Introduction
In recent years, with the aging population, the
likelihood of breast cancer with various complications and other
malignancies has increased. Notably, associations between chronic
disease and cancer risk have been emphasized at various levels. For
example, patients with systemic lupus erythematosus (SLE) have a
higher susceptibility to cancer than the general population
(1). Furthermore, treatment for
breast cancer with collagen disorder is complicated due to the use
of various concomitant drugs and its clinical course is thus
interesting.
Collagen disorders are chronic autoimmune diseases
with complex clinical courses. Moreover, breast cancer with
comorbid collagen disorders involving steroids and
immunosuppressants complicates treatment protocols. Although the
use of immunosuppressants increases the risk of opportunistic
infections, their influence on prognosis remains unclear (1).
As reported in previous studies, collagen disorders
comorbid with breast cancer include polymyositis (PM) and
dermatomyositis (DM) (2,3). Moreover, the risk of malignant disease
was the highest at time of myositis diagnosis in both PM and DM;
specifically, the former was associated with a raised risk of
non-Hodgkin lymphoma, lung, and bladder cancers, while the latter
was strongly associated with malignant disease, particularly
ovarian, lung, pancreatic, stomach, and colorectal cancers, and
non-Hodgkin lymphoma (3). Patients
with SLE are at a high risk of developing hematopoietic
malignancies, especially non-Hodgkin's lymphoma, but at a low risk
of developing solid carcinomas, such as breast, ovarian,
endometrial, and prostate cancers (1,4).
Previous studies have investigated the risk factors for malignancy
in patients with DM and PM (5).
However, studies on patients with breast cancer and collagen
disorders had small sample sizes and did not elucidate the
biological and treatment status of patients with breast cancer
(6,7). Therefore, this study aimed to clarify
the clinicopathological characteristics and long-term prognoses of
patients with breast cancer and collagen disorders.
Materials and methods
Patients
Between January 2004 and December 2011, 25 patients
with histologically diagnosed invasive cancer who underwent surgery
for primary breast lesions and were concomitantly diagnosed with
collagen disorders were included in the study. Patients with stage
IV disease, those who had received systemic chemotherapy, and male
patients were excluded from the study. Furthermore, a control group
of patients with breast cancer but without collagen disorders
(n=58) matched for approximately contemporaneous surgery, age,
disease stage, and nodal status was included. On April 7, 2021, the
Ethics Committee of Saitama Medical School General Medical Center
approved this retrospective study (approval no. 2021-006). Given
the retrospective nature of this study, the requirement for
informed consent was waived.
Methods
We obtained the following clinicopathological
factors from hospital medical records: estrogen receptor (ER),
progesterone receptor (PgR), human epidermal growth factor receptor
type 2 (HER2), and Ki-67 labeling index (LI) (using monoclonal
mouse anti-human antigen clone MIB-1; Dako Denmark A/S). ER and PgR
positivity was determined when ≥1% of the nuclei in the tumor were
stained using immunohistochemical staining. HER2 positivity was
defined as an immunohistochemistry (VENTANA I–VIEW, clone 6F11)
score of 3+ or a positive result on fluorescence in situ
hybridization.
Statistical analysis
The differences in the background factors between
the groups were analyzed using the χ2, Fisher's exact,
or unpaired two-group t-test. The results are summarized in
Table I. Regarding survival
analyses, we performed intergroup comparisons for recurrence-free
survival (RFS), measured from the time of surgery until the
recurrence of breast cancer, and overall survival (OS), calculated
from the date of surgery until the date of death or last patient
contact. RFS and OS were assessed using the Kaplan-Meier and
log-rank tests. A multivariate Cox regression model was used to
analyze the RFS. We defined the model using the forced entry
method, including age, invasive tumor size, nodal status,
histological tumor grade, lymphovascular invasion, comorbidity with
collagen disorder, and steroid use. The independent effect of each
variable was described using hazard ratios with 95% confidence
intervals (CIs). All P values were two-sided, and P<0.05 was
considered to indicate a statistically significant difference. IBM
SPSS Statistics for Windows version 27 (IBM Corp., Armonk, NY, USA)
was used for all statistical analyses. The date of outcome
evaluation was February 25, 2022. The median potential observation
periods for recurrence-free endpoints and overall survival were 103
months and 114 months, respectively.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Variable | Patients with
collagen disorder (n=25) | Patients without
collagen disorder (n=58) | P-value |
|---|
| Mean age, years
(range) | 56 (31–75) | 55 (32–84) | N.S. |
| Stage, n (%) |
|
| N.S. |
| I | 14 (56.0) | 25 (43.1) |
|
| IIA | 5 (20.0) | 21 (36.2) |
|
| IIB | 3 (12.0) | 9 (15.5) |
|
| III | 3 (12.0) | 3 (5.2) |
|
| Tumor size, n
(%) |
|
| N.S. |
| T1 (≤2
cm) | 15 (60.0) | 35 (60.3) |
|
| T2-3
(>2 cm) | 10 (40.0) | 23 (39.7) |
|
| Nodal status, n
(%) |
|
| N.S. |
|
Negative | 13 (52.0) | 36 (62.1) |
|
|
Positive | 10 (40.0) | 21 (36.2) |
|
|
Missing | 2 (8.0) | 1 (1.7) |
|
| Lymphatic
invasion, |
|
| 0.015 |
| n
(%) |
|
|
|
| 0 | 17 (68.0) | 47 (81.0) |
|
| 1 | 2 (8.0) | 9 (15.5) |
|
| 2-3 | 5 (20.0) | 2 (3.4) |
|
|
Missing | 1 (4.0) | 0 (0.0) |
|
| Histological grade, n
(%) |
|
| 0.029 |
| I | 8 (32.0) | 23 (39.7) |
|
| II | 10 (40.0) | 30 (51.7) |
|
| III | 6 (24.0) | 2 (3.4) |
|
|
Missing | 1 (4.0) | 3 (5.2) |
|
| Estrogen receptor, n
(%) |
|
|
|
|
Positive | 20 (80.0) | 44 (75.9) | N.S. |
|
Negative | 5 (20.0) | 14 (24.1) |
|
| Progesterone
receptor, n (%) |
|
| N.S. |
|
Positive | 12 (48.0) | 35 (60.3) |
|
|
Negative | 13 (52.0) | 23 (39.7) |
|
| HER2, n (%) |
|
| N.S. |
|
Positive | 4 (16.0) | 7 (12.1) |
|
|
Negative | 20 (80.0) | 51 (87.9) |
|
|
Missing | 1 (4.0) | 0 (0.0) |
|
| Adjuvant
chemotherapy, n (%) |
|
| N.S. |
|
Yes | 5 (20.0) | 14 (24.1) |
|
| No | 20 (80.0) | 44 (75.9) |
|
Results
Background of patients
The mean age of the patients was 56.4 (±12.6) years.
In addition, 14, 8, and 3 patients had disease of clinical stages
I, II, and III, respectively. The status of lymph node metastasis
was N0 and N1 in 15 and 10 patients, respectively (Table I). The comorbidities included
rheumatoid arthritis (11 patients), systemic lupus erythematosus
(four patients), PM/DM (four patients), mixed connective tissue
disease (two patients), Sjögren's syndrome (four patients),
scleroderma (one patient), and adult-onset Still's disease (one
patient) (Table II). The status of
hormone receptor (HR) and HER2 expression was as follows: HR(+), 20
(80.0%) patients; HER2(+), four (16.0%) patients; and HR(−)HER2(−),
four (16.0%) patients. All but two patients in the collagen
disorder group who were scheduled for chemotherapy owing to nodal
positivity, histological grade 3 disease, or both received
chemotherapy. Although there was no between-group difference in the
clinicopathological factors (Table
I), 22 (88.0%), 14 (56%), 12 (48%), and 3 (12%) patients in the
collagen disorder group received medications, steroids,
immunosuppressants, and biological agents (tumor necrosis factor-α
inhibitor), respectively, for treatment (Table II).
 | Table II.Comorbid collagen disorders and
treatment. |
Table II.
Comorbid collagen disorders and
treatment.
| Collagen
disorder | No. of patients
(%) | No. of patients
with treatmenta |
|---|
| Rheumatoid
arthritis | 11 (44.0) | 10 |
| Systemic lupus
erythematosus | 4 (16.0) | 4 |
|
Polymyositis/Dermatomyositis | 4 (16.0) | 3 |
| Mixed connective
tissue disease | 2 (8.0) | 2 |
| Sjögren's
syndrome | 2 (8.0) | 1 |
| Scleroderma | 1 (4.0) | 1 |
| Adult-onset Still's
disease | 1 (4.0) | 1 |
Subtypes and Ki-67 values
Regarding breast cancer subtypes and growth factors,
Table III shows the number of
cases stratified according to biological factors and Ki-67 LI
values. There was no between-group difference in subtype
distribution. The collagen disorder group had a higher mean Ki-67
LI value than the control group (41.1% vs. 20.8%). Of the 20
patients with recurrent disease, 11 (55%) were in the collagen
disorder group. Among the patients with recurrent disease in the
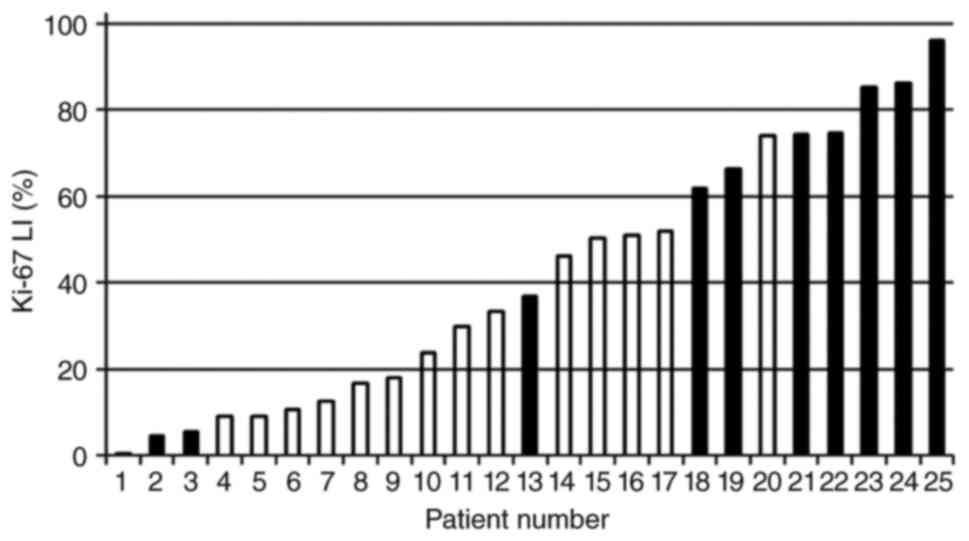
collagen disorder group, eight (73%) had a Ki-67 LI value of ≥20%
(Fig. 1).
 | Table III.Subtypes and Ki-67 values in patients
with breast cancer stratified according to collagen disorder
comorbidity (n=83). |
Table III.
Subtypes and Ki-67 values in patients
with breast cancer stratified according to collagen disorder
comorbidity (n=83).
| Variable | Patients with
collagen disorder (n=25) | Patients without
collagen disorder (n=58) | P-value |
|---|
| Subtypes |
|
| N.S. |
|
Luminal, n (%)a | 17 (68.0) | 42 (72.4) |
|
|
Luminal·HER2+, n (%) | 3 (12.0) | 2 (3.4) |
|
| HER2+,
n (%) | 1 (4.0) | 5 (8.6) |
|
| Triple
negative, n (%) | 4 (16.0) | 9 (15.6) |
|
| Ki-67 LI (%) |
|
|
|
|
Range | 0.4–96.1 |
1.9–75.6b |
|
|
Mean | 41.1 | 20.8 | 0.007 |
|
Median | 36.9 | 16.9 |
|
Prognostic analyses
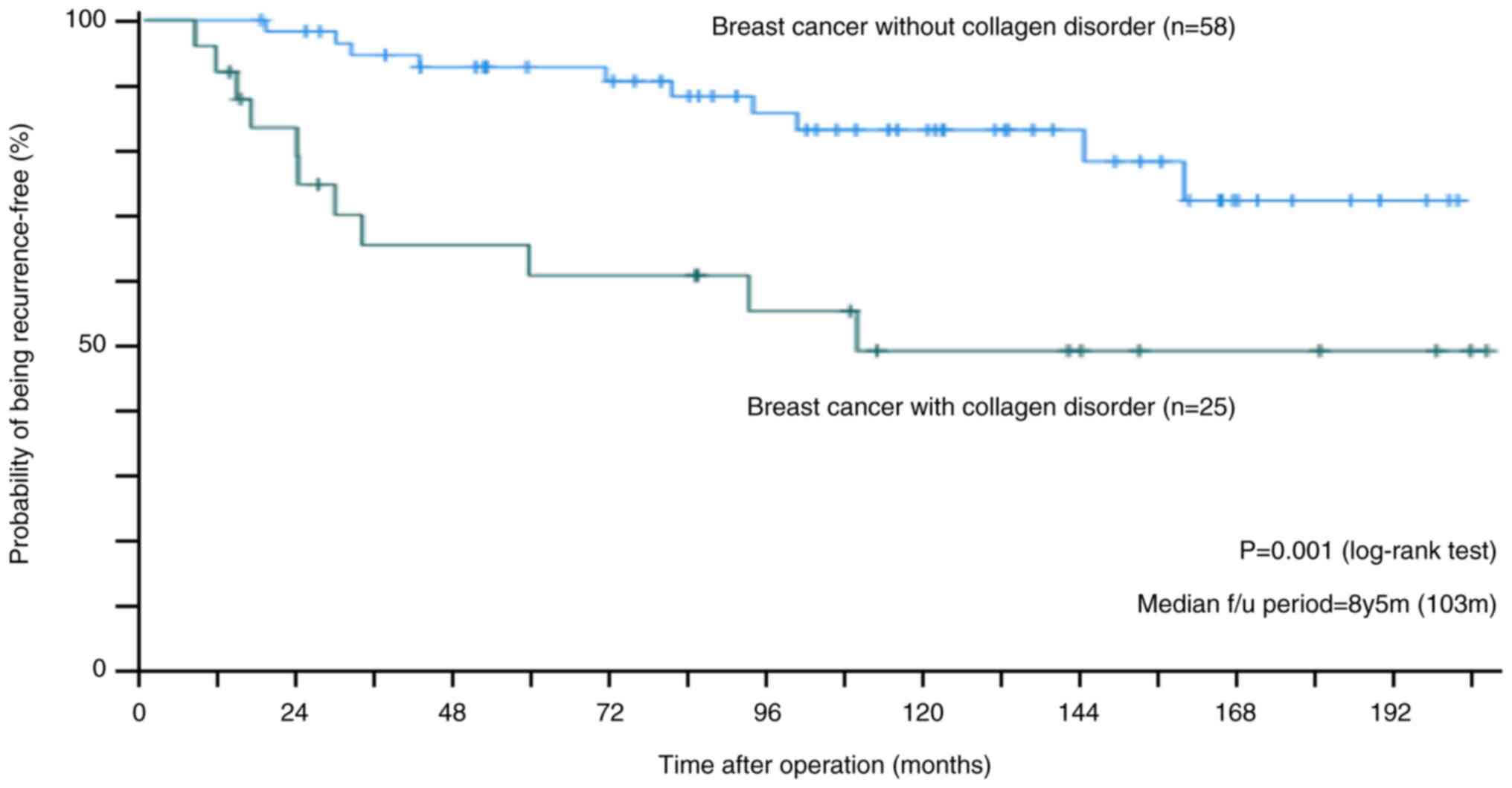
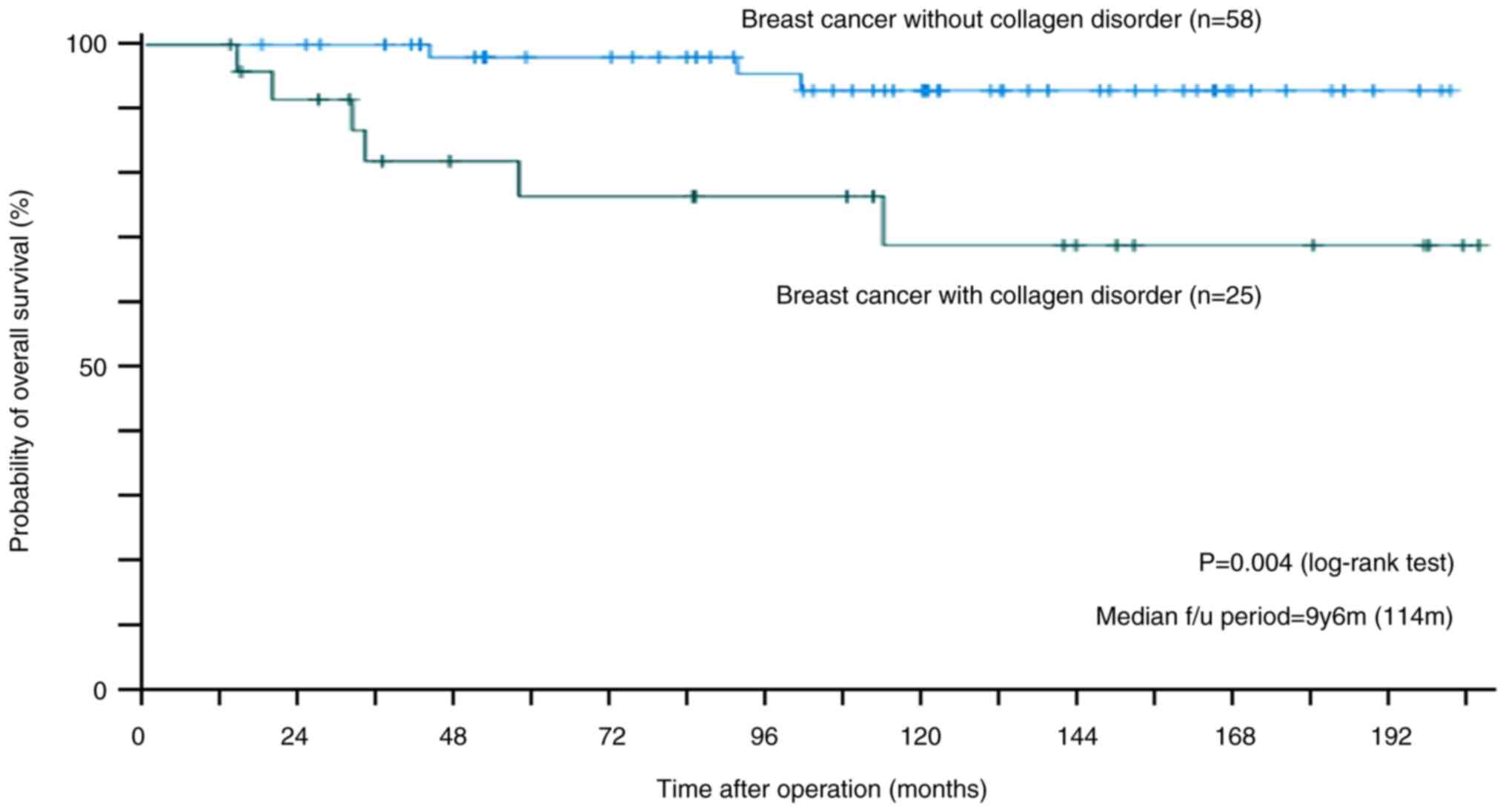
After a median observation period of 103 and 114
months, the RFS and OS rates in the collagen disorder group were
49.0% (Fig. 2) and 68.8% (Fig. 3), respectively, which were lower
than those in the control group (RFS, 73.9%; OS, 92.9%). Among
other clinicopathological factors evaluated in the RFS, tumor
diameter of >2 cm and lymphatic invasion were significantly
associated (Figs. S1 and S2), while node positivity and
histological grade 2 or 3 were marginally but significantly
associated with poor prognosis (Figs.
S3 and S4). In the
multivariate analysis, four patients with missing data (two
patients each with and without collagen disease) were excluded, as
shown in Table IV, in addition to
tumor diameter of >2 cm (P=0.034; hazard ratio, 3.076; 95% CI,
1.091–8.674), comorbidity of collagen disorder was a significant
risk factor for breast cancer recurrence (P=0.014; hazard ratio,
4.855; 95% CI, 1.369–17.223).
 | Table IV.Multivariate analysis of factors
related to recurrence of breast cancer. |
Table IV.
Multivariate analysis of factors
related to recurrence of breast cancer.
| Parameter | P-value | Hazard ratio
(95%CI) |
|---|
| Age (increase per
year) | 0.293 | 0.979
(0.941–1.019) |
| Tumor size (≤2 cm
vs. >2 cm) | 0.034 | 3.076
(1.091–8.674) |
| Lymph node
metastasis (negative vs. positive) | 0.854 | 0.889
(0.255–3.103) |
| Tumor grade (1 vs.
2–3) | 0.054 | 3.107
(0.980–9.846) |
| Lymphovascular
invasion (negative vs. positive) | 0.090 | 3.160
(0.837–11.932) |
| Comorbidity with
collagen disorder (no vs. yes) | 0.014 | 4.855
(1.369–17.223) |
| Use of steroids (no
vs. yes) | 0.737 | 0.795
(0.209–3.030) |
Discussion
Our findings demonstrated relatively low RFS and OS
rates in patients with breast cancer and collagen disorder who
frequently received steroids or other immunosuppressants for the
treatment of collagen disorder.
The survival rate of patients with SLE, a typical
collagen disorder, has dramatically improved from >40% in the
1950s to >90% since the 1980s (8,9). The
main causes of death for these patients are disease activity,
infections, and cardiovascular complications (10), although cancer is not one of the
comorbidities. Furthermore, the 10-year survival rate among
patients with PM or DM has been reported to be 62%, with the major
causes of death being cardiac complications, pulmonary
complications, infections, and cancer (11). Patients with rheumatoid arthritis,
the most common collagen disorder in our study, had a lower
mortality rate than the general population. Moreover, the major
risk factor for mortality is accelerated cardiovascular disease,
especially in patients with high disease activity and chronic
inflammation (12). In our study,
the 10-year survival rate was lower in the collagen disorder group
than in the control group (68.8% vs. 92.9%). Furthermore, in our
study, the cause of death was the progression of breast cancer
rather than the progression or complications of collagen
disorders.
Steroids and immunosuppressants can adversely affect
the immune system of patients with collagen disorders. Moreover,
patients with collagen disorders may receive insufficient or
moderate treatment for breast cancer, given their systemic
fragility. Cairat et al (13) reported that systemic glucocorticoid
use is associated with an increased risk of in situ breast cancer
and a decreased risk of invasive breast cancer and progression to
stage 3/4. Although glucocorticoid-induced immunosuppression can
theoretically increase the risk of cancer (14,15),
the relationship between systemic glucocorticoid use and cancer may
differ according to tumor type and stage (16). In our study, 76% of the patients in
the collagen disorder group received steroid medications or other
immunosuppressants, suggesting that immunological fragility leads
to poor prognosis. However, multivariate analysis revealed that
steroid use was not a risk factor for recurrence.
Regarding the possibility of undertreatment for
breast cancer, five out of seven patients who had indications for
chemotherapy received standard chemotherapy, indicating an
insignificant influence of undertreatment. The relatively poor
prognosis in the collagen disorder group could be attributed to the
biological characteristics of breast cancer tissues. Regarding
background characteristics, the collagen disorder group had a
higher proportion of patients with moderate or high lymphatic
invasion and histological grade 3 disease than the control group.
There was no significant difference in the proportion of patients
with triple-negative and HER2-type breast cancer. In the collagen
disorder group, eight of the 11 patients with recurrent breast
cancer had Ki-67 LI values of ≥20%. Moreover, the collagen disorder
group showed higher average and median Ki-67 LI values than the
control group (Table III).
Extensive studies have demonstrated the prognostic utility of Ki-67
LI values (17). Although the
optimal cutoff value is yet to be established, values ≥30% and ≤10%
can be considered high and low, respectively (18). A high Ki-67 LI value may be related
to the malignant potential of breast cancer in patients with
collagen disorders. However, our intergroup comparison of Ki-67 LI
was restricted because older patients were not included in the
control group; Ki-67 LI examination was not performed as a routine
examination before July 2006, and it was not possible to collect
formalin-fixed paraffin-embedded samples for Ki-67 LI because of
the lack of prior research on this topic. These results can be
verified if similar case and control data were collected in 2012
and afterward.
In conclusion, patients with breast cancer and
collagen disorders have relatively high growth factors and
relatively low RFS and OS rates. Thorough follow-up is necessary
for patients with breast cancer who have collagen disorders and
high Ki-67 value.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KM, AO, YI, AF, AS, AN, HY, HS, AA, MO, HI, TT and
TS provided the clinical data included in the text, performed the
literature search and data analysis, and confirm the authenticity
of all the raw data. KM wrote the manuscript draft. AO, HI and TS
contributed to the conceptualization of the work and interpreted
and revised the laboratory test results included in this report. MO
and TT revised the manuscript critically and modified the text. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Saitama Medical University International Medical Center (approval
no. 2021-006; April 7, 2021). The requirement of informed consent
was waived given the retrospective nature of the study.
Patient consent for publication
Not applicable.
Competing interests
Akihiko Osaki reports grants and personal fees from
Astra Zeneca, Eisai, Chugai, Nippon Kayaku, and Eli Lilly; grants
from Kyowa Kirin, Daiichi Sankyo, Taiho, Novartis, MSD, Sawai,
Covance, Maruho, Sanofi, Takeda, WJOG, and LabCorp; and personal
fees from Shionogi and Pfizer, outside the submitted work. Toshiaki
Saeki reports personal fees from Taiho, Eisai, Kyowa Kirin, Chugai,
Ono, ASKA, Novartis, Astra Zeneca, Takeda, Eli Lilly, Pfizer,
MiRteL, and Meiji Seika, outside the submitted work and grants from
Nippon Kayaku. Hiroshi Ishiguro reports grants and personal fees
from Eisai and Chugai; grants from Astra Zeneca, MSD, Eli Lilly,
Daiichi Sankyo, Takeda, and Epcrsu; and personal fees from Pfizer,
Kyowa Kirin, EP-Force, and Cancer and Chemotherapy Publishers,
outside the submitted work. Takao Takahashi reports personal fees
from Daiichi Sankyo, Tsumura, Hisamitsu, and Shionogi, outside the
submitted work. Kazuo Matsuura, Yuki Ichinose, Akihiro Fujimoto,
Ayaka Sakakibara, Asami Nukui, Hideki Yokogawa, Hiroko Shimada, and
Aya Asano declare that they have no competing interests.
Glossary
Abbreviations
Abbreviations:
|
RFS
|
recurrence-free survival
|
|
OS
|
overall survival
|
|
HR
|
hormone receptors
|
|
HER2
|
human epidermal growth factor receptor
2
|
|
PM
|
polymyositis
|
|
DM
|
dermatomyositis
|
|
SLE
|
systemic lupus erythematosus
|
|
ER
|
estrogen receptor
|
|
PgR
|
progesterone receptor
|
|
LI
|
labeling index
|
References
|
1
|
Bernatsky S, Kale M, Ramsey-Goldman R,
Gordon C and Clarke AE: Systemic lupus and malignancies. Curr Opin
Rheumatol. 24:177–181. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Barnes BE and Mawr B: Dermatomyositis and
malignancy: A review of the literature. Ann Intern Med. 84:68–76.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hill CL, Zhang Y, Sigurgeirsson B, Pukkala
E, Mellemkjaer L, Airio A, Evans SR and Felson DT: Frequency of
specific cancer types in dermatomyositis and polymyositis: A
population-based study. Lancet. 357:96–100. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Choi MY, Flood K, Bernatsky S,
Ramsey-Goldman R and Clarke AE: A review on SLE and malignancy.
Best Pract Res Clin Rheumatol. 31:373–396. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fardet L, Dupuy A, Gain M, Kettaneh A,
Chérin P, Bachelez H, Dubertret L, Lebbe C, Morel P and Rybojad M:
Factors associated with underlying malignancy in a retrospective
cohort of 121 patients with dermatomyositis. Medicine (Baltimore).
88:91–97. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mebazaa A, Boussen H, Nouira R, Gamoudi A,
Rahal K, Kamoun MR and Osman AB: Dermatomyositis and breast cancer:
A multicenter Tunisian retrospective study of 13 cases. Tunis Med.
89:18–22. 2011.PubMed/NCBI
|
|
7
|
András C, Bodoki L, Nagy-Vincze M, Griger
Z, Csiki E and Dankó K: Retrospective analysis of cancer-associated
myositis patients over the past 3 decades in a Hungarian myositis
cohort. Pathol Oncol Res. 26:1749–1755. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pons-Estel GJ, Alarcón GS, Scofield L,
Reinlib L and Cooper GS: Understanding the epidemiology and
progression of systemic lupus erythematosus. Semin Arthritis Rheum.
39:257–268. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Singh RR and Yen EY: SLE mortality remains
disproportionately high, despite improvements over the last decade.
Lupus. 27:1577–1581. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ocampo-Piraquive V, Nieto-Aristizábal I,
Cañas CA and Tobón GJ: Mortality in systemic lupus erythematosus:
Causes, predictors and interventions. Expert Rev Clin Immunol.
14:1043–1053. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Findlay AR, Goyal NA and Mozaffar T: An
overview of polymyositis and dermatomyositis. Muscle Nerve.
51:638–656. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wasserman AM: Diagnosis and management of
rheumatoid arthritis. Am Fam Physician. 84:1245–1252.
2011.PubMed/NCBI
|
|
13
|
Cairat M, Al Rahmoun M, Gunter MJ, Heudel
PE, Severi G, Dossus L and Fournier A: Use of systemic
glucocorticoids and risk of breast cancer in a prospective cohort
of postmenopausal women. BMC Med. 19:1862021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
de Visser KE, Eichten A and Coussens LM:
Paradoxical roles of the immune system during cancer development.
Nat Rev Cancer. 6:24–37. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Herr I and Marme A: Glucocorticoids and
progression of breast cancer. Cancer Biol Ther. 4:1415–1416. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Keith BD: Systematic review of the
clinical effect of glucocorticoids on nonhematologic malignancy:.
BMC Cancer. 8:842008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Coates AS, Winer EP, Goldhirsch A, Gelber
RD, Gnant M, Piccart-Gebhart M, Thürlimann B and Senn HJ; Panel
Members, : Tailoring therapies-improving the management of early
breast cancer: St Gallen International Expert Consensus on the
Primary Therapy of Early Breast Cancer 2015. Ann Oncol.
26:1533–1546. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Polley MY, Leung SC, Gao D, Mastropasqua
MG, Zabaglo LA, Bartlett JM, McShane LM, Enos RA, Badve SS and Bane
AL: An international study to increase concordance in Ki67 scoring.
Mod Pathol. 28:778–786. 2015. View Article : Google Scholar : PubMed/NCBI
|