Introduction
Pulmonary enteric adenocarcinoma (PEAC) is a rare
primary lung adenocarcinoma with histological morphologies similar
to those of metastatic colorectal cancer, but its etiology remains
elusive. Severe intestinal lesions accompanied by necrosis patterns
were observed in more than half of the cases reported (1,2), which
expressed at least one of the typical immunohistochemical markers
of intestinal differentiation, including caudal type homeobox 2
(CDX2), cytokeratin 20 (CK20) or mucin 2 (MUC2) (2–4).
Another review including 127 patients showed that the median
overall survival (OS) of patients with early PEAC was 56 months,
while the median OS of patients with advanced or metastatic
diseases was 14 months (5). PEAC is
often difficult to distinguish from metastasis of colon cancer, so
the differential diagnosis between these two types of cancer is a
challenging clinical issue (6).
Secondary bone malignancies are usually caused by
metastasis to the bone of other malignant tumors (7,8), while
secondary lymph malignancies are more common in intermediate and
advanced cancers. In the present case, it was hypothesized that the
metastasis of the bone and lymphatic system was caused by
deteriorated lung cancer. Of note, bone and lymphatic metastasis of
PEAC are rare to be observed.
In addition, most of the previously reported PEACs
are metastasized from colorectal cancer (6). However, in the present case, no
abnormalities were found during colorectal examination, which means
that this is a rare case of primary PEAC. There was no obvious
discomfort in the early and middle stages of cancer.
Case report
A 58-year-old male, a long-term smoker with ~8
cigarettes per day, was admitted to the Department of Orthopedics
of the Second Affiliated Hospital of Xi'an Jiaotong University
(Xi'an, China) in December 2022 due to pain in the lower back and
right iliac region with no obvious inductance. The patient denied
any history of chronic diseases, including hypertension, coronary
heart disease, diabetes, hepatitis, typhoid fever, malaria and
other infectious diseases. The case information and images
published in this case report were obtained with the patient's
written informed consent.
Physical examination indicated the following:
Positive for thoracolumbra spinous process tenderness, no
abnormalities were found in the auscultation of the heart and
lungs, abdominal signs and symptoms were negative and no
abnormalities were found in the limbs and nervous system
examination.
The first laboratory examination (blood sample)
showed that carcinoembryonic antigen, carbohydrate antigen 125
(CA125) (1,250.00 U/ml; normal range, 0–24 U/ml) and CA72-4 (74.50
U/ml; normal range, 0–6.9 U/ml) were markedly increased, while
neuron-specific enolase and non-small cell lung cancer-associated
antigen (CYFRA) were also increased to a certain extent. In a
repeat examination after 2 weeks, the above-mentioned indexes had
further increased to varying degrees (CA125: 3,706.00 U/ml; normal
range, 0–24 U/ml; CYFRA: 19.10 ng/ml; normal range, 0–3.3 ng/ml),
while the level of CA72-4 had decreased (53.70 U/ml; normal range,
0–6.9 U/ml) (Table I).
 | Table I.Laboratory examination results at
different time points. |
Table I.
Laboratory examination results at
different time points.
| Characteristics | First
examination | At 2 weeks after the
first examination | Reference value |
|---|
| CEA, ng/ml | 185.00 | 500.00 | 0-5.00 |
| NSE, ng/ml | 16.40 | 24.80 | 0-16.3 |
| CYFRA, ng/ml | 7.51 | 19.10 | 0-3.3 |
| CA125, U/ml | 1255.00 | 3706.00 | 0-24 |
| CA72-4, U/ml | 74.50 | 53.70 | 0-6.9 |
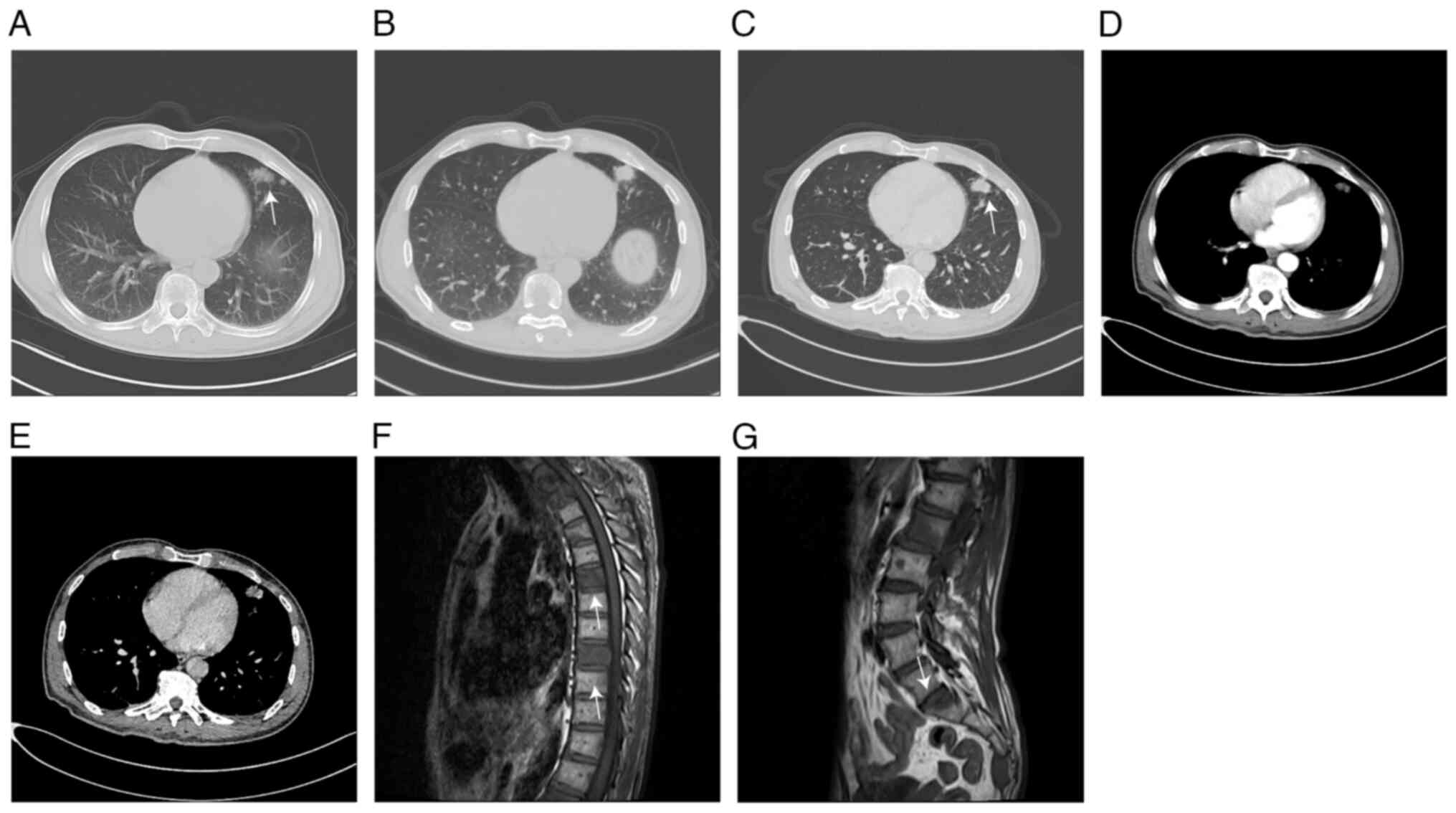
Chest CT and enhanced CT revealed space-occupying
lesions in the upper lobe of the left lung with enlarged lymph
nodes in the mediastinum and left hilum, bone destruction in
certain vertebral bodies, multiple nodules in both lungs,
interstitial lesions in both lower lobes, fibrous foci in the lower
lobe of the right lung and the upper lobe of the left lung, and
localized thickening of the bilateral pleura (Fig. 1A-D). CT plain scan of the brain,
liver, bile, pancreas and spleen showed no obvious abnormalities,
while bone destruction was found in the right femur, left inferior
rami of pubis, right ischial bone, right iliac bone, sacrum and a
proportion of the lumbosacral vertebrae, and part of the
lumbosacral vertebrae showed compact shadows.
Thoracic MR plain scan showed multiple abnormal
signals in the thoracolumbosacral vertebra and certain adnexal
areas, indicating the formation of metastatic foci (Fig. 1E-G).
In the early stage of treatment, it was planned to
perform lung mass puncture biopsy on the patient, but the
preoperative examination showed that the coagulation D-2 polymer
was as high as 11,220 ng/ml (reference range, 0–1,000 ng/ml), a
significant contraindication for puncture. In this case, according
to the condition and imaging examination results, it was decided to
puncture the iliac tissue instead of puncturing the left upper lobe
tumor. CT-guided fine needle aspiration (FNA) and endoscopic
ultrasound-guided FNA were performed to obtain the right iliac
tissue tumor specimens, suggesting a metastatic adenocarcinoma.
Immunohistochemical test was performed according to
standard procedures and results of iliac bone puncture tissues were
found to be positive for CK7, CK19, CDX2, villin, tumor protein p53
(p53; 60%) and Ki-67 (70%), and negative for CK20, p16 (0%),
transcription termination factor 1 (TTF-1), napsin A aspartic
peptidase (NapsinA), prostate-specific antigen (PSA) and
carbohydrate antigen 19–9 (CA19-9). All the antibodies were
purchased from Xi'an Dingguo Trading Co., Ltd., dilutions were set
at 1:100 according to the manufacturer's recommendations for
immunohistochemistry for each antibody and all antibodies were
rabbit anti-human monoclonal antibodies. Consecutive parallel
sections were stained with the following antibodies: CK7 (cat. no.
DG300545), CK19 (cat. no. DG300347), CDX2 (cat. no. DG300171),
villin (cat. no. DG302057), p53 (cat. no. DG302700), Ki-67 (cat.
no. DG300540), CK20 (cat. no. DG300366), p16 (cat. no. DG302620),
TTF-1 (cat. no. DG300313), NapsinA (mouse anti-human monoclonal
antibody; cat. no. DG169), PSA (cat. no. DG302428) and CA19-9
(mouse anti-human monoclonal antibody; cat. no. DG208). The test
tissue was sliced into 5-µm thick sections and fixed at room
temperature for 4 h using 10% neutral formalin. H&E staining
was performed using standard reagents at room temperature for 7 min
(hematoxylin staining for 5 min and eosin staining for 2 min;
Guidechem). The patient was diagnosed with metastatic
adenocarcinoma based on the histopathological and
immunohistochemistry results, assessed using light microscopy at
×400 magnification (Fig. 2).
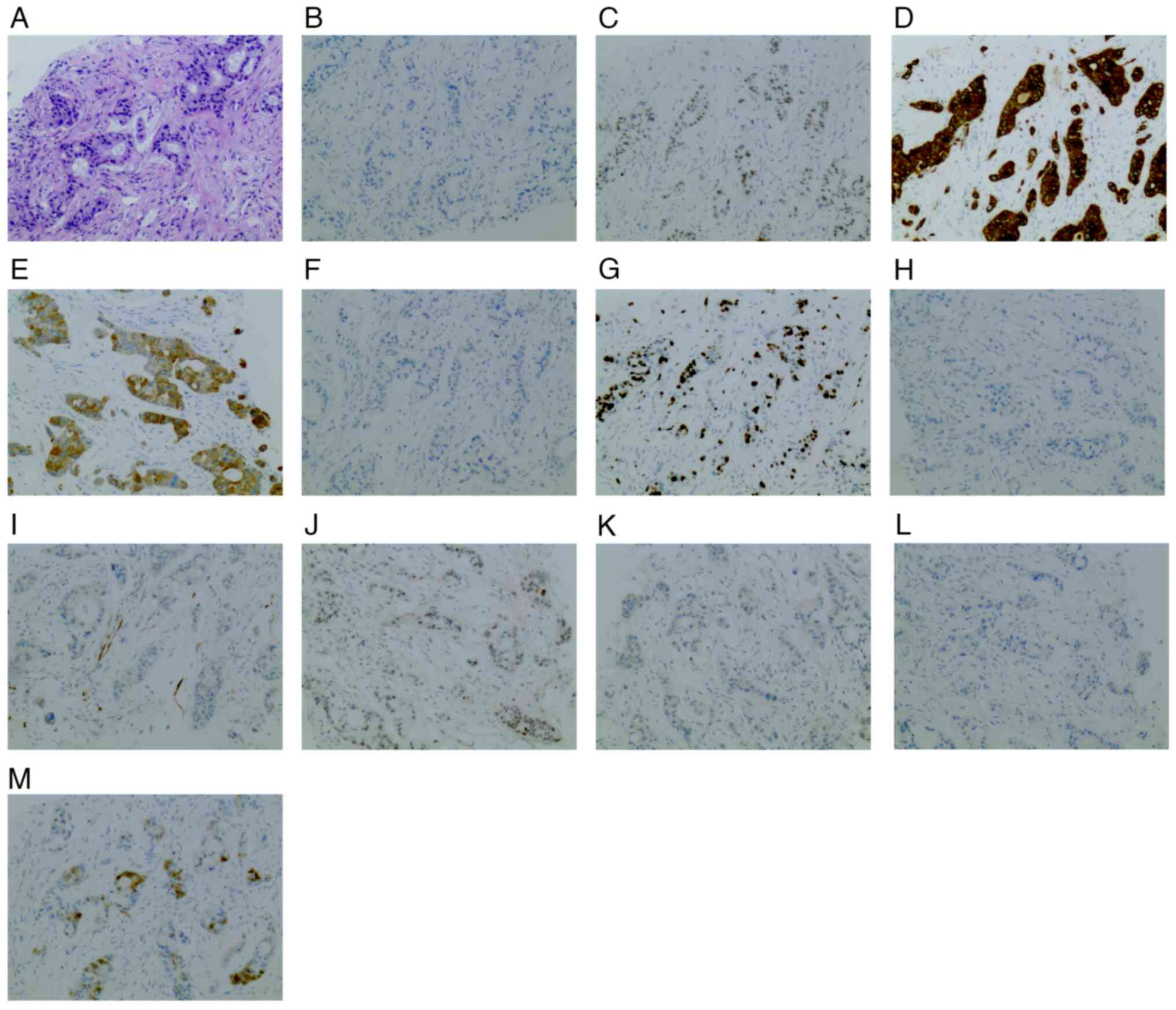 | Figure 2.Immunohistochemistry and
histopathology results of right iliac bone puncture tissue. (A) The
histopathological section stained with H&E showed significant
cell necrosis, which indicated metastatic adenocarcinoma.
Immunohistochemistry indicated thetissues to be (B) carbohydrate
antigen 19-9-negative, (C) caudal type homeobox 2-positive, (D)
CK7-positive, (E) CK19-positive, (F) CK20-negative, (G)
Ki-67-positive (70%), (H) napsin A aspartic peptidase-negative, (I)
p16-negative (0%), (J) tumor protein p53-positive (60%), (K)
prostate-specific antigen-negative, (L) transcription termination
factor 1-negative and (M) Villin-positive (magnification, ×100).
CK, cytokeratin. |
Positron emission tomography/CT was performed to
detect the tumor metabolic status. The metabolism level of
18F-fluorodeoxyglucose was increased in the tubercle on
the left superior lobe of the lung and the tubercle was considered
to be a malignant tumor. Metastasis was observed in the left hilar
lymph node and mediastinal lymph node, and multiple bone metastases
were observed throughout the body. Multiple small nodules in both
lungs showed no increase in glucose metabolism. The left adrenal
junction was slightly thickened and no increase in glucose
metabolism was observed. Soft-tissue masses had formed around
multiple vertebrae of the neck, chest and lumbar spine and
accessories, bilateral iliac crest and sacrum, and an increase of
nuclide uptake was observed in these soft-tissue masses (Fig. 3). There was no obvious abnormality
in the craniocerebral parenchyma.
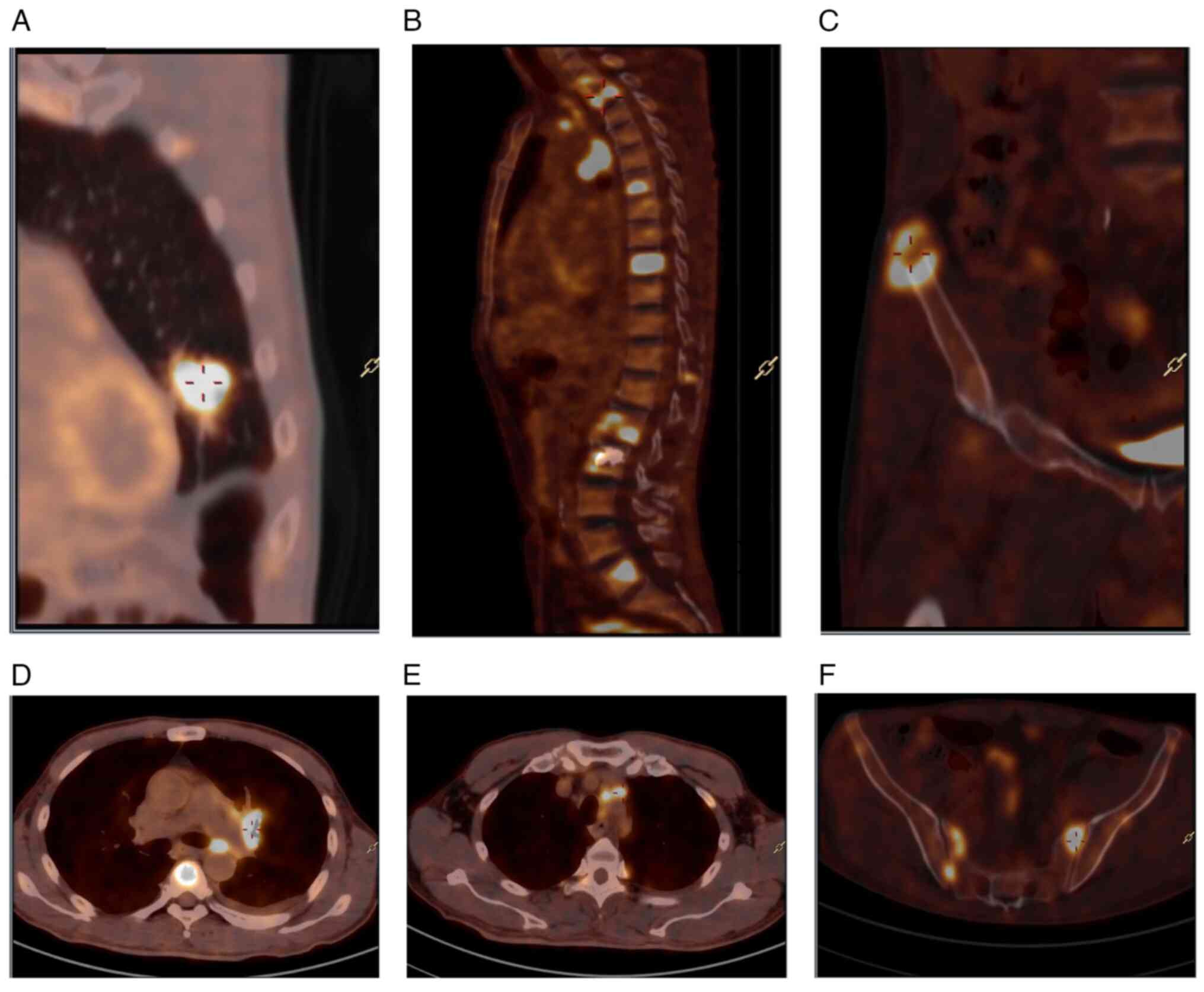 | Figure 3.Characteristics of positron emission
tomography/computed tomography lesions. (A) Increased uptake of
18F-FDG in the tubercle of the left superior lingual
segment, SUV max 16.6. (B) Increased uptake of 18F-FDG
in multiple vertebral bodies of neck, thoracic, lumbar vertebrae
and accessories, SUV max 18.08. (C) Increased uptake of
18F-FDG in iliac crest, SUV max 15.44. (D) Increased
uptake of 18F-FDG in left hilar lymph nodes, SUV max
13.08. (E) Increased uptake of 18F-FDG in mediastinal
lymph nodes, SUV max 8.97. (F) Increased uptake of
18F-FDG in the sacrum, SUV max 16.73. FDG,
fluorodeoxyglucose. SUV, standardized uptake value. |
To rule out the probability of colorectal
adenocarcinoma, rectal colonoscopy was performed and no
abnormalities were found.
To explore the mutant spectrum of tumor samples,
consent from the patient was obtained to perform genetic analysis
with next-generation sequencing, aiming to identify somatic
variations among 571 cancer-associated genes, including epidermal
growth factor receptor, ALK receptor tyrosine kinase, ROS
proto-oncogene 1 receptor tyrosine kinase, ret proto-oncogene, KRAS
proto-oncogene (KRAS) and KIT proto-oncogene. Genetic analysis of
tumor samples was performed by Ide Medical Laboratory Ltd. using
the Illumina NovaSeq 6000 platform (Illumina, Inc.). Of the 571
genes examined, only the KRAS and kelch-like ECH-associated protein
1 (KEAP1) genes were mutated. KRAS showed a missense mutation in
exon 3 (c.183A> T, p.Q61H) and KEAP1 exhibited a frameshift
mutation in exon 6 (c.1796del, p. S599Wfs*73) (Table II).
 | Table II.Results of gene mutation analysis. |
Table II.
Results of gene mutation analysis.
| Gene | Exon | Nucleotide
variation | Aminoacid
variation | Variant allele
frequency, % |
|---|
| KRAS | 3 | c.183A>T | p.Q61H | 5.44 |
| KEAP1 | 6 | c.1796del | p.S599Wfs*73 | 4.60 |
The final diagnosis of the patient with primary
pulmonary intestinal adenocarcinoma was submitted to the oncology
department of our hospital for subsequent treatment. However, due
to personal reasons, the patient and their family decided to give
up on subsequent treatment. The prognosis was extremely poor. Prior
to this, a palliative treatment plan based on basic symptomatic and
supportive treatment was carried out, including anticoagulant
treatment, protein supplementation, amino acid supplementation, fat
emulsion and electrolyte treatment. As the patient suffered from
moderate to severe cancer pain, morphine and OxyContin were
administered for pain relief. After follow-up investigations, the
patient passed away ~3 months after discharge.
Discussion
According to the 2011 International Association for
the Study of Lung Cancer/American Thoracic Society/European
Respiratory Society International Multidisciplinary Classification
of Lung Adenocarcinoma (9), PEAC is
defined as a type of lung adenocarcinoma with enteral
differentiation by >50%. This type of cancer frequently exhibits
characteristics of colorectal adenocarcinoma, such as glandular,
papillary and/or sieve structures with luminal necrosis, tall
columnar cells with pseudostratification, atypical nuclei and
eosinophil cytoplasm, making PEAC difficult to be distinguished
from colorectal adenocarcinoma (10,11).
Currently, the differential diagnosis is mainly based on the
combination of immunohistochemical markers. CK7 is positive in most
PEAC cases and is often expressed in breast, endometrium, pancreas,
biliary tract and lung tissues (12), but not in the gastrointestinal
tract. TTF-1 and NapsinA are specific biomarkers of lung
adenocarcinoma, which were reported to be generally positive in
PEAC (13,14), while being negative in lung
metastatic gastrointestinal adenocarcinoma. CDX2, CK20 and MUC2 are
generally expressed in intestinal tissues, and at least one of them
is positive in PEAC (15,16). However, PEAC cases with lung
cancer-specific immunophenotypically negative status, such as
negative results for TTF-1 and NapsinA, have been previously
reported, suggesting that the understanding of PEAC remains
incomplete (5).
The onset of PEAC is usually insidious and most
patients have no specific clinical symptoms, which makes it
difficult for patients to receive an early diagnosis and treatment;
the condition has usually been developing for a period of time when
admitted to the hospital, which may relate to the fact that PEAC
mostly manifests as peripheral lung cancer that mostly originates
from small bronchi and grows slowly (17–19).
Zhao et al (20) summarized
the clinical symptoms of 28 patients with primary PEAC. They
determined that 42.9% of the patients initially presented with a
cough, including 3 patients with cough accompanied by hemoptysis, 1
patient with cough and fever accompanied by chest and back pain, 1
patient with cough accompanied by fever, and 1 patient with
persistent chest and back pain (20). In the case reported in the present
study, the patient presented with advanced symptoms of distant
metastasis at diagnosis, accompanied by multiple bone and lymphatic
metastases, which is very rare in clinical practice, but no
gastrointestinal symptoms were observed throughout the course of
the disease and no abnormalities were observed under colonoscopy.
Malignancies with lung metastasis not only show respiratory
symptoms, but also exhibit symptoms associated with the primary
disease, which may be distinguished from lung metastatic colorectal
cancer (21).
The imaging findings of PEAC are different from
those of typical invasive lung adenocarcinoma, in which the lesions
are often larger, but there are no significant differences in the
lobular sign, burr sign and pleural effusion, which manifest as
patches, compact lesions or ground glass lesions (21). It is difficult to distinguish PEAC
from the imaging perspective only, and histological and
immunohistochemical phenotyping are also required.
Immunohistochemical results are of great significance in the
diagnosis of this disease, and in spite of its low incidence and
the small number of cases, more efforts should be made to find
specific diagnostic markers (22).
Previous studies have identified smoking as a
predisposing factor for PEAC, and the WNT-SOX2 signaling regulation
pathway is over-activated under the stimulation of smoking and
other factors, which may lead to intestinal metaplasia of airway
basal cells. However, a recent study suggested that smoking may not
be an important risk factor for the development of PEAC (23). In addition, several studies have
shown that males are more likely to have PEAC, while other studies
have come to the opposite conclusion (24). These controversies may be related to
the limited number of reported cases so far, which were not able to
support such large-scale statistics and analysis. However, it is
widely thought that most patients with PEAC are elderly
individuals.
In previous studies, it was noted that the
occurrence of PEAC is often accompanied by the mutation of KRAS
(2,5,25).
Furthermore, KEAP1, a drug-resistant gene mutation, was also found
in the present case.
Of the 46 PEAC cases examined by Nottegar et
al (26), more than half had
KRAS mutations and similar results were found in a retrospective
cohort study by Xie et al (27). The KRAS gene is a proto-oncogene in
the RAS family (28) and the most
frequently mutated sites are mostly concentrated in codon 12 of
exon 2, accounting for 90% (29).
In the present case, the KRAS gene mutation comprised a missense
mutation of codon 61 in exon 3, which led to the mutation of amino
acid 61 of the gene-encoded protein from glutamine to histidine.
This mutation is located in the GTp-binding region of the KRAS
protein, a hot spot mutation that leads to the decrease of KRAS
GTase activity. It preferentially interacts with Raf-1
proto-oncogene and activates ERK signaling, leading to cell
transformation (30).
KEAP1 binds to Nrf2 and targets ubiquitin-mediated
degradation, thereby negatively regulating the downstream cell
protective activity of Nrf2 (31).
In the present case, the mutation was a pipework mutation, which
led to the mutation of the amino acid at position 599 of the
gene-encoded protein from serine to tryptophan and its termination
at position 671, thus leading to cancerous cell transformation
(32). KEAP1 mutations are common
in both lung adenocarcinoma (~17%) and squamous cell carcinoma
(10–12%) (33), although, to the
best of our knowledge, there have been no reports of KEAP1 gene
mutations in PEAC. Lung adenocarcinomas with co-mutated KEAP1
driver genes do not respond to immunotherapy despite a high tumor
mutation load, which may be associated with a poorer prognosis in
patients with PEAC, according to a study published in 2020 in
Annals of Oncology (34).
Covariant KRAS/KEAP1 may cause conventional
immunotherapy to be ineffective. A retrospective study has reported
that patients with advanced lung adenocarcinoma treated with a
programmed cell death 1 (ligand 1) inhibitor in the context of
KRAS/KEAP1 frequently exhibit poor disease-free survival and OS
(35).
Currently, the treatment plan for primary pulmonary
intestinal adenocarcinoma is similar to that for other types of
primary pulmonary adenocarcinoma. Based on clinical staging, a
comprehensive treatment plan with surgery as the main treatment,
supplemented by radiotherapy, chemotherapy and/or targeted therapy,
is adopted, and classic thoracic surgical intervention is the
preferred treatment method (5). It
has been reported that after 2–3 weeks of treatment with
pemetrexed, carboplatin and monoclonal antibody against
pabolizumab, as well as pemetrexed, carboplatin and chemotherapy,
the condition remained stable (5,36).
To the best of our knowledge, the present study was
the first case report of PEAC with systemic multiple bone and
lymphatic metastasis. At present, treatment guidelines for this
rare type of cancer are lacking. Considering that mutations in
drug-resistant genes are frequently found in PEAC and immunotherapy
may not be able to effectively control the progression of cancer,
it may be concluded that an effective alternative and personalized
treatment is necessary.
Acknowledgements
Not applicable.
Funding
This work was funded in part by the National Science Foundation
of China (grant no. 82204854).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL, SZ and WY conceived and designed the study, and
wrote the manuscript. ZF and XW obtained MRI and CT images. PY, XL
and YJ collected and analyzed the data. WJ performed the
pathological examination and provided experimental support. SZ and
WY confirm the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for the case information and images to be published in this
case report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Travis WD, Brambilla E, Burke AP, Marx A
and Nicholson AG: Introduction to The 2015 World Health
Organization Classification of Tumors of the Lung, Pleura, Thymus,
and Heart. J Thorac Oncol. 10:1240–1242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Inamura K, Satoh Y, Okumura S, Nakagawa K,
Tsuchiya E, Fukayama M and Ishikawa Y: Pulmonary adenocarcinomas
with enteric differentiation: Histologic and immunohistochemical
characteristics compared with metastatic colorectal cancers and
usual pulmonary adenocarcinomas. Am J Surg Pathol. 29:660–665.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Montezuma D, Azevedo R, Lopes P, Vieira R,
Cunha AL and Henrique R: A panel of four immunohistochemical
markers (CK7, CK20, TTF-1, and p63) allows accurate diagnosis of
primary and metastatic lung carcinoma on biopsy specimens. Virchows
Arch. 463:749–754. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Travis WD and Rekhtman N: Pathological
diagnosis and classification of lung cancer in small biopsies and
cytology: Strategic management of tissue for molecular testing.
Semin Respir Crit Care Med. 32:22–31. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fassi E, Mandruzzato M, Zamparini M,
Bianchi S, Petrelli F, Baggi A, Alberti A, Grisanti S and Berruti
A: Clinical presentation and outcome of patients with enteric-type
adenocarcinoma of the lung: A pooled analysis of published cases.
Lung Cancer. 179:1071762023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maeda R, Isowa N, Onuma H and Miura H:
Pulmonary intestinal-type adenocarcinoma. Interact Cardiovasc
Thorac Surg. 7:349–351. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yin JJ, Pollock CB and Kelly K: Mechanisms
of cancer metastasis to the bone. Cell Res. 15:57–62. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Serfaty A and Samim M: Bone tumors:
Imaging features of the most common primary osseous malignancies.
Radiol Clin North Am. 60:221–238. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Travis WD, Brambilla E, Noguchi M,
Nicholson AG, Geisinger K, Yatabe Y, Powell CA, Beer D, Riely G,
Garg K, et al: International Association for the Study of Lung
Cancer/American Thoracic Society/European Respiratory Society:
International multidisciplinary classification of lung
adenocarcinoma: Executive summary. Proc Am Thorac Soc. 8:381–385.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun WW, Xu ZH, Wang CF, Wu F, Cao JM, Cui
PJ, Huang W, Jin XL, Li B, Chen KM, et al: Pulmonary enteric
adenocarcinoma with pancreatic metastasis: A case report. Oncol
Lett. 13:4651–4656. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gong J, Fan Y and Lu H: Pulmonary enteric
adenocarcinoma. Transl Oncol. 14:1011232021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ricciuti B, Alessi JV, Elkrief A, Wang X,
Cortellini A, Li YY, Vaz VR, Gupta H, Pecci F, Barrichello A, et
al: Dissecting the clinicopathologic, genomic, and immunophenotypic
correlates of KRASG12D-mutated non-small-cell lung
cancer. Ann Oncol. 33:1029–1040. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu H, Li L, Liu D and Li WM: Expression of
TTF-1, NapsinA, P63, CK5/6 in lung cancer and its diagnostic values
for histological classification. Sichuan Da Xue Xue Bao Yi Xue Ban.
48:336–341. 2017.(In Chinese). PubMed/NCBI
|
|
14
|
Sharma T, Das P, Panigrahi R, Rao CM and
Rath J: Immunocytochemical evaluation of TTF-1, Napsin-A, and p-63
for Subtyping of non-small cell lung carcinoma and
clinicopathological correlation. J Cytol. 39:180–187. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shin JH, Bae JH, Lee A, Jung CK, Yim HW,
Park JS and Lee KY: CK7, CK20, CDX2 and MUC2 Immunohistochemical
staining used to distinguish metastatic colorectal carcinoma
involving ovary from primary ovarian mucinous adenocarcinoma. Jpn J
Clin Oncol. 40:208–213. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Haraldsson S, Klarskov L, Nilbert M,
Bernstein I, Bonde J and Holck S: Differential expression of CK20,
β-catenin, and MUC2/5AC/6 in Lynch syndrome and familial colorectal
cancer type X. BMC Clin Pathol. 17:112017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin D, Zhao Y, Li H and Xing X: Pulmonary
enteric adenocarcinoma with villin brush border immunoreactivity: A
case report and literature review. J Thorac Dis. 5:E17–E20.
2013.PubMed/NCBI
|
|
18
|
László T, Lacza A, Tóth D, Molnár TF and
Kálmán E: Pulmonary enteric adenocarcinoma indistinguishable
morphologically and immunohistologically from metastatic colorectal
carcinoma. Histopathology. 65:283–287. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu X, Chen D, Wu X and Wang Q: A pulmonary
enteric adenocarcinoma patient harboring a rare EGFR exon 19 P753S
mutation: Case report and review. Front Oncol. 12:9886252022.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao L, Huang S, Liu J, Zhao J, Li Q and
Wang HQ: Clinicopathological, radiographic, and oncogenic features
of primary pulmonary enteric adenocarcinoma in comparison with
invasive adenocarcinoma in resection specimens. Medicine
(Baltimore). 96:e81532017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li H and Cao W: Pulmonary enteric
adenocarcinoma: A literature review. J Thorac Dis. 12:3217–3226.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Q, Zhang L, Li H, Liu L, Sun X and
Liu H: Clinical features and prognosis of pulmonary enteric
adenocarcinoma: A retrospective study in China and the SEER
database. Front Oncol. 13:10991172023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Okada F, Takeda M, Fujii T, Uchiyama T,
Sasaki S, Matsuoka M, Nitta Y, Terada C, Maebo K, Morita K, et al:
Clinicopathological and genetic analyses of pulmonary enteric
adenocarcinoma. J Clin Pathol. Dec 1–2022.(Epub ahead of print).
View Article : Google Scholar
|
|
24
|
Zuo Y, Bai H, Ying JM and Wang J: Progress
in pulmonary enteric adenocarcinoma. Zhonghua Zhong Liu Za Zhi.
44:321–325. 2022.(In Chinese). PubMed/NCBI
|
|
25
|
Gu L, Wang XZ, Wen W, Lin J, Chen XF, Lai
GX, Chen L, Ouyang XJ, Zhang L, Ye J, et al: Clinical analysis of
23 patients pathologically diagnosed with primary and secondary
pulmonary enteric adenocarcinoma. Chin Med J (Engl). 132:1368–1369.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nottegar A, Tabbò F, Luchini C, Brunelli
M, Bria E, Veronese N, Santo A, Cingarlini S, Gilioli E, Ogliosi C,
et al: Pulmonary adenocarcinoma with enteric differentiation:
Immunohistochemistry and molecular morphology. Appl Immunohistochem
Mol Morphol. 26:383–387. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xie M, Chen D, Li Y, Liu X, Kuang D and Li
X: Genetic mutation profiles and immune microenvironment analysis
of pulmonary enteric adenocarcinoma. Diagn Pathol. 17:302022.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Smith G, Bounds R, Wolf H, Steele RJ,
Carey FA and Wolf CR: Activating K-Ras mutations outwith ‘hotspot’
codons in sporadic colorectal tumours-implications for personalised
cancer medicine. Br J Cancer. 102:693–703. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dong ZY, Zhong WZ, Zhang XC, Su J, Xie Z,
Liu SY, Tu HY, Chen HJ, Sun YL, Zhou Q, et al: Potential predictive
value of TP53 and KRAS mutation status for response to PD-1
blockade immunotherapy in lung adenocarcinoma. Clin Cancer Res.
23:3012–3024. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jeanson A, Tomasini P, Souquet-Bressand M,
Brandone N, Boucekine M, Grangeon M, Chaleat S, Khobta N, Milia J,
Mhanna L, et al: Efficacy of immune checkpoint inhibitors in
KRAS-Mutant non-small cell lung cancer (NSCLC). J Thorac Oncol.
14:1095–1101. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhou C, Wang Y, Zhao J, Chen G, Liu Z, Gu
K, Huang M, He J, Chen J, Ma Z, et al: Efficacy and biomarker
analysis of camrelizumab in combination with apatinib in patients
with advanced nonsquamous NSCLC previously treated with
chemotherapy. Clin Cancer Res. 27:1296–1304. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jaramillo MC and Zhang DD: The emerging
role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev.
27:2179–2191. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Menegon S, Columbano A and Giordano S: The
dual roles of NRF2 in cancer. Trends Mol Med. 22:578–593. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Marinelli D, Mazzotta M, Scalera S,
Terrenato I, Sperati F, D'Ambrosio L, Pallocca M, Corleone G,
Krasniqi E, Pizzuti L, et al: KEAP1-driven co-mutations in lung
adenocarcinoma unresponsive to immunotherapy despite high tumor
mutational Burden. Ann Oncol. 31:1746–1754. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ricciuti B, Arbour KC, Lin JJ, Vajdi A,
Vokes N, Hong L, Zhang J, Tolstorukov MY, Li YY, Spurr LF, et al:
Diminished efficacy of programmed death-(Ligand)1 inhibition in
STK11- and KEAP1-Mutant lung adenocarcinoma is affected by KRAS
mutation status. J Thorac Oncol. 17:399–410. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cresto N, Forner-Piquer I, Baig A,
Chatterjee M, Perroy J, Goracci J and Marchi N: Pesticides at brain
borders: Impact on the blood-brain barrier, neuroinflammation, and
neurological risk trajectories. Chemosphere. 324:1382512023.
View Article : Google Scholar : PubMed/NCBI
|