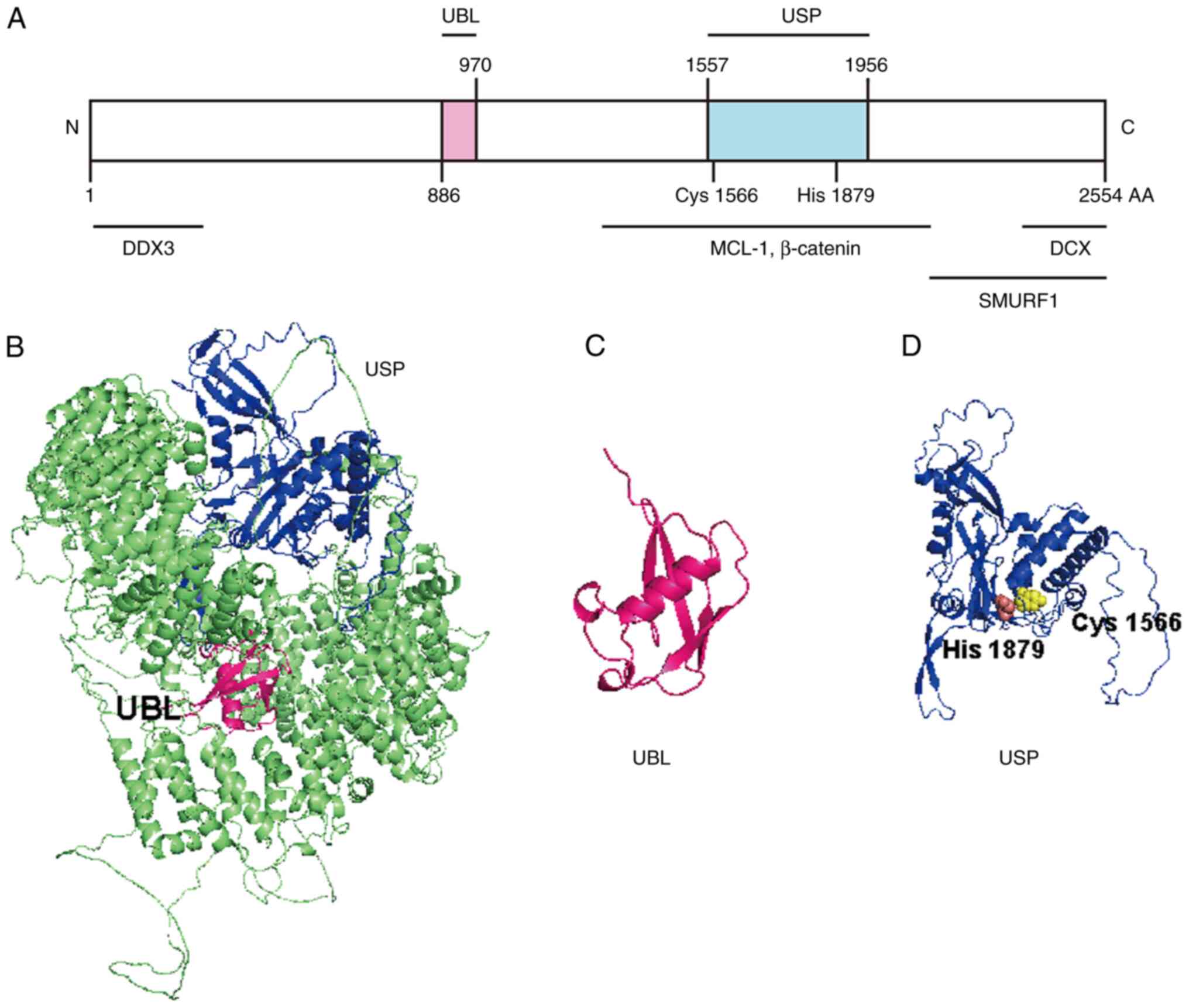
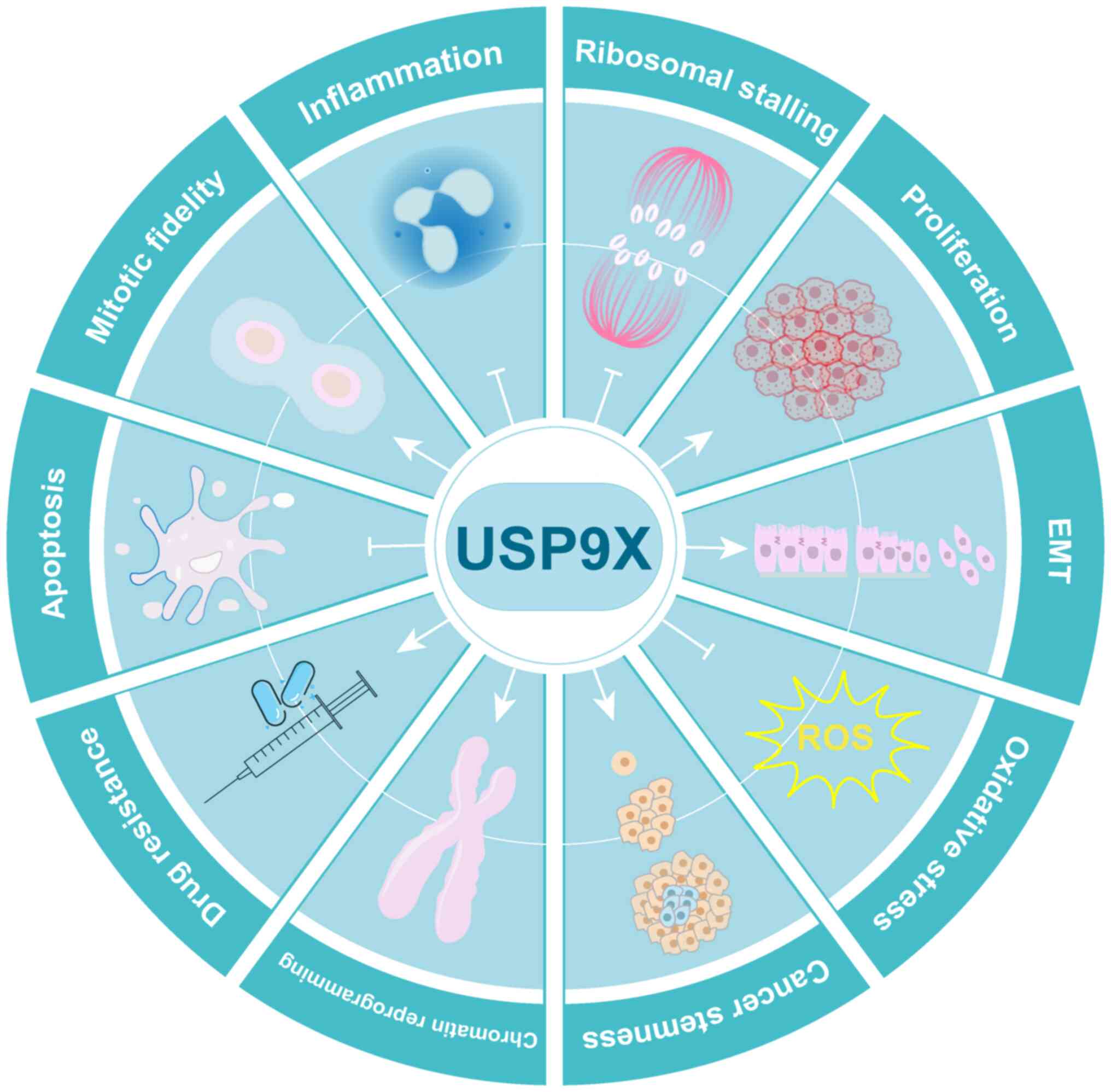
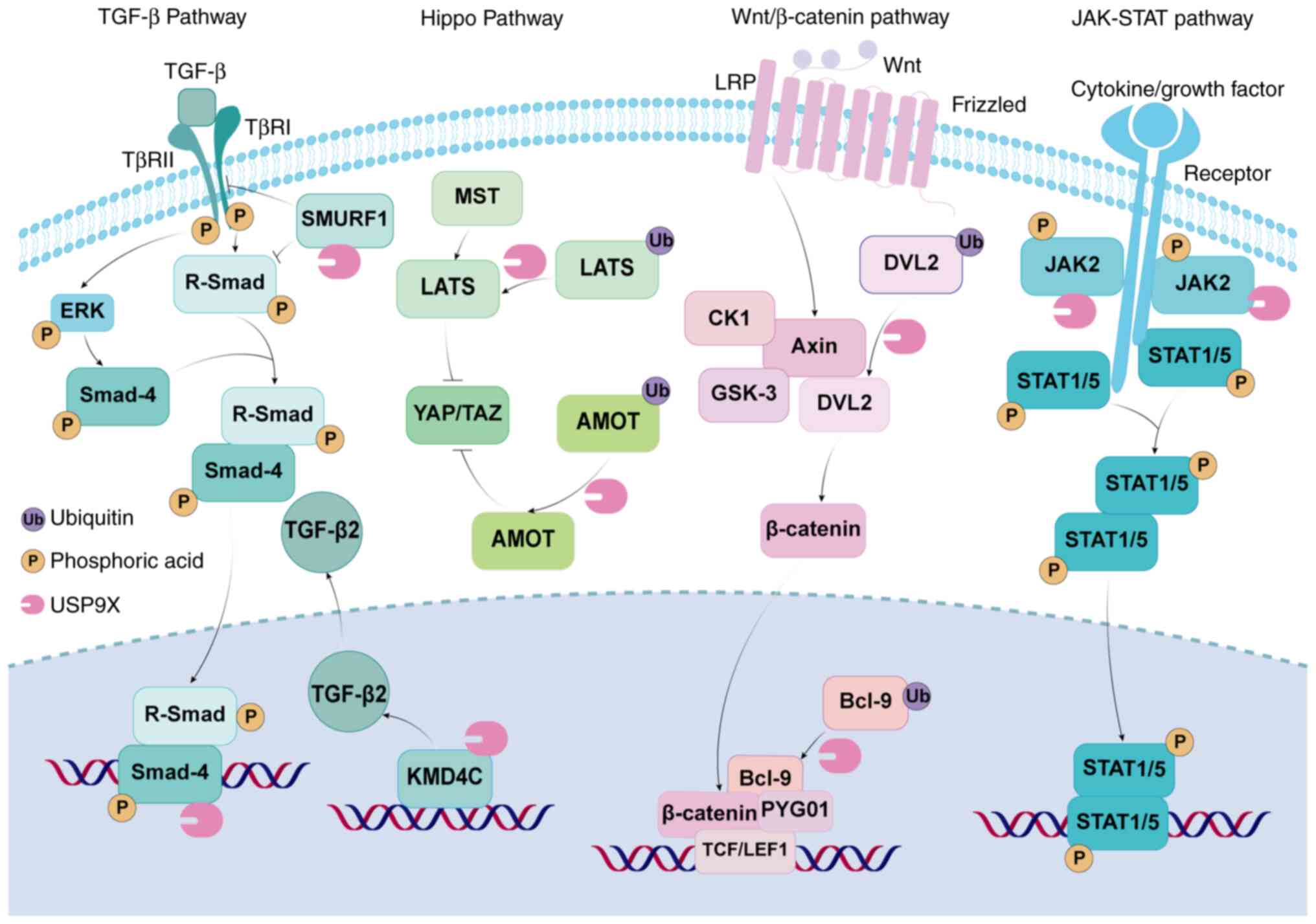
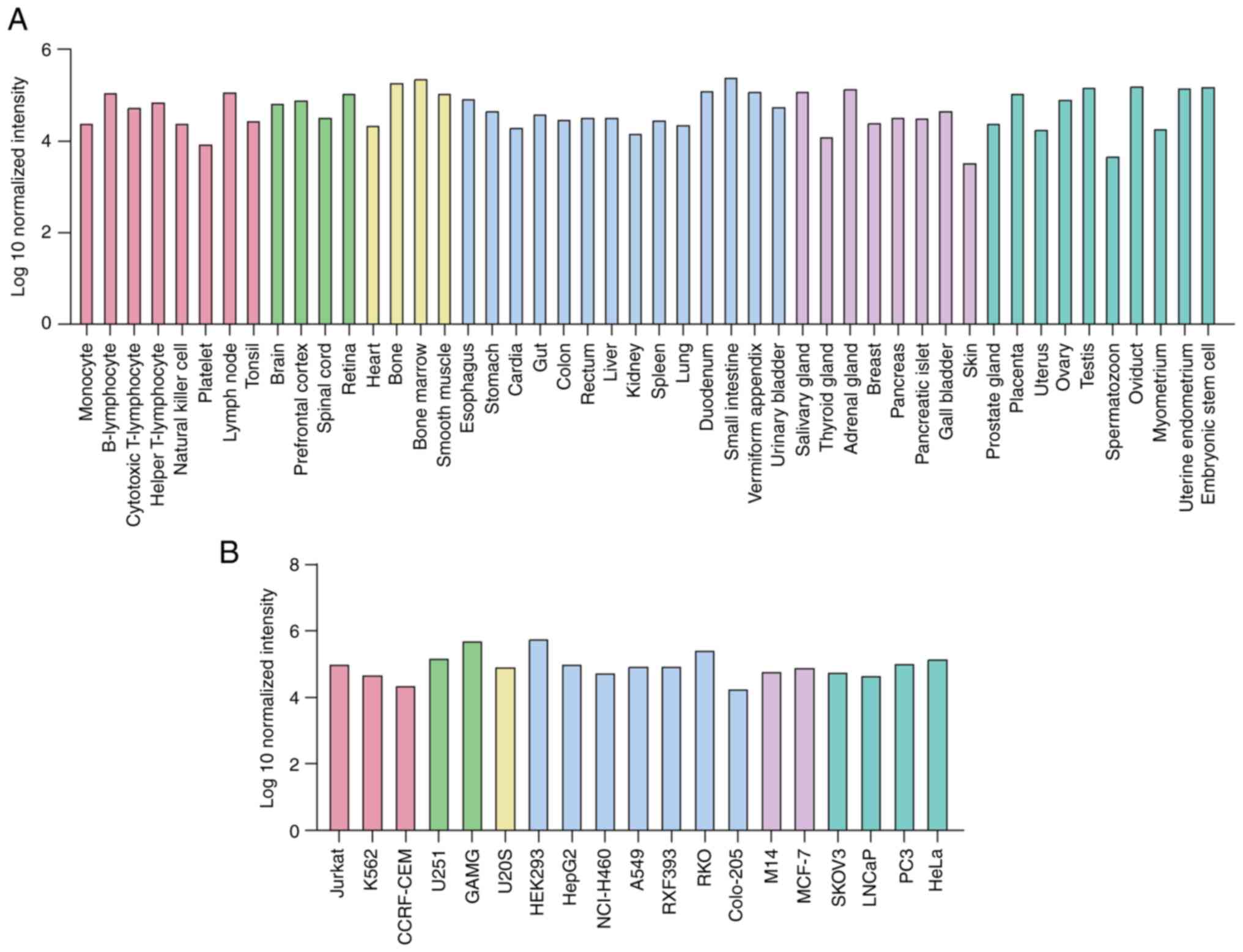
|
1
|
Ermolaeva M, Neri F, Ori A and Rudolph KL:
Cellular and epigenetic drivers of stem cell ageing. Nat Rev Mol
Cell Biol. 19:594–610. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Holguin-Cruz JA, Foster LJ and Gsponer J:
Where protein structure and cell diversity meet. Trends Cell Biol.
32:996–1007. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Toyama BH and Hetzer MW: Protein
homeostasis: Live long, won't prosper. Nat Rev Mol Cell Biol.
14:55–61. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Beynon RJ and Bond JS: Catabolism of
intracellular protein: Molecular aspects. Am J Physiol.
251:141–152. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Komander D and Rape M: The ubiquitin code.
Annu Rev Biochem. 81:203–229. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li X, Elmira E, Rohondia S, Wang J, Liu J
and Dou QP: A patent review of the ubiquitin ligase system:
2015–2018. Expert Opin Ther Pat. 28:919–937. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jang HH: Regulation of protein degradation
by proteasomes in cancer. J Cancer Prev. 23:153–161. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Becker JR, Clifford G, Bonnet C, Groth A,
Wilson MD and Chapman JR: BARD1 reads H2A lysine 15 ubiquitination
to direct homologous recombination. Nature. 596:433–437. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Roberts JZ, Crawford N and Longley DB: The
role of ubiquitination in apoptosis and necroptosis. Cell Death
Differ. 29:272–284. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun T, Liu Z and Yang Q: The role of
ubiquitination and deubiquitination in cancer metabolism. Mol
Cancer. 19:1462020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schmidt MF, Gan ZY, Komander D and Dewson
G: Ubiquitin signalling in neurodegeneration: Mechanisms and
therapeutic opportunities. Cell Death Differ. 28:570–590. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fhu CW and Ali A: Dysregulation of the
ubiquitin proteasome system in human malignancies: A window for
therapeutic intervention. Cancers (Basel). 13:15132021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Grabbe C, Husnjak K and Dikic I: The
spatial and temporal organization of ubiquitin networks. Nat Rev
Mol Cell Biol. 12:295–307. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shimizu K, Gi M, Suzuki S, North BJ,
Watahiki A, Fukumoto S, Asara JM, Tokunaga F, Wei W and Inuzuka H:
Interplay between protein acetylation and ubiquitination controls
MCL1 protein stability. Cell Rep. 37:1099882021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Murtaza M, Jolly LA, Gecz J and Wood SA:
La FAM fatale: USP9X in development and disease. Cell Mol Life Sci.
72:2075–2089. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Johnson BV, Kumar R, Oishi S, Alexander S,
Kasherman M, Vega MS, Ivancevic A, Gardner A, Domingo D, Corbett M,
et al: Partial loss of USP9X function leads to a male
neurodevelopmental and behavioral disorder converging on
transforming growth factor beta signaling. Biol Psychiatry.
87:100–112. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Delbridge ARD, Kueh AJ, Ke F, Zamudio NM,
El-Saafin F, Jansz N, Wang GY, Iminitoff M, Beck T, Haupt S, et al:
Loss of p53 causes stochastic aberrant x-chromosome inactivation
and female-specific neural tube defects. Cell Rep. 27:442–454.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Homan CC, Kumar R, Nguyen LS, Haan E,
Raymond FL, Abidi F, Raynaud M, Schwartz CE, Wood SA, Gecz J, et
al: Mutations in USP9X are associated with X-linked intellectual
disability and disrupt neuronal cell migration and growth. Am J Hum
Genet. 94:470–478. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang B, Tang X, Yao L, Wang Y, Chen Z, Li
M, Wu N, Wu D, Dai X, Jiang H and Ai D: Disruption of USP9X in
macrophages promotes foam cell formation and atherosclerosis. J
Clin Invest. 132:e1542172022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Greenhill C: Pancreatic cancer: USP9X can
be used to predict pancreatic cancer outcomes. Nat Rev
Gastroenterol Hepatol. 9:3022012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jones MH, Furlong RA, Burkin H, Chalmers
IJ, Brown GM, Khwaja O and Affara NA: The Drosophila developmental
gene fat facets has a human homologue in Xp11.4 which escapes
X-inactivation and has related sequences on Yq11.2. Hum Mol Gene.
5:1695–1701. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wood SA, Pascoe WS, Ru K, Yamada T,
Hirchenhain J, Kemler R and Mattick JS: Cloning and expression
analysis of a novel mouse gene with sequence similarity to the
Drosophila fat facets gene. Mech Dev. 63:29–38. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen X, Overstreet E, Wood SA and Fischer
JA: On the conservation of function of the Drosophila fat facets
deubiquitinating enzyme and Fam, its mouse homolog. Dev Genes Evol.
210:603–610. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fischer-Vize JA, Rubin GM and Lehmann R:
The fat facets gene is required for Drosophila eye and embryo
development. Development. 116:985–1000. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Khut PY, Tucker B, Lardelli M and Wood SA:
Evolutionary and expression analysis of the zebrafish
deubiquitylating enzyme, usp9. Zebrafish. 4:95–101. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nijman SM, Luna-Vargas MP, Velds A,
Brummelkamp TR, Dirac AM, Sixma TK and Bernards R: A genomic and
functional inventory of deubiquitinating enzymes. Cell.
123:773–786. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Amerik AY and Hochstrasser M: Mechanism
and function of deubiquitinating enzymes. Biochim Biophys Acta.
1695:189–207. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Paudel P, Zhang Q, Leung C, Greenberg HC,
Guo Y, Chern YH, Dong A, Li Y, Vedadi M, Zhuang Z and Tong Y:
Crystal structure and activity-based labeling reveal the mechanisms
for linkage-specific substrate recognition by deubiquitinase USP9X.
Proc Natl Acad Sci USA. 116:7288–7297. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Marx C, Held JM, Gibson BW and Benz CC:
ErbB2 trafficking and degradation associated with K48 and K63
polyubiquitination. Cancer Res. 70:3709–3717. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Al-Hakim AK, Zagorska A, Chapman L, Deak
M, Peggie M and Alessi DR: Control of AMPK-related kinases by USP9X
and atypical Lys(29)/Lys(33)-linked polyubiquitin chains. Biochem
J. 411:249–260. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dupont S, Mamidi A, Cordenonsi M,
Montagner M, Zacchigna L, Adorno M, Martello G, Stinchfield MJ,
Soligo S, Morsut L, et al: FAM/USP9×, a deubiquitinating enzyme
essential for TGFbeta signaling, controls Smad4 monoubiquitination.
Cell. 136:123–135. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mouchantaf R, Azakir BA, McPherson PS,
Millard SM, Wood SA and Angers A: The ubiquitin ligase itch is
auto-ubiquitylated in vivo and in vitro but is protected from
degradation by interacting with the deubiquitylating enzyme
FAM/USP9X. J Biol Chem. 281:38738–38747. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Taya S, Yamamoto T, Kano K, Kawano Y,
Iwamatsu A, Tsuchiya T, Tanaka K, Kanai-Azuma M, Wood SA, Mattick
JS and Kaibuchi K: The Ras target AF-6 is a substrate of the fam
deubiquitinating enzyme. J Cell Biol. 142:1053–1062. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vong QP, Cao K, Li HY, Iglesias PA and
Zheng Y: Chromosome alignment and segregation regulated by
ubiquitination of survivin. Science. 310:1499–1504. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Narayanan N, Wang Z, Li L and Yang Y:
Arginine methylation of USP9X promotes its interaction with TDRD3
and its anti-apoptotic activities in breast cancer cells. Cell
Discov. 3:160482017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Murray RZ, Jolly LA and Wood SA: The FAM
deubiquitylating enzyme localizes to multiple points of protein
trafficking in epithelia, where it associates with E-cadherin and
beta-catenin. Mol Biol Cell. 15:1591–1599. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Theard D, Labarrade F, Partisani M,
Milanini J, Sakagami H, Fon EA, Wood SA, Franco M and Luton F:
USP9×-mediated deubiquitination of EFA6 regulates de novo tight
junction assembly. EMBO J. 29:1499–1509. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schwickart M, Huang X, Lill JR, Liu J,
Ferrando R, French DM, Maecker H, O'Rourke K, Bazan F,
Eastham-Anderson J, et al: Deubiquitinase USP9X stabilizes MCL1 and
promotes tumour cell survival. Nature. 463:103–107. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Trinkle-Mulcahy L, Boulon S, Lam YW, Urcia
R, Boisvert FM, Vandermoere F, Morrice NA, Swift S, Rothbauer U,
Leonhardt H and Lamond A: Identifying specific protein interaction
partners using quantitative mass spectrometry and bead proteomes. J
Cell Biol. 183:223–239. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li X, Song N, Liu L, Liu X, Ding X, Song
X, Yang S, Shan L, Zhou X, Su D, et al: USP9X regulates centrosome
duplication and promotes breast carcinogenesis. Nat Commun.
8:148662017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Engel K, Rudelius M, Slawska J, Jacobs L,
Abhari BA, Altmann B, Kurutz J, Rathakrishnan A, Fernandez-Saiz V,
Brunner A, et al: USP9X stabilizes XIAP to regulate mitotic cell
death and chemoresistance in aggressive B-cell lymphoma. EMBO Mol
Med. 8:851–862. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Grou CP, Francisco T, Rodrigues TA,
Freitas MO, Pinto MP, Carvalho AF, Domingues P, Wood SA,
Rodriguez-Borges JE, Sa-Miranda C, et al: Identification of
ubiquitin-specific protease 9X (USP9X) as a deubiquitinase acting
on ubiquitin-peroxin 5 (PEX5) thioester conjugate. J Biol Chem.
287:12815–12827. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Akiyama H, Umezawa Y, Ishida S, Okada K,
Nogami A and Miura O: Inhibition of USP9X induces apoptosis in
FLT3-ITD-positive AML cells cooperatively by inhibiting the mutant
kinase through aggresomal translocation and inducing oxidative
stress. Cancer Lett. 453:84–94. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang P, Wang J, Yao S, Cui M, Cheng Y, Liu
W, Gao Z, Hu J, Zhang J and Zhang H: Deubiquitinase USP9X
stabilizes RNA m(6)A demethylase ALKBH5 and promotes acute myeloid
leukemia cell survival. J Biol Chem. 299:1050552023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Akiyama H, Umezawa Y, Watanabe D, Okada K,
Ishida S, Nogami A and Miura O: Inhibition of USP9X downregulates
JAK2-V617F and induces apoptosis synergistically with BH3 mimetics
preferentially in ruxolitinib-persistent JAK2-V617F-positive
leukemic cells. Cancers (Basel). 12:4062020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wu Y, Yu X, Yi X, Wu K, Dwabe S, Atefi M,
Elshimali Y, Kemp KT II, Bhat K, Haro J, et al: Aberrant
phosphorylation of SMAD4 Thr277-mediated USP9×-SMAD4 interaction by
free fatty acids promotes breast cancer metastasis. Cancer Res.
77:1383–1394. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li L, Liu T, Li Y, Wu C, Luo K, Yin Y,
Chen Y, Nowsheen S, Wu J, Lou Z and Yuan J: The deubiquitinase
USP9X promotes tumor cell survival and confers chemoresistance
through YAP1 stabilization. Oncogene. 37:2422–2431. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Guan T, Yang X, Liang H, Chen J, Chen Y,
Zhu Y and Liu T: Deubiquitinating enzyme USP9X regulates metastasis
and chemoresistance in triple-negative breast cancer by stabilizing
Snail1. J Cell Physiol. 237:2992–3000. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lu H, Lyu Y, Tran L, Lan J, Xie Y, Yang Y,
Murugan NL, Wang YJ and Semenza GL: HIF-1 recruits NANOG as a
coactivator for TERT gene transcription in hypoxic breast cancer
stem cells. Cell Rep. 36:1097572021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Jie X, Fong WP, Zhou R, Zhao Y, Zhao Y,
Meng R, Zhang S, Dong X, Zhang T, Yang K, et al: USP9X-mediated
KDM4C deubiquitination promotes lung cancer radioresistance by
epigenetically inducing TGF-β2 transcription. Cell Death Differ.
28:2095–2111. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chen X, Yu C, Gao J, Zhu H, Cui B, Zhang
T, Zhou Y, Liu Q, He H, Xiao R, et al: A novel USP9X substrate TTK
contributes to tumorigenesis in non-small-cell lung cancer.
Theranostics. 8:2348–2360. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kushwaha D, O'Leary C, Cron KR, Deraska P,
Zhu K, D'Andrea AD and Kozono D: USP9X inhibition promotes
radiation-induced apoptosis in non-small cell lung cancer cells
expressing mid-to-high MCL1. Cancer Biol Ther. 16:392–401. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sulkshane P, Pawar SN, Waghole R, Pawar
SS, Rajput P, Uthale A, Oak S, Kalkar P, Wani H, Patil R, et al:
Elevated USP9X drives early-to-late-stage oral tumorigenesis via
stabilisation of anti-apoptotic MCL-1 protein and impacts outcome
in oral cancers. Br J Cancer. 125:547–560. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Cheng P, Wang J, Waghmare I, Sartini S,
Coviello V, Zhang Z, Kim SH, Mohyeldin A, Pavlyukov MS, Minata M,
et al: FOXD1-ALDH1A3 signaling is a determinant for the
self-renewal and tumorigenicity of mesenchymal glioma stem cells.
Cancer Res. 76:7219–7230. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Khan OM, Carvalho J, Spencer-Dene B,
Mitter R, Frith D, Snijders AP, Wood SA and Behrens A: The
deubiquitinase USP9X regulates FBW7 stability and suppresses
colorectal cancer. J Clin Invest. 128:1326–1337. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Potu H, Peterson LF, Kandarpa M, Pal A,
Sun H, Durham A, Harms PW, Hollenhorst PC, Eskiocak U, Talpaz M and
Donato NJ: Usp9× regulates Ets-1 ubiquitination and stability to
control NRAS expression and tumorigenicity in melanoma. Nat Commun.
8:144492017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chen W, Song J, Liu S, Tang B, Shen L, Zhu
J, Fang S, Wu F, Zheng L, Qiu R, et al: USP9X promotes apoptosis in
cholangiocarcinoma by modulation expression of KIF1Bbeta via
deubiquitinating EGLN3. J Biomed Sci. 28:442021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Czabotar PE, Lessene G, Strasser A and
Adams JM: Control of apoptosis by the BCL-2 protein family:
Implications for physiology and therapy. Nat Rev Mol Cell Biol.
15:49–63. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Nagai H, Noguchi T, Homma K, Katagiri K,
Takeda K, Matsuzawa A and Ichijo H: Ubiquitin-like sequence in ASK1
plays critical roles in the recognition and stabilization by USP9X
and oxidative stress-induced cell death. Mol Cell. 36:805–818.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Huntwork-Rodriguez S, Wang B, Watkins T,
Ghosh AS, Pozniak CD, Bustos D, Newton K, Kirkpatrick DS and
Lewcock JW: JNK-mediated phosphorylation of DLK suppresses its
ubiquitination to promote neuronal apoptosis. J Cell Biol.
202:747–763. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Lai MC, Chen YP, Li DA, Yu JS, Hung HY and
Tarn WY: DDX3 interacts with USP9X and participates in
deubiquitination of the anti-apoptotic protein MCL1. FEBS J.
289:1043–1061. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Hogh-Binder SA, Klein D, Wolfsperger F,
Huber SM, Hennenlotter J, Stenzl A and Rudner J: Protein levels of
anti-apoptotic Mcl-1 and the deubiquitinase USP9× are cooperatively
upregulated during prostate cancer progression and limit response
of prostate cancer cells to radiotherapy. Cancers (Basel).
15:24962023. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Sun H, Kapuria V, Peterson LF, Fang D,
Bornmann WG, Bartholomeusz G, Talpaz M and Donato NJ: Bcr-Abl
ubiquitination and Usp9× inhibition block kinase signaling and
promote CML cell apoptosis. Blood. 117:3151–3162. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kapuria V, Peterson LF, Fang D, Bornmann
WG, Talpaz M and Donato NJ: Deubiquitinase inhibition by
small-molecule WP1130 triggers aggresome formation and tumor cell
apoptosis. Cancer Res. 70:9265–9276. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Karpel-Massler G, Ishida CT, Bianchetti E,
Shu C, Perez-Lorenzo R, Horst B, Banu M, Roth KA, Bruce JN, Canoll
P, et al: Inhibition of mitochondrial matrix chaperones and
antiapoptotic Bcl-2 family proteins empower antitumor therapeutic
responses. Cancer Res. 77:3513–3526. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kim S, Woo SM, Min KJ, Seo SU, Lee TJ,
Kubatka P, Kim DE and Kwon TK: WP1130 enhances TRAIL-induced
apoptosis through USP9X-dependent miR-708-mediated downregulation
of c-FLIP. Cancers (Basel). 11:3442019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Liu Y, Xu X, Lin P, He Y, Zhang Y, Cao B,
Zhang Z, Sethi G, Liu J, Zhou X and Mao X: Inhibition of the
deubiquitinase USP9× induces pre-B cell homeobox 1 (PBX1)
degradation and thereby stimulates prostate cancer cell apoptosis.
J Biol Chem. 294:4572–4582. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Dominguez-Brauer C, Thu KL, Mason JM,
Blaser H, Bray MR and Mak TW: Targeting mitosis in cancer: Emerging
strategies. Mol Cell. 60:524–536. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Motofei IG: Biology of cancer:
Understanding the supracellular control of mitosis in physiological
processes and malignancy. Semin Cancer Biol. 92:42–44. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Schoonen PM, Talens F, Stok C, Gogola E,
Heijink AM, Bouwman P, Foijer F, Tarsounas M, Blatter S, Jonkers J,
et al: Progression through mitosis promotes PARP inhibitor-induced
cytotoxicity in homologous recombination-deficient cancer cells.
Nat Commun. 8:159812017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Skowyra A, Allan LA, Saurin AT and Clarke
PR: USP9X limits mitotic checkpoint complex turnover to strengthen
the spindle assembly checkpoint and guard against chromosomal
instability. Cell Rep. 23:852–865. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Dietachmayr M, Rathakrishnan A, Karpiuk O,
von Zweydorf F, Engleitner T, Fernandez-Saiz V, Schenk P, Ueffing
M, Rad R, Eilers M, et al: Antagonistic activities of CDC14B and
CDK1 on USP9X regulate WT1-dependent mitotic transcription and
survival. Nat Commun. 11:12682020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Wang Q, Tang Y, Xu Y, Xu S, Jiang Y, Dong
Q, Zhou Y and Ge W: The X-linked deubiquitinase USP9X is an
integral component of centrosome. J Biol Chem. 292:12874–12884.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Clancy A, Heride C, Pinto-Fernandez A,
Elcocks H, Kallinos A, Kayser-Bricker KJ, Wang W, Smith V, Davis S,
Fessler S, et al: The deubiquitylase USP9X controls ribosomal
stalling. J Cell Biol. 220:2020042112021. View Article : Google Scholar
|
|
75
|
Weimer JM and Anton ES: Doubling up on
microtubule stabilizers: Synergistic functions of doublecortin-like
kinase and doublecortin in the developing cerebral cortex. Neuron.
49:3–4. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Gleeson JG, Lin PT, Flanagan LA and Walsh
CA: Doublecortin is a microtubule-associated protein and is
expressed widely by migrating neurons. Neuron. 23:257–271. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Kharitidi D, Apaja PM, Manteghi S, Suzuki
K, Malitskaya E, Roldan A, Gingras MC, Takagi J, Lukacs GL and
Pause A: Interplay of endosomal pH and ligand occupancy in integrin
alpha5beta1 ubiquitination, endocytic sorting, and cell migration.
Cell Rep. 13:599–609. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Zhu H, Kavsak P, Abdollah S, Wrana JL and
Thomsen GH: A SMAD ubiquitin ligase targets the BMP pathway and
affects embryonic pattern formation. Nature. 400:687–693. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Ebisawa T, Fukuchi M, Murakami G, Chiba T,
Tanaka K, Imamura T and Miyazono K: Smurf1 interacts with
transforming growth factor-beta type I receptor through Smad7 and
induces receptor degradation. J Biol Chem. 276:12477–12480. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Xie Y, Avello M, Schirle M, McWhinnie E,
Feng Y, Bric-Furlong E, Wilson C, Nathans R, Zhang J, Kirschner MW,
et al: Deubiquitinase FAM/USP9X interacts with the E3 ubiquitin
ligase SMURF1 protein and protects it from ligase
activity-dependent self-degradation. J Biol Chem. 288:2976–2985.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Wang T, Jing B, Sun B, Liao Y, Song H, Xu
D, Guo W, Li K, Hu M, Liu S, et al: Stabilization of PTGES by
deubiquitinase USP9X promotes metastatic features of lung cancer
via PGE(2) signaling. Am J Cancer Res. 9:1145–1160. 2019.PubMed/NCBI
|
|
82
|
Harrigan JA, Jacq X, Martin NM and Jackson
SP: Deubiquitylating enzymes and drug discovery: Emerging
opportunities. Nat Rev Drug Discov. 17:57–78. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Negrini S, Gorgoulis VG and Halazonetis
TD: Genomic instability-an evolving hallmark of cancer. Nat Rev Mol
Cell Biol. 11:220–228. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Kang JW, Zhan Z, Ji G, Sang Y, Zhou D, Li
Y, Feng H and Cheng T: PUMA facilitates EMI1-promoted cytoplasmic
Rad51 ubiquitination and inhibits DNA repair in stem and progenitor
cells. Signal Transduct Target Ther. 6:1292021. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
McGarry E, Gaboriau D, Rainey MD,
Restuccia U, Bachi A and Santocanale C: The deubiquitinase USP9X
maintains DNA replication fork stability and DNA damage checkpoint
responses by regulating CLASPIN during S-Phase. Cancer Res.
76:2384–2393. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Wolfsperger F, Hogh-Binder SA,
Schittenhelm J, Psaras T, Ritter V, Bornes L, Huber SM, Jendrossek
V and Rudner J: Deubiquitylating enzyme USP9× regulates
radiosensitivity in glioblastoma cells by Mcl-1-dependent and
-independent mechanisms. Cell Death Dis. 7:20392016. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Lautenbacher L, Samaras P, Muller J,
Grafberger A, Shraideh M, Rank J, Fuchs ST, Schmidt TK, The M,
Dallago C, et al: ProteomicsDB: Toward a FAIR open-source resource
for life-science research. Nucleic Acids Res. 50:D1541–D1552. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Zhang C, Cai TY, Zhu H, Yang LQ, Jiang H,
Dong XW, Hu YZ, Lin NM, He QJ and Yang B: Synergistic antitumor
activity of gemcitabine and ABT-737 in vitro and in vivo through
disrupting the interaction of USP9X and Mcl-1. Mol Cancer Ther.
10:1264–1275. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Oosterkamp HM, Hijmans EM, Brummelkamp TR,
Canisius S, Wessels LF, Zwart W and Bernards R: USP9X
downregulation renders breast cancer cells resistant to tamoxifen.
Cancer Res. 74:3810–3820. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. 45:W98–W102.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Zhang C, Qian H, Liu K, Zhao W and Wang L:
A feedback loop regulation of LINC01433 and YAP promotes malignant
behavior in gastric cancer cells. Onco Targets Ther. 12:7949–7962.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Chen H, Yang F, Li X, Gong ZJ and Wang LW:
Long noncoding RNA LNC473 inhibits the ubiquitination of survivin
via association with USP9X and enhances cell proliferation and
invasion in hepatocellular carcinoma cells. Biochem Biophys Res
Commun. 499:702–710. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Xu X, Wang S, Wang H, Pan C, Yang W and Yu
J: Hsa_circ_0008434 regulates USP9X expression by sponging
miR-6838-5p to promote gastric cancer growth, migration and
invasion. BMC Cancer. 21:12892021. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Shen G, Lin Y, Yang X, Zhang J, Xu Z and
Jia H: MicroRNA-26b inhibits epithelial-mesenchymal transition in
hepatocellular carcinoma by targeting USP9X. BMC Cancer.
14:3932014. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Chen W, Huang Y, Zhang S, Zheng X, Xie S,
Mao J, Cai Y, Lu X, Hu L, Shen J, et al: MicroRNA-212 suppresses
nonsmall lung cancer invasion and migration by regulating
ubiquitin-specific protease-9. J Cell Biochem. 120:6482–6489. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Chen W, Zhou Y, Zhi X, Ma T, Liu H, Chen
BW, Zheng X, Xie S, Zhao B, Feng X, et al: Delivery of miR-212 by
chimeric peptide-condensed supramolecular nanoparticles enhances
the sensitivity of pancreatic ductal adenocarcinoma to doxorubicin.
Biomaterials. 192:590–600. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Guo H, Zhang X, Chen Q, Bao Y, Dong C and
Wang X: miR-132 suppresses the migration and invasion of lung
cancer cells by blocking USP9X-induced epithelial-mesenchymal
transition. Am J Transl Res. 10:224–234. 2018.PubMed/NCBI
|
|
98
|
Chen E, Li E, Liu H, Zhou Y, Wen L, Wang
J, Wang Y, Ye L and Liang T: miR-26b enhances the sensitivity of
hepatocellular carcinoma to Doxorubicin via USP9X-dependent
degradation of p53 and regulation of autophagy. Int J Biol Sci.
17:781–795. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Madan V, Li J, Zhou S, Teoh WW, Han L,
Meggendorfer M, Malcovati L, Cazzola M, Ogawa S, Haferlach T, et
al: Distinct and convergent consequences of splice factor mutations
in myelodysplastic syndromes. Am J Hematol. 95:133–143. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Aqaqe N, Yassin M, Yassin AA, Ershaid N,
Katz-Even C, Zipin-Roitman A, Kugler E, Lechman ER, Gan OI,
Mitchell A, et al: An ERG enhancer-based reporter identifies
leukemia cells with elevated leukemogenic potential driven by
ERG-USP9X feed-forward regulation. Cancer Res. 79:3862–3876. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Shen SM, Zhang C, Ge MK, Dong SS, Xia L,
He P, Zhang N, Ji Y, Yang S, Yu Y, et al: PTENalpha and PTENbeta
promote carcinogenesis through WDR5 and H3K4 trimethylation. Nat
Cell Biol. 21:1436–1448. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Kapoor S, Natarajan K, Baldwin PR, Doshi
KA, Lapidus RG, Mathias TJ, Scarpa M, Trotta R, Davila E, Kraus M,
et al: Concurrent inhibition of pim and FLT3 kinases enhances
apoptosis of FLT3-ITD acute myeloid leukemia cells through
increased Mcl-1 proteasomal degradation. Clin Cancer Res.
24:234–247. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Sisoudiya SD, Mishra P, Li H, Schraw JM,
Scheurer ME, Salvi S, Doddapaneni H, Muzny D, Mitchell D, Taylor O,
et al: Identification of USP9X as a leukemia susceptibility gene.
Blood Adv. 7:4563–4575. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Schwartzman O, Savino AM, Gombert M, Palmi
C, Cario G, Schrappe M, Eckert C, von Stackelberg A, Huang JY,
Hameiri-Grossman M, et al: Suppressors and activators of JAK-STAT
signaling at diagnosis and relapse of acute lymphoblastic leukemia
in Down syndrome. Proc Natl Acad Sci USA. 114:4030–4039. 2017.
View Article : Google Scholar
|
|
105
|
Moroishi T, Hansen CG and Guan KL: The
emerging roles of YAP and TAZ in cancer. Nat Rev Cancer. 15:73–79.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Demark-Wahnefried W, Platz EA, Ligibel JA,
Blair CK, Courneya KS, Meyerhardt JA, Ganz PA, Rock CL, Schmitz KH,
Wadden T, et al: The role of obesity in cancer survival and
recurrence. Cancer Epidemiol Biomarkers Prev. 21:1244–1259. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Jaiswal A, Murakami K, Elia A, Shibahara
Y, Done SJ, Wood SA, Donato NJ, Ohashi PS and Reedijk M:
Therapeutic inhibition of USP9×-mediated Notch signaling in
triple-negative breast cancer. Proc Natl Acad Sci USA.
118:e21015921182021. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Yan J, Zhong N, Liu G, Chen K, Liu X, Su L
and Singhal S: Usp9×- and Noxa-mediated Mcl-1 downregulation
contributes to pemetrexed-induced apoptosis in human non-small-cell
lung cancer cells. Cell Death Dis. 5:e13162014. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Perez-Mancera PA, Rust AG, van der Weyden
L, Kristiansen G, Li A, Sarver AL, Silverstein KA, Grutzmann R,
Aust D, Rummele P, et al: The deubiquitinase USP9X suppresses
pancreatic ductal adenocarcinoma. Nature. 486:266–270. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Pal A, Dziubinski M, Di Magliano MP,
Simeone DM, Owens S, Thomas D, Peterson L, Potu H, Talpaz M and
Donato NJ: Usp9× promotes survival in human pancreatic cancer and
its inhibition suppresses pancreatic ductal adenocarcinoma in vivo
tumor growth. Neoplasia. 20:152–164. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Guo M, Luo G, Jin K, Long J, Cheng H, Lu
Y, Wang Z, Yang C, Xu J, Ni Q, et al: Somatic genetic variation in
solid pseudopapillary tumor of the pancreas by whole exome
sequencing. Int J Mol Sci. 18:812017. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Perurena N, Lock R, Davis RA, Raghavan S,
Pilla NF, Ng R, Loi P, Guild CJ, Miller AL, Sicinska E, et al:
USP9X mediates an acute adaptive response to MAPK suppression in
pancreatic cancer but creates multiple actionable therapeutic
vulnerabilities. Cell Rep Med. 4:1010072023. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Chen Z, Wang HW, Wang S, Fan L, Feng S,
Cai X, Peng C, Wu X, Lu J, Chen D, et al: USP9X deubiquitinates
ALDH1A3 and maintains mesenchymal identity in glioblastoma stem
cells. J Clin Invest. 129:2043–2055. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Nguyen HT, Andrejeva D, Gupta R, Choudhary
C, Hong X, Eichhorn PJ, Loya AC and Cohen SM: Deubiquitylating
enzyme USP9× regulates hippo pathway activity by controlling
angiomotin protein turnover. Cell Discov. 2:160012016. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Bartholomeusz G, Talpaz M, Bornmann W,
Kong LY and Donato NJ: Degrasyn activates proteasomal-dependent
degradation of c-Myc. Cancer Res. 67:3912–3918. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Luo H, Jing B, Xia Y, Zhang Y, Hu M, Cai
H, Tong Y, Zhou L, Yang L, Yang J, et al: WP1130 reveals USP24 as a
novel target in T-cell acute lymphoblastic leukemia. Cancer Cell
Int. 19:562019. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Peterson LF, Sun H, Liu Y, Potu H,
Kandarpa M, Ermann M, Courtney SM, Young M, Showalter HD, Sun D, et
al: Targeting deubiquitinase activity with a novel small-molecule
inhibitor as therapy for B-cell malignancies. Blood. 125:3588–3597.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Cui J, Sun W, Hao X, Wei M, Su X, Zhang Y,
Su L and Liu X: EHMT2 inhibitor BIX-01294 induces apoptosis through
PMAIP1-USP9X-MCL1 axis in human bladder cancer cells. Cancer Cell
Int. 15:42015. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Boise LH: DUB-ling down on B-cell
malignancies. Blood. 125:3522–3523. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Chou DH, Vetere A, Choudhary A, Scully SS,
Schenone M, Tang A, Gomez R, Burns SM, Lundh M, Vital T, et al:
Kinase-independent small-molecule inhibition of JAK-STAT signaling.
J Am Chem Soc. 137:7929–7934. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Lawson AP, Long MJC, Coffey RT, Qian Y,
Weerapana E, El Oualid F and Hedstrom L: Naturally occurring
isothiocyanates exert anticancer effects by inhibiting
deubiquitinating enzymes. Cancer Res. 75:5130–5142. 2015.
View Article : Google Scholar : PubMed/NCBI
|