Introduction
Thyroid cancer (TC), although accounting for only 2%
of all malignancies, is the most common type of endocrine cancer
(1). The occurrence of TC is
rising, mainly because of improved diagnosis due to widespread
ultrasound examination (2). It was
estimated that TC would lead to >2,200 deaths in the US in 2022
(3). TC affects mostly women, while
in men, although less prevalent, it leads to a more aggressive
disease and poor prognosis (1).
According to the fifth World Health Organization classification
system, histological types of TC include papillary TC (PTC),
follicular TC (FTC), oncocytic carcinoma, poorly differentiated TC,
anaplastic follicular cell-derived TC (ATC) and medullary TC, as
well as several other, less prominent subtypes including
mucoepidermoid carcinoma, ectopic thymoma or spindle epithelial
tumor with thymus-like differentiation (4,5). PTC
is the most common TC subtype, contributing to 80–90% of all
diagnosed thyroid malignancies (1,6). The
incidence of PTC has increased rapidly, making it the
fastest-growing cancer in East Asia (prevalence of 76%) (1,7). A
total of 40–80% of PTC cases bear the BRAF V600E mutation that
contributes to increased cell proliferation (1,7,8). The
prognosis for patients with TC depends on the subtype of cancer
(9). PTC and FTC are usually
associated with good prognosis (91.1 and 79.9% 5-year survival,
respectively), while ATC has an extremely poor outcome (7–12.2%
5-year survival) (10,11). PTC spreads mostly (99%) regionally
to the neck, while distant metastases (lung and bones) affect only
1–7% of patients (12–15). FTC is the second most frequently
diagnosed type of TC, accounting for 10–30% of cases (16). Compared with PTC, FTC is
characterized by worse prognosis and more frequent metastasis to
distant organs, affecting 6–23% of patients (16–18).
ATC is an undifferentiated tumor, with disease-specific mortality
approaching 100%. The metastatic disease most commonly (≤90%)
affects lungs and pleura, with less frequent involvement of bone
(5–15%) and brain (5% of patients with distant metastasis)
(3). ATC responds poorly to
conventional therapy including surgery, radiation therapy,
radioactive iodine therapy, chemotherapy and hormone therapy
(3). Palliative and supportive care
should be introduced early in the disease (3).
Agrin (AGRN) is a matricellular glycoprotein
involved in multiple physiological and pathological processes,
including neuromuscular signaling (19–21),
cardiac regeneration (22) and
autoimmunity (23,24). Several studies reported enhanced
AGRN expression in cancers including glioblastoma (25), non-small cell lung cancer (NSCLC)
(26), oral squamous cell carcinoma
(27) as well as its involvement in
tumor-promoting signaling pathways (25–28).
AGRN stimulates tumorigenesis and metastasis via focal adhesion and
mitogen-activated protein kinase activation (26); to the best of our knowledge,
however, the role of AGRN in the pathobiology of TC has not been
analyzed and the present study aimed to explore the potential roles
of AGRN in the functioning of TC cells.
Materials and methods
Cell lines
Thyroid follicular epithelial cells (Nthy-ori 3-1;
NTHY) and FTC cells (FTC-133) were obtained from the European
Collection of Authenticated Cell Cultures. Thyroid gland squamous
cell carcinoma (CGTH-W-1), a cell line derived from SW-579 (CGTH;
cat. no. ACC 360) and TC cells (BcPAP) were obtained from the
Leibniz Institute (DSMZ-German Collection of Microorganisms and
Cell Cultures GmbH). A cell line derived from anaplastic carcinoma
(8505C) was generously provided by Dr Cuong Hoang-Vu (Martin Luther
University; Halle, Germany). All cell lines were authenticated by
immunofluorescence with tetraspanins including CD9, CD63, CD81,
CD82 and CD151 (29). Analyzed cell
lines were cultured according to the supplier's protocols as
follows: i) NTHY and CGTH cell lines cultured in complete RPMI-1640
medium; ii) FTC-133 and 8505C cell lines in DMEM/F12 and iii) BcPAP
cell line in complete DMEM/GlutaMAX™ (all Thermo Fisher Scientific,
Inc.). All analyzed cell lines were cultured in medium supplemented
with 10% heat-inactivated fetal bovine serum (FBS; Thermo Fisher
Scientific, Inc.), at 37°C in a humidified 5% CO2
atmosphere. The number of cells was estimated using Trypan Blue
exclusion test with automatic counting on an EVA Automatic Cell
counter (NanoEnTek, Inc.). All cell lines were tested for presence
of mycoplasma.
Small interfering (si)RNA
transfection
AGRN silencing was performed using the following
siRNA to target AGRN (siAGRN): Sense, 5′-CGUAUGACAGUGAUUGCUGTT-3′;
antisense, 3′-CAGCAAUCACUGUCAUACGTG-5′ (Thermo Fisher Scientific,
Inc.). Briefly, following trypsinization, cells were diluted in
RPMI-1640 or DMEM/GlutaMAX™ (both Thermo Fisher Scientific, Inc.).
The transfection mixture comprised 30 nM siRNA in OptiMem and
Lipofectamine™ 2000 (both Thermo Fisher Scientific, Inc.). The
mixture was added to the cells for 10 min incubation at room
temperature. The cells were seeded on a 6
(1.25×105/well)-, 12 (5×104/well)- or 96
(1,200 cells/well)-well plate and incubated at 37°C for 48 h prior
to RNA isolation and 72 h for protein isolation. As a control,
MISSION siRNA Universal Negative Control (siNEG; cat. no. SIC001;
Sigma-Aldrich; Merck KGaA) was used for transfection. The
experiments were performed in triplicate.
Reverse transcritpion-quantitative
(RT-q)PCR
Total RNA from TC cell lines was isolated using a
Universal RNA Purification kit (EURx, Ltd.), according to the
manufacturer's protocol. Both concentration and purity were
evaluated by measuring absorbance at 260 and 280 nm with a Synergy
2 Multi-Mode Reader (BioTek Instruments, Inc.). PrimeScript™ RT
reagent kit (Takara Bio, Inc.) with oligo dT primers and random
hexamers were used to transcribe 200 ng RNA into cDNA. RT was
performed on a T100™ Thermal Cycler (Bio-Rad Laboratories, Inc.),
according to the manufacturer's protocol. Subsequently, gene
expression was analyzed by RT-qPCR using Maxima SYBR
Green/Fluorescein qPCR Master Mix (Thermo Fisher Scientific, Inc.),
5 nM specific oligonucleotide primers (Genomed, Ltd.) and 5-fold
diluted cDNA samples. Amplification and data analysis were
performed using CFX96 Detection System (Bio-Rad Laboratories, Inc.)
under the following thermocycling conditions: Initial denaturation
at 95°C for 30 sec; 95°C for 5 sec (40 cycles); 58°C for 15 sec and
72°C for 10 sec. The expression of AGRN was normalized to that of
β-actin and 18S and quantified using the 2−ΔΔCq method
(30). Primer sequences were as
follows: 18S forward, 5′ CCAGTAAGTGCGGGTCATAAG3′ and reverse, 5′
CCATCCAATCGGTAGTAGCG3′; β-actin forward, 5′ GCCGAGGACTTTGATTGC3′
and reverse, 5′ CTGTGTGGACTTGGGAGAG3′ and AGRN forward,
5′ACACCGTCCTCAACCTGAAG3′ and reverse, 5′ CCAGGTTGTAGCTCAGTTGC-3′
(31).
Western blotting
Total protein was extracted using RIPA cell lysis
buffer (Pierce RIPA Buffer; Thermo Fisher Scientific, Inc.) that
contained phosphatase and protease inhibitors. Following 30 min
incubation on ice and centrifugation at 12,092 × g at 4°C for 10
min, supernatant was boiled in 5X SDS loading buffer for 5 min.
Pierce BCA Protein Assay kit (Thermo Fisher Scientific, Inc.) was
used to quantify the protein concentration. Subsequently, 30 µg
protein/lane was separated by SDS-PAGE on a 10 or 8% gel and
transferred onto a PVDF membrane. The membrane was blocked with 5%
non-fat milk in TBST (TBS with 0.1% Tween) overnight at 4°C and
incubated with monoclonal mouse anti-human AGRN antibody (1:100;
cat. no. sc-374117; Santa Cruz Biotechnology, Inc.) at room
temperature for 1 h. Rabbit polyclonal vimentin (1:500; cat. no.
sc-7558, Santa Cruz Biotechnology, Inc.) and N-cadherin (1:3,000;
cat. no. GTX127345; GeneTex, Inc.) and mouse monoclonal E-cadherin
(1:3,000; cat. no. GTX629691, GeneTex, Inc.) antibodies were
incubated overnight at 4°C after blocking membranes in 5% non-fat
milk in TBST for 1 h at room temperature. After washing in TBST,
the membranes were incubated with HRP-conjugated mouse (1:10,000;
cat. no. 115-035-146; Jackson ImmunoResearch Laboratoies, Inc.) or
rabbit antibody (1:10,000; cat. no. P0448; Dako; Agilent
Technologies, Inc.) at room temperature for 1 h. The expression of
proteins was normalized to β-actin (1:10,000; cat. no. ab6276;
Abcam) and GAPDH (1:5,000; cat. no. MAB374; Merck KGaA.).
Immunoreactive bands were detected using the Super-Signal™ West
Dura Extended Duration Substrate kit (Thermo Fisher Scientific,
Inc.) on Carestream membranes. Quantification of scanned images was
performed with ImageJ version 1.53k software (National Institute of
Health).
Immunofluorescence
The cells were fixed on 24×24 mm glass slides with
methanol for 10 min at −20°C. Following washing and blocking with
2% BSA (cat. no. A9418; Sigma-Aldrich; Merk KGaA), in TBS + 0.1%
Tween-20 at room temperature for 1 h, the cells were incubated with
monoclonal mouse anti-human AGRN antibody (1:150; cat. no.
sc-374117; Santa Cruz Biotechnology, Inc.) at 4°C overnight. Nuclei
were stained with DAPI (1:50,000) and F-actin was stained with
phalloidin-FITC (1:500; Sigma-Aldrich; Merk KGaA) for 30 min at
room temperature. The images were obtained using a scanning
confocal microscope LSM 800 AxioObserver Z.1 using ZEN 2.6 software
(both Zeiss AG).
Viability assay
Cell viability was analyzed using CellTiter 96
Aqueous One Solution Cell Proliferation Assay (Promega
Corporation). MTS reagent was added to cells seeded in a 96-well
plate at ~1,200 cells/well and incubated at 37°C for 1 h. The
quantity of formazan was assessed by measuring absorbance at 490 nm
on an L Synergy 2 Multi-Mode Reader (BioTek Instruments, Inc.).
Proliferation assay
Cell proliferation was analyzed using a
bromodeoxyuridine (BrdU; colorimetric) assay kit (cat. no.
11647229001, Roche Diagnostics GmbH), according to the
manufacturer's instructions. Briefly, cells (1,200 cells/well) were
labeled at 37°C with BrdU reagent for 2 h, then dried at 65°C for 1
h and stored for 2 days in the fridge. Subsequently, cells were
incubated for 30 min with fixing solution (70% ethanol) at room
temperature followed by a mouse anti-BrdU antibody (1:100; cat. no.
11647229001; Roche Diagnostics GmbH) incubation for 1.5 h at room
temperature. Following brief washing, cells were stained at room
temperature with peroxidase substrate (100 µl/well) for 30 min. The
absorbance was measured according to the manufacturer's
instructions at 450 and 550 nm on the Synergy 2 Multi-Mode Reader
(BioTek Instruments, Inc.).
Modified Boyden chamber assay
NTHY or BcPAP cells (2×104 cells/well)
were suspended in RPMI-1640 medium or DMEM/GlutaMAX (both from
Thermo Fisher Scientific, Inc.), respectively, without 5% FBS and
plated into the upper chambers of a Transwell with a membrane with
8-µm pores (cat. no. 353097; Falcon; Corning Life Sciences) for
migration or Transwell with a membrane with 8-µm pores coated with
Matrigel (cat. no. 354480; Corning Life Sciences) for invasion
assay. RPMI-1640 or DMEM/GlutaMAX with 5% FBS was added to the
lower chamber. Following incubation for 24 h at 37°C, cells in the
upper surface of the chamber were wiped using a cotton swab. The
membranes were stained at room temperature with Diff-Quick reagent
(Medion Diagnostics, Ltd.) for 2 min for invasion assay and 30 sec
for the migration assay. Cells were counted manually and
photographed at ×200 magnification using a light microscope Olympus
BX41 (Olympus Corporation).
Bioinformatics analysis
AGRN expression was evaluated using The Cancer
Genome Atlas (TCGA) transcriptomic data of TC cohort (THCA; n=505)
and normal tissue controls (n=59) provided by The University of
Alabama at Birmingham Cancer Data Analysis Portal (UALCAN;
ualcan.path.uab.edu/analysis.html) (32–34).
The association between AGRN expression and the survival of
patients with TC was analyzed using the THCA cohort (n=412)
provided by UALCAN. Immune infiltration was evaluated using TIMER
2.0 (timer.cistrome.org/) and TCGA data of the THCA cohort
(35–37).
Statistical analysis
Statistical analysis was performed using unpaired t
test or one-way ANOVA followed by Dunnett's post hoc test (GraphPad
Prism 6.00 for Windows; GraphPad Software, Inc.; Dotmatics). Data
are presented as the mean ± SD from at least three independent
experiments. P<0.05 was considered to indicate a statistically
significant difference.
Results
AGRN is overexpressed in TC
Analysis of TCGA data revealed AGRN upregulation in
TC samples (n=505) compared with that in the adjacent normal
thyroid tissues (n=59; Fig. 1A;
P<0.0001). AGRN upregulation was observed in classical (n=358)
and tall PTC (n=36). In FTC (n=102), AGRN expression was lower than
in PTC (Fig. 1B; P<0.0001 vs.
Classical and Tall PTC and FTC). Patients of African-American
descent with higher AGRN expression had lower long-term survival
rates (Fig. 1C; P=0.051).
Consistent with clinical samples, RT-qPCR (Fig. 1D; P<0.0001), immunoblots
(Fig. 1E) and immunofluorescence
(Fig. 1F) confirmed high AGRN
levels in the BcPAP TC cell line. At the protein level, AGRN was
increased in the cells of FTC (FTC-133). In the CGTH cell line,
AGRN expression was lower compared with that in the NTHY cell line
(Fig. 1E and F). The nuclear
localization of AGRN was confirmed by immunofluorescence assay
(Fig. 1F).
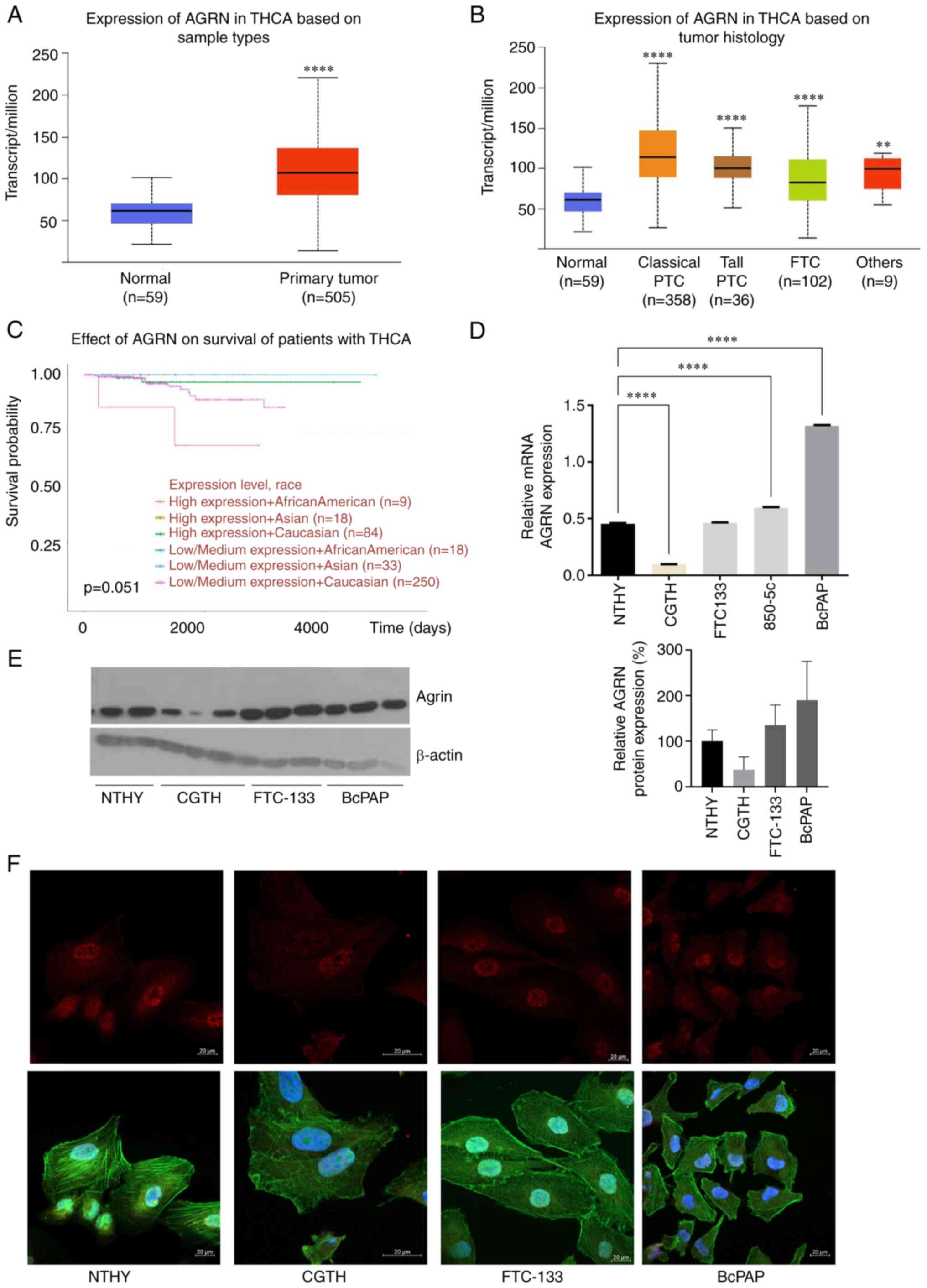 | Figure 1.AGRN is overexpressed in TC. (A) AGRN
expression in TC (n=505) vs. normal thyroid tissue (n=59). (B) AGRN
expression in THCA subtypes. The plot shows analysis of the TCGA
THCA cohort data performed using UALCAN. **P<0.005,
****P<0.0001 vs. normal. (C) Expression of AGRN is associated
with survival of patients with TC. The analysis was performed using
UALCAN. (D) Reverse transcription-quantitative PCR analysis of AGRN
mRNA expression in cell lines derived from NTHY and TC (CGTH,
FTC-133 and BcPAP). Expression was analyzed in RNA isolated from
one cell culture plate/cell line and three technical replicates
****P<0.0001. (E) Western blotting of AGRN protein expression in
cell line derived from NTHY and TC (CGTH, FTC-133, BcPAP). Western
blotting was performed on three biological replicates using
monoclonal mouse anti-human AGRN antibody. The plot shows changes
in AGRN protein expression normalized to β-actin. (F)
Representative images of AGRN immunostaining in cell lines derived
from TC (AGRN, red; phalloidin-FITC, green and DAPI, blue). Scale
bar, 20 µm. TC, thyroid cancer; TCGA, The Cancer Genome Atlas;
AGRN, agrin; THCA, thyroid carcinoma; UALCAN, University of Alabama
at Birmingham Cancer Data Analysis Portal; PTC, papillary thyroid
carcinoma; FTC, follicular thyroid carcinoma. |
AGRN contributes to tumorigenesis of
TC
To analyze the impact of AGRN on TC cells, AGRN
expression was silenced in normal thyroid cells and BcPAP cell line
in which AGRN was most highly expressed. Knockdown of AGRN
expression was confirmed by RT-qPCR (Fig. 2A; P<0.0016 and P<0.0006) and
immunoblotting (Fig. 2B).
Suppression of AGRN expression attenuated migration, invasion,
viability and proliferation of BcPAP cells (Fig. 2C, D, F and G). By contrast, in NTHY
cells only migration and proliferation were significantly decreased
following AGRN silencing (Fig. 2C and
G). To investigate the involvement of AGRN in cell migration
and invasion, expression of epithelial-mesenchymal transition
markers, including vimentin, N-cadherin and E-cadherin, were
analyzed. Increased protein levels of vimentin (mesenchymal cell
marker) and decreased levels of E-cadherin (epithelial cell marker)
in siAGRN-transfected BcPAP cells were observed (Fig. 2; P<0.005 and P<0.05,
respectively). N-cadherin levels (mesenchymal cell marker)
decreased in siAGRN-transfected NTHY cell line (Fig. 2E; P<0.025). These results
suggested that AGRN served an important role in TC tumorigenesis by
stimulating key cellular processes that contribute to cancer
progression.
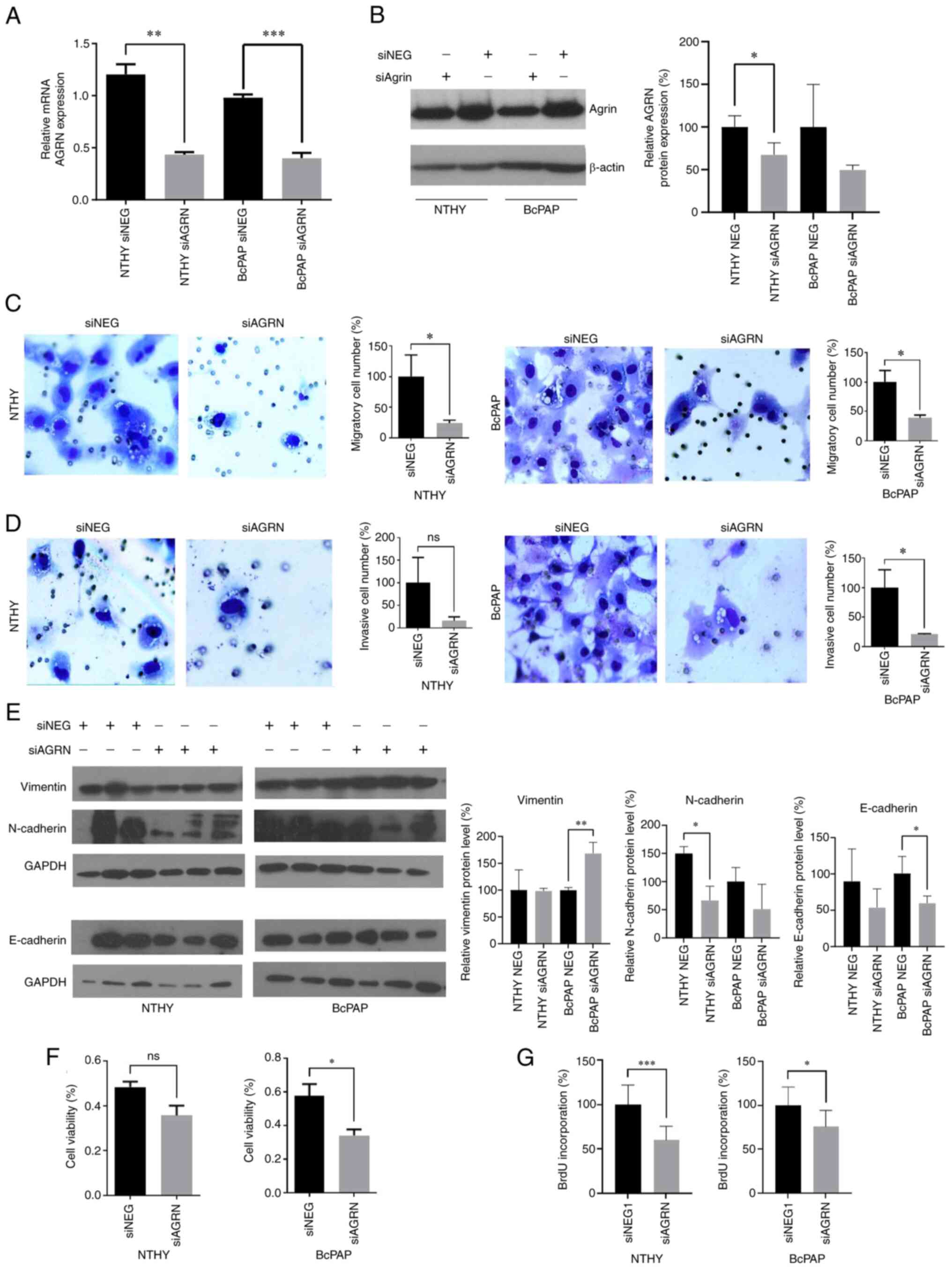 | Figure 2.AGRN silencing suppresses migration,
invasion, viability and proliferation of TC cells. (A) mRNA and (B)
protein expression of AGRN in NTHY and BcPAP cells transfected with
siAGRN or NEG were determined using reverse
transcription-quantitative PCR and western blotting, respectively.
*P<0.05, **P<0.0016 and ***P<0.0006. The plot shows
changes in protein expression that were normalized to β-actin. The
effects of siAGRN on (C) migration and (D) invasion of NTHY and
BcPAP cells. The plots show modified Boyden chamber migration and
invasion assays performed in three independent biological
experiments. Magnification, ×200. (E) Effects of siAGRN
transfection on vimentin, N-cadherin and E-cadherin protein levels
in NTHY and BcPAP cells. *P<0.05 and **P<0.005.
Representative western blotting of protein expression normalized
GAPDH. (F) Effects of siAGRN transfection on the viability of NTHY
and BcPAP cells. The plots show the results of the MTS assay
performed in three independent biological experiments. *P<0.05.
(G) Effects of siAGRN on the proliferation of NTHY and BcPAP cells.
The plots show BrdU assay performed in three independent biological
experiments. ***P<0.0002 and *P<0.01. TC, thyroid cancer;
siRNA, small interfering RNA; NEG, negative control; AGRN,
agrin. |
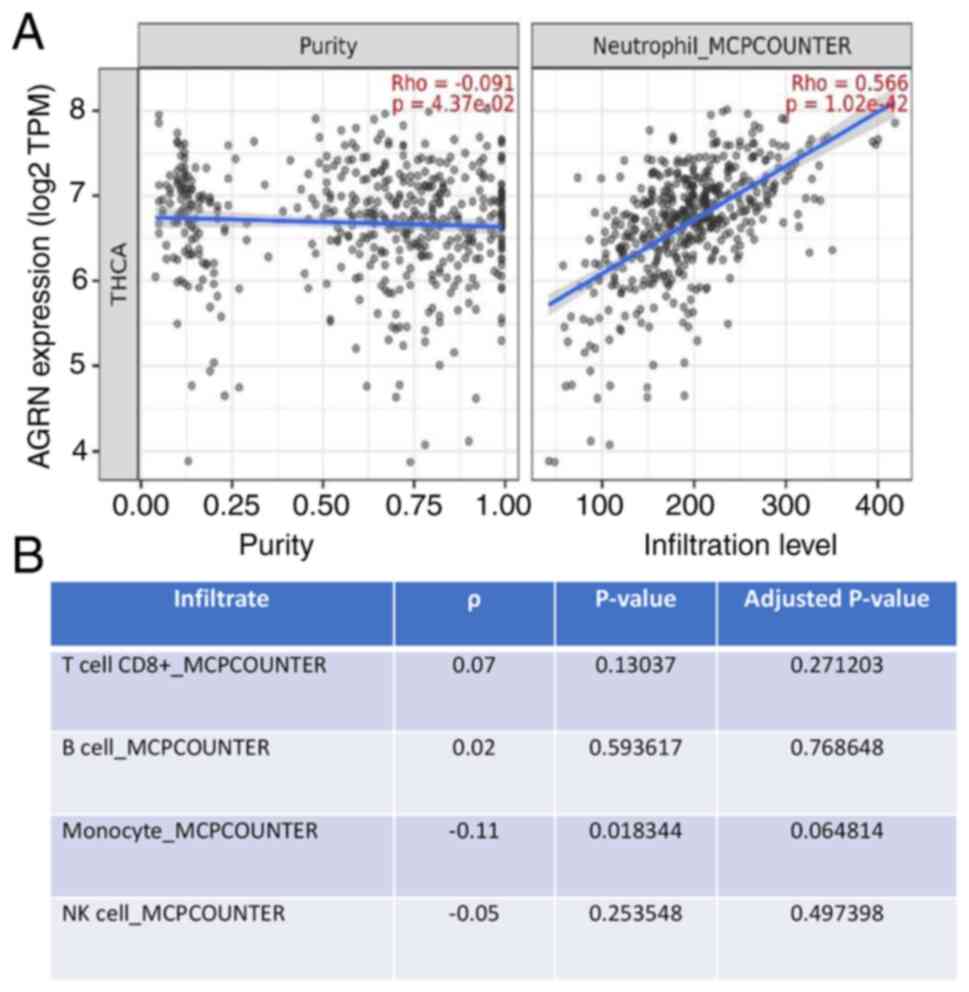
AGRN expression is associates with
neutrophil infiltration in thyroid tumors
The present study analyzed the association between
AGRN expression and the presence of immune infiltrates in thyroid
tumors (Table SI). Analysis
revealed variable weak-to-moderate correlations between the
expression of agrin and various immune cell types, including T
cells, plasma or macrophages. AGRN expression was significantly
correlated with the infiltration of neutrophils (ρ=0.566 and
P=1.02×10−42; Fig.
3).
Discussion
The present study showed that AGRN was overexpressed
in TC and promoted viability, proliferation, migration and invasion
of TC cells. For functional analysis of AGRN involvement in TC,
BcPAP cell line was selected, which is characterized by a mutation
in the BRAF gene (V600E) that occurs in the majority of cases of
PTC (up to 80%) (1). Moreover, AGRN
expression was associated with neutrophil infiltration in thyroid
tumors.
The results of the present study are consistent with
the established AGRN roles in other types of cancer (25–28).
AGRN is a proteoglycan component of the extracellular matrix (ECM)
that serves as a ligand for the co-receptors lipoprotein-related
receptor 4 (Lrp4) and muscle-specific kinase (MuSK) (38). The tumor-promoting effects of AGRN
are primarily mediated by the activation of the integrin/focal
adhesion/Lrp4/MuSK receptor pathway (39) In liver and breast cancer, AGRN
triggers this pathway to activate transcriptional coactivator
Yes-associated protein, thereby contributing to tumorigenesis
(38,39). In hepatocellular carcinoma (HCC),
AGRN promotes proliferation, migration and invadopodia formation by
stimulating epithelial-mesenchymal transition via Lrp4/MuSK/focal
adhesion kinase (FAK) signaling (39). Furthermore, AGRN activates
Lrp4/MuSK/FAK signaling to promote the VEGFR2 pathway to recruit
endothelial cells and facilitate adhesion to cancer cells to
promote liver tumor angiogenesis (40). Other AGRN-stimulated signaling
pathways include PI3K/AKT-mediated stimulation of IL-6 secretion to
promote growth and immune infiltration of non-small cell lung
cancer (26) and Wnt-dependent
promotion of proliferation, migration and invasion of rectal cancer
(41). AGRN promotes cancer
progression and in vivo tumor formation in
cholangiocarcinoma (42),
pancreatic ductal adenocarcinoma (28) and oral squamous cell carcinoma
(27,43). Finally, in HCC, AGRN depletion
decreases expression of mesenchymal markers including N-cadherin,
vimentin and snail and increases the expression of epithelial
marker E-cadherin (39). To the
best of our knowledge, the role of AGRN in non-cancerous thyroid
tissue has not been studied. Studies have showed that AGRN is
highly expressed in normal thyroid tissue (44,45);
however, its role is unknown. It is hypothesized that AGRN serves a
role in normal thyroid tissue by interacting with dystroglycan
(DC), a thyroid-stimulating hormone-regulated transmembrane
glycoprotein, providing interactions with the ECM (45). Furthermore, endodermal cells
expressing thyroid transcription factor 1, competent to form
thyroid epithelial lineages, are enriched in AGRN interactions at
neuromuscular junction pathways, suggesting a potential role in
thyroid development (46).
Furthermore, it is hypothesized that the thyroid hormone controls
the expression of highly glycosylated proteoglycans, including the
family of heparan sulfate, which AGRN belongs to (47). Heparan sulfate proteoglycans (HSPG)
participate in Indian hedgehog and fibroblast growth factor
signaling by regulating degradation, sequestration and diffusion of
growth factors and morphogens (47). HSPG role is essential for bone
development when thyroid hormones coordinate the progression of
endochondral ossification (48).
Basset et al (47) showed
that HSPG expression is upregulated in hypothyroidism. Therefore,
homeostasis of thyroid hormone is key to maintain the proper
expression of HSPGs such as AGRN.
Previous studies have reported involvement of AGRN
in immune regulation or autoimmunity (23,24).
AGRN autoantibodies are detected in patients with myasthenia gravis
(23). In T cells, T cell receptor
activation triggers expression of AGRN, which acts as a receptor
activator and leads to intracellular signaling, resulting in actin
polymerization and changes in T cell responsiveness (24). This mechanism is pronounced in
patients with lupus in whom AGRN is overexpressed in T lymphocytes
(24). In non-small cell lung
cancer, AGRN expression is correlated with regulatory T cell (Treg)
infiltration (26). AGRN is
required for proper maturation and viability of monocytes and
macrophages, with α-DC acting as a receptor (49). When activated by AGRN, α-DC triggers
intracellular signaling involving Erk1/2 kinase, which results in
changes in actin polymerization, contributing to proper conduction
of phagocytosis (49). TCs are
infiltrated by at least 22 types of immune cells that contribute to
tumor progression (50). The
aforementioned study showed that a high stroma score, low
CD8+ T cell infiltration and increased presence of
static memory CD4+ T cells, as well as active dendritic
cells, are associated with poor prognosis for patients with TC
(50). The presence of
tumor-associated macrophages is associated with lymph node
metastasis and larger tumor size, as well as poor survival of
patients with TC (51,52). PTC progression is associated with
higher tumor infiltration by Tregs and decreased presence of NK
cells (53). The present study
found that AGRN expression was correlated with neutrophil
infiltration in thyroid tumors but not other types of immune cell.
Several studies described the presence of a correlation between
tumor-associated neutrophils (TANs) in cancer and clinical outcomes
of patients (54–56). In some studies, TANs are shown to be
involved in the promotion of cancer cell proliferation, invasive
behavior or angiogenesis (57,58).
Furthermore, in TC, neutrophils serve tumor-promoting roles by
modulating immune and inflammatory responses (59–61).
For example, the conditioned media derived from TC induces
neutrophil chemotaxis by releasing CXCL8 and IL-8, which are
ligands of neutrophil receptors, such as C-X-C Motif Chemokine
Receptor (CXCR) 1 and CXCR2, and induces the production of reactive
oxygen species, the release of MMP-9 and the expression of
proinflammatory and angiogenic factors (62). Therefore, TC cells release soluble
factors that induce neutrophil chemotaxis and survival (62). These correlations suggest that AGRN
may be involved in the regulation of the immune environment of
thyroid tumors by inducing neutrophil recruitment. This hypothesis
and the mechanisms by which AGRN contribute to immune infiltration
require experimental verification. AGRN localizes to the nuclei of
thyroid cells, suggesting that its role in TC may not rely on
extracellular-mediated signaling (63). Furthermore, AGRN nuclear
localization in lung cancer with high nuclear AGRN localization is
associated not only with the clinical stage and poor
differentiation of lung adenocarcinoma, but also with lymph node
metastases (63). These data
suggest possible mechanisms of actions of AGRN in the nuclei which
should be evaluated in future studies.
The present study did not identify the mechanism of
action used by AGRN to modulate cancer progression and the immune
environment. However, the present study showed that AGRN was
overexpressed in thyroid tumors and contributed to the
proliferation, viability, migration and invasion of TC cells.
Moreover, AGRN silencing upregulated epithelial-mesenchymal
transition marker and downregulated N-cadherin and E-cadherin. The
association of AGRN expression with presence of neutrophil
infiltration in thyroid tumors suggested that AGRN may contribute
to the cancer immune environment.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Dr Cuong Hoang-Vu
(Martin Luther University (Halle, Germany) for the 8505C cell line
and Professor Marlena Godlewska (Department of Cell Biology and
Immunology, Centre of Postgraduate Medical Education, Warsaw,
Poland) for N-cadherin and E-cadherin antibodies (purchased from
GeneTex, Inc.).
Funding
The present study was supported by The National Science Center,
Poland (grant no. 2016/21/B/NZ5/00063) and The Centre of
Postgraduate Medical Education, Poland (grant no.
501-1-025-01-23).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BC conceived and supervised the study, and wrote the
manuscript. AAO and MG perfomed the experiments and wrote the
manuscript. AAO and MG confirm the authenticity of all the raw
data. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Scheffel RS, Dora JM and Maia AL: BRAF
mutations in thyroid cancer. Curr Opin Oncol. 34:9–18. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
European Network of Cancer Registries, .
Thyroid cancer (TC) Factsheet, January 2017. https://www.encr.eu/sites/default/files/factsheets/ENCR_Factsheet_Thyroid_2017-2.pdfDecember
4–2022
|
|
3
|
Haddad RI, Bischoff L, Ball D, Bernet V,
Blomain E, Busaidy NL, Campbell M, Dickson P, Duh QY, Ehya H, et
al: Thyroid carcinoma, version 2.2022, NCCN clinical practice
guidelines in oncology. J Natl Compr Canc Netw. 20:925–951. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bai Y, Kakudo K and Jung CK: Updates in
the pathologic classification of thyroid neoplasms: A review of the
World Health Organization Classification. Endocrinol Metab (Seoul).
35:696–715. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jung CK, Bychkov A and Kakudo K: Update
from the 2022 World Health Organization Classification of Thyroid
Tumors: A standardized diagnostic approach. Endocrinol Metab
(Seoul). 37:703–718. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cantwell-Dorris ER, O'Leary JJ and Sheils
OM: BRAFV600E: Implications for carcinogenesis and molecular
therapy. Mol Cancer Ther. 10:385–394. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang P, Guan H, Yuan S, Cheng H, Zheng J,
Zhang Z, Liu Y, Yu Y, Meng Z, Zheng X and Zhao L: Author
Correction: Targeting myeloid derived suppressor cells reverts
immune suppression and sensitizes BRAF-mutant papillary thyroid
cancer to MAPK inhibitors. Nat Commun. 13:70252022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Longheu A, Canu GL, Cappellacci F, Erdas
E, Medas F and Calò PG: Tall cell variant versus conventional
papillary thyroid carcinoma: A retrospective analysis in 351
consecutive patients. J Clin Med. 10:702020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Śmiech M, Leszczyński P, Kono H, Wardell C
and Taniguchi H: Emerging BRAF mutations in cancer progression and
their possible effects on transcriptional networks. Genes (Basel).
11:13422020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Prete A, Matrone A, Gambale C, Torregrossa
L, Minaldi E, Romei C, Ciampi R, Molinaro E and Elisei R: Poorly
differentiated and anaplastic thyroid cancer: Insights into
genomics, microenvironment and new drugs. Cancers (Basel).
13:32002021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou Y, Zhao Y, Ding X, Liang J, Xu H, Lin
Y, Khan HH and Shi B: A new way out of the predicament of
anaplastic thyroid carcinoma from existing data analysis. Front
Endocrinol (Lausanne). 13:8879062022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iñiguez-Ariza NM, Bible KC and Clarke BL:
Bone metastases in thyroid cancer. J Bone Oncol. 21:1002822020.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Erden ES, Babayigit C, Davran R, Akin M,
Karazincir S, Isaogullari N, Demirkose M and Genc S: Papillary
thyroid carcinoma with lung metastasis arising from
dyshormonogenetic goiter: A case report. Case Rep Med.
2013:8131672013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Basnet A, Pandita A, Fullmer J and
Sivapiragasam A: Squamous cell carcinoma of the thyroid as a result
of anaplastic transformation from BRAF-positive papillary thyroid
cancer. Case Rep Oncol Med. 2017:42764352017.PubMed/NCBI
|
|
15
|
Toraih EA, Hussein MH, Zerfaoui M, Attia
AS, Marzouk Ellythy A, Mostafa A, Ruiz EML, Shama MA, Russell JO,
Randolph GW and Kandil E: Site-Specific metastasis and survival in
papillary thyroid cancer: The importance of brain and multi-organ
disease. Cancers (Basel). 13:16252021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Varadarajan VV, Pace EK, Patel V, Sawhney
R, Amdur RJ and Dziegielewski PT: Follicular thyroid carcinoma
metastasis to the facial skeleton: A systematic review. BMC Cancer.
17:2252017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Parameswaran R, Shulin Hu J, Min En N, Tan
WB and Yuan NK: Patterns of metastasis in follicular thyroid
carcinoma and the difference between early and delayed
presentation. Ann R Coll Surg Engl. 99:151–154. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu MH, Lee YY, Lu YL and Lin SF: Risk
factors and prognosis for metastatic follicular thyroid cancer.
Front Endocrinol (Lausanne). 13:7918262022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim N, Stiegler AL, Cameron TO, Hallock
PT, Gomez AM, Huang JH, Hubbard SR, Dustin ML and Burden SJ: Lrp4
is a receptor for Agrin and forms a complex with MuSK. Cell.
135:334–342. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ruegg MA and Bixby JL: Agrin orchestrates
synaptic differentiation at the vertebrate neuromuscular junction.
Trends Neurosci. 21:22–27. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang B, Luo S, Wang Q, Suzuki T, Xiong WC
and Mei L: LRP4 serves as a coreceptor of agrin. Neuron.
60:285–297. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bassat E, Mutlak YE, Genzelinakh A,
Shadrin IY, Baruch Umansky K, Yifa O, Kain D, Rajchman D, Leach J,
Riabov Bassat D, et al: The extracellular matrix protein agrin
promotes heart regeneration in mice. Nature. 547:179–184. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lazaridis K and Tzartos SJ: Myasthenia
Gravis: Autoantibody specificities and their role in MG management.
Front Neurol. 11:5969812020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jury EC, Eldridge J, Isenberg DA and
Kabouridis PS: Agrin signalling contributes to cell activation and
is overexpressed in T lymphocytes from lupus patients. J Immunol.
179:7975–7983. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sethi MK, Downs M, Shao C, Hackett WE,
Phillips JJ and Zaia J: In-Depth matrisome and glycoproteomic
analysis of human brain glioblastoma versus control tissue. Mol
Cell Proteomics. 21:1002162022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Han L, Shi H, Ma S, Luo Y, Sun W, Li S,
Zhang N, Jiang X, Gao Y, Huang Z, et al: Agrin promotes non-small
cell lung cancer progression and stimulates regulatory T cells via
increasing IL-6 secretion through PI3K/AKT pathway. Front Oncol.
11:8044182022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kawahara R, Granato DC, Carnielli CM,
Cervigne NK, Oliveria CE, Rivera C, Yokoo S, Fonseca FP, Lopes M,
Santos-Silva AR, et al: Agrin and perlecan mediate tumorigenic
processes in oral squamous cell carcinoma. PLoS One. 9:e1150042014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tian C, Öhlund D, Rickelt S, Lidström T,
Huang Y, Hao L, Zhao RT, Franklin O, Bhatia SN, Tuveson DA and
Hynes RO: Cancer cell-derived matrisome proteins promote metastasis
in pancreatic ductal adenocarcinoma. Cancer Res. 80:1461–1474.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Grzanka M, Stachurska-Skrodzka A,
Adamiok-Ostrowska A, Gajda E and Czarnocka B: Extracellular
vesicles as signal carriers in malignant thyroid tumors? Int J Mol
Sci. 23:32622022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li X, Wang X, Song W, Xu H, Huang R, Wang
Y, Zhao W, Xiao Z and Yang X: Oncogenic properties of NEAT1 in
prostate cancer cells depend on the CDC5L-AGRN transcriptional
regulation circuit. Cancer Res. 78:4138–4149. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chandrashekar DS, Karthikeyan SK, Korla
PK, Patel H, Shovon AR, Athar M, Netto GJ, Qin ZS, Kumar S, Manne
U, et al: UALCAN: An update to the integrated cancer data analysis
platform. Neoplasia. 25:18–27. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chandrashekar DS, Bashel B, Balasubramanya
SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi BVSK and
Varambally S: UALCAN: A portal for facilitating tumor subgroup gene
expression and survival analyses. Neoplasia. 19:649–658. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen F, Chandrashekar DS, Varambally S and
Creighton CJ: Pan-cancer molecular subtypes revealed by
mass-spectrometry-based proteomic characterization of more than 500
human cancers. Nat Commun. 10:56792019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li T, Fan J, Wang B, Traugh N, Chen Q, Liu
JS, Li B and Liu XS: TIMER: A web server for comprehensive analysis
of tumor-infiltrating immune cells. Cancer Res. 77:e108–e110. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li T, Fu J, Zeng Z, Cohen D, Li J, Chen Q,
Li B and Liu XS: TIMER2.0 for analysis of tumor-infiltrating immune
cells. Nucleic Acids Res. 48((W1)): W509–W514. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li B, Severson E, Pignon JC, Zhao H, Li T,
Novak J, Jiang P, Shen H, Aster JC, Rodig S, et al: Comprehensive
analyses of tumor immunity: Implications for cancer immunotherapy.
Genome Biol. 17:1742016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chakraborty S, Lakshmanan M, Swa HL, Chen
J, Zhang X, Ong YS, Loo LS, Akıncılar SC, Gunaratne J, Tergaonkar
V, et al: An oncogenic role of Agrin in regulating focal adhesion
integrity in hepatocellular carcinoma. Nat Commun. 6:61842015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chakraborty S, Njah K, Pobbati AV, Lim YB,
Raju A, Lakshmanan M, Tergaonkar V, Lim CT and Hong W: Agrin as a
Mechanotransduction Signal Regulating YAP through the Hippo
Pathway. Cell Rep. 18:2464–2479. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Njah K, Chakraborty S, Qiu B, Arumugam S,
Raju A, Pobbati AV, Lakshmanan M, Tergaonkar V, Thibault G, Wang X
and Hong W: A role of agrin in maintaining the stability of
vascular endothelial growth factor receptor-2 during tumor
angiogenesis. Cell Rep. 28:949–965.e7. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang ZQ, Sun XL, Wang YL and Miao YL:
Agrin promotes the proliferation, invasion and migration of rectal
cancer cells via the WNT signaling pathway to contribute to rectal
cancer progression. J Recept Signal Transduct Res. 41:363–370.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
He M, Cheng C, Tu J, Ji SS, Lou D and Bai
B: Agrin expression is correlated with tumor development and poor
prognosis in cholangiocarcinoma. J Int Med Res.
49:30006052110097222021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rivera C, Zandonadi FS, Sánchez-Romero C,
Soares CD, Granato DC, González-Arriagada WA and Paes Leme AF:
Agrin has a pathological role in the progression of oral cancer. Br
J Cancer. 118:1628–1638. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Groffen AJ, Buskens CA, van Kuppevelt TH,
Veerkamp JH, Monnens LA and van den Heuvel LP: Primary structure
and high expression of human agrin in basement membranes of adult
lung and kidney. Eur J Biochem. 254:123–128. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Collins BJ, Gorelick G and Schneider AB:
Dystroglycan is present in rat thyroid and rat thyroid cells and
responds to thyrotropin. Endocrinology. 142:3152–3162. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kurmann AA, Serra M, Hawkins F, Rankin SA,
Mori M, Astapova I, Ullas S, Lin S, Bilodeau M, Rossant J, et al:
Regeneration of thyroid function by transplantation of
differentiated pluripotent stem cells. Cell Stem Cell. 17:527–542.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bassett JH, Swinhoe R, Chassande O,
Samarut J and Williams GR: Thyroid hormone regulates heparan
sulfate proteoglycan expression in the growth plate. Endocrinology.
147:295–305. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bassett JH and Williams GR: Role of
thyroid hormones in skeletal development and bone maintenance.
Endocr Rev. 37:135–187. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Mazzon C, Anselmo A, Soldani C, Cibella J,
Ploia C, Moalli F, Burden SJ, Dustin ML, Sarukhan A and Viola A:
Agrin is required for survival and function of monocytic cells.
Blood. 119:5502–5511. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gong J, Jin B, Shang L and Liu N:
Characterization of the immune cell infiltration landscape of
thyroid cancer for improved immunotherapy. Front Mol Biosci.
8:7140532021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kim S, Cho SW, Min HS, Kim KM, Yeom GJ,
Kim EY, Lee KE, Yun YG, Park DJ and Park YJ: The expression of
tumor-associated macrophages in papillary thyroid carcinoma.
Endocrinol Metab (Seoul). 28:192–198. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ryder M, Ghossein RA, Ricarte-Filho JC,
Knauf JA and Fagin JA: Increased density of tumor-associated
macrophages is associated with decreased survival in advanced
thyroid cancer. Endocr Relat Cancer. 15:1069–1074. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Gogali F, Paterakis G, Rassidakis GZ,
Kaltsas G, Liakou CI, Gousis P, Neonakis E, Manoussakis MN and
Liapi C: Phenotypical analysis of lymphocytes with suppressive and
regulatory properties (Tregs) and NK cells in the papillary
carcinoma of thyroid. J Clin Endocrinol Metab. 97:1474–1482. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Galdiero MR, Bianchi P, Grizzi F, Di Caro
G, Basso G, Ponzetta A, Bonavita E, Barbagallo M, Tartari S,
Polentarutti N, et al: Occurrence and significance of
tumor-associated neutrophils in patients with colorectal cancer.
Int J Cancer. 139:446–456. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wikberg ML, Ling A, Li X, Öberg A, Edin S
and Palmqvist R: Neutrophil infiltration is a favorable prognostic
factor in early stages of colon cancer. Hum Pathol. 68:193–202.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhou L, Xu L, Chen L, Fu Q, Liu Z, Chang
Y, Lin Z and Xu J: Tumor-infiltrating neutrophils predict benefit
from adjuvant chemotherapy in patients with muscle invasive bladder
cancer. Oncoimmunology. 6:e12932112017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Masucci MT, Minopoli M and Carriero MV:
Tumor associated neutrophils. Their role in tumorigenesis,
metastasis, prognosis and therapy. Front Oncol. 9:11462019.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wu L and Zhang XH: Tumor-Associated
neutrophils and macrophages-heterogenous but not chaotic. Front
Immunol. 11:5539672020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Jablonska J, Leschner S, Westphal K,
Lienenklaus S and Weiss S: Neutrophils responsive to endogenous
IFN-beta regulate tumor angiogenesis and growth in a mouse tumor
model. J Clin Invest. 120:1151–1164. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Queen MM, Ryan RE, Holzer RG, Keller-Peck
CR and Jorcyk CL: Breast cancer cells stimulate neutrophils to
produce oncostatin M: Potential implications for tumor progression.
Cancer Res. 65:8896–8904. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Scapini P and Cassatella MA: Social
networking of human neutrophils within the immune system. Blood.
124:710–719. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Galdiero MR, Varricchi G, Loffredo S,
Bellevicine C, Lansione T, Ferrara AL, Iannone R, di Somma S,
Borriello F, Clery E, et al: Potential involvement of neutrophils
in human thyroid cancer. PLoS One. 13:e01997402018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Li D, Gu Q, Xie Z, Shen Q and Li H:
Clinical significance of nuclear localisation of agrin in lung
adenocarcinoma. Pol J Pathol. 70:198–204. 2019. View Article : Google Scholar : PubMed/NCBI
|