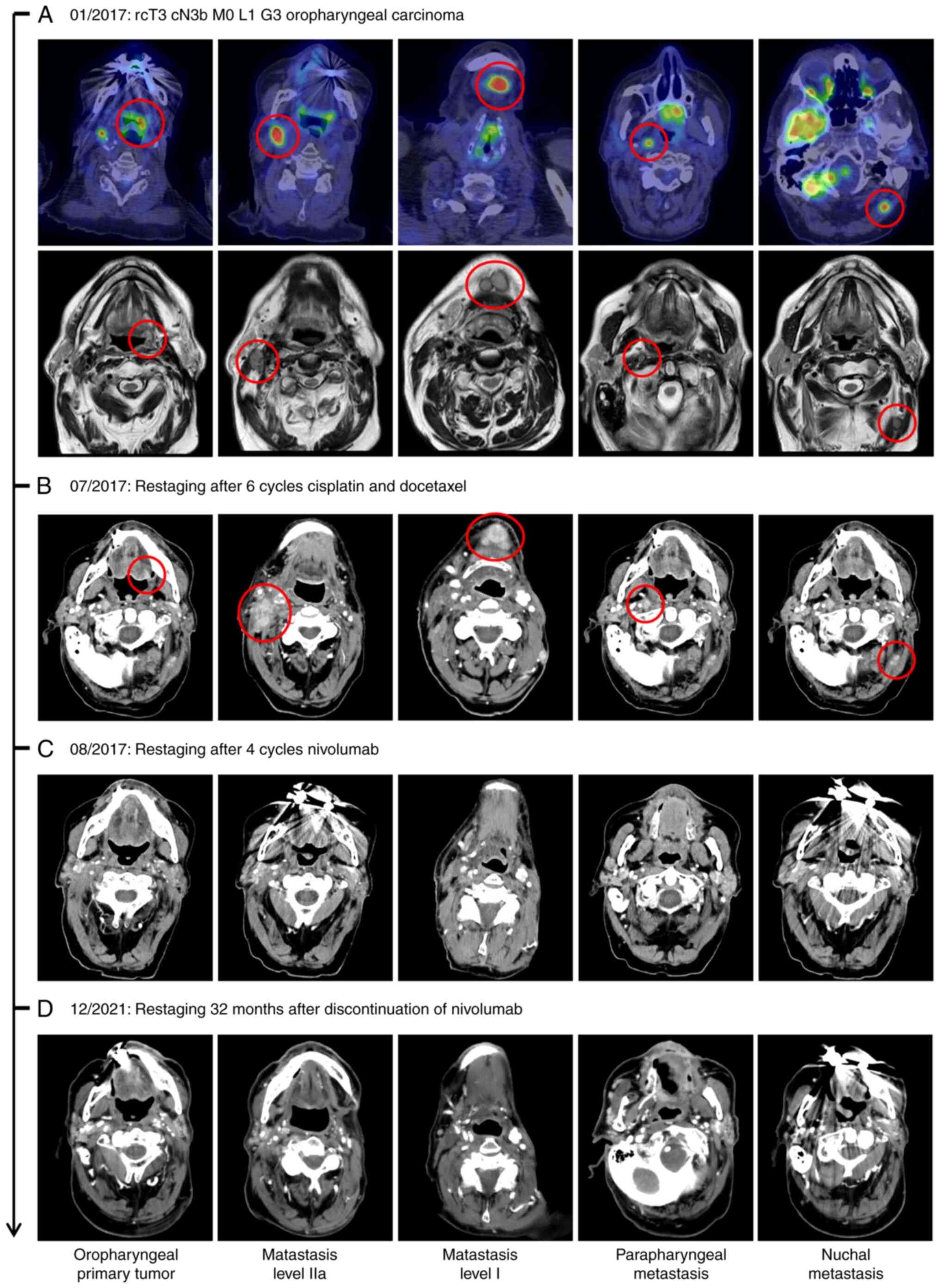
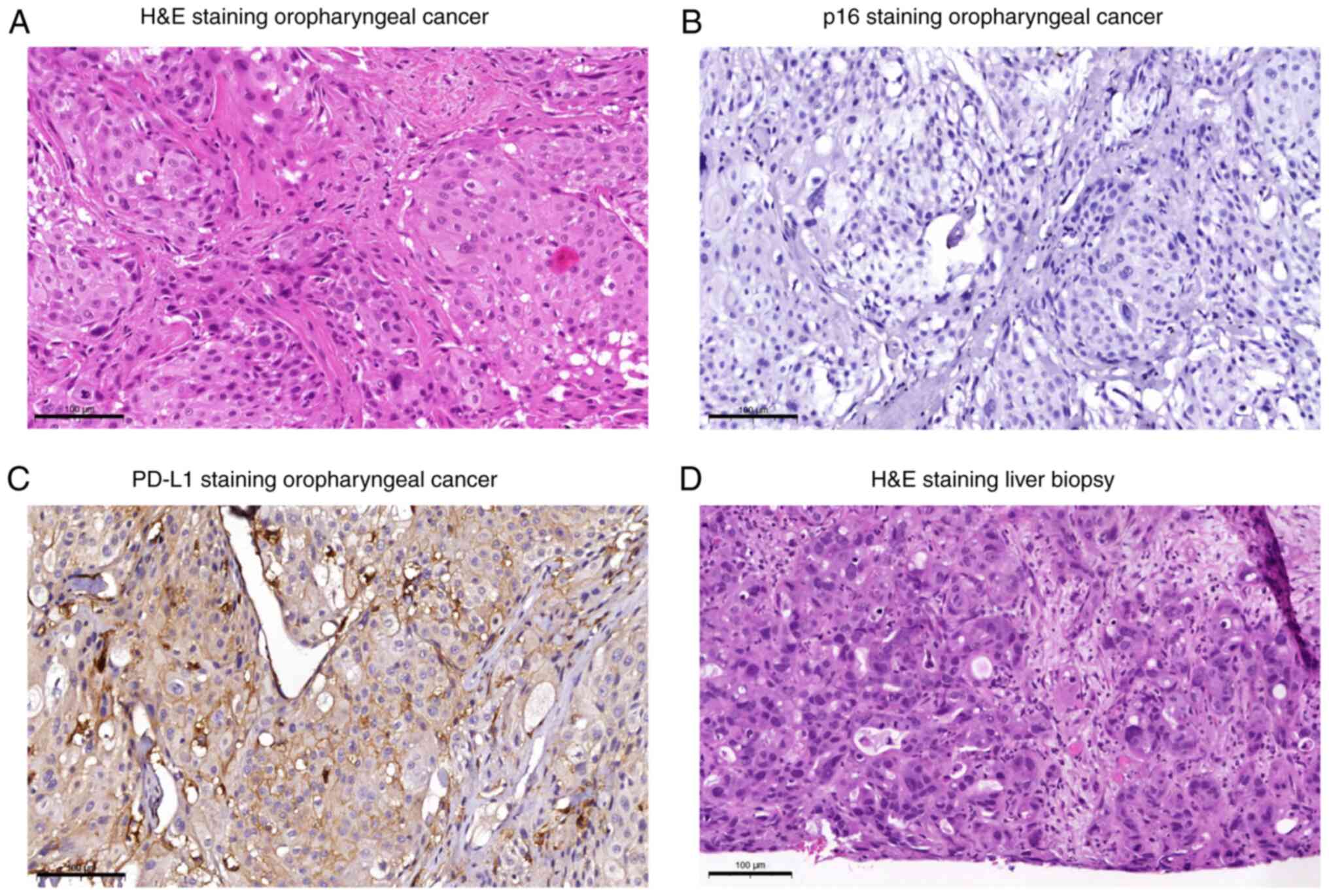
|
1
|
Johnson DE, Burtness B, Leemans CR, Lui
VWY, Bauman JE and Grandis JR: Head and neck squamous cell
carcinoma. Nat Rev Dis Primers. 6:922020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Decker J and Goldstein JC: Risk factors in
head and neck cancer. N Engl J Med. 306:1151–1155. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
zur Hausen H: Papillomaviruses in the
causation of human cancers-a brief historical account. Virology.
384:260–265. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Forastiere A, Koch W, Trotti A and
Sidransky D: Head and neck cancer. N Engl J Med. 345:1890–1900.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ferris RL, Blumenschein G Jr, Fayette J,
Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE,
Even C, et al: Nivolumab for recurrent squamous-cell carcinoma of
the head and neck. N Engl J Med. 375:1856–1867. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Burtness B, Harrington KJ, Greil R,
Soulières D, Tahara M, de Castro G Jr, Psyrri A, Basté N, Neupane
P, Bratland Å, et al: Pembrolizumab alone or with chemotherapy
versus cetuximab with chemotherapy for recurrent or metastatic
squamous cell carcinoma of the head and neck (KEYNOTE-048): A
randomised, open-label, phase 3 study. Lancet. 394:1915–1928. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Scheel AH, Dietel M, Heukamp LC, Jöhrens
K, Kirchner T, Reu S, Rüschoff J, Schildhaus HU, Schirmacher P,
Tiemann M, et al: Predictive PD-L1 immunohistochemistry for
non-small cell lung cancer: Current state of the art and
experiences of the first German harmonization study. Pathologe.
37:557–567. 2016.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thinn MM and Hsueh CT and Hsueh CT:
Sustained complete response to erlotinib in squamous cell carcinoma
of the head and neck: A case report. World J Clin Cases. 7:616–622.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Roh KW, Jang JS, Kim MS, Sun DI, Kim BS,
Jung SL, Kang JH, Yoo EJ, Yoon SC, Jang HS, et al: Fractionated
stereotactic radiotherapy as reirradiation for locally recurrent
head and neck cancer. Int J Radiat Oncol Biol Phys. 74:1348–1355.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Albers AE, Grabow R, Qian X, Jumah MD,
Hofmann VM, Krannich A and Pecher G: Efficacy and toxicity of
docetaxel combination chemotherapy for advanced squamous cell
cancer of the head and neck. Mol Clin Oncol. 7:151–157. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
de Ruiter EJ, Mulder FJ, Koomen BM, Speel
EJ, van den Hout MFCM, de Roest RH, Bloemena E, Devriese LA and
Willems SM: Comparison of three PD-L1 immunohistochemical assays in
head and neck squamous cell carcinoma (HNSCC). Mod Pathol.
34:1125–1132. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rasmussen JH, Lelkaitis G, Hakansson K,
Vogelius IR, Johannesen HH, Fischer BM, Bentzen SM, Specht L,
Kristensen CA, von Buchwald C, et al: Intratumor heterogeneity of
PD-L1 expression in head and neck squamous cell carcinoma. Br J
Cancer. 120:1003–1006. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ferris RL, Spanos WC, Leidner R, Gonçalves
A, Martens UM, Kyi C, Sharfman W, Chung CH, Devriese LA, Gauthier
H, et al: Neoadjuvant nivolumab for patients with resectable
HPV-positive and HPV-negative squamous cell carcinomas of the head
and neck in the CheckMate 358 trial. J Immunother Cancer.
9:e0025682021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Patel JJ, Levy DA, Nguyen SA, Knochelmann
HM and Day TA: Impact of PD-L1 expression and human papillomavirus
status in anti-PD1/PDL1 immunotherapy for head and neck squamous
cell carcinoma-Systematic review and meta-analysis. Head Neck.
42:774–786. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Botticelli A, Cirillo A, Strigari L,
Valentini F, Cerbelli B, Scagnoli S, Cerbelli E, Zizzari IG, Rocca
CD, D'Amati G, et al: Anti-PD-1 and Anti-PD-L1 in head and neck
cancer: A network meta-analysis. Front Immunol. 12:7050962021.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Miyauchi S, Sanders PD, Guram K, Kim SS,
Paolini F, Venuti A, Cohen EEW, Gutkind JS, Califano JA and Sharabi
AB: HPV16 E5 mediates resistance to PD-L1 blockade and can be
targeted with rimantadine in head and neck cancer. Cancer Res.
80:732–746. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Massarelli E, William W, Johnson F, Kies
M, Ferrarotto R, Guo M, Feng L, Lee JJ, Tran H, Kim YU, et al:
Combining immune checkpoint blockade and tumor-specific vaccine for
patients with incurable human papillomavirus 16-related cancer: A
phase 2 clinical trial. JAMA Oncol. 5:67–73. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mehanna H, Taberna M, von Buchwald C, Tous
S, Brooks J, Mena M, Morey F, Grønhøj C, Rasmussen JH,
Garset-Zamani M, et al: Prognostic implications of p16 and HPV
discordance in oropharyngeal cancer (HNCIG-EPIC-OPC): A
multicentre, multinational, individual patient data analysis.
Lancet Oncol. 24:239–251. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Park JC, Krishnakumar HN and Saladi SV:
Current and future biomarkers for immune checkpoint inhibitors in
head and neck squamous cell carcinoma. Curr Oncol. 29:4185–4198.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Matsuki T, Okamoto I, Fushimi C, Takahashi
H, Okada T, Kondo T, Sato H, Ito T, Tokashiki K, Tsukahara K, et
al: Real-World, long-term outcomes of nivolumab therapy for
recurrent or metastatic squamous cell carcinoma of the head and
neck and impact of the magnitude of best overall response: A
retrospective multicenter study of 88 patients. Cancers (Basel).
12:34272020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Foster CC, Couey MA, Kochanny SE, Khattri
A, Acharya RK, Tan YC, Brisson RJ, Leidner RS and Seiwert TY:
Immune-related adverse events are associated with improved
response, progression-free survival, and overall survival for
patients with head and neck cancer receiving immune checkpoint
inhibitors. Cancer. 127:4565–4573. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bilger G, Girard N, Doubre H, Levra MG,
Giroux-Leprieur E, Giraud F, Decroisette C, Carton M and Massiani
MA: Discontinuation of immune checkpoint inhibitor (ICI) above 18
months of treatment in real-life patients with advanced non-small
cell lung cancer (NSCLC): INTEPI, a multicentric retrospective
study. Cancer Immunol Immunother. 71:1719–1731. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Perez L, Samlowski W and Lopez-Flores R:
Outcome of elective checkpoint inhibitor discontinuation in
patients with metastatic melanoma who achieved a complete
Remission: Real-World data. Biomedicines. 10:11442022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wahl RL, Jacene H, Kasamon Y and Lodge MA:
From RECIST to PERCIST: Evolving considerations for PET response
criteria in solid tumors. J Nucl Med. 50 (Suppl 1):122S–150S. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tan AC, Emmett L, Lo S, Liu V, Kapoor R,
Carlino MS, Guminski AD, Long GV and Menzies AM: FDG-PET response
and outcome from anti-PD-1 therapy in metastatic melanoma. Ann
Oncol. 29:2115–2120. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sato K, Akamatsu H, Murakami E, Sasaki S,
Kanai K, Hayata A, Tokudome N, Akamatsu K, Koh Y, Ueda H, et al:
Correlation between immune-related adverse events and efficacy in
non-small cell lung cancer treated with nivolumab. Lung Cancer.
115:71–74. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Martini DJ, Hamieh L, McKay RR, Harshman
LC, Brandao R, Norton CK, Steinharter JA, Krajewski KM, Gao X,
Schutz FA, et al: Durable clinical benefit in metastatic renal cell
carcinoma patients who discontinue PD-1/PD-L1 therapy for
immune-related adverse events. Cancer Immunol Res. 6:402–408. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yatsuda Y, Hirose S, Ito Y, Onoda T,
Sugiyama Y, Nagafuchi M, Suzuki H, Niisato Y, Tange Y, Ikeda T, et
al: A durable response after the discontinuation of nivolumab in an
advanced gastric cancer patient. Intern Med. 60:1011–1017. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Davies MA: Is it safe to stop Anti-PD-1
immunotherapy in patients with metastatic melanoma who achieve a
complete response? J Clin Oncol. 38:1645–1647. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Andrews A: Treating with checkpoint
inhibitors-figure $1 million per patient. Am Health Drug Benefits.
8((Spec Issue)): 92015.PubMed/NCBI
|
|
31
|
Gauci ML, Lanoy E, Champiat S, Caramella
C, Ammari S, Aspeslagh S, Varga A, Baldini C, Bahleda R, Gazzah A,
et al: Long-Term survival in patients responding to Anti-PD-1/PD-L1
therapy and disease outcome upon treatment discontinuation. Clin
Cancer Res. 25:946–956. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pons-Tostivint E, Latouche A, Vaflard P,
Ricci F, Loirat D, Hescot S, Sablin MP, Rouzier R, Kamal M, Morel
C, et al: Comparative analysis of durable responses on immune
checkpoint inhibitors versus other systemic therapies: A pooled
analysis of phase III trials. JCO Precis Oncol. 3:1–10. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sekido K, Imaue S, Tomihara K, Tachinami
H, Yamagishi K, Okazawa S, Ikeda A, Fujiwara K and Noguchi M:
Durable complete response to immunotherapy with anti-PD-1 antibody
nivolumab in a patient with oral squamous cell carcinoma presenting
with lung metastasis: A case report. Clin Case Rep. 9:e045452021.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yasumatsu R, Matsuo M, Wakasaki T, Masuda
M, Takeuchi T, Manako T, Jiromaru R, Uchi R, Hashimoto K and
Nakagawa T: Clinical outcome in recurrent and/or metastatic head
and neck cancer patients after discontinuation of nivolumab
monotherapy due to immune-related adverse events. Acta Otolaryngol.
140:1043–1048. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lopez-Flores R, Samlowski W and Perez L:
Elective checkpoint inhibitor discontinuation in metastatic solid
tumor patients: A case series. Ann Case Rep. 7:8942022.PubMed/NCBI
|
|
36
|
Zambrana F, Carril-Ajuria L, Gomez de
Liano A, Martinez Chanza N, Manneh R, Castellano D and de Velasco
G: Complete response and renal cell carcinoma in the immunotherapy
era: The paradox of good news. Cancer Treat Rev. 99:1022392021.
View Article : Google Scholar : PubMed/NCBI
|