slowed and plateaued. If the cut-off point was
set to 167.9 U/l, this was lower than previous studies (12,13),
because one patient was operable earlier in the present study.
Previous studies have reported heterogeneous cut-offs for LDH
(12,13,30). A
meta-analysis incorporating data from 68 studies included 31,857
patients with CC reported that high levels of LDH were associated
with a poor prognosis in solid tumors, whereas variations in LDH
cut-off do not affect its prognosis (30).
The present study had several limitations. First, it
was a retrospective study, which might have led to selection bias.
Second, five-year overall survival rate was high due to the short
follow-up and early-stage tumor patients. Therefore, the link
between LDH and LNM did not reflect the benefit of survival
analysis. Finally, data regarding serial dynamic serum LDH levels
are lacking.
In conclusion, results from the present study
suggested that higher LDH levels were independently associated with
CC and LNM. LDH values may serve as a potential tumor marker, and
these convenient clinical indicators may be combined to guide the
future personalized treatment of patients with CC.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by Education and Teaching Reform
Research Project of Fujian Medical University (grant no. J21055)
and Fujian Provincial Health Technology Project (grant no.
2022CX0101).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XZ and SL conceptualized and designed the work. QH,
SL and XC participated in the study design and wrote the
manuscript. CH, YC, YH and YL performed data analyses. QH prepared
the manuscript. YW contributed to the analysis of the data and
critically revised the manuscript for important intellectual
content. XZ and YW confirm the authenticity of all the raw data.
All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The study was conducted in accordance with The
Declaration of Helsinki and was approved by The Ethics Committee of
the Fujian Maternity and Child Health Hospital, an Affiliated
Hospital of Fujian Medical University (Fuzhou, China; approval no.
SFY2022KYLLR1206). Informed consent was waived, considering the
retrospective nature of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Koh W, Abu-Rustum N, Bean S, Bradley K,
Campos SM, Cho KR, Chon HS, Chu C, Clark R, Cohn D, et al: Cervical
cancer, version 3.2019, NCCN clinical practice guidelines in
oncology. J Natl Compr Canc Netw. 17:64–84. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sedlis A, Bundy BN, Rotman MZ, Lentz SS,
Muderspach LI and Zaino RJ: A randomized trial of pelvic radiation
therapy versus no further therapy in selected patients with stage
IB carcinoma of the cervix after radical hysterectomy and pelvic
lymphadenectomy: A gynecologic oncology group study. Gynecol Oncol.
73:177–183. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chao B, Ju X, Zhang L, Xu X and Zhao Y: A
novel prognostic marker systemic inflammation response index (SIRI)
for operable cervical cancer patients. Front Oncol. 10:7662020.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Deng Q, Long Q, Liu Y, Yang Z, Du Y and
Chen X: Prognostic value of preoperative peripheral blood mean
platelet volume/platelet count ratio (MPV/PC) in patients with
resectable cervical cancer. BMC Cancer. 21:12822021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang P, Kong W, Gong C, Chen Y, Li F, Xu
L, Yang Y, Gou S and Hu Z: Predicting the recurrence of operable
cervical cancer patients based on hemoglobin, albumin, lymphocyte,
and platelet (HALP) score and classical clinicopathological
parameters. J Inflamm Res. 15:5265–5281. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Markert C: Lactate dehydrogenase isozymes:
Dissociation and recombination of subunits. Science. 140:1329–1330.
1963. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Passardi A, Scarpi E, Tamberi S, Cavanna
L, Tassinari D, Fontana A, Pini S, Bernardini I, Accettura C, Ulivi
P, et al: Impact of pre-treatment lactate dehydrogenase levels on
prognosis and bevacizumab efficacy in patients with metastatic
colorectal cancer. PLoS One. 10:e01347322015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Valvona CJ, Fillmore HL, Nunn PB and
Pilkington GJ: The regulation and function of lactate dehydrogenase
A: Therapeutic potential in brain tumor. Brain Pathol. 26:3–17.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Koch A, Fohlin H and Sörenson S:
Prognostic significance of C-reactive protein and smoking in
patients with advanced non-small cell lung cancer treated with
first-line palliative chemotherapy. J Thorac Oncol. 4:326–332.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim CH, Oh HG, Lee SY, Lim JK, Lee YH, Seo
H, Yoo SS, Lee SY, Cha SI, Park JY and Lee J: Differential
diagnosis between lymphoma-associated malignant pleural effusion
and tuberculous pleural effusion. Ann Transl Med. 7:3732019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li J, Wu MF, Lu HW, Chen Q, Lin ZQ and
Wang LJ: Pretreatment serum lactate dehydrogenase is an independent
prognostic factor for patients receiving neoadjuvant chemotherapy
for locally advanced cervical cancer. Cancer Med. 5:1863–1872.
2016. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang H, Wang MS, Zhou YH, Shi JP and Wang
WJ: Prognostic values of LDH and CRP in cervical cancer. Onco
Targets Ther. 13:1255–1263. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ye Y, Chen M, Chen X, Xiao J, Liao L and
Lin F: Clinical significance and prognostic value of lactate
dehydrogenase expression in cervical cancer. Genet Test Mol
Biomarkers. 26:107–117. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tholen DW, Musiolkroll EM, Kroll M, Astles
JR, Caffo AL, Happe TM and Lasky F: Evaluation of the linearity of
quantitative measurement procedures: A statistical approach;
approved guideline. Clinical and Laboratory Standards Institute;
2003
|
|
16
|
Tholen DW, Linnet K, Kondratovich M,
Armbruster DA, Garrett PE, Jones RL, Kroll MH, Lequin RM, Pankratz
TJ, Scassellati GA, et al: Protocols for determination of limits of
detection and limits of quantitation; approved guidelines. Clinical
and Laboratory Standards Institute; 2004
|
|
17
|
White IR, Royston P and Wood AM: Multiple
imputation using chained equations: Issues and guidance for
practice. Stat Med. 30:377–399. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Meira-Machado L, Cadarso-Suárez C, Gude F
and Araújo A: smoothHR: An R package for pointwise nonparametric
estimation of hazard ratio curves of continuous predictors. Comput
Math Methods Med. 2013:7457422013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang Y, Cao JZ, Lan SM, Wu JX, Wu T, Zhu
SY, Qian LT, Hou XR, Zhang FQ, Zhang YJ, et al: Association of
improved locoregional control with prolonged survival in
early-stage extranodal nasal-type natural killer/T-cell lymphoma.
JAMA Oncol. 3:83–91. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Koukourakis M, Kontomanolis E,
Giatromanolaki A, Sivridis E and Liberis V: Serum and tissue LDH
levels in patients with breast/gynaecological cancer and benign
diseases. Gynecol Obstet Invest. 67:162–168. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Burgner JW II and Ray WJ Jr: On the origin
of the lactate dehydrogenase induced rate effect. Biochemistry.
23:3636–3648. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Harris A: Hypoxia-a key regulator factor
in tumor growth. Nat Rev Cancer. 2:38–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu S, Zhou HY, Deng SC, Deng SJ, He C, Li
X, Chen JY, Jin Y, Hu ZL, Wang F, et al: ASIC1 and ASIC3 contribute
to acidity-induced EMT of pancreatic cancer through activating
Ca2+/RhoA pathway. Cell Death Dis. 8:e28062017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Giatromanolaki A, Sivridis E, Gatter K,
Turley H, Harris A, Koukourakis M and Tumour; Angiogenesis Research
Group, : Lactate dehydrogenase 5 (LDH-5) expression in endometrial
cancer relates to the activated VEGF/VEGFR2(KDR) pathway and
prognosis. Gynecol Oncol. 103:912–918. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Scartozzi M, Giampieri R, Maccaroni E, Del
Prete M, Faloppi L, Bianconi M, Galizia E, Loretelli C, Belvederesi
L, Bittoni A and Cascinu S: Pre-treatment lactate dehydrogenase
levels as predictor of efficacy of first-line bevacizumab-based
therapy in metastatic colorectal cancer patients. Br J Cancer.
106:799–804. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tewari KS, Sill MW, Long HJ III, Penson
RT, Huang H, Ramondetta LM, Landrum LM, Oaknin A, Reid TJ, Leitao
MM, et al: Improved survival with bevacizumab in advanced cervical
cancer. N Engl J Med. 370:734–743. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li Y, Liao X and Ma L: ERCC1 is a
potential biomarker for predicting prognosis, immunotherapy,
chemotherapy efficacy, and expression validation in HER2
over-expressing breast cancer. Front Oncol. 12:9557192022.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Doherty JR and Cleveland JL: Targeting
lactate metabolism for cancer therapeutics. J Clin Invest.
123:3685–3692. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hummitzsch L, Zitta K, Rusch R, Cremer J,
Steinfath M, Gross J, Fandrich F, Berndt R and Albrecht M:
Characterization of the angiogenic potential of human regulatory
macrophages (Mreg) after ischemia/reperfusion injury in vitro. Stem
Cells Int. 2019:37258632019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang J, Yao YH, Li BG, Yang Q, Zhang PY
and Wang HT: Prognostic value of pretreatment serum lactate
dehydrogenase level in patients with solid tumors: A systematic
review and meta-analysis. Sci Rep. 5:98002015. View Article : Google Scholar : PubMed/NCBI
|