Introduction
Renal cell carcinoma (RCC) accounts for 2–3% of all
cancer cases, while clear cell RCC (ccRCC) accounts for ~75% of all
RCC cases worldwide (1,2). It is estimated that 20–50% of patients
who are diagnosed with localized RCC tumors will still encounter
local recurrence or tumor metastasis after surgical resection, and
that their 5-year survival rate is <10% (2,3). This
therefore calls for the urgent development of effective and less
toxic drugs for treating RCC tumors, and identifying the molecular
targets and underlying mechanisms of RCC is an important step in
developing these treatments. Signal transducer and activator of
transcription (STAT) 3 is phosphorylated and activated by various
hormones, growth factors and cytokines (4). Upon activation, phosphorylated STAT3
(p-STAT3) dimerizes and translocates to the nucleus, where it
regulates transcription of a broad spectrum of target genes
involved in the regulation of critical functions, including cell
proliferation (5), apoptosis
(6), metastasis (7), angiogenesis (8) and immune responses (9). Numerous studies have shown that high
STAT3 levels were associated with the poor prognosis of patients
with RCC (10–13). Notably, inhibition of STAT3
inhibited cell proliferation and induced the apoptosis of RCC cells
(14), indicating that STAT3 could
be a potential and effective target for RCC therapy.
Prospecting for novel anticancer agents derived from
traditional Chinese medicine has gained research interest. Notably,
the glycoalkaloid compound, solamargine (SM), the main active
ingredient in Solanum nigrum L, was found to possess significant
inhibitory effects against several types of cancer cells. For
example, preliminary results from a number of studies have revealed
its efficacy and potential underlying mechanisms of action against
prostate cancer (15), hepatoma
(16), melanoma (17) and ovarian cancer cells (18). However, to the best of our
knowledge, the effect and underlying molecular mechanism of action
of SM on human RCC cells remains unknown. Therefore, the main
purpose of the present study was to investigate the effect of SM on
the viability and apoptosis of RCC cells, and to elucidate the
underlying molecular mechanisms.
Materials and methods
Cell lines and culture
The ACHN cell line was obtained from The Cell Bank
of Type Culture Collection of The Chinese Academy of Sciences,
while the 786-O cell line was purchased from American Type Culture
Collection. ACHN cells were cultured in DMEM (HyClone; Cytiva) and
786-O cells were cultured in 1640 medium (HyClone; Cytiva). The
media were supplemented with 10% heat-inactivated fetal bovine
serum (Shanghai ExCell Biology, Inc.), 100 U/ml penicillin and 100
mg/ml streptomycin (HyClone; Cytiva). Both cell lines were
maintained in a humidified atmosphere at 37°C and 5%
CO2.
Drug and chemicals
SM was purchased from MedChemExpress (cat. no.
HY-N0069) and dissolved in dimethyl sulfoxide (DMSO). The dose was
prepared as a 10 mM stock solution (stored at −80°C) and freshly
diluted to a final concentration using cell culture medium prior to
use. BAY2353 was obtained from Selleck Chemicals.
Cell toxicity experiments
Cells were seeded in 96-well plates (Corning Inc.)
containing culture media at a density of 2,000 cells/well and were
cultured for a further 24 h. Then, the media was replaced with
fresh media containing different SM concentrations (0, 0.1, 1.0,
10.0 and 100.0 µM) and incubated for a further 0, 24, 48 or 72 h,
respectively. After incubation, 20 µl of MTT reagent (Promega
Corporation) was added to each well, and the samples were incubated
at 37°C for a further 20 min. The optical density was measured at
492 nm using a Multiskan GO spectrophotometer (Thermo Fisher
Scientific, Inc.). The experiments were repeated three times.
Flow cytometry
The rate of cell apoptosis was analyzed using an
Annexin V/propidium iodide (PI) apoptosis detection kit [Hangzhou
MultiSciences (Lianke) Biotech, Co., Ltd.] according to the
manufacturer's instructions. Briefly, cells were trypsinized,
washed three times with PBS and then resuspended in 0.5 ml binding
buffer containing 5 µl Annexin-V-FITC and 10 µl PI for 30 min at
room temperature. Then, the contents were subjected to a
FACSCalibur Flow Cytometer using CellQuest Pro software (Version
5.1, BD Biosciences). The experiments were repeated three
times.
Detection of caspase activity
The activities of caspase-3, caspase-8 and caspase-9
in cells treated with SM were detected using the caspase activity
assay kit, according to the manufacturer's instructions (Beyotime
Institute of Biotechnology). Absorbance values were measured at 405
nm using a spectrophotometer (Multiscan skyhigh; Thermo Fisher
Scientific, Inc.). The experiments were repeated three times.
Colony formation assay
Cells were seeded into 6-well plates at a density of
500 cells/well, then treated with 0, 0.05, 0.10 and 0.20 µM SM. The
cells were then incubated for 15 days at 37°C and 5%
CO2, fixed with methanol solution for 15 min at room
temperature, and finally stained with Giemsa for 30 min at room
temperature, and colonies consisting of ≥50 cells were manually
counted. The experiments were repeated three times.
Fluorescence staining
Cells were first treated with either SM or 0.1%
DMSO, washed with PBS and then fixed with 4% paraformaldehyde
solution for 15 min at room temperature. Then, the cells were again
washed with PBS and the chromosomes were stained with 5 µg/ml DAPI
for 5 min at room temperature. Following staining, the cells were
washed with PBS and then subjected to fluorescence microscopy
(Nikon Corporation) for visualization and image capture. The
experiments were repeated three times.
Western blot assay
RCC cells were treated with different doses of SM in
6-well plates, then total proteins were extracted using the RIPA
Lysis Buffer (Beijing Solarbio Science & Technology Co., Ltd.).
A cell nuclear and cytoplasmic protein extraction kit (Beyotime
Institute of Biotechnology) was used to extract the nuclear and
cytoplasmic proteins. Protein concentrations were determined using
the BCA protein assay kit (Beyotime Institute of Biotechnology),
then equal amounts of protein (25 µg) were separated on a 12%
SDS-PAGE. The protein bands were subsequently transferred onto PVDF
membranes (MilliporeSigma), which were then blocked for 1 h with 5%
(w/v) not-fat dry milk at room temperature. The membranes were then
incubated overnight with appropriate dilutions of the following
primary antibodies at 4°C: Mouse monoclonal anti-Bax (cat. no.
#89477; 1:1,000; Cell Signaling Technology, Inc.), mouse monoclonal
anti-Bcl-2 (cat. no. 15071; 1:1,000; Cell Signaling Technology,
Inc.), rabbit monoclonal anti-GAPDH (cat. no. 2118; 1:2,000; Cell
Signaling Technology, Inc.), rabbit monoclonal anti-p-STAT3 (cat.
no. 9145; 1:2,000; Cell Signaling Technology, Inc.), mouse
monoclonal anti-total STAT3 (cat. no. 9139; t-STAT3; 1:1,000; Cell
Signaling Technology, Inc.), rabbit monoclonal anti-cleaved
caspase-3 (cat. no. 9664; 1:1,000; Cell Signaling Technology,
Inc.), rabbit monoclonal anti-cleaved caspase-9 (cat. no. 7237;
1:1,000; Cell Signaling Technology, Inc.), rabbit monoclonal
anti-Histone H3 (cat. no. 4499; 1:2,000; Cell Signaling Technology,
Inc.) and rabbit monoclonal anti-cleaved caspase-8 (cat. no. 38680;
1:2,000; Invitrogen; Thermo Fisher Scientific, Inc.). Next, the
membranes were washed three times with TBST (containing 1‰
Tween-20), then probed with a secondary HRP conjugated goat
anti-mouse (cat. no. BA1050; 1:5000; Boster) or HRP conjugated goat
anti-rabbit antibodies (cat. no. BA1054; 1:5000; Boster) for 1.5 h
at room temperature. Finally, protein bands were visualized using
enhanced chemiluminescence reagent (Advansta) and analyzed by Tanon
Image software (Version 1.00, Tanon Science & Technology Co.,
Ltd., Shanghai, China). The experiments were repeated three
times.
Mouse model
Male 6-week-old BALB/c nude mice were purchased from
Shanghai SLAC Laboratory Animal Co., Ltd, and maintained in an
air-conditioned specific pathogen-free room (temperature: 23±3°C;
humidity: 40–65%) with a 12:12-h light-dark cycle. A total of 10
nude mice were randomly divided into two equal groups (5 per
group), then tumors were induced by subcutaneously injecting ACHN
cells (5.0×106 cells/mice) into the right flank area of
each mouse on day 0. Mice in the experimental group were
intragastrically administered 25 mg/kg SM once daily (19), while those in the control group were
given PBS. The animal health and behavior was checked once a day.
The initial average weight of the SM group and control group were
16.55 and 16.35 g respectively. The length of the experiment lasted
one month. All ten nude mice eventually developed tumors during
this period. Tumor growth and animal body weight were measured
after every 3 days, and the size of the tumors were calculated
using the following formula: π/6 × length × weight2. At
the end of the experiment, all mice were sacrificed through
cervical dislocation after intraperitoneal injection of 2% sodium
pentobarbital (25 mg/kg) anesthetic. Tumor tissues were collected
and weighed prior to immunohistochemistry. The major organs (heart,
liver, spleen, lung and kidney) were also collected.
Immunohistochemistry
Tumor tissues were fixed for 12 h with 4%
paraformaldehyde at room temperature, cut into 4-µm sections and
then embedded using paraffin. The slides were heated at 60°C for 1
h and deparaffinized in xylene solution, rehydrated by descending
concentrations of ethanol, washed three times with PBS, blocked
with 5% bovine serum albumin (cat. no. SW3015; Solarbio) at room
temperature for 1 h, and then incubated with primary antibody
against p-STAT3 (cat. no. 9145; 1:400; Cell Signaling Technology,
Inc.) at room temperature for 1 h. Next, the sections were washed
three times with PBS and incubated with Biotin-HRP labeled
secondary antibody (cat. no. BA1018; 1:200; Boster) at room
temperature for 1 h. The immunostained cells were counted from five
randomly selected fields, viewed under ×400 magnification using an
inverted light microscope (Version ECLIPSE NI-U, Nikon
Corporation). The number of positively stained cells was measured
using the Image-Pro Plus analysis software (Version 6.0, Media
Cybernetics).
Haematoxylin and eoxin (H&E)
staining
Paraffin-embedded sections were prepared as
aforementioned, then stained with hematoxylin for 1 min at room
temperature. The sections were washed for 10 min with water and
then stained with eosin for 1 min at room temperature. The slides
were visualized using an optical microscope under ×100
magnification.
Statistical analysis
All statistical analyses were performed using SPSS
version 18.0 (SPSS, Inc.) and GraphPad Prism version 5.0
(Dotmatics) software. Data are presented as the mean ± standard
deviation. The unpaired two-tailed Student's t-test was used to
analyze the statistical difference between two groups. A one-way
ANOVA analysis followed by Dunnett's test was used to calculate the
statistical difference between multiple groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
SM suppresses viability of RCC
cells
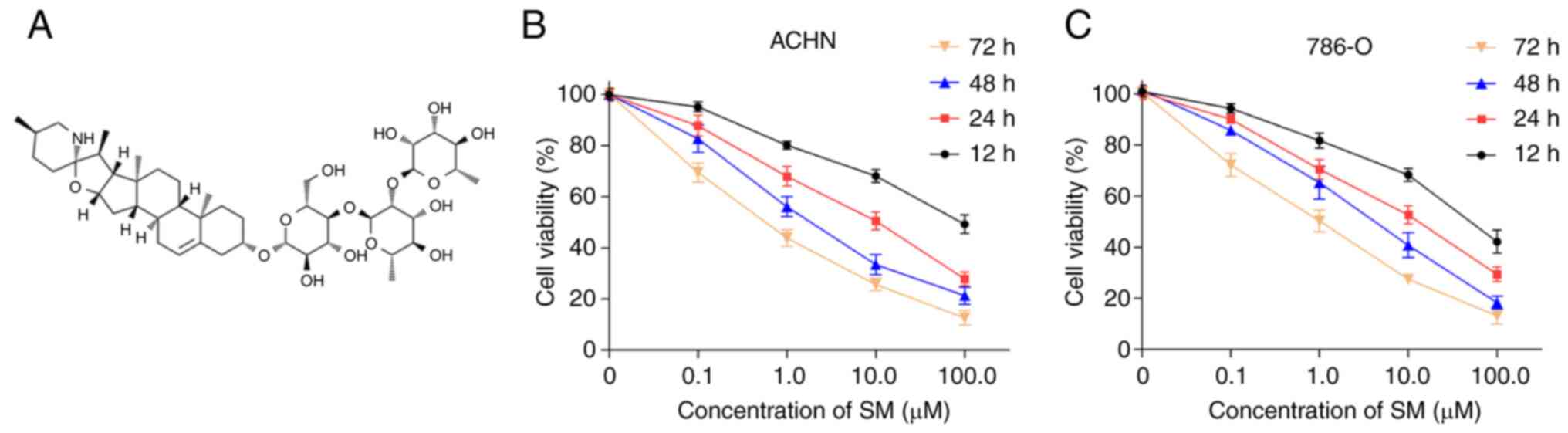
The chemical structure of SM is shown in Fig. 1A. The results of the MTT assay
demonstrated that SM inhibited the viability of both ACHN and 786-O
cells in a dose- and time-dependent manner (Fig. 1B and C). A summary of the SM
IC50 values in ACHN and 786-O cells across each
incubation period is shown in Table
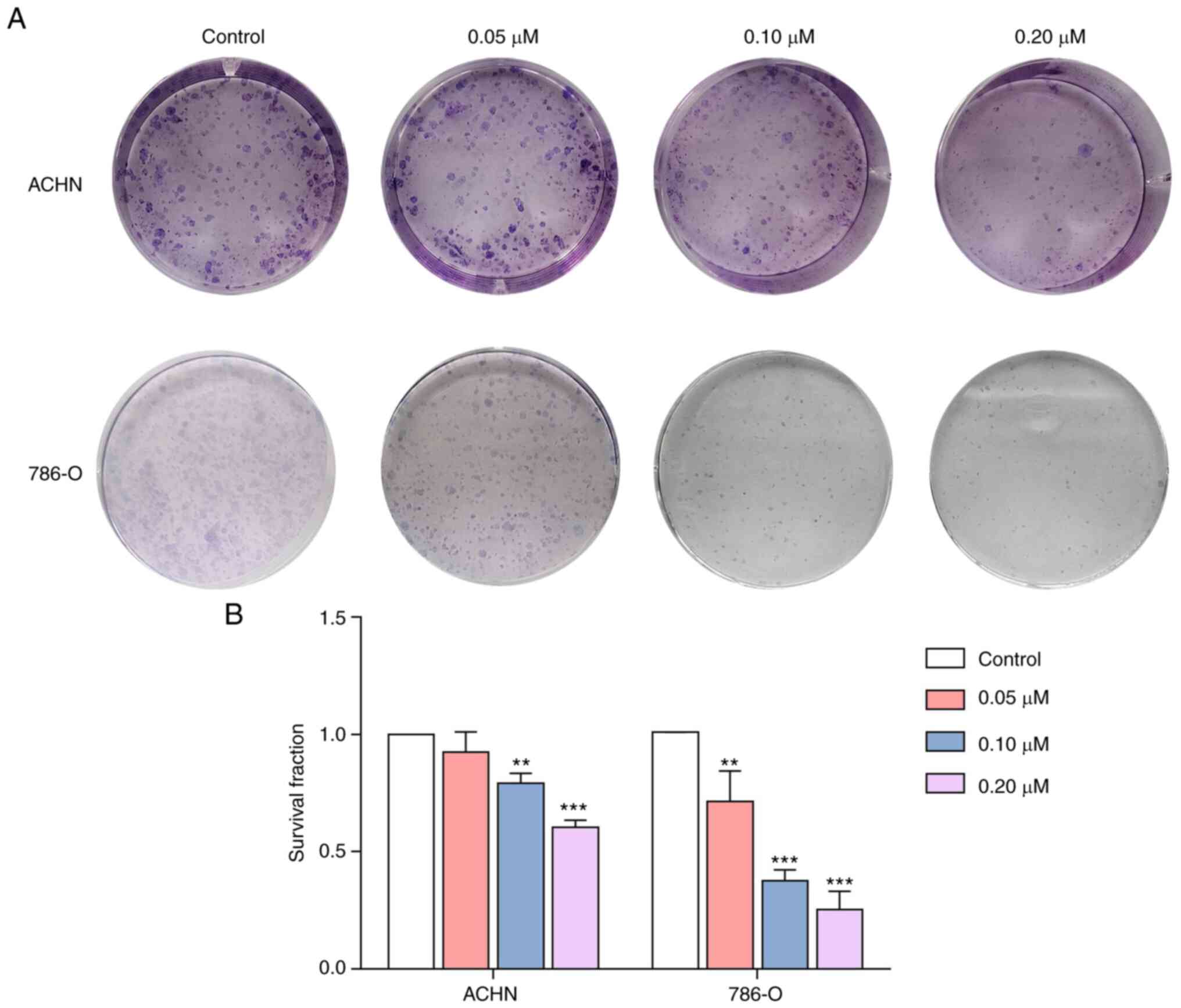
I. Moreover, SM treatment suppressed the clonogenicity of ACHN
and 786-O cells (Fig. 2). Notably,
the density and number of cell colonies significantly decreased
with increasing SM concentrations.
 | Table I.IC50 values of solamargine
in ACHN and 786-O cells. |
Table I.
IC50 values of solamargine
in ACHN and 786-O cells.
|
| IC50,
µM |
|---|
|
|
|
|---|
| Cell line | 12 h | 24 h | 48 h | 72 h |
|---|
| ACHN | 6.888 | 0.895 | 0.311 | 0.105 |
| 786-O | 5.190 | 1.124 | 0.527 | 0.249 |
SM induces the apoptosis of RCC
cells
A previous study has demonstrated an association
between inhibition of cell viability with the induction of
apoptosis in cancer cells (20). To
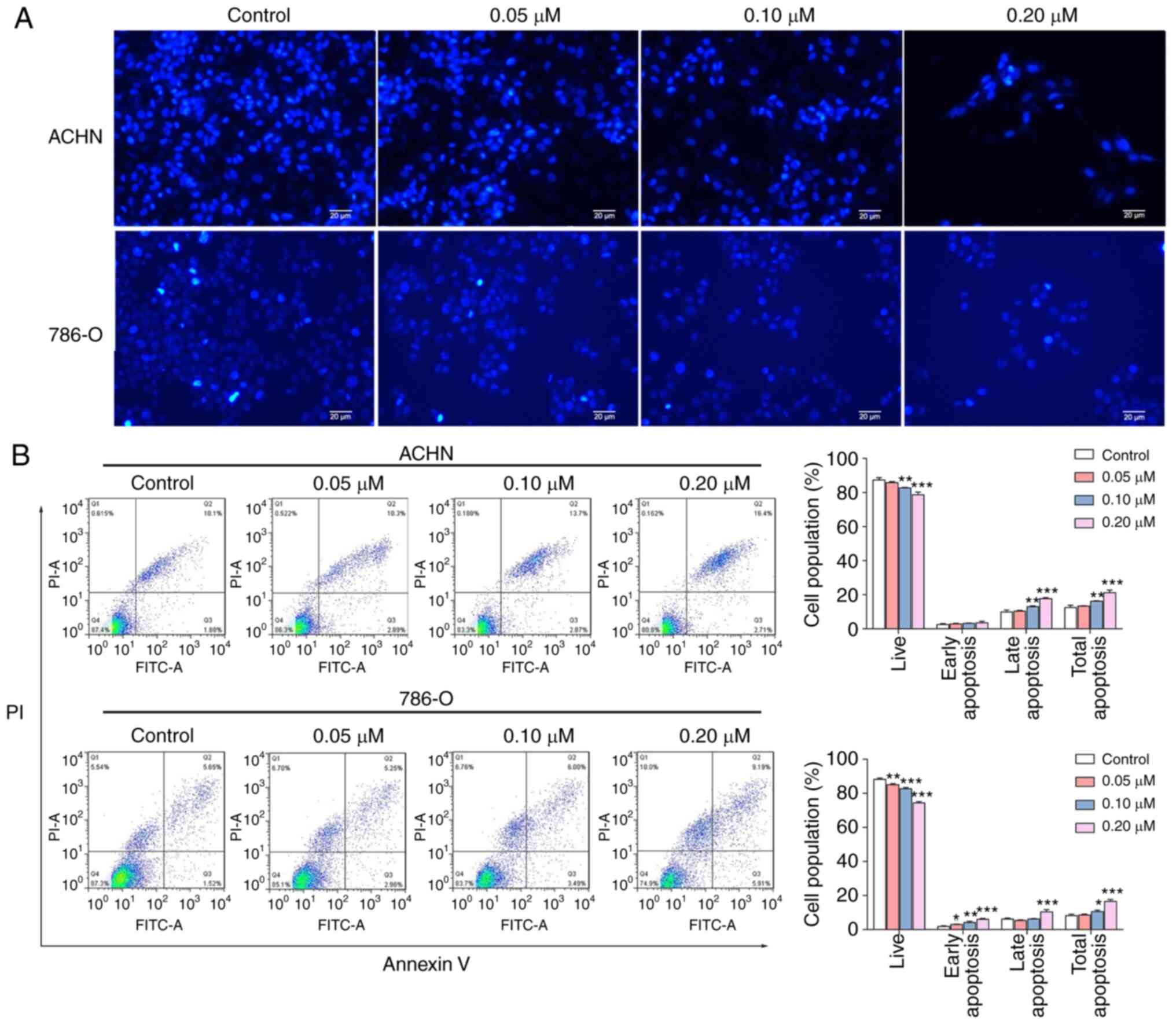
evaluate whether SM induces apoptosis, the nuclei of RCC cells were
stained with DAPI. The nuclear chromatin condensation and
fragmentation in cells were notably increased with increasing
concentrations of SM (Fig. 3A).
Next, the rate of SM-induced apoptosis was explored using an
Annexin V/PI assay. The results of the assay demonstrated that SM
treatment increased total cell apoptosis in a dose-dependent manner
(Fig. 3B). Moreover, SM-treated
cells exhibited higher rates of apoptosis at both the early and
late stages compared with the control group, however this was not
significant for early apoptosis. To elucidate the underlying
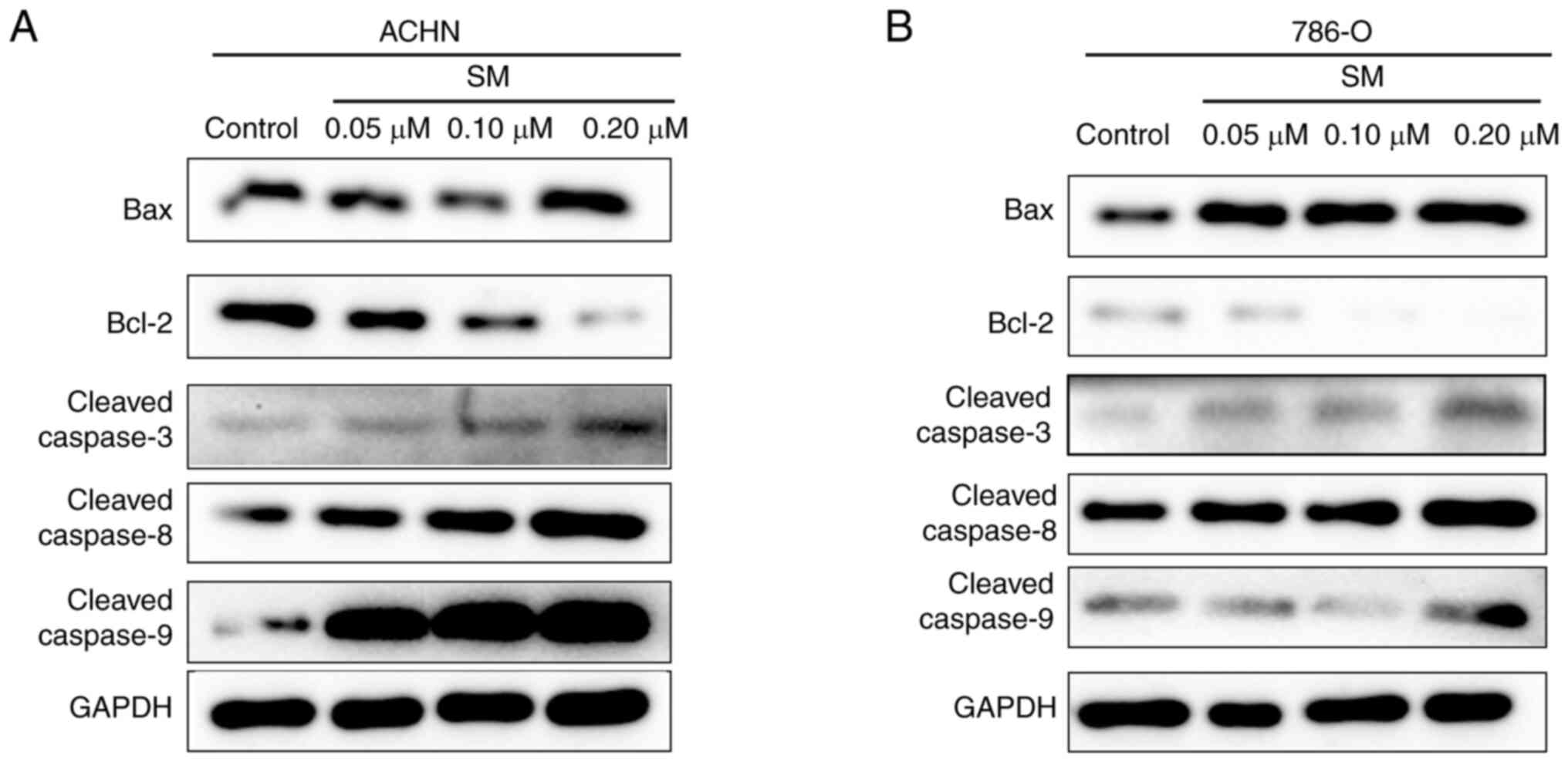
mechanism of SM-induced apoptosis, the activities of caspase-3,
caspase-8 and caspase-9 were determined. It was found that the
activities of all three caspases were significantly upregulated at
higher SM concentrations (Table
II). In addition, the expression levels of cleaved
caspase-3,-8,-9 and Bax were markedly upregulated while the level
of Bcl-2 was downregulated following 24 h SM treatment (Fig. 4).
 | Table II.Effects of SM on the activities of
caspases in treated 786-O and ACHN cells. |
Table II.
Effects of SM on the activities of
caspases in treated 786-O and ACHN cells.
| Cell line | SM concentration,
µM | Caspase-3 activity,
IU | Caspase-8 activity,
IU | Caspase-9 activity,
IU |
|---|
| 786-O | 0 (control) | 1.800±0.081 | 1.757±0.095 | 1.367±0.100 |
|
| 0.05 | 1.803±0.029 | 1.803±0.086 | 1.473±0.072 |
|
| 0.10 | 2.133±0.116 | 1.897±0.110 | 1.657±0.215 |
|
| 0.20 |
2.323±0.291a |
2.310±0.274a |
2.100±0.326b |
| ACHN | 0 (control) | 2.183±0.144 | 1.507±0.111 | 1.803±0.127 |
|
| 0.05 | 2.210±0.104 | 1.623±0.127 | 2.103±0.253 |
|
| 0.10 |
2.597±0.038a | 1.770±0.085 |
2.367±0.093a |
|
| 0.20 |
2.813±0.264b |
2.513±0.510b |
3.123±0.201c |
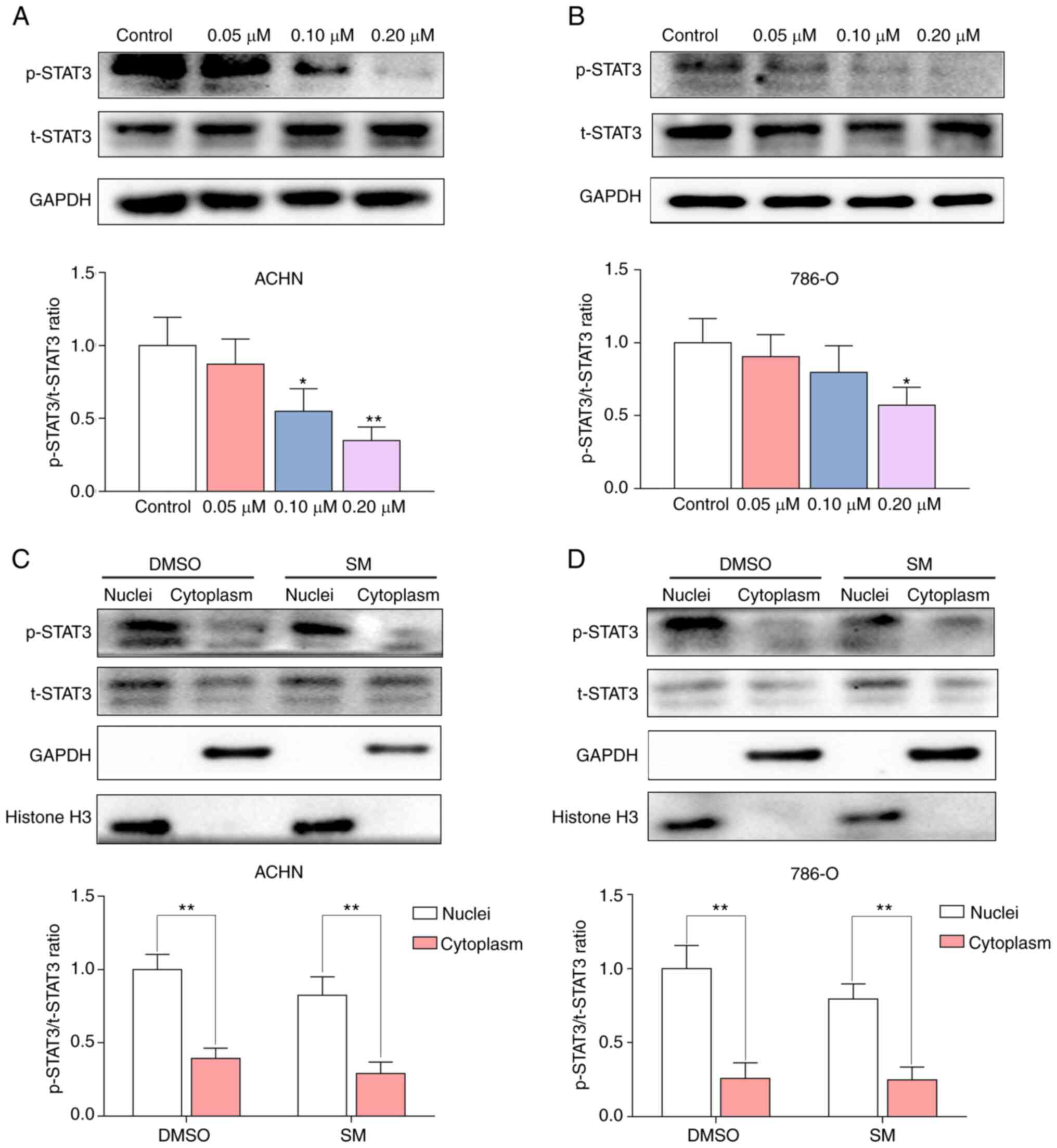
SM downregulates expression of p-STAT3
and inhibits its translocation into the cytoplasm
Accumulating evidence has underscored the critical
role of STAT3 in modulating the viability and proliferation of
cancer cells (21–23). Therefore, it was next determined
whether STAT3 was involved in SM-induced cell apoptosis. It was
found that SM mediated a significant reduction in the
p-STAT3/t-STAT3 ratio in a dose-dependent manner (Fig. 5A and B). Phosphorylation of STAT3 is
required for STAT3 dimerization, which enables it to translocate
from the cytoplasm to the nucleus, thus regulating expression of
its target genes (24,25). To further confirm the localization
of p-STAT3, proteins from SM-treated RCC cells were fractionated
using a nuclear and cytoplasmic protein extraction kit, following
24 h of treatment. The results demonstrated that SM treatment
downregulated p-STAT3 levels in the nuclear fraction of RCC cells
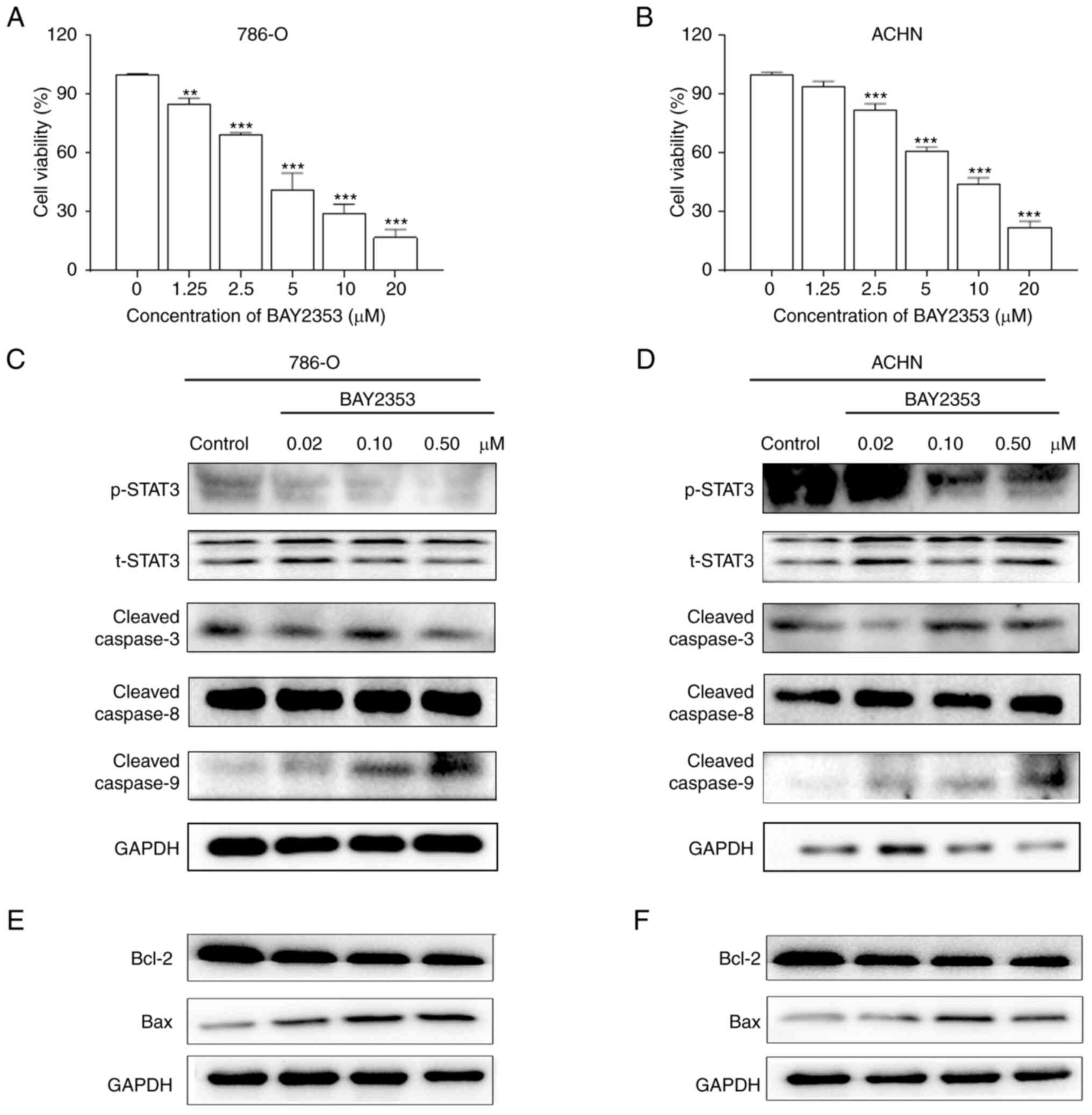
(Fig. 5C and D). In addition,
treatment with the p-STAT3 inhibitor, BAY2353, inhibited the
viability of ACHN and 786-O cells (Fig.
6A and B). Furthermore, BAY2353 increased the expression levels
of cleaved caspase-9 and Bax but decreased levels of p-STAT3 and
Bcl-2 (Fig. 6C-F).
SM inhibits tumor growth in ACHN
xenograft mice
Whether SM could suppress tumor growth in
vivo was next determined by analyzing its effect on an
ACHN-bearing nude mouse model. Treatment with SM caused a
significant decrease in the volume and weight of tumor tissues
(Fig. 7A-C) compared with the
control group. Moreover, there was no significant difference in
body weight between the experimental group and control group
(Fig. 7D). H&E staining of the
major organs (heart, liver, spleen, lung and kidney) revealed no
notable acute or chronic physiological toxicity following SM
treatment (Fig. 7E). Furthermore,
SM treatment significantly decreased the number of
p-STAT3+ cells compared with the control group,
indicating that SM deactivated STAT3 phosphorylation in ACHN
xenograft mice (Fig. 7F and G).
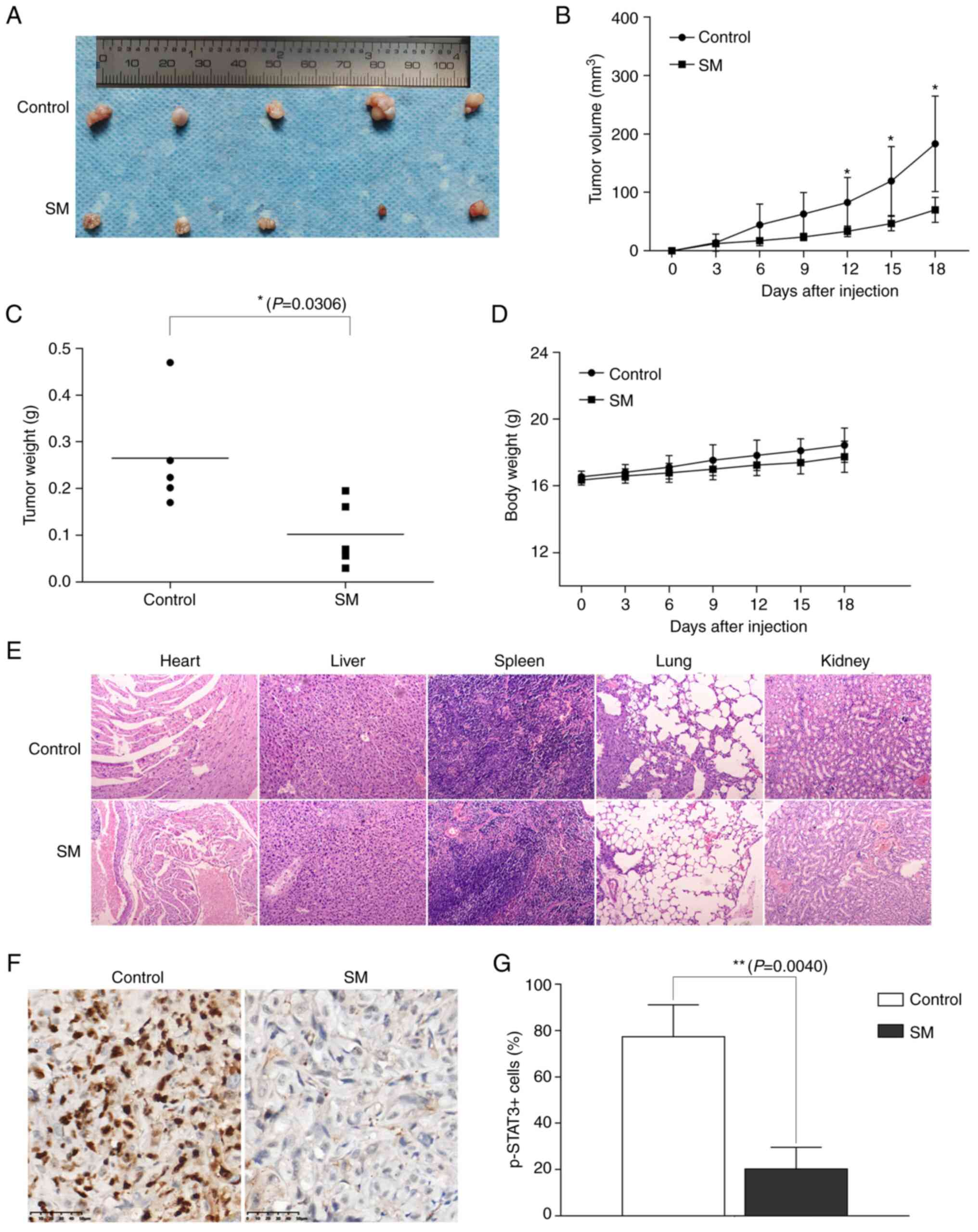 | Figure 7.Effects of SM on tumor growth in
ACHN-bearing nude mice. (A) tumors, (B) tumor volume, (C) tumor
weight, (D) body weight, (E) Haematoxylin and eoxin staining of
major organs (heart, liver, spleen, lung and kidney) (×100) and (F)
Immunohistochemical staining with the p-STAT3 antibody of tumor
tissues from control and SM group mice (×400). (G) Quantification
of pSTAT3+ cells. Data are presented as the mean ±
standard deviation, n=5. *P<0.05, **P<0.01 vs. control group.
STAT3, signal transducer and activator of transcription-3; p-STAT3,
phosphorylated STAT3, t-STAT3, total STAT3; SM, solamargine. |
Discussion
RCC is the third most common malignant tumor of the
urinary system (26). Although
numerous treatment options exist for advanced RCC, the disease
remains incurable. Notably, natural products have exhibited more
favorable outcomes and lower side effects compared with chemical
synthetic drugs, such as andrographis paniculata and phomaketides A
(27–29). In the present study, results of the
MTT assay revealed that SM treatment inhibited RCC cell viability
in a dose- and time-dependent manner, while findings from the
colony formation assay indicated that SM inhibited the growth and
clonogenicity of RCC cells in a dose-dependent manner. These
results were consistent with previous studies that demonstrated
that SM reduced the viability of various cancer cell types in a
dose-dependent manner (15,30,31).
Apoptosis, a process of programmed cell death,
occurs through both extrinsic and intrinsic pathways. While the
extrinsic pathway is triggered by death receptors that result in
caspase-8 activation, the intrinsic pathway is triggered by
organelle injury leading to caspase-9 activation (32,33).
Both pathways have been shown to activate the same downstream
caspase-3 molecules, to eventually trigger cell apoptosis (25,34,35).
Notably, the Bcl-2 family plays a critical role in controlling the
intrinsic apoptotic pathway. Both pathways have been previously
reported in SM-induced apoptosis (25). Liang et al (36) found that tumor necrosis factor
receptor 1-associated DEATH domain protein and FAS-associated death
domain protein were recruited, while caspase-8 and caspase-3 were
activated in SM-treated A549 cells. Moreover, it was demonstrated
that SM treatment induced the release of cytochrome c from the
mitochondria, downregulation of Bcl-2 and Bcl-x(L), upregulation of
Bax and upregulation of caspase-9 activities in A549 cells
(36). In addition, Xie et
al (31) found that SM
treatment mediated a significant downregulation of proliferation
associated (Ki-67 and proliferating cell nuclear antigen) and
anti-apoptotic (Bcl-2) proteins but promoted the activity of
apoptosis-associated proteins (Bax, caspase-3 and caspase-9) in
hepatocellular cells. Zhang et al (37) also reported that SM downregulated
Bcl-2 and poly ADP ribose polymerase (PARP) proteins, but
upregulated Bax, cleaved PARP, caspase 3, cleaved caspase 3 and
caspase 7 proteins in QBC939 human cholangiocarcinoma cells. The
results of the present study demonstrated that SM significantly
increased apoptosis (accomplished by the upregulation of caspase-3,
caspase-8, caspase-9 and Bax), whilst decreasing Bcl-2 levels in
RCC cells. These results indicated that both the extrinsic and
intrinsic pathways were involved in SM-induced apoptosis, which is
consistent with previous studies.
A number of studies have reported the antitumor
activities of SM. Notably, one study demonstrated that SM exerted
its antitumor activity by inhibiting the MAPK signaling pathway
(30), while another demonstrated
that SM inhibited cell growth by suppressing PI3K/Akt signaling
(15). In addition, Liu et
al (38) concluded that the
Notch signaling pathway was suppressed in SM-treated CM-319 human
chordoma cells, while Zhou et al (39) demonstrated that SM not only
inhibited proliferation but also induced the apoptosis of lung
cancer cells through the p38 MAPK-mediated suppression of
phosphorylation and protein expression of STAT3, followed by
induction of the STAT3 downstream effector, p21. As a
proto-oncogenic transcription factor, constitutive activation of
STAT3 induces tumor development by promoting cell proliferation and
inhibiting apoptosis (40,41). This implies that SM-mediated
suppression of p-STAT3 may facilitate apoptosis. The results of the
present study demonstrated that SM downregulated expression of
p-STAT3 in a dose-dependent manner. Since tumor-promotion is
ultimately regulated by STAT3-dependent transcriptional regulation
of downstream oncogenes, the present study further focused on the
localization of p-STAT3 and found that SM diminished its nuclear
localization in cells. Therefore, it was hypothesized that SM
suppressed translocation of STAT3 to the nucleus in RCC cells.
These results indicated that the decreased p-STAT3 levels were
associated with SM-induced apoptosis. Furthermore, intragastric
administration of SM to ACHN-bearing nude mice at a ratio of 25
mg/kg suppressed tumor growth and decreased the number of
p-STAT3+ cells in tumor tissue from the established
xenograft mouse model, and it had no notable toxicity to the main
organs. However, it is unclear whether higher SM drug
concentrations will cause damages in vivo, and we will
continue to explore in our further studies. In summary, the results
of the present study indicated that SM triggered the apoptosis of
RCC cells. This event was associated with the inactivation of STAT3
phosphorylation. Taken together, these results indicated that SM
has potential inhibitory effects on RCC cells and may be used in
clinical practice in the future.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from The Natural Science
Foundation of Ningbo (grant nos. 202003N4295 and 2023J233), The
General Health Foundation of Zhejiang Province (grant nos.
2021KY1067 and 2022KY1177), The Zhejiang Key Laboratory of
Pathophysiology (grant no. 201910) and The Science and Technology
Project of Yinzhou (grant nos. 2022AS033, 2023AS062 and
2023AS064).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DC, YR and GW designed the study. SH wrote the
manuscript; SH, MS, TL and XW performed the experiments; XW
analyzed the data. All authors read and approved the final version
of the manuscript. SH, DC and GW confirm the authenticity of all
the raw data.
Ethics approval and consent to
participate
The animal study was approved by The Ethical
Committee on Animal Research of Ningbo University (Ningbo, China;
approval no. 10533).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Capitanio U and Montorsi F: Renal cancer.
Lancet. 387:894–906. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huang J, Wang X, Wen G and Ren Y:
miRNA2055p functions as a tumor suppressor by negatively regulating
VEGFA and PI3K/Akt/mTOR signaling in renal carcinoma cells. Oncol
Rep. 42:1677–1688. 2019.PubMed/NCBI
|
|
4
|
Hillmer EJ, Zhang H, Li HS and Watowich
SS: STAT3 signaling in immunity. Cytokine Growth Factor Rev.
31:1–15. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun Y, Liu L, Wang Y, He A, Hu H, Zhang J,
Han M and Huang Y: Curcumin inhibits the proliferation and invasion
of MG-63 cells through inactivation of the p-JAK2/p-STAT3 pathway.
Onco Targets Ther. 12:2011–2021. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Song M, Wang C, Yang H, Chen Y, Feng X, Li
B and Fan H: P-STAT3 inhibition activates endoplasmic reticulum
stress-induced splenocyte apoptosis in chronic stress. Front
Physiol. 11:6802020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tan B, Chen X, Fan Y, Yang Y, Yang J and
Tan L: STAT3 phosphorylation is required for the HepaCAM-mediated
inhibition of castration-resistant prostate cancer cell viability
and metastasis. Prostate. 81:603–611. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang ZH, Li MY, Wang Z, Zuo HX, Wang JY,
Xing Y, Jin C, Xu G, Piao L, Piao H, et al: Convallatoxin promotes
apoptosis and inhibits proliferation and angiogenesis through
crosstalk between JAK2/STAT3 (T705) and mTOR/STAT3 (S727) signaling
pathways in colorectal cancer. Phytomedicine. 68:1531722020.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jahangiri A, Dadmanesh M and Ghorban K:
STAT3 inhibition reduced PD-L1 expression and enhanced antitumor
immune responses. J Cell Physiol. 235:9457–9463. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lorente D, Arevalo J, Salcedo MT, Trilla
E, de Torres I, Meseguer A and Morote J: Analysis of the nuclear
expression of pSer727-STAT3 as a prognostic factor in patients with
clear cell renal carcinoma. Actas Urol Esp (Engl Ed). 44:245–250.
2020.(In English, Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhan C, Xu C, Chen J, Shen C, Li J, Wang
Z, Ying X, Luo Z, Ren Y, Wu G, et al: Development and Validation of
an IL6/JAK/STAT3-Related gene signature to predict overall survival
in clear cell renal cell carcinoma. Front Cell Dev Biol.
9:6869072021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Arevalo J, Lorente D, Trilla E, Salcedo
MT, Morote J and Meseguer A: Nuclear and cytosolic pS727-STAT3
levels correlate with overall survival of patients affected by
clear cell renal cell carcinoma (ccRCC). Sci Rep. 11:69572021.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lorente D, Trilla E, Meseguer A, Arevalo
J, Nemours S, Planas J, Placer J, Celma A, Salvador C, Regis L, et
al: The role of STAT3 protein as a prognostic factor in the clear
cell renal carcinoma. Systematic review. Actas Urol Esp (Engl Ed).
43:118–123. 2019.(In English, Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li S, Priceman SJ, Xin H, Zhang W, Deng J,
Liu Y, Huang J, Zhu W, Chen M, Hu W, et al: Icaritin inhibits
JAK/STAT3 signaling and growth of renal cell carcinoma. PLoS One.
8:e816572013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ge J, Wang P, Ma H and Zhang J:
Solamargine inhibits prostate cancer cell growth and enhances the
therapeutic efficacy of docetaxel via Akt signaling. J Oncol.
2022:90559542022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sani IK, Marashi SH and Kalalinia F:
Solamargine inhibits migration and invasion of human hepatocellular
carcinoma cells through down-regulation of matrix
metalloproteinases 2 and 9 expression and activity. Toxicol In
Vitro. 29:893–900. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Furtado RA, Ozelin SD, Ferreira NH, Miura
BA, Almeida Junior S, Magalhaes GM, Nassar EJ, Miranda MA, Bastos
JK and Tavares DC: Antitumor activity of solamargine in mouse
melanoma model: Relevance to clinical safety. J Toxicol Environ
Health A. 85:131–142. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu YH, Chiu WT, Young MJ, Chang TH, Huang
YF and Chou CY: Solanum Incanum extract downregulates aldehyde
dehydrogenase 1-mediated stemness and inhibits tumor formation in
ovarian cancer cells. J Cancer. 6:1011–1019. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Al Chami L, Mendez R, Chataing B,
O'Callaghan J, Usubillaga A and Lacruz L: Toxicological effects of
alpha-solamargine in experimental animals. Phytother Res.
17:254–258. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sezer ED, Oktay LM, Karadadas E, Memmedov
H, Selvi Gunel N and Sozmen E: Assessing anticancer potential of
blueberry flavonoids, quercetin, kaempferol, and gentisic acid,
through oxidative stress and apoptosis parameters on HCT-116 cells.
J Med Food. 22:1118–1126. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang HQ, Man QW, Huo FY, Gao X, Lin H, Li
SR, Wang J, Su FC, Cai L, Shi Y, et al: STAT3 pathway in cancers:
Past, present, and future. MedComm (2020). 3:e1242022.PubMed/NCBI
|
|
22
|
Fan M, Sun W, Gu X, Lu S, Shen Q, Liu X
and Zhang X: The critical role of STAT3 in biogenesis of
tumor-derived exosomes with potency of inducing cancer cachexia in
vitro and in vivo. Oncogene. 41:1050–1062. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qin JJ, Yan L, Zhang J and Zhang WD: STAT3
as a potential therapeutic target in triple negative breast cancer:
A systematic review. J Exp Clin Cancer Res. 38:1952019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu Y, Liao S, Bennett S, Tang H, Song D,
Wood D, Zhan X and Xu J: STAT3 and its targeting inhibitors in
osteosarcoma. Cell Prolif. 54:e129742021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang J, Ren Y, Lou ZG, Wan X, Weng GB and
Cen D: Paeoniflorin inhibits the growth of bladder carcinoma via
deactivation of STAT3. Acta Pharm. 68:211–222. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Deleuze A, Saout J, Dugay F, Peyronnet B,
Mathieu R, Verhoest G, Bensalah K, Crouzet L, Laguerre B,
Belaud-Rotureau MA, et al: Immunotherapy in Renal cell carcinoma:
The future is now. Int J Mol Sci. 21:25322020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Islam MR, Akash S, Rahman MM, Nowrin FT,
Akter T, Shohag S, Rauf A, Aljohani ASM and Simal-Gandara J: Colon
cancer and colorectal cancer: Prevention and treatment by potential
natural products. Chem Biol Interact. 368:1101702022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shams Ul Hassan S, Ishaq M, Zhang WD and
Jin HZ: An overview of the mechanisms of marine fungi-derived
anti-inflammatory and anti-tumor agents and their novel role in
drug targeting. Curr Pharm Des. 27:2605–2614. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Su M, Qin B, Liu F, Chen Y and Zhang R:
Andrographolide enhanced 5-fluorouracil-induced antitumor effect in
colorectal cancer via inhibition of c-MET pathway. Drug Des Devel
Ther. 11:3333–3341. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fu R, Wang X, Hu Y, Du H, Dong B, Ao S,
Zhang L, Sun Z, Zhang L, Lv G and Ji J: Solamargine inhibits
gastric cancer progression by regulating the expression of
lncNEAT1_2 via the MAPK signaling pathway. Int J Oncol.
54:1545–1554. 2019.PubMed/NCBI
|
|
31
|
Xie X, Zhu H, Yang H, Huang W, Wu Y, Wang
Y, Luo Y, Wang D and Shao G: Solamargine triggers hepatoma cell
death through apoptosis. Oncol Lett. 10:168–174. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Porubsky M, Reznickova E, Krupkova S,
Krystof V and Hlavac J: Development of fluorescent dual-FRET probe
for simultaneous detection of caspase-8 and caspase-9 activities
and their relative quantification. Bioorg Chem. 129:1061512022.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mcglorthan L, Paucarmayta A, Casablanca Y,
Maxwell GL and Syed V: Progesterone induces apoptosis by activation
of caspase-8 and calcitriol via activation of caspase-9 pathways in
ovarian and endometrial cancer cells in vitro. Apoptosis.
26:184–194. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Goldar S, Khaniani MS, Derakhshan SM and
Baradaran B: Molecular mechanisms of apoptosis and roles in cancer
development and treatment. Asian Pac J Cancer Prev. 16:2129–2144.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jin Z and El-Deiry WS: Overview of cell
death signaling pathways. Cancer Biol Ther. 4:139–163. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liang CH, Liu LF, Shiu LY, Huang YS, Chang
LC and Kuo KW: Action of solamargine on TNFs and
cisplatin-resistant human lung cancer cells. Biochem Biophys Res
Commun. 322:751–758. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang X, Yan Z, Xu T, An Z, Chen W, Wang
X, Huang M and Zhu F: Solamargine derived from Solanum nigrum
induces apoptosis of human cholangiocarcinoma QBC939 cells. Oncol
Lett. 15:6329–6335. 2018.PubMed/NCBI
|
|
38
|
Liu J, Wang Z, Xu C, Qi Y and Zhang Q:
Solamargine inhibits proliferation and promotes apoptosis of CM-319
human chordoma cells through suppression of notch pathway. Transl
Cancer Res. 8:509–519. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhou Y, Tang Q, Zhao S, Zhang F, Li L, Wu
W, Wang Z and Hann S: Targeting signal transducer and activator of
transcription 3 contributes to the solamargine-inhibited growth and
-induced apoptosis of human lung cancer cells. Tumour Biol.
35:8169–8178. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mohassab AM, Hassan HA, Abdelhamid D and
Abdel-Aziz M: STAT3 transcription factor as target for anti-cancer
therapy. Pharmacol Rep. 72:1101–1124. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fathi N, Rashidi G, Khodadadi A, Shahi S
and Sharifi S: STAT3 and apoptosis challenges in cancer. Int J Biol
Macromol. 117:993–1001. 2018. View Article : Google Scholar : PubMed/NCBI
|