Introduction
Esophageal cancer ranks seventh worldwide in terms
of morbidity and is the sixth leading cause of cancer-associated
mortality (1). Esophageal squamous
cell carcinoma (ESCC) accounts for 90% of esophageal cancer cases
globally (2). Endoscopic treatment
is now well constituted as a favorable technique for early-stage
cancer, while esophagectomy remains the mainstay for locally
advanced ESCC (3). When the tumor
reaches a more advanced stage, neoadjuvant chemoradiotherapy,
chemotherapy, immunotherapy or immunotherapy plus chemotherapy are
also used (3–5). Furthermore, patients with distant
metastases (non-regional lymph node involvement or
T4b-stage cancer) are considered unresectable, and these
patients cannot be treated with surgery but only with
chemoradiotherapy or chemotherapy (3,6). As
regards to patients with non-distant metastatic ESCC, it is
essential to determine the resectability in order to select the
optimal treatment strategy to improve prognosis.
The most common diagnostic technique for ESCC is
mainly based on endoscopic biopsy followed by multidetector
computed tomography (MDCT) (7).
Computed tomography (CT) is mainly applied for disease diagnosis
and treatment guidance (8,9). A previous study suggested that CT is a
reliable method with which to measure tumor volume (10). CT-derived tumor volume associates
well with treatment failure rate, nodal metastases and the disease
survival rate. Previous researchers have confirmed that gross tumor
volume (GTV) measured using CT may be an indicator for predicting
the T category and/or N stage of ESCC (11,12).
Ou et al (8) reported that
the longer length and greater sphericalness indicates more tumor
invasions based on CT radiomic features, which increases the
possibility of unresectable ESCC. However, their study (8) did not provide an easy-to-understand
method, but only a CT radiomics model to determine resectability.
To the best of our knowledge, a simple and practicable procedure
without the requirement of additional computational resources is
not yet available to determine the resectability of non-distant
metastatic ESCC when compared with the radiomics model. Thus, the
aim of the present study was to explore a simple and practicable
quantitative model based on the GTV of non-distant metastatic ESCC.
This was measured using multidetector CT corresponding to the
anatomical distribution for the pre-operative determination of
resectability in order to facilitate the selection of personalized
treatment options by clinicians.
Materials and methods
Patients
The present retrospective study was approved by the
constituted Ethics Committee of the Affiliated Hospital of North
Sichuan Medical College (Nanchong, China; approval no.
2021ER044-1). The ethics committee waived the need for informed
consent before all patients participated in the study.
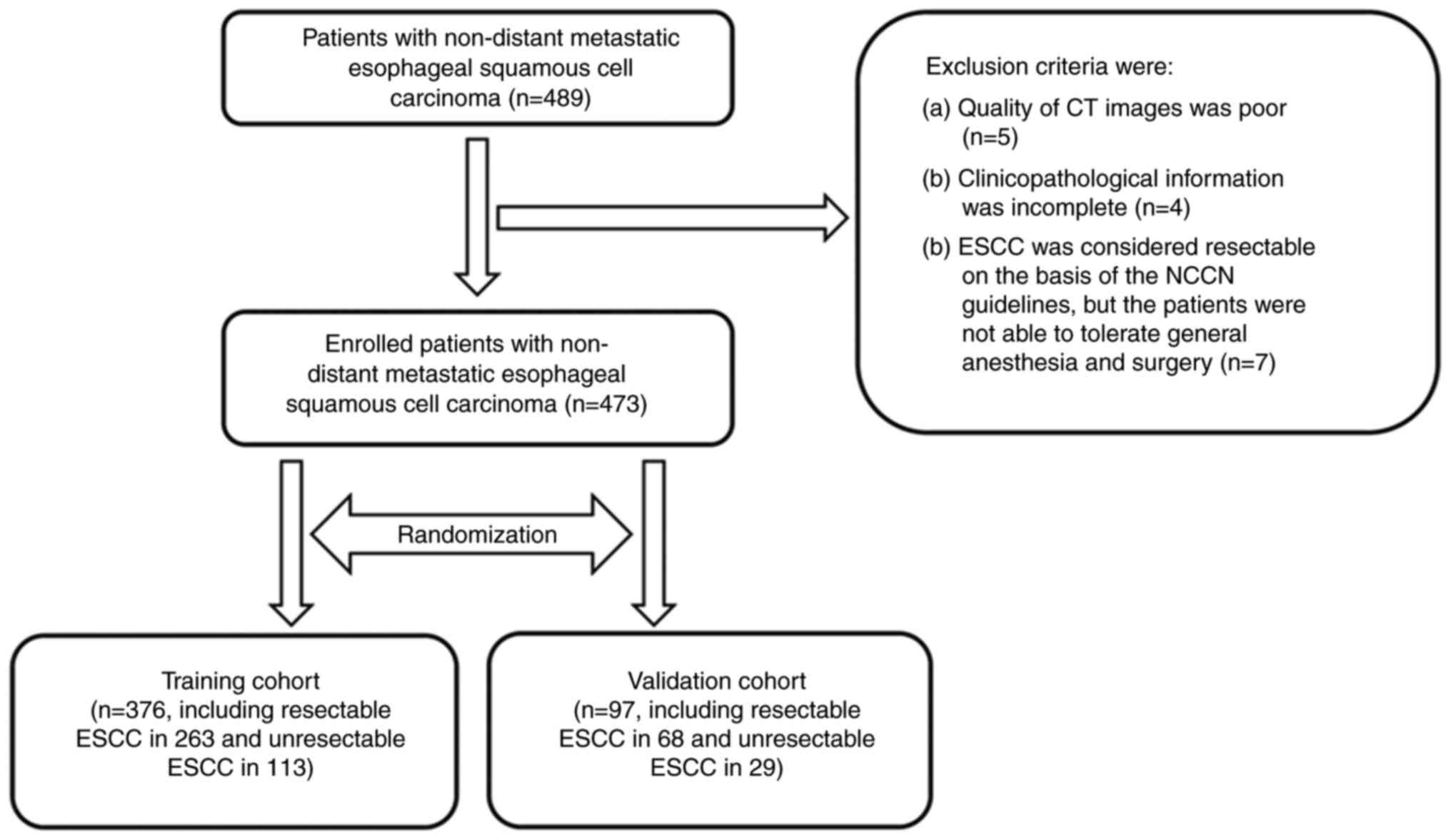
From January 2017 to December 2020, at total of 489
consecutive patients with biopsy-confirmed thoracic ESCC who
underwent MDCT scans were analyzed. In accordance with the National
Comprehensive Cancer Network (NCCN) guidelines (6), the diagnostic criteria for
unresectable esophageal cancer on CT were as follows: i)
cT4b tumors with the involvement of the trachea, heart,
great vessels or adjacent organs including the lungs, liver,
pancreas and spleen were considered unresectable; ii) ESCC with
multi-station, bulky lymphadenopathy was considered unresectable;
or iii) ESCC with distant metastases, including non-regional lymph
nodes (stage IV) was considered unresectable. In the case that ESCC
was not considered unresectable based on the criteria, the tumor
was regarded as resectable. Among the 489 patients, 342 and 147
patients had been classified as resectable and unresectable ESCCs,
respectively.
The patients with resectable and unresectable ESCC
were recruited based on the following inclusion criteria (8): i) The patients were classified as
having resectable and unresectable ESCC according to the NCCN
guidelines, as depicted using MDCT (6); ii) the patients did not accept any
pre-operative tumor-related treatments (e.g., radiotherapy or
chemotherapy) prior to undergoing the CT scans; iii) the patients
did not have distant metastases from ESCC on the CT findings; and
iv) patients with a single primary tumor of non-distant metastatic
thoracic ESCC. The exclusion criteria in the present study were as
follows: i) The quality of the CT images was poor (n=5); ii) the
clinicopathological information was incomplete (n=4); or iii) ESCC
was considered resectable on the basis of the NCCN guidelines, but
the patients were not able to tolerate general anesthesia and
surgery (n=7). As a result, 16 of the 489 patients were excluded,
and a total of 473 patients were included in the study.
Among the 473 patients, 331 and 142 were classified
as resectable and unresectable ESCCs, respectively. In the
resectable group, the resectability of ESCC was confirmed using a
histopathological biopsy during surgery, and the margin was
confirmed to be negative after the surgery. Of the 331 patients
with resectable ESCC, 20 patients accepted neoadjuvant therapy
after the CT scans and prior to surgery; the tumor size then
markedly decreased after therapy and the cases then became
resectable tumors and underwent surgical treatment, and the
resectability was also confirmed using a histopathological biopsy
during surgery. Ultimately, 331 patients with resectable ESCC and
142 patients with unresectable cancer were randomly divided into
the training cohort (TC; n=376) and the validation cohort (VC;
n=97). The clinicopathological information of the patients in the
TC and VC is presented in Table I.
A schematic diagram of the selection process used in the present
study is presented in Fig. 1.
 | Table I.Table
I. Clinical information of the patients in the training
(resectable vs. unresectable, 263 vs. 113) and validation cohorts
(resectable vs. unresectable, 68 vs. 29). |
Table I.
Table
I. Clinical information of the patients in the training
(resectable vs. unresectable, 263 vs. 113) and validation cohorts
(resectable vs. unresectable, 68 vs. 29).
| Variable | Training cohort
(n=376) | Validation cohort
(n=97) |
|---|
| Median age, years
(range) | 66 (42–86) | 65 (49–86) |
| Sex
(male:female) | 287:89 | 65:32 |
| Anatomic
distribution, n (%) |
|
|
| Upper
thoracic portion | 43 (11.5) | 23 (23.7) |
| Middle
thoracic portion | 272 (72.3) | 44 (45.4) |
| Lower
thoracic portion | 61 (16.2) | 30 (30.9) |
| T stage, n (%) |
|
|
|
cT1 | 56 (14.9) | 16 (16.5) |
|
cT2 | 59 (15.7) | 16 (16.5) |
|
cT3 | 151 (40.2) | 46 (47.4) |
|
cT4a | 25 (6.6) | 4 (4.1) |
|
cT4b | 85 (22.6) | 15 (15.5) |
| N stage, n (%) |
|
|
|
N0 | 168 (44.7) | 41 (42.3) |
|
N1 | 102 (27.1) | 24 (24.7) |
|
N2 | 67 (17.8) | 17 (17.5) |
|
N3 | 39 (10.4) | 15 (15.5) |
| GTV, cm3
(mean ± SD) | 22.88±20.95 | 22.40±19.26 |
Contrast-enhanced CT
All patients underwent thoracic contrast-enhanced
scans with a 64-row MDCT scanner (LightSpeed VCT; GE Medical
Systems). The interval time between CT and surgery ranged from 2 to
14 days (mean, 8 days). Prior to the acquisition of CT data, the
patients were required to drink 100 to 200 ml water as esophageal
negative contrast material. Following a conventional unenhanced CT
scan with the patients in the supine position, the
contrast-enhanced data acquisition commenced 25 to 30 sec after the
beginning of contrast material injection [Omnipaque™
(Iohexol); GE Healthcare; Cytiva] at a rate of 3.0 ml/sec for a
total of 70 to 100 ml via a 20-gauge needle inserted into an
antecubital vein with an automated injector (Vistron CT Injection
System; Medrad, Inc.). The dosage of the injected contrast agent
was tailored to the body weight of the patient at a ratio of 1.5
ml/kg body weight and was then flushed with 20 ml saline.
Examinations were executed during one breath hold at full suspended
inspiration for 10–15 sec. The parameters of MDCT scanning were as
follows: A tube voltage of 120 kV, tube current of 200 mA, detector
collimation of 64×0.6 mm, a rotation time of 0.5 sec, a pitch of
0.9, slice thickness of 5-mm and a matrix of 512×512 mm. The
anatomic coverage of the CT scan was from the thoracic entrance to
the middle level of the left kidney. All the image data were then
directly transferred to the General Electric Advantage Workstation
4.4 at the mediastinal window settings (conventional window level,
40 HU; window width, 400 HU).
GTV measurement
According to the NCCN guidelines (6), the thoracic esophagus was divided into
the upper, middle and lower portions through the lower edge of the
azygos vein and the lower edge of the lower pulmonary vein. GTV
values of resectable and unresectable ESCC based on the involved
thoracic portions in TC and VC were measured using the General
Electric Advantage Workstation 4.4 at the mediastinal window. In
order to obtain GTV, the esophageal wall was considered as abnormal
when the thickness exceeded 5 mm on the axial image (13). GTV was calculated by multiplying the
sum of all the tumor areas by the layer thickness based on a
previously published method (11,12).
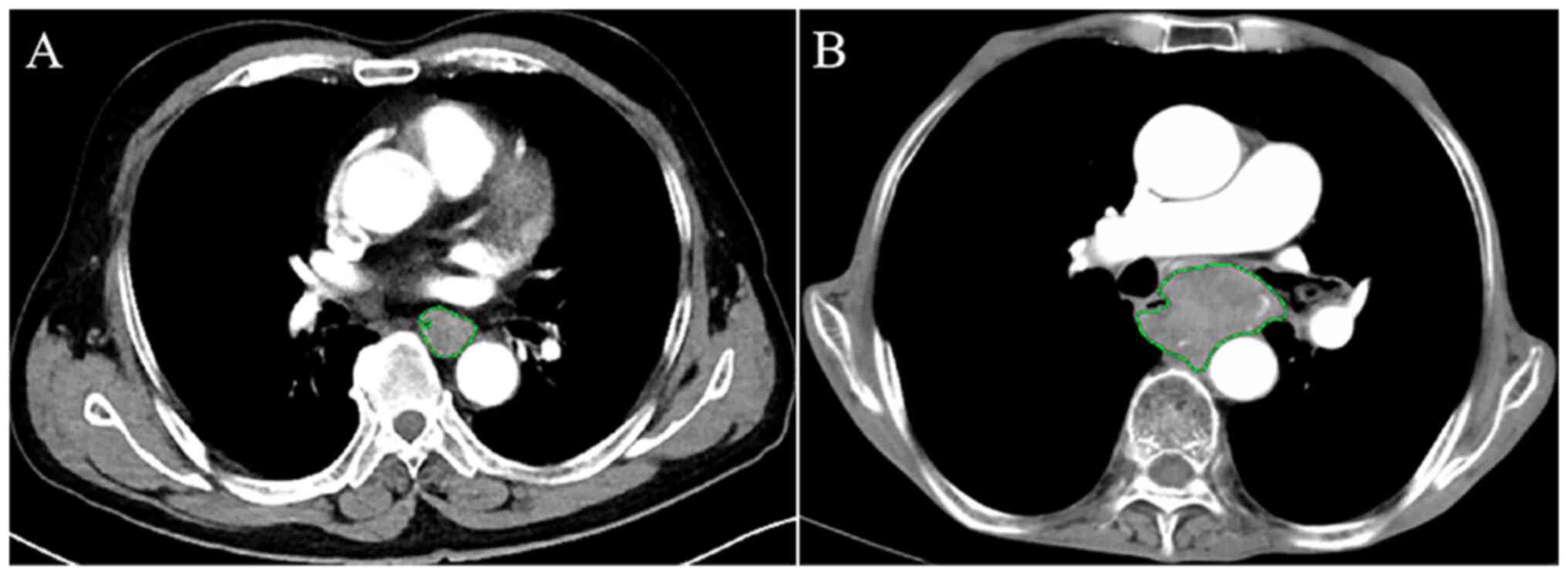
The circumference of the ESCC was manually depicted along the
visible margin of the thickened esophageal wall on each axial
contrast-enhanced CT scan in order to automatically obtain the
cross-sectional area of the tumor (Fig.
2). The aforementioned procedure and analysis were repeated on
each contiguous axial slice where the tumor was visible. To
accurately measure the tumor areas of ESCC, care was taken to avoid
the liquid and air in the lumen of the esophagus.
Subsequently, two radiologists (reader 1, DG with 4
years of experience in radiology and Reader 2, TWC with 25 years of
experience in radiology) measured the GTV of all patients with
non-distant metastatic ESCC independently in the TC and VC, without
any knowledge of the histopathological results in order to
determine the interobserver reproducibility of the measurement.
Prior to the aforementioned CT measurements, Reader 1 was trained
in measurements randomly in 20 patients of the TC by Reader 2. To
verify the intraobserver reproducibility of GTV, measurements in
the TC and VC were repeated 1 month later by Reader 1.
Statistical analysis
All statistical analyses were performed using SPSS
(version 26.0 for windows; IBM Corp.). The intraclass correlation
coefficient (ICC) was used to evaluate the interobserver and
intraobserver reliability of the repeated measurements of GTV. ICC
values <0.5, between 0.5 and 0.75, between 0.75 and 0.9, and
>0.90 represented poor, moderate, good and excellent
reliability, respectively (14).
Univariate and multivariate analyses were performed
using the TC dataset. P<0.05 was considered to indicate a
statistically significant difference. The univariate analysis of
possible determinants of non-distant metastatic ESCC resectability
was performed using the χ2 or Fisher's exact tests in
the TC. The variables with significant differences were then
enrolled into the binary logistic regression analysis to clarify
the independent determinants. Subsequently, the Mann-Whitney U test
was applied to compare the GTV between patients with resectable and
unresectable ESCC corresponding to different anatomic
distributions. In the case that a significant difference was
demonstrated, receiver operating characteristic (ROC) analysis was
performed to ascertain whether the cut-off values of GTV based on
anatomic distributions could determinate the resectability.
Finally, the performance of the models derived from the TC was
validated using unweighted Cohen's Kappa tests in the VC (15). Cohen k-values between 0.61 and 0.8,
and >0.81 were indicative of good and excellent agreements,
respectively; otherwise, the agreement is considered unsatisfactory
(16).
Results
Inter- and intraobserver agreements of
GTV measurements
The inter- and intra-observer ICC values of the
repeated measurements of GTV were 0.988 and 0.994, respectively
(Table II); this indicated the
excellent reliability of the GTV measurements by Reader 1.
Consequently, the values of the initial measurement by Reader 1
were regarded as the final results for further analyses.
 | Table II.Inter- and intra-observer agreements
of GTV measurements. |
Table II.
Inter- and intra-observer agreements
of GTV measurements.
| Agreement
analysis | ICC value | 95% CI | P-value |
|---|
| Inter-observer
agreement | 0.988 | 0.982-0.992 | <0.001 |
| Intra-observer
agreement | 0.994 | 0.991-0.996 | <0.001 |
Univariate analysis of GTV and
clinicopathological factors: Resectable vs. unresectable ESCC in
the TC
The GTV and possible clinicopathological factors
associated with the resectability of non-distant metastatic
thoracic ESCC in TC are presented in Table III. According to univariate
analysis, sex, anatomical distribution, cT stage, cN stage and the
GTV of non-distant metastatic thoracic ESCC in TC were more likely
to be related to the resectability (all P<0.05). However, no
significant difference in age was demonstrated between the
resectable and unresectable groups.
 | Table III.Univariate analysis of GTV and
clinicopathological determinants of non-distant metastatic ESCC in
the training cohort. |
Table III.
Univariate analysis of GTV and
clinicopathological determinants of non-distant metastatic ESCC in
the training cohort.
| Parameter | Resectable ESCC
(n=263) | Unresectable ESCC
(n=113) | P-value |
|---|
| Age (mean,
years) | 65.02±7.60 | 66.61±8.11 | 0.327 |
| Sex (%) |
|
| 0.002 |
|
Male | 189 (71.9) | 98 (86.7) |
|
|
Female | 74 (28.1) | 15 (13.3) |
|
| Anatomical
distribution, n (%) |
|
| <0.001 |
| Upper
thoracic portion | 19 (7.2) | 24 (21.2) |
|
| Middle
thoracic portion | 193 (73.4) | 79 (69.9) |
|
| Lower
thoracic portion | 51 (19.4) | 10 (8.9) |
|
| T stage, n (%) |
|
| <0.001 |
|
cT1 | 56 (21.3) | 0 (0) |
|
|
cT2 | 55 (20.9) | 4 (3.6) |
|
|
cT3 | 140 (53.2) | 11 (9.7) |
|
|
cT4a | 12 (4.6) | 13 (11.5) |
|
|
cT4b | 0 (0) | 85 (75.2) |
|
| N stage, n (%) |
|
| <0.001 |
|
cN0 | 161 (61.2) | 7 (6.2) |
|
|
cN1 | 75 (28.5) | 27 (23.9) |
|
|
cN2 | 27 (10.3) | 40 (35.4) |
|
|
cN3 | 0 (0) | 39 (34.5) |
|
| GTV, mean ± SD
(cm3) | 13.84±7.96 | 43.91±26.12 | <0.001 |
Multivariate analysis of the
resectability of non-distant metastatic thoracic ESCC in TC
According to the aforementioned univariate analysis,
binary logistic regression analysis was performed for sex,
anatomical distribution, cT stages, cN stages and GTV to identify
the independent determinants that could be used to evaluate the
resectability of non-distant metastatic thoracic ESCC. The logistic
regression analysis demonstrated that the GTV [P<0.001; odds
ratio (OR), 1.158; 95% CI, 1.039-1.290] and anatomic distribution
(P=0.027; OR, 1.924; 95% CI, 0.344-10.773) were independent
determinants of the resectability in the TC, as in Table IV.
 | Table IV.Multivariate analysis of the
resectability of non-distant metastatic thoracic ESCC in the
training cohort. |
Table IV.
Multivariate analysis of the
resectability of non-distant metastatic thoracic ESCC in the
training cohort.
| Parameter | P-value | OR | 95% CI |
|---|
| Sex | 0.795 | 1.418 | 0.782-5.179 |
| Anatomical
distribution | 0.027 | 1.924 | 0.344-10.773 |
| T stage | 0.551 | 1.502 | 0.490-12.951 |
| N stage | 0.605 | 1.831 | 0.647-7.845 |
| GTV | <0.001 | 1.158 | 1.039-1.290 |
Association of GTV of non-distant
metastatic thoracic ESCC based on anatomic distributions with the
resectability in TC
Mann-Whitney U test was used to investigate the
association of GTV with the resectability of non-distant metastatic
ESCC individually in the upper, middle and lower thoracic portions
of esophagus. The results demonstrated that the GTV of non-distant
metastatic ESCC based on anatomic distributions could help
determine the resectability (all P<0.001; Table V).
 | Table V.The Mann-Whitney U tests for
investigating the association of GTV with the resectability of
non-distant metastatic ESCC based on anatomic distributions in
TC. |
Table V.
The Mann-Whitney U tests for
investigating the association of GTV with the resectability of
non-distant metastatic ESCC based on anatomic distributions in
TC.
|
| GTV, cm3
(mean ± SD) |
|
|---|
|
|
|
|
|---|
| Anatomic
distribution | Resectable ESCC
(n=263) | Unresectable ESCC
(n=113) | P-value |
|---|
| Upper thoracic
portion | 10.61±4.71 | 31.97±13.14 | <0.001 |
| Middle thoracic
portion | 13.79±8.17 | 47.81±28.26 | <0.001 |
| Lower thoracic
portion | 15.25±7.86 | 41.81±24.72 | <0.001 |
ROC analysis of GTV of non-distant
metastatic thoracic ESCC based on anatomic distributions to
quantitatively determine resectability in the TC
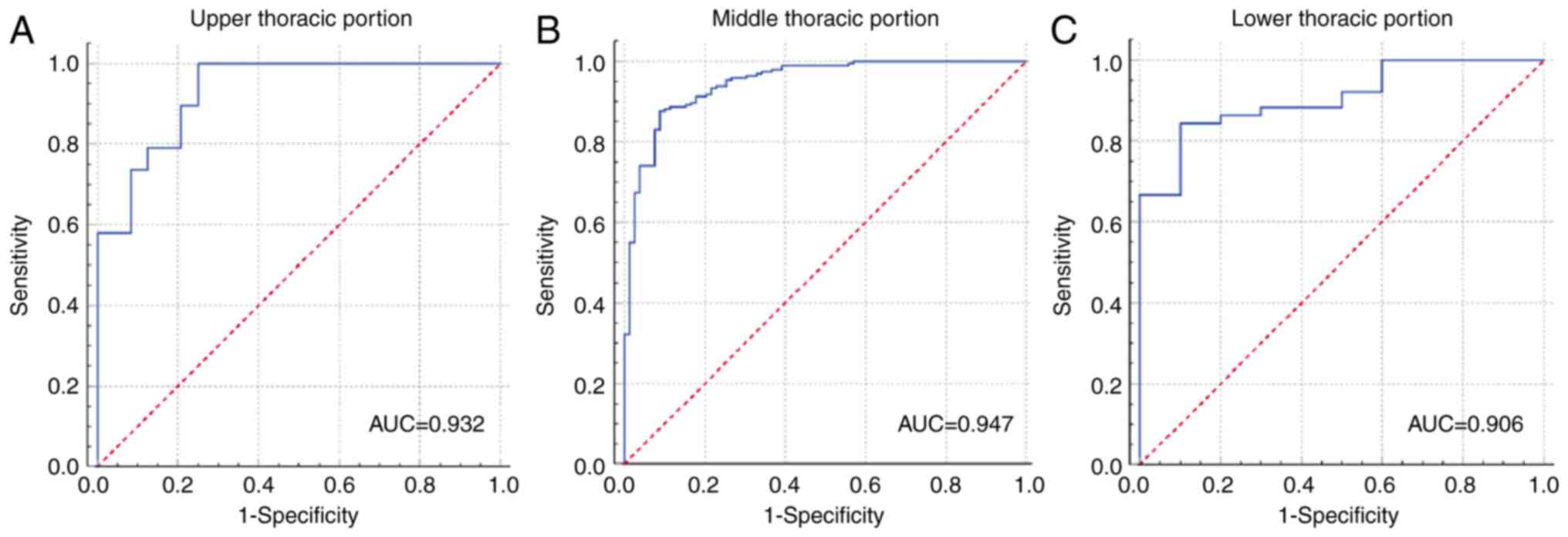
ROC analysis was performed in the TC to identify the
accuracy of GTV of non-distant metastatic thoracic ESCC
corresponding to anatomic distributions for determining the
resectability. The GTV cut-off values of 23.57, 22.89 and 22.58
cm3 were enabled to identify the resectability in
patients at the upper, middle and lower thoracic portions,
respectively. It was discovered that the GTV of non-distant
metastatic ESCC could help determine the resectability with areas
under the ROC curve (AUCs) of 0.932, 0.947 and 0.906 in the upper,
middle and lower thoracic portions (Fig. 3), respectively. The GTV of
non-distant metastatic thoracic ESCC based on an anatomic
distribution less than the cut-off value indicated that the tumor
would be more likely to be resectable. The cut-off value, AUC,
sensitivity, specificity, positive predictive value, negative
predictive value and accuracy of GTV of non-distant metastatic ESCC
for determining resectability are summarized in Table VI.
 | Table VI.ROC analysis of the ability of
anatomy-based GTV to quantitatively determine the resectability of
non-distant metastatic ESCC in TC. |
Table VI.
ROC analysis of the ability of
anatomy-based GTV to quantitatively determine the resectability of
non-distant metastatic ESCC in TC.
| Anatomic
Distribution | GTV cut-off value
(cm3) | AUC | Sensitivity
(%) | Specificity
(%) | PPV (%) | NPV (%) | Accuracy (%) |
|---|
| Upper thoracic
portion | 23.57 | 0.932 | 100.0 | 75.0 | 76.0 | 100.0 | 86.1 |
| Middle thoracic
portion | 22.89 | 0.947 | 87.6 | 91.1 | 96.0 | 75.0 | 88.6 |
| Lower thoracic
portion | 22.58 | 0.906 | 84.3 | 90.0 | 97.7 | 52.9 | 85.2 |
Unweighted Cohen's Kappa tests to
validate the performance of the ROC models in VC
Unweighted Cohen's Kappa tests in the VC were
executed to validate the agreement in the diagnostic efficiency of
the ROC models of GTV corresponding to anatomic distributions to
determine the resectability of non-distant metastatic thoracic
ESCC. The results revealed that the models obtained excellent
agreements in VC with all Cohen k-values >0.87, as presented in
Table VII.
 | Table VII.Results of unweighted Cohen's Kappa
tests in the validation cohort for the validation of the
performance of the receiver operating characteristic models. |
Table VII.
Results of unweighted Cohen's Kappa
tests in the validation cohort for the validation of the
performance of the receiver operating characteristic models.
| Anatomic
distribution | Cohen k-value | 95% CI | P-value |
|---|
| Upper thoracic
portion | 0.913 | 0.746-1.080 | <0.001 |
| Middle thoracic
portion | 0.879 | 0.716-1.042 | <0.001 |
| Lower thoracic
portion | 0.871 | 0.699-1.043 | <0.001 |
Discussion
The present study demonstrated that GTV and anatomic
distribution could be potential independent factors for determining
the resectability of non-distant metastatic thoracic ESCC. Thus,
the present study subsequently investigated whether GTV
corresponding to the anatomic distributions of thoracic ESCC can be
used to determine the resectability and to determine how this can
be achieved.
As demonstrates in the present study, the
resectability of ESCC decreased as the GTV increased. Previous
studies (11,12,15)
have reported that GTV measurement on a CT scan may be associated
with tumor TNM staging. The GTV can be a comprehensive index that
can reflect the depth of tumor invasion, tumor diameter and tumor
length (15). According to American
Joint Committee on Cancer criteria (17), the T stage of ESCC is mainly defined
based on the depth of tumor infiltration. With the increase in the
depth of tumor invasion, it is easier to detect the local tumor
invasion of adjacent structures (18). Another previous study demonstrated
that the longer length and greater sphericalness indicates an
increased number of tumor invasions, which leads to the increased
possibility of unresectable ESCC (8). It can be hypothesized that the greater
GTV based on the longer length and deeper infiltration of ESCC
indicates an increased possibility of unresectability. Therefore,
GTV was selected as an alternative factor for analysis in the
present study. To the best of our knowledge, the present study is
the first to demonstrate that the GTV may be strongly related to
the resectability of non-distant metastatic ESCC in the TC.
The present study also indicated that the anatomic
distribution of non-distant metastatic thoracic ESCC may be another
independent determinant of resectability in the TC. The esophagus
is divided into the cervical region, and the upper, middle and
lower thoracic regions by the thoracic entrance, the inferior edge
of the azygos vein arch and the inferior edge of inferior pulmonary
vein, respectively (19). ESCC is
most commonly observed in the middle thoracic portion, followed by
the lower and upper thoracic portions (20). According to a previous study
(21), upper thoracic ESCC may be
diagnosed as advanced-stage cancer with a high possibility of
invading adjacent organs; patients with this type cannot undergo
esophagectomy, which indicates that the treatment strategies of
ESCC may vary based on different anatomical distributions.
Clinically, the upper edge of the resected esophagus should be 5 cm
away from the upper edge of the tumor. Patients with upper thoracic
esophageal cancer within 5 cm from the cricopharyngeal muscle
should receive radical chemoradiotherapy, with no consideration of
surgical treatment according to NCCN guidelines (6). If the proximal end of the upper
thoracic esophageal cancers is <5 cm from the cricopharyngeal
muscle, the resection margin may be insufficient (22). The surgical resection of the middle
and lower thoracic ESCC cannot be limited by the tumor anatomic
location, as the upper edge of the tumor anatomic location is >5
cm away from the cricopharyngeal muscle (6). Thus, the anatomical distribution of
non-distant metastatic thoracic ESCC can be an independent
determinant for esophagectomy.
The cT and cN stages of non-distant metastatic ESCC
did not exhibit any independent effects on resectability in the
present study. The approximate representation of one-dimensional
data and the interaction could interpret the loss of the impact of
the cT and cN stages of ESCC in the multivariate analysis (23,24),
which has been proven to be related to the GTV of ESCC in previous
studies (11,12).
Based on the independent determinants in TC, the
present study subsequently took both GTV and tumor location into
consideration to perform the ROC analysis, in order to explore a
novel quantitative model for determining the resectability of
non-distant metastatic thoracic ESCC for the first time. The
results of ROC analysis demonstrated that GTV corresponding to
anatomic distributions measured using MDCT could well determine the
resectability of non-distant metastatic ESCC with AUC values of
>0.9. Higher AUCs were obtained to determine the resectability
of thoracic ESCC using the current ROC models when compared with
the previous CT radiomics model (maximum AUC, 0.947 vs. 0.924)
(8). The likely reason for this may
be that the present study specifically involved GTV corresponding
to esophageal anatomical distribution and excluded the patients
with distant metastasis. In addition, the results of unweighted
Cohen's Kappa tests revealed that the results obtained had
excellent reliability with Cohen k-values >0.87. The clinical
significance of the aforementioned quantitative ROC models
corresponding to the combination of GTV and anatomic distribution
may thus help to determine the resectability of non-distant
metastatic ESCC and may aid in the selection of optimal treatment
strategies.
The present study had several limitations which
should be mentioned. Firstly, the present study was a retrospective
single-center research and included the data of patients obtained
from January 2017 to December 2020. However, the present study
demonstrated a good performance for the resectability of ESCC due
to the large sample size; in addition, the authors aim to collect
data from the previous 1 to 2 years to carry out related research
in the future to confirm the current findings. Secondly, all
samples were from patients with ESCC. In the future, the authors
also aim to further investigate whether the findings obtained in
the present study are also applicable to patients with esophageal
adenocarcinoma. Thirdly, the GTV of ESCC was obtained by manually
depicting the abnormally thickened esophageal wall as opposed to
using a machine learning algorithm. However, the present study
exhibits repeatability in measuring the GTV of ESCC. Fourthly, the
methods used in the present study may be not applicable to patients
with a decreased renal function or allergies, as these patients
cannot undergo contrast-enhanced CT to identify smaller ESCC
lesions or distinguish between the tumor itself and surrounding
tissues. The authors thus aim to conduct relevant research using
magnetic resonance imaging in the future. Lastly, the present study
did not compare the prognosis of patients with non-distant
metastatic thoracic ESCC shown to be eligible for surgery as per
the NCCN guidelines and that of cases for which eligibility for
surgery was calculated using the GTV, in order to determine which
method is associated with an improved prognosis. Thus, further
studies are required to determine prognosis in future.
In conclusion, the present study demonstrated that
the GTV and anatomic distribution may be potential independent
determinants of the resectability of non-distant metastatic
thoracic ESCC. GTV based on anatomic distributions can effectively
quantitatively determine resectability with AUCs >0.9. It is
hoped that the findings presented in the present study may provide
a novel quantitative procedure that can be used to help clinicians
formulate the optimal treatment strategy for patients.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. 82271959), and the
Nanchong-University Cooperative Research Project (grant no.
20SXQT0329).
Availability of data and materials
The datasets used and/or analyzed during the current
study available from the corresponding author on reasonable
request.
Authors' contributions
TWC, RL and XMZ participated in the design of the
study. DG, BGT, JO, HYZ, ZYY and KYL contributed to data analysis.
TWC, DG, BGT and JO drafted and revised the manuscript. TWC, DG,
BGT and JO proofread the manuscript. TWC submitted the manuscript.
TWC and DG confirm the authenticity of all the raw data. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics Review
Committee of the Affiliated Hospital of North Sichuan Medical
College (approval no. 2021ER044-1). The ethics committee waived the
need for informed consent due to the retrospective nature of the
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ESCC
|
esophageal squamous cell carcinoma
|
|
MDCT
|
multidetector computed tomography
|
|
GTV
|
gross tumor volume
|
|
TC
|
training cohort
|
|
VC
|
validation cohort
|
|
ROC
|
receiver operating characteristic
|
|
NCCN
|
National Comprehensive Cancer
Network
|
|
ICC
|
intraclass correlation coefficient
|
|
AUC
|
area under the receiver operating
characteristic curve
|
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Smyth EC, Lagergren J, Fitzgerald RC,
Lordick F, Shah MA, Lagergren P and Cunningham D: Oesophageal
cancer. Nat Rev Dis Primers. 3:170482017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kato H and Nakajima M: Treatments for
esophageal cancer: A review. Gen Thorac Cardiovasc Surg.
61:330–335. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jiao R, Luo H, Xu W and Ge H: Immune
checkpoint inhibitors in esophageal squamous cell carcinoma:
Progress and opportunities. Onco Targets Ther. 12:6023–6032. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ma X, Zhao W, Li B, Yu Y, Ma Y, Thomas M
and Zhang Y, Xiang J and Zhang Y: Neoadjuvant immune checkpoint
inhibitors plus chemotherapy in locally advanced esophageal
squamous cell carcinoma: Perioperative and survival outcomes. Front
Oncol. 12:8108982022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ajani JA, D'Amico TA, Bentrem DJ, Chao J,
Corvera C, Das P, Denlinger CS, Enzinger PC, Fanta P, Farjah F, et
al: Esophageal and esophagogastric junction cancers, version
2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr
Canc Netw. 17:855–883. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Giganti F, Ambrosi A, Petrone MC, Canevari
C, Chiari D, Salerno A, Arcidiacono PG, Nicoletti R, Albarello L,
Mazza E, et al: Prospective comparison of MR with
diffusion-weighted imaging, endoscopic ultrasound, MDCT and
positron emission tomography-CT in the pre-operative staging of
oesophageal cancer: Results from a pilot study. Br J Radiol.
89:201600872016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ou J, Li R, Zeng R, Wu CQ, Chen Y, Chen
TW, Zhang XM, Wu L, Jiang Y, Yang JQ, et al: CT radiomic features
for predicting resectability of oesophageal squamous cell carcinoma
as given by feature analysis: A case control study. Cancer Imaging.
19:662019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Umeoka S, Koyama T, Togashi K, Saga T,
Watanabe G, Shimada Y and Imamura M: Esophageal cancer: Evaluation
with triple-phase dynamic CT-initial experience. Radiology.
239:777–783. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kuriakose MA, Loree TR, Hicks WL, Welch
JJ, Wang H and DeLacure MD: Tumour volume estimated by computed
tomography as a predictive factor in carcinoma of the tongue. Br J
Oral Maxillofac Surg. 38:460–465. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li H, Chen TW, Li ZL, Zhang XM, Chen XL,
Wang LY, Zhou L, Li R, Li CP and Huang XH: Tumour size of
resectable oesophageal squamous cell carcinoma measured with
multidetector computed tomography for predicting regional lymph
node metastasis and N stage. Eur Radiol. 22:2487–2493. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li H, Chen TW, Zhang XM, Li ZL, Chen XL,
Tang HJ, Huang XH, Chen N, Yang Q and Hu JN: Computed tomography
scan as a tool to predict tumor T category in resectable esophageal
squamous cell carcinoma. Ann Thorac Surg. 95:1749–1755. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moss AA, Schnyder P, Thoeni RF and
Margulis AR: Esophageal carcinoma: Pretherapy staging by computed
tomography. AJR Am J Roentgenol. 136:1051–1056. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Koo TK and Li MY: A guideline of selecting
and reporting intraclass correlation coefficients for reliability
research. J Chiropr Med. 15:155–163. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu YP, Tang S, Tan BG, Yang LQ, Lu FL,
Chen TW, Ou J, Zhang XM, Gao D, Li KY, et al: Tumor stage-based
gross tumor volume of resectable esophageal squamous cell carcinoma
measured on CT: Association with early recurrence after
esophagectomy. Front Oncol. 11:7537972021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qiao J, Qin J, Xing D, Li S and Wu D:
Diagnosis of retrolingual obstruction during drug-induced sleep
endoscopy versus polysomnography with nasopharyngeal tube in
patients with obstructive sleep apnea. Ann Otol Rhinol Laryngol.
130:1285–1291. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rice TW, Ishwaran H, Blackstone EH,
Hofstetter WL, Kelsen DP and Apperson-Hansen C; Worldwide
Esophageal Cancer Collaboration Investigators, : Recommendations
for clinical staging (cTNM) of cancer of the esophagus and
esophagogastric junction for the 8th edition AJCC/UICC staging
manuals. Dis Esophagus. 29:913–919. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Panebianco V, Grazhdani H, Iafrate F,
Petroni M, Anzidei M, Laghi A and Passariello R: 3D CT protocol in
the assessment of the esophageal neoplastic lesions: Can it improve
TNM staging? Eur Radiol. 16:414–421. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shi H, Zhang K, Niu ZX, Wang WP, Gao Q and
Chen LQ: Does tumour location influence postoperative long-term
survival in patients with oesophageal squamous cell carcinoma? Eur
J Cardiothorac Surg. 48:266–272. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rice TW, Rusch VW, Apperson-Hansen C,
Allen S, Chen LQ, Hunter JG, Kesler KA, Law S, Lerut TE, Reed CE,
et al: Worldwide esophageal cancer collaboration. Dis Esophagus.
22:1–8. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kato H, Tachimori Y, Watanabe H, Yamaguchi
H, Ishikawa T and Kagami Y: Thoracic esophageal carcinoma above the
carina: A more formidable adversary? J Surg Oncol. 65:28–33. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kuwano H, Nishimura Y, Oyama T, Kato H,
Kitagawa Y, Kusano M, Shimada H, Takiuchi H, Toh Y, Doki Y, et al:
Guidelines for diagnosis and treatment of carcinoma of the
esophagus April 2012 edited by the Japan esophageal society.
Esophagus. 12:1–30. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim S, Han K, Seo N, Kim HJ, Kim MJ, Koom
WS, Ahn JB and Lim JS: T2-weighted signal intensity-selected
volumetry for prediction of pathological complete response after
preoperative chemoradiotherapy in locally advanced rectal cancer.
Eur Radiol. 28:5231–5240. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao Q, Wan L, Zou S, Zhang C E T, Yang Y,
Ye F, Zhao X, Ouyang H and Zhang H: Prognostic risk factors and
survival models for T3 locally advanced rectal cancer: What can we
learn from the baseline MRI? Eur Radiol. 31:4739–4750. 2021.
View Article : Google Scholar : PubMed/NCBI
|