Introduction
Primary renal epithelial tumors are typically
malignant, whereas benign adenomas are rare. Metanephric adenomas
(MAs) are a rare type of tumor, accounting for 0.2% of adult renal
epithelial tumors. Although MA can occur at any age, it is more
common in women aged 50–60 years. MA is extremely rare in children,
with only a few cases reported to date. Most patients with MA
display no obvious clinical symptoms and they often seek medical
treatment due to physical examination or incidental findings. Only
a small number of patients report lower back pain, presence of an
abdominal mass or gross or microscopic hematuria (1). The present study aimed to report a
case of a 5-year-old female patient found to have a space-occupying
mass in the right kidney during a routine pre-enrollment physical
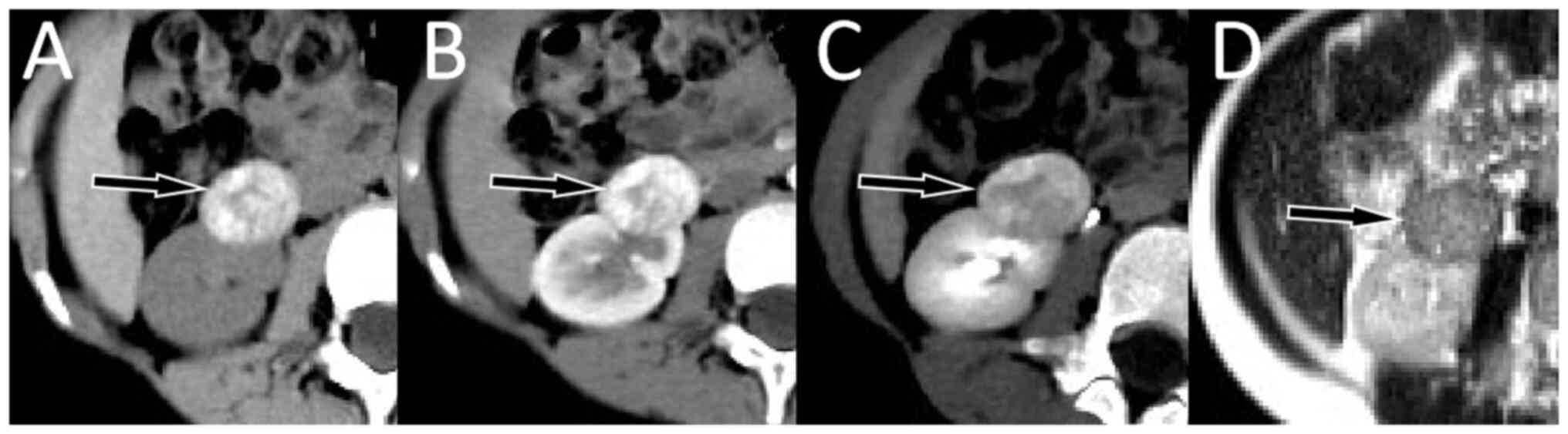
examination. Computed tomography (CT) scan images revealed multiple
high-density calcifications in the mass and contrast-enhanced CT
and magnetic resonance imaging (MRI) demonstrated that the mass was
significantly enhanced in the cortical phase and decreased in the
medullary phase. Based on these findings, the mass was first
diagnosed as an angiomyolipoma before surgery, while postoperative
pathology confirmed it to be a MA.
Case report
A 5-year-old female presented to The Affiliated
Hospital of Zunyi Medical University for a routine physical
examination. The physical examination revealed a hard palpable mass
on the right side of the abdomen, without tenderness, rebound pain
or muscle tension. The patient had no previous clinical signs such
as waist pain, frequent urination, urgency, dysuria or gross
hematuria. A kidney B-ultrasound demonstrated a mass in the
patient's right kidney in August 2019. Laboratory testing revealed
an elevated level of carbohydrate antigen 19–9 level at a value of
128.4 (expected value <25). Additional tumor markers and routine
blood examination values were within the expected reference value
range. CT scan showed that the mass was clearly demarcated from the
surrounding normal renal parenchyma and multiple high-density
calcifications were observed inside. Contrast-enhanced CT scan and
MRI showed that the tumor in the cortical stage was greatly
enhanced, while in the medullary stage, it was significantly
weakened (Fig. 1). These imaging
findings were different from those of the majority of renal
malignant tumors, including nephroblastoma, neuroblastoma, and
renal carcinoma, with cystic necrosis often visible in the tumors,
and therefore a fat-poor renal angiomyolipoma (RAML) was
considered. A preoperative needle biopsy was not performed as the
family of the patient opted for an intraoperative frozen biopsy and
the patient subsequently underwent a tumor resection under general
anesthesia at 13 days post-admission.
During the operation, a piece of tumor tissue was
removed, washed and dried with distilled water, and the tumor
tissue sections were placed on the frozen section machine table.
The temperature was adjusted to −20°C and the tissues were frozen
for ~3 min. The diseased tissue was cut into 3- to 5-µm slices, and
the frozen slices were treated using a hematoxylin-eosin staining
method. After staining, the slices were placed under an optical
microscope for observation at ×400 magnification. Under the
microscope, the size of the tumor cells was relatively consistent
and they were mainly arranged in an acinar-like pattern (Fig. 2). The tumor cells were small in
size, with little cytoplasm, round or oval nuclei and no nucleoli
and mitoses. Based on these pathological findings, the patient was
considered to have a benign tumor. After the surgery, the excised
tumor tissue was sent to the pathology department for further
immunohistochemistry (all specimens were fixed with 10% neutral
formalin, dehydrated at room temperature for ~24 h and paraffin
embedded. The 3- to 4-µm thick sections were stained for creatine
kinase, vimentin, paired box protein 8, Wilm's tumor 1, cluster of
differentiation 10, epithelial membrane antigen and CD56, with
antibodies purchased from MXB Biotechnologies, and viewed at ×400
magnification under an optical microscope) and the results showed
that tumor cells positively expressed CK, vimentin, PAX-8, WT-1 and
CD10, but did not express EMA and CD56, and the Ki-67 index was
<1%. Based on these results, the patient was diagnosed with MA.
The patient underwent no further treatment following surgical
resection of the tumor due to the benign nature of MAs. The patient
attended follow-up appointments for 2 years and the patient's
parents reported that the patient had no discomfort.
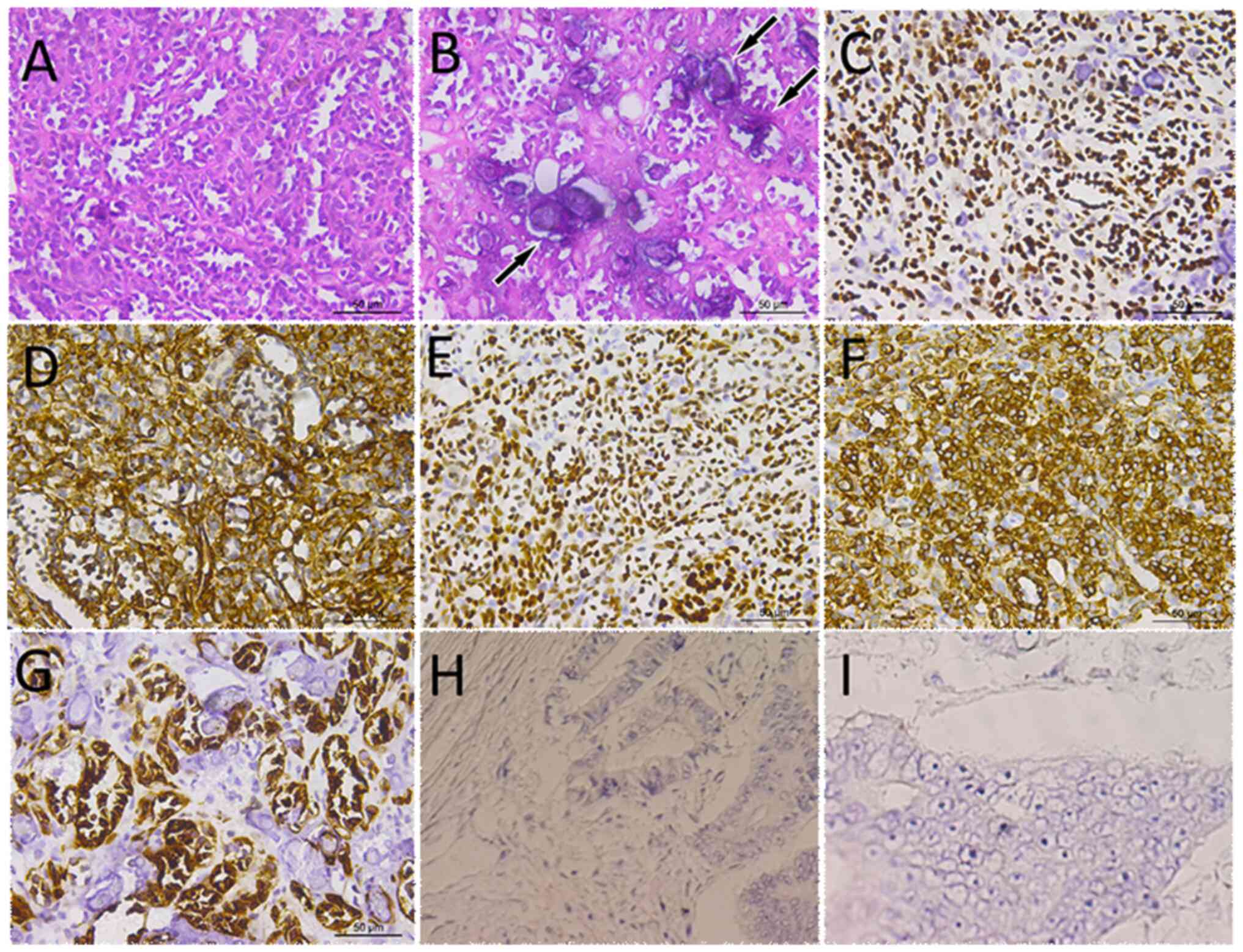 | Figure 2.Staining of patient tumor tissue. (A)
H&E staining showed that the size of the tumor cells was
relatively consistent, mainly in acinar-like arrangement, and the
tumor cells were small in size, with little cytoplasm, round or
oval nuclei, and no nucleoli and mitoses. (B) H&E staining
showed blue-stained calcifications (arrows) observed under high
magnification. Immunohistochemical staining of tumor tissues
showing cells expressing (C) Wilms tumor 1, (D) vimentin, (E)
PAX-8, (F) WT-1 and (G) CD10, but negatively expressing (H) EMA and
(I) CD56. H&E, hematoxylin and eosin. |
Literature review
The PubMed (https://pubmed.ncbi.nlm.nih.gov/), Embase (https://www.embase.com/) and Web of Science databases
(https://www.webofscience.com/wos/woscc/basic–search)
(as of September 1, 2022), were searched for case reports and case
series of pediatric patients with MA. Language restrictions were
limited to English. For each relevant case report, the first
author, publication year and country, as well as the patient's age,
sex, main clinical symptoms, tumor location (left or right), CT and
MRI imaging findings, immunohistochemical results, treatment
methods and follow-up results were recorded (Table I).
 | Table I.Clinical and imaging features of the
cases of children with MA based on the literature review and the
present study. |
Table I.
Clinical and imaging features of the
cases of children with MA based on the literature review and the
present study.
| First author,
year | Country | Patient sex, age,
years) | Main symptoms | Tumor location | CT/MRI | CECT/MRI | Maximum diameter,
cm |
Immunohisto-chemistry results | Treatment | Follow-up
results | (Refs.) |
|---|
|
|---|
| Density/signal | Calcification | Cystic | T1WI | T2WI | DWI |
|---|
| Benson et
al, 2018 | USA | F, 10 | Abdominal pain for
1 week | R | Heterogeneous | + | + | Hypo | Hypo | Hyper |
Hypo-enhancement | 3.3 | WT-1 (+), CD57 (+),
CD56 (+), TTF1 (+), CK7 (−), EMA (−), S100 (−) | PN | NA | (2) |
| Küpeli et
al, 2009 | Turkey | F, 6 | Abdominal pain,
polyuria | R | Heterogeneous | + | + | NA | NA | NA | NA | 6.5 | WT-1 (+), NSE (+),
CD57 (+), CD56 (+), K7 (−) | PN | 24 months without
recurrence | (3) |
| Sarhan et
al, 2022 | Egypt | M, 1.75 | Abdominal
distension, polyuria | L | Homogenous | - | - | NA | NA | NA |
Hypo-enhancement | 2.0 | WT-1 (+), CD57
(+) | PN | 12 months without
recurrence | (4) |
| Amodio et
al, 2005 | USA | F, 8 | Nausea, vomiting,
diarrhea for 2 days | L | Heterogeneous | + | + | Hypo | Hypo | NA | NA | 3.0 | NA | TN | NA | (5) |
| Bastos et
al, 2007 | Brazil | F, 2 | Urinary tract
infection | L | Heterogeneous | + | - | NA | NA | NA | NA | 4.0 | NA | TN | 15 months without
recurrence | (6) |
| Drut et al,
2001 | Argentina | F, 11 | Hematuria | L | NA | NA | NA | NA | NA | NA | NA | 4.5 | NA | TN | NA | (8) |
| Hu et al,
2022 | P.R. China | M, 2 | Occasionally
found | L | Homogenous | - | - | Iso | Slightly hyper | Hyper | Delayed
reinforcement | 5.4 | Vim (+), CK (+),
WT-1 (+), CD99 (+), INI-1 (+), PAX-8 (+), CD56 (+), CD57 (+), CD10
(−), S100 (−) | PN + Ch | NA | (9) |
| Jiang et al,
2019 | P.R. China | M, 3 | Back pain | R | Heterogeneous | - | + | Hypo | Iso | Hyper | Delayed
reinforcement | 4.5 | NA | PN | 48 months without
recurrence | (10) |
| Kohashi et
al, 2009 | Japan | M, 9 | Hematuria | L | NA | NA | NA | NA | NA | NA | NA | 1.9 | Vim (+), CK (+),
WT-1 (+), CK7 (−), EMA (−) | TN | 12 months without
recurrence | (11) |
| Loeser et
al, 2009 | Germany | F, 2 | Hematuria | L | Heterogeneous | NA | NA | NA | NA | NA |
Hypo-enhancement | 2.0 | WT-1 (+), CD56 (+),
CD57 (+) | PN | No recurrence | (12) |
| Portugal and
Barroca, 2008 | Portugal | F, 8 | Abdominal mass | R | NA | NA | NA | NA | NA | NA | NA | 10.0 | CD56 (+), CD57 (+),
EMA (−), Syn (−) | TN | NA | (13) |
| McNeil et
al, 2008 | USA | M, 5 | Chyluria | L | NA | NA | NA | NA | NA | NA | NA | 2.0 | NA | TN | 12 months without
recurrence | (14) |
| Rakheja et
al, 2005 | USA | M, 10 | Hematuria | R | NA | NA | NA | NA | NA | NA | NA | 1.7 | WT-1 (+), CD56 (+),
CD57 (+), CK (+), CK7 (+), P53 (−) | PN | NA | (15) |
| Ünal et al,
2020 | Turkey | M, 7.5 | Anorexia | NA | NA | NA | NA | NA | NA | NA | NA | 4.0 | NA | TN | No recurrence | (16) |
| Zhang et al,
2020 | P.R. China | M, 11 | Occasionally
found | R | NA | NA | NA | NA | NA | NA | NA | 3.0 | NA | PN | 92 months without
recurrence | (17) |
| Keshani de Silva
et al, 2000 | USA | M, 9 | Proteinuria | L | NA | NA | NA | NA | NA | NA | NA | 1.0 | Vim (+), CAM5.2
(+), AE1/3 (+), EMA (−), CK7 (−) | TN | NA | (18) |
| Renshaw et
al, 2000 | USA | F, 7 | Hematuria | L | Heterogeneous | - | + | NA | NA | NA | NA | 10.5 | CAM5.2 (+), CK7
(+), EMA (−), AE1 (−) | TN + Ch | 2 months without
recurrence | (19) |
| Navarro et
al, 1999 | Canada | F, 9 | Urinary tract
infection | R | Heterogeneous | - | + | NA | NA | NA |
Hyperenhancement | 5.0 | NA | PN | No recurrence | (20) |
| Mahoney et
al, 1997 | USA | F, 2 | Lethargy,
polydipsia | R | Homogenous | - | - | NA | NA | NA | Did not
enhance | 2.1 | NA | PN | No recurrence | (21) |
| Le Nué et
al, 2011 | France | F, 4 | Increased red blood
cells | R | Heterogeneous | + | - | Iso |
Hypo-enhancement | NA | Hypo- | 5.3 | WT-1 (+), AE1/3
(+), EMA (−), CK7 (−), CD56 (−), desmin (−) | PN | 24 months without
recurrence | (22) |
| Barroca et
al, 2016 | Portugal | M, 8 | Vomiting, abdominal
pain | R | Homogenous | - | - | NA | NA | NA | NA | 3.1 | WT-1 (+), CD56 (+),
CD57 (+), EMA (−), CK7 (−) | TN | NA | (23) |
| Mei et al,
2010 | P.R. China | F, 2 | Occasionally
found | R | Heterogeneous | + | + | Hypo | Hyper | NA |
Hypo-enhancement | 2.5 | CD56 (+), AE1/AE3
(+), EMA (+), Vim (+) | PN | 14 months without
recurrence | (24) |
| Davis et al,
1995 | USA | F, 10 | NA | R | NA | NA | NA | NA | NA | NA | NA | 2.5 | NA | PN | No recurrence | (7) |
| Ozden et al,
2015 | Turkey | M, 10 | Occasionally
found | R | Homogenous | - | - | Hypo | NA | NA |
Hypo-enhancement | 2.0 | NA | PN | 18 months without
recurrence | (25) |
| Pasricha et
al, 2012 | India | M, 4 | Abdominal pain and
distension for 2 months | Bilateral | Homogenous | - | + | NA | NA | NA |
Hypo-enhancement | 15.5 | Vim (+), CD57 (+),
EMA (−), CD56 (−) | TN | 8 months without
recurrence | (26) |
| Spaner et
al, 2014 | Canada | M, 7 | Occasionally
found | L | Heterogeneous | + | + | NA | NA | NA | Delayed
reinforcement | 3.0 | WT-1 (+), Vim (+),
S100 (+), EMA (−), CD34 (−), Syn (−) | PN | NA | (27) |
| Yin et al,
2015 | P.R. China | F, 0.2 | Prenatal
check-up | L | Heterogeneous | - | + | Hypo | NA | NA |
Hypo-enhancement | 9.0 | WT-1 (+), Vim (+),
CD10 (+), CD57 (+), CK7 (+), EMA (−), TFE3 (−) | TN | 18 months without
recurrence | (28) |
| Present study | P.R. China | F, 5 | Occasionally
found | R | Heterogeneous | + | + | Hypo | Hypo | Hypo | Fast in fast
out | 3.0 | CK (+), Vimentin
(+), PAX-8 (+), WT-1 (+), CD10 (+), SMA (−), CD56 (−) | PN | 24 months without
recurrence |
|
SPSS software (version 18.0; IBM Corp.) was used for
data analysis. The measurement data conforming to the normal
distribution were presented as the mean ± standard deviation. The
non-normal distribution was presented as the median (upper and
lower quartiles). The chi-squared test was used to compare the data
between groups. P<0.05 was considered to indicate a
statistically significant difference.
A systematic database search showed that 27 studies
reported MA in pediatric patients prior to the case reported in the
present study (2–28), resulting in a total of 28 pediatric
patients with MA. Of these, 13 patients were male and 15 were
female. The proportion of Caucasian patients (n=20) was
significantly higher compared with Xanthoderm patients (n=8). No
significant difference was demonstrated between patients with a
left or right tumor location. One patient had MAs in both kidneys
(26). In most patients, MA was
found after a routine physical examination, abdominal pain or
diarrhea. Rare clinical symptoms and signs included hematuria,
urinary tract infection, polycythemia, chyluria and proteinuria.
The maximum diameter of tumors ranged from 1.0-15.5 cm, but most
tumors were <5.0 cm in diameter at the time of diagnosis. In the
available data, the density and signal of most tumors on CT or MRI
was heterogenous (13/19) and the probability of cystic change was
slightly higher than that of calcification (Table II). Only a few studies reported MRI
findings related to MA, which usually showed a low signal on
T1-weighted imaging (T1WI) and hypo-to-hypersignal on T2-weighted
imaging (T2WI). Most tumors displayed hypo-enhancement on
contrast-enhanced CT or T1WI and some patients showed progressive
enhancement during delayed scanning.
 | Table II.Distribution and imaging findings of
metanephric adenoma in children. |
Table II.
Distribution and imaging findings of
metanephric adenoma in children.
| Parameters | Proportion of
patients, n/total n (%) | P-value |
|---|
| Patient sex |
| 0.496 |
|
Male | 13/28 (46.4) |
|
|
Female | 15/28 (53.6) |
|
| Patient
ethnicity |
| <0.001 |
|
Xanthoderm | 8/28 (28.6) |
|
|
Caucasian | 20/28 (71.4) |
|
| Tumor density |
| 0.027 |
|
Heterogeneity | 13/19 (68.4) |
|
|
Homogeneity | 6/19 (31.6) |
|
| Calcification |
| 0.816 |
|
Yes | 8/18 (44.4) |
|
| No | 10/18 (55.6) |
|
| Cystic change |
| 0.342 |
|
Yes | 11/18 (61.1) |
|
| No | 7/18 (38.9) |
|
| Maximum diameter,
cm |
| <0.001 |
|
<5.0 | 20/28 (71.4) |
|
|
≥5.0 | 8/28 (28.6) |
|
The immunohistochemistry staining results of the
tissues of 17 patients with MA in 17 cases were analyzed. These
results demonstrated that almost all tumors positively expressed
WT-1 and most of the tumors positively expressed vimentin, CD56 and
CD57 and almost negatively expressed EMA, S100, and so forth. The
majority of the 28 patients with MA underwent partial nephrectomy.
In some cases, the tumors were considered as nephroblastoma or
could not be identified before surgery. These patients underwent
radical nephrectomy. None of these patients showed signs of
recurrence during follow-up because of the benign nature of MA.
Discussion
MA was first reported by Bove et al in 1979
(29) and named by Brisigotti et
al (30) in 1992. MA tends to
occur in the renal cortex and its histological origin remains
unclear. The World Health Organization's histopathological
classification of renal tumors classified MA, metanephric
adenofibroma and metanephric stromal tumors as metanephric tumors
and their biological symptoms as benign (31). The disease can occur at any age but
is more common in women aged 50–60 years (32). The incidence of MA in children is
extremely rare (9,31), with only 27 cases reported in the
literature before the present study. Most of the patients with MA
have no obvious clinical symptoms and they often seek medical
treatment after a physical examination or incidental findings. Only
a few patients may have low back pain, abdominal mass or gross or
microscopic hematuria (1). The
patient in the present study was a 5-year-old female whose kidney
mass was discovered incidentally during a routine pre-enrollment
physical examination.
Imaging examinations, including B-ultrasound, CT and
MRI, serve a significant role in the diagnosis and differential
diagnosis of renal tumors. The ultrasonographic appearance of MAs
may be a well-circumscribed hyperechoic, anechoic or hypoechoic
solid mass, with few intratumoral blood vessels (33). CT images indicate that most of these
tumors are soft tissue masses with a clear boundary and uniform
density, the mass has cystic or/and calcification and the density
is not uniform. Larger masses protrude from the kidney outline and
grow outward. On contrast-enhanced scans, the tumors mostly
demonstrate mild enhancement in the cortical phase and progressive
enhancement in the medullary and delayed phases and the enhancement
degree is lower than that of the surrounding normal renal
parenchyma. Tumors appear hypointense on T1WI and hypointense to
hyperintense on T2WI compared with the adjacent renal parenchyma on
MRI. The tumors are predominantly hyperintense compared with
adjacent renal parenchyma on fat-suppressed T2WI and
diffusion-weighted imaging (DWI) (10,33).
The patient in the present study presented with a
high-density mass protruding out of the kidney contour on CT and
decreased enhancement of delayed phase on contrast-enhanced scan,
which was similar to the imaging findings of fat-poor RAML. The
T1WI, T2WI and DWI sequences of MRI showed heterogeneous low-signal
changes, which were different from the imaging findings of typical
MA as the mass of the patient in the present study had diffuse
calcification. As the patient in the present study had a mass with
diffuse calcification, it was necessary to differentiate the mass
from fat-poor RAML. RAML comprises varying proportions of fat,
smooth muscle and abnormal blood vessels. It is sometimes difficult
to diagnose on imaging because of the lack of fat or less fat.
Fat-poor RAMLs have a slightly high density on CT and enhanced scan
usually shows uniform enhancement, the degree of enhancement in the
delayed phase is reduced and T2WI shows uniform, slightly low
signals or a few patchy high signals (34,35).
In addition, MA should also be differentiated from renal carcinoma,
Wilms tumor (WT), renal oncocytoma (RO) and neuroblastoma.
Renal carcinoma is the most common type of clear
cell carcinoma. It is more common in adults and mostly occurs in
the renal cortex. CT plain scan images demonstrate that these
tumors are mostly isodense and low-density cystic degeneration,
necrosis and high-density calcification are often seen in the
tumor. Contrast-enhanced scans demonstrate obvious uneven
enhancement in the early stage. This enhancement gradually
decreases in the middle and late stages, which is a typical
‘fast-in, fast-out’ performance (36). Chromophobe carcinoma and clear cell
carcinoma demonstrate similar imaging manifestations, but central
spoke scars can also be seen in MRI images with larger tumor
volumes being associated with increased numbers of spoke scars
(36). Renal papillary carcinoma
has a lower degree of enhancement on contrast-enhanced scans than
the renal parenchyma, but the enhancement lasts longer and is
progressive (36–38). WT is the most common renal malignant
tumor in children. According to statistics, in the 10 years from
2005 to 2014, the incidence of nephroblastoma in developing
countries was ~1 in 8,000 (39). On
CT or MRI images, WT mostly appears as a large mass with equal or
slightly low density and signal (40). Necrosis, cystic degeneration and
hemorrhage are common in the tumor and calcification can be seen in
some lesions (40). RO is another
type of rare benign tumor of the kidney, accounting for only 7% of
kidney tumors in the United States between 1980 and 1995, which
tends to occur in middle-aged and elderly men and mostly originates
from the renal cortex (41). On CT
images, RO appears as a well,-circumscribed soft tissue mass which
protrudes beyond the renal contour. Contrast-enhanced scans show
lower enhancement than normal renal parenchyma in both early and
late phases (42). Some lesions
show central stellate scar sign and segmental enhancement inversion
sign, which are relatively specific to RO (43). Neuroblastoma is a malignant tumor
that occurs more frequently in children and is less common in the
kidneys, the incidence rate in the United States was
10.5/106 between 1990 and 2002 (44). A typical renal neuroblastoma is a
large, lobulated soft tissue mass that is prone to necrosis, cystic
degeneration and hemorrhage. It is likely to be accompanied by
calcification, which is usually characterized by sandy or
large-area calcification (45). The
mass is likely to surround and bury the renal blood vessels
(46).
The diagnosis of MA is mainly based on
histopathological examination. Microscopically, small and uniform
tumor cells are seen in an acinar-like arrangement, with little
cytoplasm, fine chromatin, no or inconspicuous nucleoli and rare
mitoses (47). Most previously
reported MA cases were positive for WT-1, CD57, CD56, AEl/AE3,
CAM5.2, CK18 and vimentin, negative for EMA, NSE, CEA, CgA, Syn and
P504S and focally positive or negative for CK7 (11,48).
The microscopic structural features of the patient in the present
study were consistent with the aforementioned literature. The
immunohistochemical staining results demonstrated that the tumor
cells positively expressed CK, vimentin, PAX-8, WT-1 and CD10, but
did not express EMA and CD56. Therefore, these results were
consistent with the diagnosis of MA.
Surgical resection of the tumor is the main
treatment method for MA and the surgical method has gradually
changed from nephrectomy to nephron-sparing surgery due to the
benign nature of the tumor (25,49).
For patients who experience difficulties obtaining a clinical
diagnosis, percutaneous biopsy cytology examination has certain
clinical significance, and radical nephrectomy cannot be blindly
performed. Intraoperative frozen pathological examination can
clarify the diagnosis and aid in selection of an appropriate
surgical method, which is an ideal diagnostic tools that can
minimize the occurrence of missed diagnosis and mistreatment and
avoid the need to perform a total nephrectomy (17). A previous study reported that MA can
be treated with a chemotherapy regimen also used to treat WT
(9). Overall, MA has a good
prognosis and even with a small number of cases of malignant MA
reported in previous studies, no recurrence has been reported
during follow-up (8,19,50).
The patient in the present study had no complaints of discomfort
during the 2-year follow-up period following surgical removal of
the mass. A limitation of current study is that the quality of the
MR images was relatively low, so only T2WI sequences are presented
in the article.
In conclusion, MA is a rare benign kidney tumor with
a low incidence in children. The typical imaging appearance of MA
is a soft tissue mass with relatively homogeneous density or signal
and delayed enhancement on contrast-enhanced scans. However, the
patient in the present study presented with heterogeneous
hyperdensity, with marked enhancement in the early phase of
contrast-enhanced scans and decreased enhancement in the delayed
phase. Therefore, the present case study demonstrated that MA
should be considered as one of the imaging differential diagnoses
of fat-poor angiomyolipoma, renal carcinoma, oncocytoma and the
like. Moreover, a preoperative correct understanding of MA can
avoid unnecessary radical nephrectomy.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XH, WL, DL, JC and JB conceived and designed the
study. PW, JC and XH acquired, analyzed and interpreted the data.
PW and JC confirm the authenticity of all the raw data. XH and WL
drafted the manuscript. JC and PW critically revised the manuscript
for important intellectual content and gave final approval of the
version to be published. All authors agreed to the journal to which
the article was submitted and agreed to take responsibility for all
aspects of the work. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Affiliated Hospital of Zunyi Medical University
(approval no. KLL-2023-102).
Patient consent for publication
Written informed consent was obtained from both
parents of the patient to publish this case report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang P, Tian Y, Xiao Y, Zhang Y, Sun FA
and Tang K: A metanephric adenoma of the kidney associated with
polycythemia: A case report. Oncol Lett. 11:352–354. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Benson M, Lee S, Bhattacharya R, Vasy V,
Zuberi J, Yasmeen S, Ahmed M and Hanna MK: Metanephric adenoma in
the pediatric population: Diagnostic challenges and follow-up.
Urology. 120:211–215. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Küpeli S, Baydar DE, Canakl F, Yalçn B,
Kösemehmetoğlu K, Tekgül S and Büyükpamukçu M: Metanephric adenoma
in a 6-year-old child with hemihypertrophy. J Pediatr Hematol
Oncol. 31:453–455. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sarhan OM, Al Farhan A, Abdallah S, Al
Ghwanmah H, Boqari D, Omar H, Al Faddagh A, Al Kanani H and Al
Kawai F: Pediatric metanephric adenoma with Fanconi-Bickel
syndrome: A case report and review of literature. Surg Case Rep.
8:862022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Amodio JB, Shapiro E, Pinkney L, Rivera R,
Strubel N, Douglas D and Fefferman N: Metanephric adenoma in an
8-year-old child: Case report and review of the literature. J
Pediatr Surg. 40:e25–e28. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Netto JM, Esteves TC, Mattos R, Tibiriçá
SH, Costa SM and Vieira LJ: Metanephric adenoma: A rare
differential diagnosis of renal tumor in children. J Pediatr Urol.
3:340–341. 2007. View Article : Google Scholar
|
|
7
|
Davis CJ Jr, Barton JH, Sesterhenn IA and
Mostofi FK: Metanephric adenoma. Clinicopathological study of fifty
patients. Am J Surg Pathol. 19:1101–1114. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Drut R, Drut RM and Ortolani C: Metastatic
metanephric adenoma with foci of papillary carcinoma in a child: A
combined histologic, immunohistochemical, and FISH study. Int J
Surg Pathol. 9:241–247. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu S, Zhao Z, Wan Z, Bu W, Chen S and Lu
Y: Chemotherapy combined with surgery in a case with metanephric
adenoma. Front Pediatr. 10:8478642022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang T, Li W, Lin D, Wang J, Liu F and
Ding Z: Imaging features of metanephric adenoma and their
pathological correlation. Clin Radiol. 74:408.e9–408.e17. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kohashi K, Oda Y, Nakamori M, Yamamoto H,
Tamiya S, Toubo T, Kinoshita Y, Tajiri T, Taguchi T and Tsuneyoshi
M: Multifocal metanephric adenoma in childhood. Pathol Int.
59:49–52. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Loeser A, Katzenberger T, Spahn M, Gerharz
EW and Riedmiller H: Metanephric adenoma in a two-year-old child.
Urol Int. 83:119–121. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Portugal R and Barroca H: Clear cell
sarcoma, cellular mesoblastic nephroma and metanephric adenoma:
Cytological features and differential diagnosis with Wilms tumour.
Cytopathology. 19:80–85. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McNeil JC, Corbett ST, Kuruvilla S and
Jones EA: Metanephric adenoma in a five-year-old boy presenting
with chyluria: Case report and review of literature. Urology.
72:545–547. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rakheja D, Lian F, Tomlinson GE, Ewalt DH,
Schultz RA and Margraf LR: Renal metanephric adenoma with
previously unreported cytogenetic abnormalities: Case report and
review of the literature. Pediatr Dev Pathol. 8:218–223. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ünal E, Yilmaz E, Özcan A, Işik B,
Karakükcü M, Turan C, Akgün H, Öztürk F, Coşkun A, Özdemir MA and
Patiroğlu T: Twenty children with non-Wilms renal tumors from a
reference center in Central Anatolia, Turkey. Turk J Med Sci.
50:18–24. 2020.PubMed/NCBI
|
|
17
|
Zhang L, Gao X, Li R, Li K, Liu B, Li J,
Zhang W and Tang M: Experience of diagnosis and management of
metanephric adenoma: Retrospectively analysis of 10 cases and a
literature review. Transl Androl Urol. 9:1661–1669. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Keshani de Silva V, Tobias V, Kainer G and
Beckwith B: Metanephric adenoma with embryonal hyperplasia of
Bowman's capsular epithelium: Previously unreported association.
Pediatr Dev Pathol. 3:472–478. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Renshaw AA, Freyer DR and Hammers YA:
Metastatic metanephric adenoma in a child. Am J Surg Pathol.
24:570–574. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Navarro O, Conolly B, Taylor G and Bägli
DJ: Metanephric adenoma of the kidney: A case report. Pediatr
Radiol. 29:100–103. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mahoney CP, Cassady C, Weinberger E,
Winters WD and Benjamin DR: Humoral hypercalcemia due to an occult
renal adenoma. Pediatr Nephrol. 11:339–342. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Le Nué R, Marcellin L, Ripepi M, Henry C,
Kretz JM and Geiss S: Conservative treatment of metanephric
adenoma. A case report and review of the literature. J Pediatr
Urol. 7:399–403. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Barroca HM, Cirnes L and Lopes JM:
Metanephric adenoma: Cytological, histological, and molecular
diagnosis of a case. Diagn Cytopathol. 44:263–266. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mei H, Zheng L, Zhou C and Tong Q:
Metanephric adenoma in a 2-year-old child: Case report and
immunohistochemical observations. J Pediatr Hematol Oncol.
32:489–493. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ozden E, Yagiz B, Atac F, Cetin H,
Bostanci Y, Yakupoglu YK and Sarikaya S: Laparoscopic
nephron-sparing surgery for metanephric adenoma in children: A
report of 2 cases. Urology. 86:165–167. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pasricha S, Gandhi JS, Gupta G, Mehta A
and Beg S: Bilateral, multicenteric metanephric adenoma associated
with Wilms' tumor in a child: A rare presentation with important
diagnostic and therapeutic implications. Int J Urol. 19:1114–1117.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Spaner SJ, Yu Y, Cook AJ and Boag G:
Pediatric metanephric adenoma: Case report and review of the
literature. Int Urol Nephrol. 46:677–680. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yin M, Cai J and Thorner PS: Congenital
renal tumor: Metanephric adenoma, nephrogenic rest, or malignancy.
Pediatr Dev Pathol. 18:245–250. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bove KE, Bhathena D, Wyatt RJ, Lucas BA
and Holland NH: Diffuse metanephric adenoma after in utero aspirin
intoxication. A unique case of progressive renal failure. Arch
Pathol Lab Med. 103:187–190. 1979.PubMed/NCBI
|
|
30
|
Brisigotti M, Cozzutto C, Fabbretti G,
Sergi C and Callea F: Metanephric adenoma. Histol Histopathol.
7:689–692. 1992.PubMed/NCBI
|
|
31
|
de Jel D, Hol JA, Ooms A, de Krijger RR,
Jongmans M, Littooij AS, Drost J, van Grotel M and van den
Heuvel-Eibrink MM: Paediatric metanephric tumours: A
clinicopathological and molecular characterization. Crit Rev Oncol
Hematol. 150:1029702020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schmelz HU, Stoschek M, Schwerer M, Danz
B, Hauck EW, Weidner W and Sparwasser C: Metanephric adenoma of the
kidney: Case report and review of the literature. Int Urol Nephrol.
37:213–217. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bastide C, Rambeaud JJ, Bach AM and Russo
P: Metanephric adenoma of the kidney: Clinical and radiological
study of nine cases. BJU Int. 103:1544–1548. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jinzaki M, Tanimoto A, Narimatsu Y, Ohkuma
K, Kurata T, Shinmoto H, Hiramatsu K, Mukai M and Murai M:
Angiomyolipoma: Imaging findings in lesions with minimal fat.
Radiology. 205:497–502. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rosenkrantz AB, Hecht EM, Taneja SS and
Melamed J: Angiomyolipoma with epithelial cysts: Mimic of renal
cell carcinoma. Clin Imaging. 34:65–68. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zokalj I, Marotti M and Kolarić B:
Pretreatment differentiation of renal cell carcinoma subtypes by
CT: The influence of different tumor enhancement measurement
approaches. Int Urol Nephrol. 46:1089–1100. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schieda N, Lim RS, McInnes M, Thomassin I,
Renard-Penna R, Tavolaro S and Cornelis FH: Characterization of
small (<4cm) solid renal masses by computed tomography and
magnetic resonance imaging: Current evidence and further
development. Diagn Interv Imaging. 99:443–455. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Young JR, Margolis D, Sauk S, Pantuck AJ,
Sayre J and Raman SS: Clear cell renal cell carcinoma:
Discrimination from other renal cell carcinoma subtypes and
oncocytoma at multiphasic multidetector CT. Radiology. 267:444–453.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bahoush G and Saeedi E: Outcome of
children with Wilms' tumor in developing countries. J Med Life.
13:484–489. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kang C, Shin HJ, Yoon H, Han JW, Lyu CJ
and Lee MJ: Differentiation between clear cell sarcoma of the
kidney and Wilms' tumor with CT. Korean J Radiol. 22:1185–1193.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Perez-Ordonez B, Hamed G, Campbell S,
Erlandson RA, Russo P, Gaudin PB and Reuter VE: Renal oncocytoma: A
clinicopathologic study of 70 cases. Am J Surg Pathol. 21:871–883.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ishigami K, Jones AR, Dahmoush L, Leite
LV, Pakalniskis MG and Barloon TJ: Imaging spectrum of renal
oncocytomas: A pictorial review with pathologic correlation.
Insights Imaging. 6:53–64. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kim JI, Cho JY, Moon KC, Lee HJ and Kim
SH: Segmental enhancement inversion at biphasic multidetector CT:
Characteristic finding of small renal oncocytoma. Radiology.
252:441–448. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Maris JM: Recent advances in
neuroblastoma. N Engl J Med. 362:2202–2211. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jiang M, Hu X, Qian K, Yang P, Tang Y,
Wang P and Cai J: Atypical CT findings of renal neuroblastoma: A
case report. Transl Pediatr. 11:1267–1273. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Akçaer V, Eren T and Taştekin E: Primary
renal neuroblastoma mimicking Wilms' tumor. Balkan Med J.
38:135–136. 2021.PubMed/NCBI
|
|
47
|
Udager AM, Pan J, Magers MJ, Palapattu GS,
Morgan TM, Montgomery JS, Weizer AZ, Hafez KS, Miller DC, Wolf JS
Jr, et al: Molecular and immunohistochemical characterization
reveals novel BRAF mutations in metanephric adenoma. Am J Surg
Pathol. 39:549–557. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Muir TE, Cheville JC and Lager DJ:
Metanephric adenoma, nephrogenic rests, and Wilms' tumor: A
histologic and immunophenotypic comparison. Am J Surg Pathol.
25:1290–1296. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Guo J, Zhou X, Fu B, Cao R, Liu W and Wang
G: Retroperitoneal laparoscopic partial nephrectomy for treatment
of metanephric adenoma (Report of 6 cases). Springerplus.
5:9962016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Pins MR, Jones EC, Martul EV, Kamat BR,
Umlas J and Renshaw AA: Metanephric adenoma-like tumors of the
kidney: Report of 3 malignancies with emphasis on discriminating
features. Arch Pathol Lab Med. 123:415–420. 1999. View Article : Google Scholar : PubMed/NCBI
|