Introduction
Intracranial primary chondrosarcomas are considered
to arise from residual embryonic cartilage tissue or chondrocytes
following endochondral ossification (1). While these tumors are rare, accounting
for ~1% of all chondrosarcomas and <0.15% of all intracranial
tumors (2,3), the recurrence risk is high; the 5-year
recurrence rate for patients with chondrosarcoma treated with
surgery alone is 44%, so a timely diagnosis is essential to
effectively treat these patients. However, an accurate diagnosis is
challenging, as this condition may manifest with diverse and
non-specific symptoms. Patients frequently present with chronic
headache and various symptoms caused by tumor compression of brain
tissues, blood vessels and nerves, but these symptoms also occur in
patients with chordoma and other brain tumors (4–6).
Furthermore, characteristics on computed tomography (CT) and
magnetic resonance imaging (MRI) scans typically resemble those of
meningioma and chordoma, further hampering an accurate preoperative
diagnosis (7–11).
The gold standard for identification of intracranial
primary chondrosarcoma is pathological examination and
immunohistochemistry (11). The
World Health Organization (WHO) divides chondrosarcoma into three
histological grades. Grade I tumors, also known as atypical
cartilaginous tumors, are well differentiated and appear similar to
normal cartilage or benign chondroma, with mild cellular atypia,
small nuclei and rare mitoses. Grade II primary chondrosarcomas are
moderately differentiated and malignant, with greater cellular
atypia, larger nuclei, higher cell density and more frequent
mitoses. Finally, grade III tumors are poorly differentiated, with
obvious cellular atypia, frequent mitoses and lobules with cells
that become less differentiated and spindled at the periphery
(4). In addition, a fourth type,
grade IV or dedifferentiated chondrosarcoma, can be defined
histologically by the presence of a high-grade, often spindled or
pleomorphic tumor without significant cartilaginous matrix
(12). However, there are also
immunohistochemical similarities among chondrosarcoma, meningioma,
chordoma and chondromyxoid fibroma that may complicate the
postoperative diagnosis (13).
Intracranial chondrosarcomas often occur in the skull base,
accounting for 6% of all skull base neoplasms (2,14),
which increases the difficulty of surgical management, particularly
complete resection, due to a close proximity with cranial nerves
and vessels.
In the present study, the case of a 41-year-old
female with WHO grade II chondrosarcoma of the clivus who was
treated surgically through an endoscopic transsphenoidal approach
was reported. In order to prevent tumor recurrence, adjuvant
radiotherapy [25 doses of 60 Gy planning gross tumor volume (PGTV)
and 50 Gy planning clinical target volume (PCTV), over 5 weeks] was
performed after the operation. Therefore, surgical resection using
an endoscopic transsphenoidal approach and postoperative adjuvant
radiotherapy is an effective method for treating intracranial
clivus chondrosarcoma.
Case report
In November 2018, a 41-year-old female was admitted
to Chongqing General Hospital (Chongqing, China) with esotropia of
the left eye, visual impairment of the left nasal field and double
vision for the previous 2 months. The patient received a
comprehensive examination, including routine blood analyses and
evaluations of liver, kidney, immune and blood coagulation
functions, but all parameters were within the expected ranges.
Neurological examination results were also as expected and there
were no obvious signs of pathology. Furthermore, the patient had no
history of trauma and no family history of hereditary illness.
Visual acuity of the left eye was 0.8 and the extent of left eye
esotropia was 10°, while the visual acuity and visual field of the
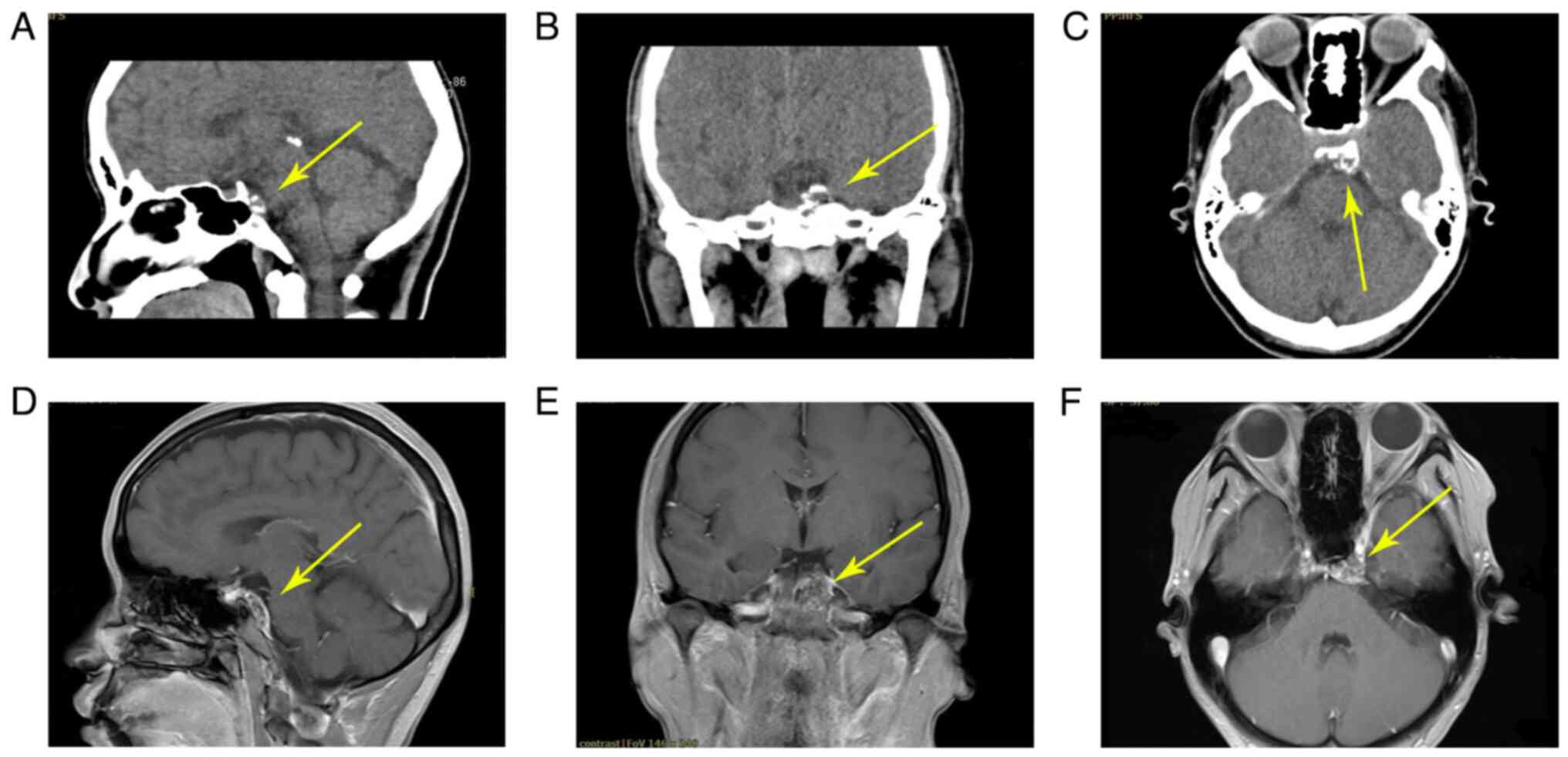
right eye were normal. Preoperative CT scan images (Fig. 1A-C) demonstrated evidence of a
calcified lesion with clear margins and enhancement in the left
clivus region. This mass appeared hypo- or isointense on
T1-weighted images (T1WI) and heterogeneously hyperintense on T2WI,
with heterogeneous enhancement (Fig.
1D-F). The mass demonstrated swelling growth but did not break
through the dura and there were no necrotic areas.
Based on preoperative imaging, chordoma was
suspected, and the patient underwent tumor resection using the
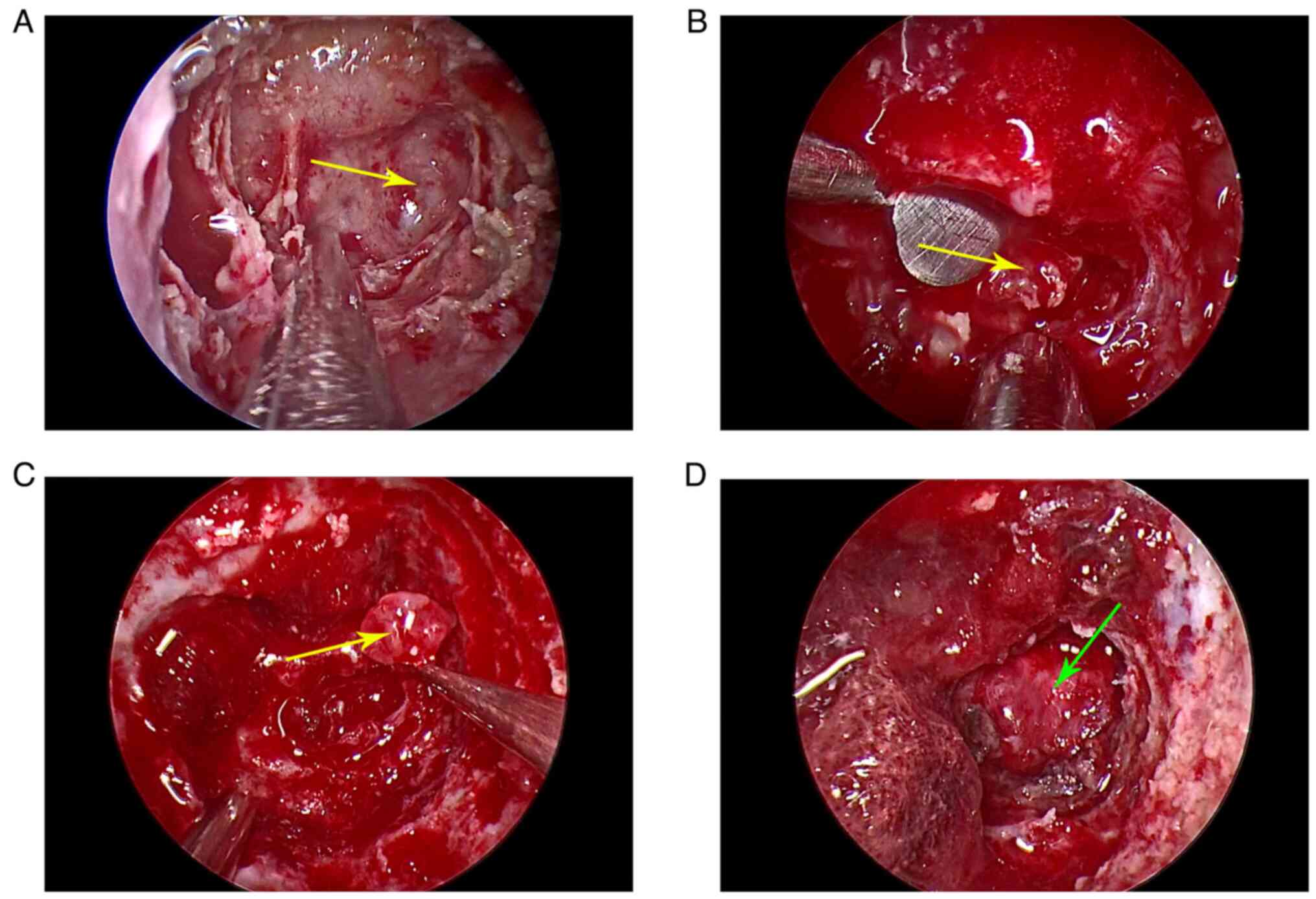
endoscopic transsphenoidal approach. After removing the mucosa at
the slope, the lesion was exposed (Fig.
2A) and resembled a chordoma. However, further exposure by
removal of the surrounding normal bone revealed a complete capsule,
which ruled out a chordoma. Puncture with a fine needle did not
induce the outflow of cystic fluid or cerebrospinal fluid. After
cutting the capsule, a white, jelly-like, shiny and clear lesion
free of bone was observed (Fig. 2B and
C). The dura was observed intact over the clivus after gross
total resection (Fig. 2D).
Surgically resected tumor tissues were analyzed by routine
pathological examination using H&E staining. Tumor specimens
were first fixed with 4% formaldehyde solution at room temperature
for 24 h and then embedded and fixed in paraffin. The specimens
were then cut into 4-µm sections and deparaffinized in xylene at
60°C for 2 h. Subsequently, at room temperature, the sections were
stained with 0.5% hematoxylin for 3 min, followed by 0.5% eosin for
3 min. Subsequently, the stained sections were observed under a
light microscope to obtain microphotographs of the histopathology.
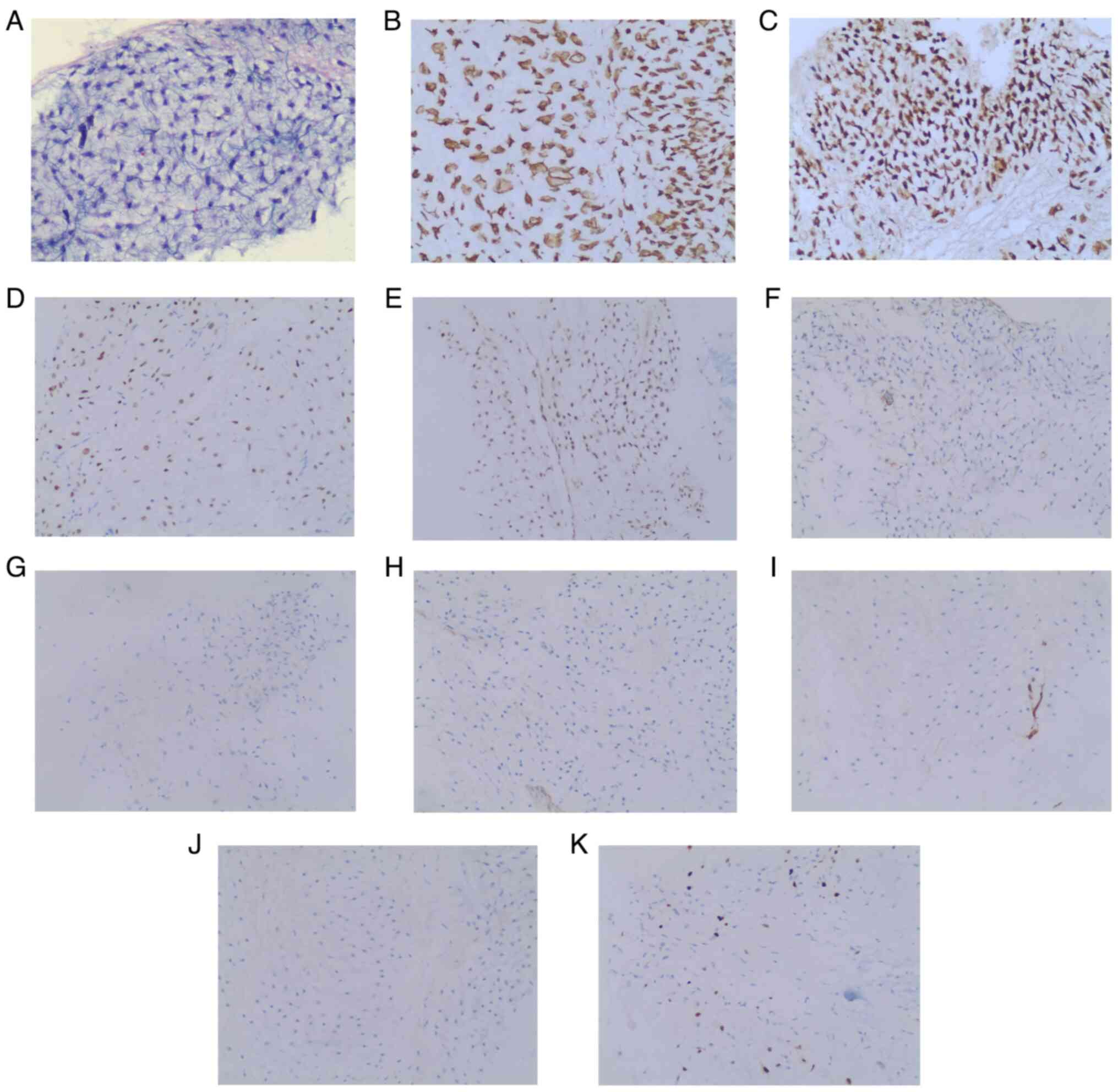
Postoperative histopathological analysis demonstrated that the mass
was composed of undifferentiated round or spindle-shaped cells and
mature cartilaginous tissue with detectable mitoses, which ruled
out dedifferentiated chondrosarcoma (Fig. 3). Immunohistochemical analysis was
performed using the DAB substrate kit (cat. no. 8059; Cell
Signaling Technology, Inc.) according to the manufacturer's
instructions. Immunohistochemical analysis results of the excised
tumor were as follows: Vimentin(+), S100(+), stabilin 2(+),
integrase interactor 1(+), smooth muscle actin(focal +), desmin(−),
calponin(−), cluster of differentiation 34(vascellum +) and
brachyury(−). In total, 8% of cells were Ki-67 (MIB-1)-positive.
Based on these histopathological and immunohistochemical features,
the mass was identified as a grade II chondrosarcoma. Postoperative
MRI confirmed that the entire tumor was removed and the patient was
discharged at 12 days post-surgery with no obvious postoperative
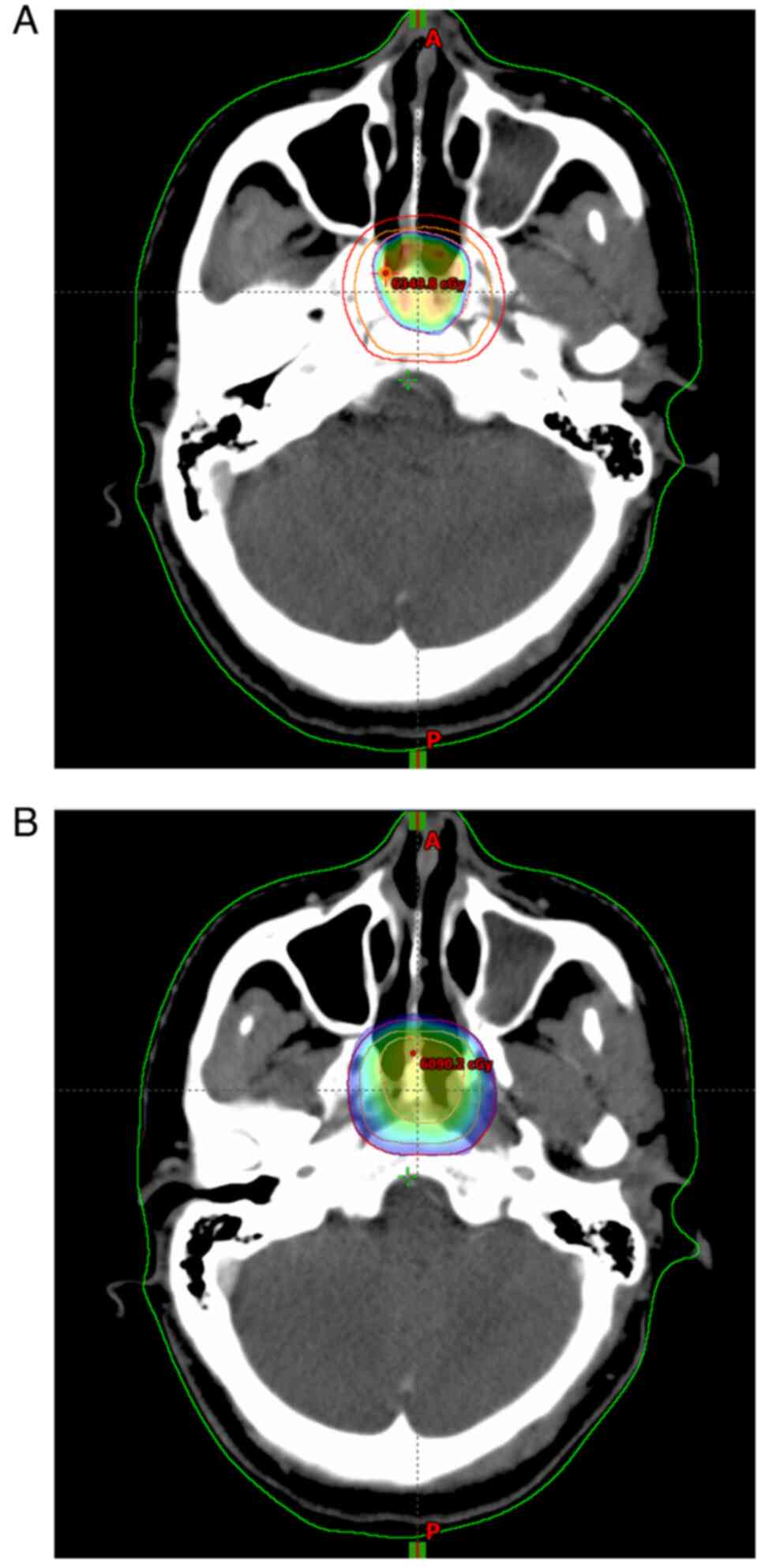
complications. After 1 month, the patient began adjuvant γ-knife
treatment with 25 doses of PGTV 60 Gy and PCTV 50 Gy over 5 weeks
(Fig. 4). A 3-month postoperative
MRI scan demonstrated no evidence of recurrence. There was no
evidence of tumor recurrence during the 28-month patient
follow-up.
Discussion
Intracranial chondrosarcoma is a rare type of
malignant cartilage tumor first described by Mott in 1899 (15). The vast majority of these tumors
originate from the skull base, including the petrous, clival,
occipital, sphenoid and parietal bones (16–19),
while a number of these tumors have also been reported in the brain
parenchyma, peripatellar region and spine (20–22).
The clinical manifestations of intracranial chondrosarcoma are
non-specific, although most patients present with a long-term
history of headache and other symptoms of increased intracranial
pressure (such as headache, nausea and papilledema) resulting from
tumor growth and compression or invasion of local intracranial
structures (19,23,24).
Neuroimaging is thus essential for preoperative planning. In most
cases, intracranial chondrosarcoma appears as high-density or
iso-intense lesions on CT, with uneven enhancement, calcification
and osteolytic destruction (14,25–28).
Compared with CT, MRI can better reveal the focus boundary and
tumor characteristics for diagnosis of chondrosarcoma. In general,
these lesions appear as low-intensity signals on T1WI, but possibly
as mixed high- and low-intensity signals on T2WI with honeycomb
enhancement, resembling the typical heterogeneous enhancement of
malignant tumors (14,25,26,28–31).
However, chordoma and chondrosarcoma exhibit similar imaging
characteristics, so a differential diagnosis is difficult prior to
surgery.
One possible distinguishing feature between
chordomas and chondrosarcomas is the anatomical location of the
tumor, as most chordomas occur in the midline of the brain, while
chondrosarcomas are usually found on one side (4). In addition, calcification is common in
chondrosarcoma (32,33), but not in chordoma, which may aid
the diagnosis before surgery. Immunohistochemistry results have
shown that chondrosarcoma is cytokeratin (CK)(−), epithelial
membrane antigen (EMA)(−) and S100 (+), while chordoma is CK(+),
EMA(+) and S100(+) (14).
Therefore, immunohistochemistry of excised tumor tissue provides
essential information for diagnosis.
There are three histological types of
chondrosarcoma: Classic, myxoid or mesenchymal type (23), each with distinct frequencies and
prognoses. Chandler et al (34) reviewed a series of chondrosarcoma
cases and reported that the classic type accounted for 62%, the
mesenchymal type for 30% and the myxoid type for only 8%. Bloch
et al (23) found that
prognosis was strongly related to histological type, with the
lowest mortality rate among patients with classic chondrosarcoma
(5%), followed by myxoid chondrosarcoma (10%) and mesenchymal
chondrosarcoma (25%). Moreover, mesenchymal type tumors also
exhibited a much higher recurrence rate (63%) compared with the
classic type (16%) (35).
Therefore, mesenchymal chondrosarcoma has a substantially poorer
prognosis compared with classic and myxoid chondrosarcoma.
Surgical resection is the preferred first-line
treatment for intracranial chondrosarcoma due to aggressive growth,
clear boundaries and few metastases. Most resection surgeries are
conducted using either a traditional transcranial approach or an
endoscope-assisted transsphenoidal approach, as these lesions are
most frequently located at the skull base (4). The most common transcranial approaches
are frontotemporal, orbital, zygomatic and pterional (36). However, these skull base tumors are
often close to cranial nerves and blood vessels, so certain
transcranial resection surgeries carry a high risk of nervous
system complications. As an alternative, endoscope technologies are
growing in popularity for skull base surgery due to the wider field
of vision, superior lighting and lack of visual obstruction
(16,17,37–41).
Hasegawa et al (42)
reported that of 19 transnasal endoscopic surgeries performed for
intracranial chondrosarcoma, 15 resulted in total resection, while
only four resulted in subtotal resection due to tumor invasion of
important nerves and vessels. Further, only 1 patient experienced
tumor recurrence over 5 years of follow-up. These findings suggest
that endoscopic skull base surgery can yield a resection rate
similar to classical transcranial approaches but with fewer
neurological complications. A systematic analysis of 33 studies,
including 1,307 patients with intracranial chondrosarcoma,
concluded that endoscopic transsphenoidal surgery can safely expose
the focus and involved nerves and vessels, enhancing the
probability of good surgical results (36). Therefore, endoscopic transsphenoidal
surgery is a good choice for the treatment of intracranial
chondrosarcoma. By contrast, for large tumors that cannot be
removed in a single operation, staged surgery is recommended
(43–45). However, if surgery alone is
performed for chondrosarcoma, the prognosis is not ideal and the
tumor may recur.
In such cases, adjuvant therapy may improve patient
outcomes as chondrosarcoma is considered relatively radiosensitive
(46–51). For instance, Bloch et al
(23,35) reported significant reductions in the
5-year recurrence rate and mortality rate among patients with
intracranial chondrosarcoma receiving surgery combined with
postoperative adjuvant radiotherapy compared with surgery alone (9
vs. 44% and 4 vs. 26%, respectively). Similarly, Rosenberg et
al (49) reported 5- and
10-year local control rates of 99 and 98% respectively and 5- and
10-year survival rates as high as 99% in a cohort of 200 patients
with intracranial chondrosarcoma receiving combined surgery and
radiotherapy. Therefore, adjuvant radiotherapy should be
recommended after maximum-achievable tumor resection for patients
with large and complex intracranial chondrosarcomas and for tumors
in close proximity to vital neural and neurovascular structures.
Chemotherapy is not recommended for patients with intracranial
chondrosarcoma, as most chemotherapeutic drugs act selectively on
rapidly dividing cells and the mitosis rate is low in most tumors
of this type (14). Ultimately,
continued developments in targeted therapies exploiting specific
molecular expression profiles hold the greatest promise for
numerous patients with intracranial chondrosarcoma.
In the present study, the case of a 41-year-old
female with WHO grade II chondrosarcoma of the clivus who was
treated surgically through an endoscopic transsphenoidal approach
was reported. In order to prevent tumor recurrence, adjuvant
radiotherapy (25 doses of 60 Gy PGTV and 50 Gy planning clinical
target volume PCTV, over 5 weeks) was performed after the
operation. The prognosis of patients with intracranial
chondrosarcoma may be affected by the degree of tumor resection,
histological type, choice of postoperative adjuvant radiotherapy
and previous treatment, such as surgery or radiotherapy. Therefore,
surgery should be the first choice for patients with intracranial
chondrosarcoma and endoscopic transsphenoidal surgery is a good
surgical option given that these lesions are usually in close
proximity to critical nerves and blood vessels. In addition to
maximal surgical resection, early adjuvant radiotherapy is
recommended for preventing recurrence.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
NW, JWW, PW, HTJ, JL, CT, GZ, JYP and HFG
participated in the conception, design and data acquisition of the
article. HTJ drafted the manuscript. HTJ, JYP and HFG revised the
manuscript. NW critically revised the article. NW ensured that
questions related to the integrity of any part of the work were
appropriately investigated and resolved. HTJ, JL, CT and GZ confirm
the authenticity of all the raw data. All authors read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
The Ethics Committee of Chongqing General Hospital
(Chonqing, China) waived the requirement for additional ethical
review, as this report is retrospective and not based on any
specific patient priorities, experiences or preferences. Informed
consent for participation in the study or use of the medical data
was obtained from the patient.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of anonymized data and any accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Oghalai JS, Buxbaum JL, Jackler RK and
McDermott MW: Skull base chondrosarcoma originates from the
petroclival junction. Otol Neurotol. 26:1052–1060. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cianfriglia F, Pompili A and Occhipinti E:
Intracranial malignant cartilaginous tumors. Report of two cases
and review of literature. Acta Neurochir (Wien). 45:163–175. 1978.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Richardson MS: Pathology of skull base
tumors. Otolaryngol Clin North Am. 34:1025–1042. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kremenevski N, Schlaffer SM, Coras R,
Kinfe TM, Graillon T and Buchfelder M: Skull base chordomas and
chondrosarcomas. Neuroendocrinology. 110:836–847. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Edem I, DeMonte F and Raza SM: Advances in
the management of primary bone sarcomas of the skull base. J
Neurooncol. 150:393–403. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alamer OB, Haider AS, Haider M, Sagoo NS,
Robertson FC, Arrey EN, Aoun SG, Yu K, Cohen-Gadol AA and El
Ahmadieh TY: Primary and radiation induced skull base osteosarcoma:
A systematic review of clinical features and treatment outcomes. J
Neurooncol. 153:183–202. 2021. View Article : Google Scholar
|
|
7
|
Oot RF, Melville GE, New PF,
Austin-Seymour M, Munzenrider J, Pile-Spellman J, Spagnoli M,
Shoukimas GM, Momose KJ and Carroll R: The role of MR and CT in
evaluating clival chordomas and chondrosarcomas. AJR Am J
Roentgenol. 151:567–575. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Müller U, Kubik-Huch RA, Ares C, Hug EB,
Löw R, Valavanis A and Ahlhelm FJ: Is there a role for conventional
MRI and MR diffusion-weighted imaging for distinction of skull base
chordoma and chondrosarcoma? Acta Radio. l57:225–232. 2016.
View Article : Google Scholar
|
|
9
|
Welzel T, Meyerhof E, Uhl M, Huang K, von
Deimling A, Herfarth K and Debus J: Diagnostic accuracy of DW MR
imaging in the differentiation of chordomas and chondrosarcomas of
the skull base: A 3.0-T MRI study of 105 cases. Eur J Radiol.
105:119–124. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Doucet V, Peretti-Viton P,
Figarella-Branger D, Manera L and Salamon G: MRI of intracranial
chordomas. Extent of tumour and contrast enhancement: Criteria for
differential diagnosis. Neuroradiology. 39:571–576. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fu LY, Han Q, Cheng P, Yang HJ and Zhao Y:
Rare case report and literature review of intracranial mesenchymal
chondrosarcoma. Ann Palliat Med. 10:12012–12017. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Limaiem F, Davis DD and Sticco KL:
Chondrosarcoma. In: StatPearls [Internet]. Treasure Island (FL):
StatPearls Publishing; 2023
|
|
13
|
Paulus W, Slowik F and Jellinger K:
Primary intracranial sarcomas: Histopathological features of 19
cases. Histopathology. 18:395–402. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Y, Huang J, Zhang C, Jiang C, Ding
C, Lin Y, Wu X, Wang C, Kang D and Lin Z: An extended endoscopic
endonasal approach for sellar area chondrosarcoma: A case report
and literature review. World Neurosurg. 127:469–477. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mott FW: Chondrosarcoma springing from the
sella turcica. Arch Neurol Psychiat. 1:432–433. 1899.
|
|
16
|
Abhinav K, Acosta Y, Wang WH, Bonilla LR,
Koutourousiou M, Wang E, Synderman C, Gardner P and
Fernandez-Miranda JC: Endoscopic endonasal approach to the optic
canal: Anatomic considerations and surgical relevance.
Neurosurgery. 11 (Suppl 3):S431–S445. 2015.
|
|
17
|
Arbolay OL, González JG, González RH and
Gálvez YH: Extended endoscopic endonasal approach to the skull
base. Minim Invasive Neurosurg. 52:114–118. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oghalai JS, Buxbaum JL, Jackler RK and
McDermott MW: Skull base chondrosarcoma originating from the
petroclival junction. Otol Neurotol. 26:1052–1060. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Korten AG, ter Berg HJ, Spincemaille GH,
van der Laan RT and Van de Wel AM: Intracranial chondrosarcoma:
Review of the literature and report of 15 cases. J Neurol Neurosurg
Psychiatry. 65:88–92. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Marshman LA, Gunasekera L, Rose PE and
Olney JS: Primary intracerebral mesenchymal chondrosarcoma with
rhabdomyosarcomatous differentiation: Case report and literature
review. Br J Neurosurg. 15:419–424. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li YH and Yao XH: Primary intradural
mesenchymal chondrosarcoma of the spine in a child. Pediatr Radiol.
37:1155–1158. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sindou M, Daher A, Vighetto A and Goutelle
A: Parasellar chondrosarcoma: Report of a case operated on through
a pteriono-temporal approach and review of the literature.
Neurochirurgie. 5:186–190. 1989.(In French).
|
|
23
|
Bloch OG, Jian BJ, Yang I, Han SJ, Aranda
D, Ahn BJ and Parsa AT: A systematic review of intracranial
chondrosarcoma and survival. J Clin Neurosci. 16:1547–1551. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Güneş M, Günaldi O, Tuğcu B, Tanriverdi O,
Güler AK and Cöllüoğlu B: Intracranial chondrosarcoma: A case
report and review of the literature. Minim Invasive Neurosurg.
52:238–241. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen F, Chen B, Wang H, Xu W, Li W and
Chen D: Intracranial nonskull-based chondrosarcoma arising from the
sagittal sinus: A case report and review of the literature. World
Neurosurg. 120:234–239. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Abele TA, Yetkin ZF, Raisanen JM, Mickey
BE and Mendelsohn DB: Non-pituitary origin sellar tumours mimicking
pituitary macroadenomas. Clin Radiol. 67:821–827. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chu J, Ma H, Wang Y, Li K, Liao C and Ding
Y: CT and MRI findings of intracranial extraskeletal mesenchymal
chondrosarcoma-a case report and literature review. Transl Cancer
Res. 11:3409–3415. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cho BK, Chi JG, Wang KC, Chang KH and Choi
KS: Intracranial mesenchymal chondrosarcoma: A case report and
literature review. Childs Nerv Syst. 9:295–299. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee YY, Van Tassel P and Raymond AK:
Intracranial dural chondrosarcoma. AJNR Am J Neuroradiol.
9:1189–1193. 1988.PubMed/NCBI
|
|
30
|
Bingaman KD, Alleyne CH Jr and Olson JJ:
Intracranial extraskeletal mesenchymal chondrosarcoma: Case report.
Neurosurgery. 46:207–211. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kothary N, Law M, Cha S and Zagzag D:
Conventional and perfusion MR imaging of parafalcine
chondrosarcoma. AJNR Am J Neuroradiol. 24:245–248. 2003.PubMed/NCBI
|
|
32
|
Tauber M, van Loveren HR, Jallo G, Romano
A and Keller JT: The enigmatic foramen lacerum. Neurosurgery.
44:386–391. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rich TA, Schiller A, Suit HD and Mankin
HJ: Clinical and pathologic review of 48 cases of chordoma. Cancer.
56:182–187. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chandler JP, Yashar P, Laskin WB and
Russell EJ: Intracranial chondrosarcoma: A case report and review
of the literature. J Neurooncol. 68:33–39. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bloch OG, Jian BJ, Yang I, Han SJ, Aranda
D, Ahn BJ and Parsa AT: Cranial chondrosarcoma and recurrence.
Skull Base. 20:149–156. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Palmisciano P, Haider AS, Sabahi M, Nwagwu
CD, Bin Alamer O, Scalia G, Umana GE, Cohen-Gadol AA, El Ahmadieh
TY, Yu K and Pathmanaban ON: Primary skull base chondrosarcomas: A
systematic review. Cancers (Basel). 13:59602021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Adappa ND, Learned KO, Palmer JN, Newman
JG and Lee JY: Radiographic enhancement of the nasoseptal flap does
not predict postoperative cerebrospinal fluid leaks in endoscopic
skull base reconstruction. Laryngoscope. 122:1226–1234. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cavallo LM, de Divitiis O, Aydin S,
Messina A, Esposito F, Iaconetta G, Talat K, Cappabianca P and
Tschabitscher M: Extended endoscopic endonasal transsphenoidal
approach to the suprasellar area: Anatomic considerations-part 1.
Neurosurgery. 61:24–33. 2007.PubMed/NCBI
|
|
39
|
Kassam AB, Prevedello DM, Carrau RL,
Snyderman CH, Gardner P, Osawa S, Seker A and Rhoton AL Jr: The
front door to meckel's cave: An anteromedial corridor via expanded
endoscopic endonasal approach- technical considerations and
clinical series. Neurosurgery. 64:on71–82. 2009.
|
|
40
|
Jho HD and Carrau RL: Endoscopy assisted
transsphenoidal surgery for pituitary adenoma. Technical note. Acta
Neurochir (Wien). 138:1416–1425. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Osawa S, Rhoton AL Jr, Seker A, Shimizu S,
Fujii K and Kassam AB: Microsurgical and endoscopic anatomy of the
vidian canal. Neurosurgery. 64 (5 Suppl 2):S385–S411. 2009.
|
|
42
|
Hasegawa H, Shin M, Kondo K, Hanakita S,
Mukasa A, Kin T and Saito N: Role of endoscopic transnasal surgery
for skull base chondrosarcoma: A retrospective analysis of 19 cases
at a single institution. J Neurosurg. 128:1438–1447. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tzortzidis F, Elahi F, Wright DC, Temkin
N, Natarajan SK and Sekhar LN: Patient outcome at long-term
follow-up after aggressive microsurgical resection of cranial base
chondrosarcomas. Neurosurgery. 58:1090–1098. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kano H, Sheehan J, Sneed PK, McBride HL,
Young B, Duma C, Mathieu D, Seymour Z, McDermott MW, Kondziolka D,
et al: Skull base chondrosarcoma radiosurgery: Report of the North
American gamma knife consortium. J Neurosurg. 123:1268–1275. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Feuvret L, Bracci S, Calugaru V, Bolle S,
Mammar H, De Marzi L, Bresson D, Habrand JL, Mazeron JJ, Dendale R
and Noël G: Efficacy and safety of adjuvant proton therapy combined
with surgery for chondrosarcoma of the skull base: A retrospective,
population-based study. Int J Radiat Oncol Biol Phys. 95:312–321.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Suit HD, Goitein M, Munzenrider J, Verhey
L, Urie M, Gragoudas E, Koehler A, Gottschalk B, Sisterson J and
Tatsuzaki H: Increased efficacy of radiation therapy by use of
proton beam. Strahlenther Onkol. 166:40–44. 1990.PubMed/NCBI
|
|
47
|
Coltrera MD, Googe PB, Harrist TJ, Hyams
VJ, Schiller AL and Goodman ML: Chondrosarcoma of the temporal
bone. Diagnosis and treatment of 13 cases and review of the
literature. Cancer. 58:2689–2696. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Castro JR, Linstadt DE, Bahary JP, Petti
PL, Daftari I, Collier JM, Gutin PH, Gauger G and Phillips TL:
Experience in charged particle irradiation of tumors of the skull
base: 1977–1992. Int J Radiat Oncol Biol Phys. 29:647–655. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Rosenberg AE, Nielsen GP, Keel SB, Renard
LG, Fitzek MM, Munzenrider JE and Liebsch NJ: Chondrosarcoma of the
base of the skull: A clinicopathologic study of 200 cases with
emphasis on its distinction from chordoma. Am J Surg Pathol.
23:1370–1378. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Austin-Seymour M, Munzenrider J, Goitein
M, Verhey L, Urie M, Gentry R, Birnbaum S, Ruotolo D, McManus P and
Skates S: Fractionated proton radiation therapy of chordoma and
low-grade chondrosarcoma of the base of the skull. J Neurosurg.
70:13–17. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Suit HD, Goitein M, Munzenrider J, Verhey
L, Davis KR, Koehler A, Linggood R and Ojemann RG: Definitive
radiation therapy for chordoma and chondrosarcoma of base of skull
and cervical spine. J Neurosurg. 56:377–385. 1982. View Article : Google Scholar : PubMed/NCBI
|