Introduction
Thyroid cancer is one of the most common types of
endocrine malignancies (1). There
are ≤43 types of thyroid cancer according to the new World Health
Organization classification of thyroid tumors (2). Of these, differentiated thyroid cancer
and papillary thyroid carcinoma (PTC) are the most frequent,
accounting for ~80% of all thyroid cancer. PTC usually has a good
clinical prognosis, with >90% of patients exhibiting a
disease-specific survival of >10 years (3,4).
However, histologically, interstitial TC may develop into
thyroid-like carcinoma, which is a biological process that can
induce a transition from a more differentiated state to a poorly
differentiated state (5).
Therefore, it is important to develop treatments for TC besides
surgical operation.
1,3,8-trihydroxy-6-methylanthraquinone (emodin),
with the chemical formula
C15H10O5, is primarily obtained
from the Polygonaceae family and is the main active
ingredient in Chinese herbal medicines rhubarb and Polygonum
cuspidatum (6). Emodin has
multiple biological activities, such as antibacterial, antitumor
and hepatoprotective effects (7).
It exhibits inhibitory effects on numerous types of malignant
tumor, including liver, stomach, breast and pancreatic cancer
(8–11). Studies have proposed that the
antitumor effect of emodin is associated with its anti-NF-κB
activity (12,13). In the present study, the effects of
emodin on levels of NF-κB components, upstream and downstream
agents of NF-κB and the in vitro carcinoma features of two
thyroid cancer cell lines were analyzed. The present study aimed to
explore the mechanism of emodin in treatment of PTC and to provide
theoretical support for the therapeutic effect of emodin on
PTC.
Materials and methods
Ethics statement
Written informed consent regarding participation in
the present study was obtained from patients. Samples were
collected from 12 human samples used in this experiment. The age
range was from 31 to 64; There were 5 male patients and 7 female
patients. All samples were determined and collected in Peking
University Cancer Hospital (Beijing, China) from January 2020 to
January 2022. Use of stored human samples was approved by the
Ethics Committees of Peking University Cancer Hospital and the
Institute and National Institute for Viral Disease Prevention and
Control, China CDC (approval no. IVDC2021-12, Beijing, China).
Reagents and antibodies
Emodin and dimethyl sulfoxide were purchased from
MilliporeSigma. The antibodies included antibodies for p65 (cat.
no. sc-8008), phosphorylated (p-)p65 (cat. no. sc-136548), p50
(cat. no. sc-114), p-p50 (cat. no. sc-271908), c-Rel (cat. no.
sc-6955), cyclin D1 (cat. no. sc-8396), c-Myc (cat. no. sc-40),
PCNA (cat. no. sc-56), TLR4 (cat. no. sc-293072), IRF3 (cat. no.
sc-33641), AKT1/2/3 (cat. no. sc-56878), MEK1/2 (cat. no.
sc-81504), Bcl-2 (cat. no. sc-7382), Bax (cat. no. sc-7480). The
above antibodies are from Santa Cruz Biotechnology, Inc., WB
dilution concentration is 1:200, IHC and IFA dilution concentration
is 1:50. MyD88 (cat. no. ab113739; Abcam, WB dilution concentration
is 1:2,000; IFA dilution concentration is 1:200) and β-actin (cat.
no. AB0145-200; OriGene Technologies; 1:5,000), HRP-AffiniPure Goat
Anti-Rabbit IgG (H+L) Secondary (cat. no. 111-035-003; Jackson
ImmunoResearch Laboratories, Inc.), HRP-AffiniPure Goat Anti-Mouse
IgG (H+L) Secondary (cat. no. 115-035-003; Jackson ImmunoResearch
Laboratories, Inc.).
Cell culture
PTC cell lines TPC-1 and IHH4 were used (14–16).
TPC-1 and IHH4 cell lines were purchased from Zhejiang Meisen Cell
Technology Co., Ltd. Cells were identified by short tandem repeat
analysis and did not exhibit contamination or infection with
mycoplasma, bacteria or fungi. Cells were cultured in DMEM (cat.
no. 11965118; Thermo Fisher Scientific Inc.) containing 10% fetal
bovine serum (cat. no. 10270106; Thermo Fisher Scientific Inc.) and
1% penicillin-streptomycin (cat. no. 15140122; all Thermo Fisher
Scientific Inc.) in an incubator at 37°C with 5% CO2.
Emodin was dissolved in buffer containing 0.1% DMSO. The cells in
treatment groups were separately exposed to 20 and 40 µM emodin;
cells in the mock group were exposed to buffer containing only 0.1%
DMSO. Cells were exposed to treatment at 37°C for 24 h. Considering
that DMSO at ≤0.1% has no significant effect in cell
activity-related experiments (17),
a blank cell control was not included.
Cell viability assay
Cell viability was assessed using a Cell Counting
Kit-8 (CCK-8; cat. no. CK04; Dojindo Molecular Laboratories, Inc.)
according to manufacturer's instructions. Briefly, after cells were
cultured with emodin (10, 20, 40, 60, 80 or 100 µM) at 37°C for 24
h, 10 µl CCK-8 was added to each well at 37°C and 5% CO2
for 1 h. The optical density (OD) at 450 nm was measured using a
microplate reader (Thermo Fisher Scientific, Inc.). Relative cell
viability was calculated by comparing OD450 of the
experimental group to that of the control group. The cell survival
rate was calculated as follows: Cell survival rate
(%)=(Experimental OD-blank OD)/(control OD-blank OD) ×100%.
Immunohistochemistry (IHC)
Surgically removed tumor tissues were fixed in 10%
buffered formalin solution at 4°C for 12 h and paraffin sections of
thickness of 2 µm were routinely prepared. The paraffin sections of
PTC tissue were dewaxed and dehydrated with xylene and alcohol and
repaired using a microwave at 100°C for 30 min. Samples were
blocked with 3% hydrogen peroxide (cat. no. AR1108; Wuhan Boster
Biological Technology, Ltd.) at room temperature (RT) for 10 min.
After blocking with 10% normal goat serum (cat. no. AR1009; Wuhan
Boster Biological Technology, Ltd.) at room temperature (RT) for 15
min, sections were incubated with primary antibodies (p65: cat. no.
sc-8008; p-p65: cat. no. sc-136548; p50: cat. no. sc-114; p-p50:
cat. no. sc-271908) at 4°C overnight, and secondary antibodies
(Enzyme-labeled goat anti-mouse IgG polymer: PV-6002,
Enzyme-labeled goat anti-rabbit IgG polymer: PV-6002; Beijing Zhong
Shan Goldenbridge Biotechnology Company Ltd.) at 37°C for 40 min.
For visualization, slices were incubated with 3,3-diaminobenzidine
tetrahydrochloride (cat. no. AR1000; Wuhan Boster Biological
Technology, Ltd.) at RT for 3 min, counterstained with hematoxylin
(cat. no. AR 0005; Wuhan Boster Biological Technology, Ltd.) at RT
for 1 min, dehydrated and seal the sample with resin and cover
glass (cat. no. 10212450C; Citotest Scientific Co., Ltd.). Then use
a light microscope to enlarge it by 400 times. Analysis was
performed using ImageJ (version 1.8.0; National Institutes of
Health) measurement results.
Western blotting
After washing cells with PBS three times, cultured
cells were collected and harvested by centrifugation at 37°C at 500
× g for 10 min. Resulting pellets were lysed at 4°C for 1 h using
Mammalian Protein Extraction kit (cat. no. CW0889; CoWin
Biosciences) with protease inhibitor Cocktail set III (1%; v/v;
cat. no. 535140; Merck KGaA), followed by centrifugation at 4°C at
500 × g for 10 min and supernatants were collected. Protein
concentration was estimated using a BCA protein assay kit (cat. no.
71285-3; Merck KGaA). Cell lysates (70–100 µg/lane) were separated
by 12% SDS-PAGE and electroblotted onto nylon membranes. The
membranes were blocked with Tris-buffered saline (pH 7.6)
containing 0.05% Tween-20 and 5% skimmed milk at RT for 1 h, then
incubated with antibodies [p65 (cat. no. sc-8008); p-p65 (cat. no.
sc-136548); p50 (cat. no. sc-114); p-p50 (cat. no. sc-271908);
c-Rel (cat. no. sc-6955); cyclin D1 (cat. no. sc-8396); c-Myc (cat.
no. sc-40); PCNA (at. no. sc-56); TLR4 (cat. no. sc-293072); IRF3
(cat. no. sc-33641); AKT1/2/3 (at. no. sc-56878); MEK1/2 (cat. no.
sc-81504); Bcl-2 (cat. no. sc-7382); Bax (cat. no. sc-7480). The
above antibodies are from Santa Cruz Biotechnology, Inc., WB
dilution concentration is 1:200. MyD88 (cat. no. ab113739; Abcam;
WB dilution concentration is 1:2,000) and β-actin (cat. no.
AB0145-200; OriGene Technologies, Inc.; 1:5,000)] overnight at 4°C.
The membranes were incubated with secondary antibodies [rabbit
secondary antibody (cat. no. 111-035-003); mouse secondary antibody
(cat. no. 115-035-003); The above antibodies are from Jackson
ImmunoResearch Laboratories, Inc., WB dilution concentration is
1:5,000] at RT for 1 h. The blots were developed using Western
Lightning Plus-ECL (cat. no. NEL105001EA; PerkinElmer, Inc.).
Semi-quantitative analysis was performed using a Clinx ChemiCapture
System with ChemiScope 6000 (Clinx Science Instruments Co., Ltd.).
Using ImageJ (version 1.8.0; National Institutes of Health)
measures gray values.
Immunofluorescence assay (IFA)
Firstly, 0.4% paraformaldehyde was used to fix cells
at RT for 20 min. Following treatment with 0.3% Triton-X100 at RT
for 30 min and blocking with 5% BSA for 1 h at RT, cells were
incubated with antibodies [p65 (cat. no. sc-8008); p-p65 (cat. no.
sc-136548); p50 (cat. no. sc-114); p-p50 (cat. no. sc-271908);
c-Rel (cat. no. sc-6955); cyclin D1 (cat. no. sc-8396); c-Myc (cat.
no. sc-40); PCNA (cat. no. sc-56); TLR4 (cat. no. sc-293072); IRF3
(cat. no. sc-33641); AKT1/2/3 (cat. no. sc-56878); MEK1/2 (cat. no.
sc-81504); Bcl-2 (cat. no. sc-7382); Bax (cat. no. sc-7480). The
above antibodies are from Santa Cruz Biotechnology, Inc., IFA
concentration is 1:50. MyD88 (cat. no. ab113739; Abcam; 1: 200)] at
4°C overnight. Cells were incubated with secondary antibodies
(1:200; Alexa Fluor 488 anti-rabbit or Alexa Fluor 568 anti-mouse;
cat. nos. A32731 and A32723, respectively; both Thermo Fisher
Scientific, Inc.) at 37°C for 1 h. Following counterstaining with 1
µg/ml DAPI (Beyotime Institute of Biotechnology) at RT for 20 min,
images were captured using a Leica TCS SP8 confocal microscope
(Leica Microsystems, Ltd.).
Colony formation assay
Cells were digested with trypsin, a total of 1,000
cells/well was seeded in six-well plates and incubated at 37°C for
12 h. Cells were treated with different concentrations of emodin
(20, 40 µM) at 37°C for 7 days. Next, 0.4% paraformaldehyde was
used to fix cells at RT for 20 min. And stained with crystal violet
at RT for 3 min and images were taken under a light microscope
(magnification, ×40 times. When there are more than 50 cells, it
can be judged as a colony. Using ImageJ (version 1.8.0; National
Institutes of Health), colonies were counted (18).
Wound healing assay
Wound healing assay was performed to evaluate cell
migration. Cells were seeded in a six-well plate and grew to
confluence rate of 100%, followed by scratching the monolayer with
a 200-µl pipette tip to create a wound. Plates were washed to
remove floating cells and debris, DMEM containing 1% fetal bovine
serum was added. Then treated with emodin (20, 40 µM) at 37°C for
24 h. The cell migration images were captured at 0 and 24 h
post-treatment using a light microscope (magnification, ×40). Each
group contained ≥3 independent wells.
Statistical analysis
Statistical analysis was performed using SPSS
(version 22.0; IBM Corp.). Quantification of IFA as integrated
option density) was performed using ImageJ (version 1.8.0; National
Institutes of Health). All experiments were conducted ≥3 times. All
data are presented as the mean ± SD. Difference among cells treated
with different concentrations of emodin and mock cells in CCK-8 was
determined using unpaired t test. Significant differences between
cells treated with different concentrations of emodin and mock
cells in western blotting, IHC, IFA, colony formation and wound
healing assay were determined using one-way ANOVA followed by
Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
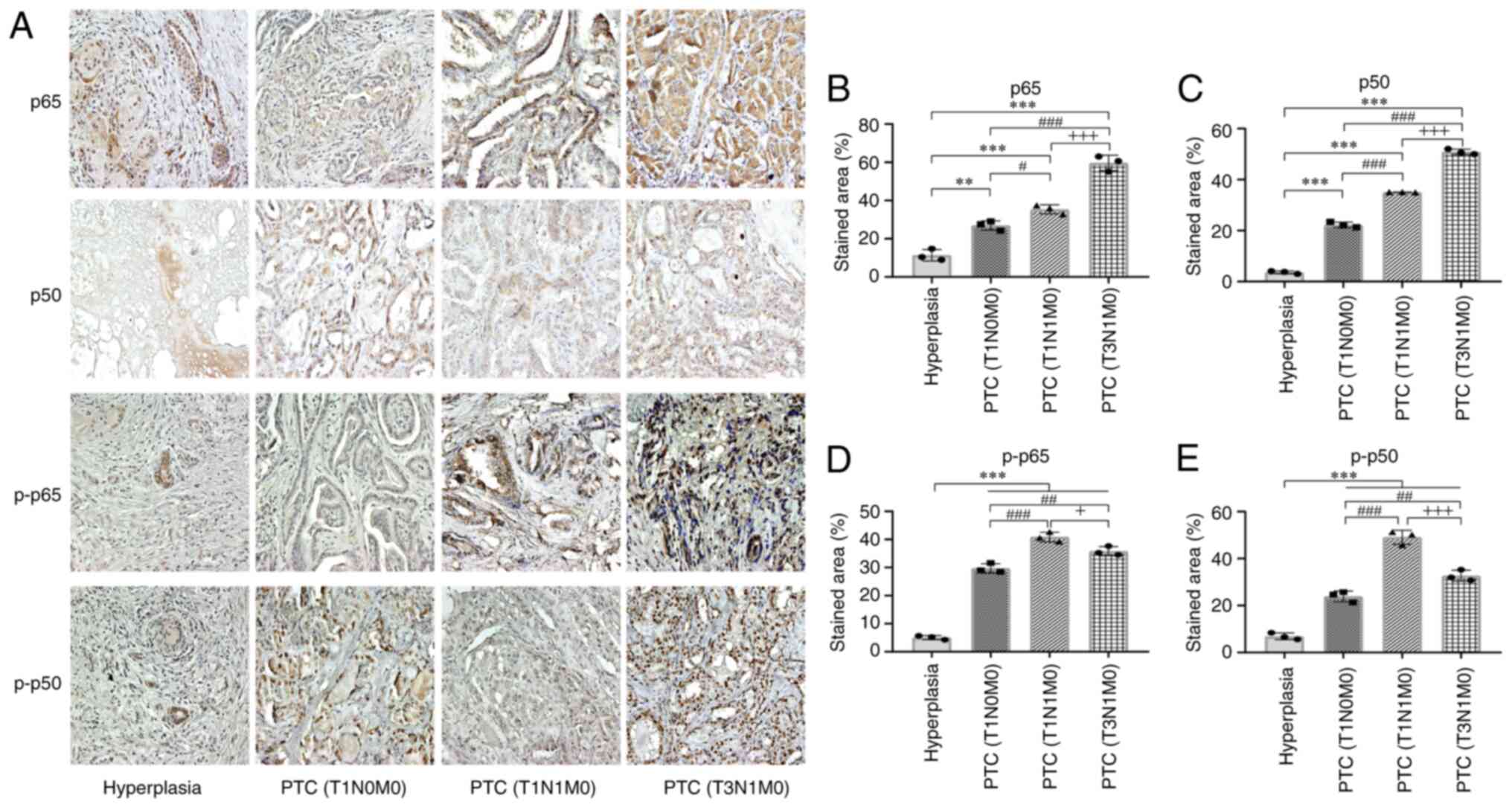
PTC tissue expresses higher levels of
NF-κB components p65 and p50, as well as p-p65 and p-p50
An increase in NF-κB activity has been described in
numerous types of malignant tumor (19). Tissue sections of PTC at different
stages, including three tumor (T)1 node (N)0 metastasis (M)0, three
T1N1M0 and three T3N1M0, as well as three benign hyperplastic
tissues, were subjected to IHC with antibodies for NF-κB components
(Fig. 1A). Compared with benign
thyroid hyperplasia tissue, more staining was observed in PTC
tissue via IHC analysis of p65 and p50 (Fig. 1B and C), particularly in PTC T1N1M0
and T3N1M0. Quantitative assay of the signal intensity revealed
increased levels of p65 and p50 in PTC tissue and the percentage of
the positive area increased with progression of tumor stages.
Subsequently, IHC of p-p65 and p-p50 demonstrated that more
staining were detected in PTC tissues (Fig. 1D and E). Significantly higher
percentages of positive areas of p65, p50, p-p65 and p-p50 were
calculated for PTC compared with benign tissues in the quantitative
assays.
Emodin treatment of thyroid cancer
cell lines downregulates NF-κB via inhibition of the TLR4 signaling
pathway
Prior to testing the effect of emodin on the NF-κB
components and upstream TLR4 signaling, thyroid cancer cell lines
IHH4 and TPC-1 were treated with various concentrations of emodin
(10, 20, 40, 60, 80 or 100 µM) to assess cytotoxicity. Cells were
harvested at 24 h post-exposure and subjected to CCK-8 assay. The
data of the individual preparations were normalized with that of
the cells without emodin, set as 100%. As emodin concentration
increased, the survival rate of cells decreased. Compared with the
preparations treated with DMSO not containing emodin (0 µM), the
viability of the cells treated with 20 and 40 µM emodin was
slightly decreased, while cells exposed to ≥60 µM emodin had
significantly decreased viability (Fig.
2A). Based on the results of CCK-8 assays, 20 and 40 µM emodin
were used in subsequent experiments. At 24 h after exposure,
emodin-treated cells and the mock cells exposed to 0.1% DMSO buffer
were harvested. Western blotting of cellular lysates revealed that
the signals of NF-κB components p65, p50 and c-Rel, as well as
p-p65 and p-p50, were significantly weaker in cells treated with
emodin compared with the mock group, the ratio of p-p65 to p65,
p-p50 to p50 were significantly decreased (Fig. 2B). IFA also revealed significantly
weaker green staining in emodin-treated IHH4 and TPC-1 cells
(Fig. 2C). The effect of 40 µM
emodin on various NF-κB components was stronger than that of 20
µM.
 | Figure 2.Expression of NF-κB components TLR4,
MyD88, IRF3, AKT and MEK decreases in thyroid cancer cell lines
IHH4 and TPC-1 following treatment with emodin. (A) Cell Counting
Kit-8 assays. TPC-1 and IHH4 cells were exposed to emodin dissolved
in the buffer containing 0.1% DMSO. The data were normalized with
that of blank control without emodin. Representative (B) western
blots and (C) IFAs for p65, p50, p-p65, p-p50 and C-Rel.
Representative (D) western blots and (E) IFAs for TLR4, MyD88,
IRF3, AKT and MEK. *P<0.05, **P<0.01, ***P<0.001 and
****P<0.0001 vs. Mock; #P<0.05,
##P<0.01 and ###P<0.001 vs. 20 µM
emodin). TLR4, toll-like receptor 4; IFA, immunofluorescence assay;
p, phosphorylated; MyD88, MYD88 innate immune signal transduction
adaptor; IRF3, interferon regulatory factor 3; IOD, integrated
option density; emodin, 1,3,8-trihydroxy-6-methylanthraquinone; NS,
not significant. |
The levels of elements in the upstream regulatory
pathway for NF-κB, including TLR4, MyD88, IRF3, AKT and MEK, in
IHH4 and TPC-1 cells were examined after exposure to emodin or
DMSO. Western blotting demonstrated that expression of TLR4, MyD88,
IRF3, AKT and MEK in the emodin-treated cells was significantly
weaker than in the mock cells exposed to DMSO (Fig. 2D). IFA revealed that the
fluorescence intensities of TLR4, MyD88, IRF3, AKT and MEK in the
emodin-treated cells was significantly weaker than in the mock
cells exposed to DMSO (Fig. 2E) in
emodin-treated cells. This indicated that emodin treatment of
thyroid cell lines inhibited the upstream regulatory TLR4 signaling
pathway of NF-κB.
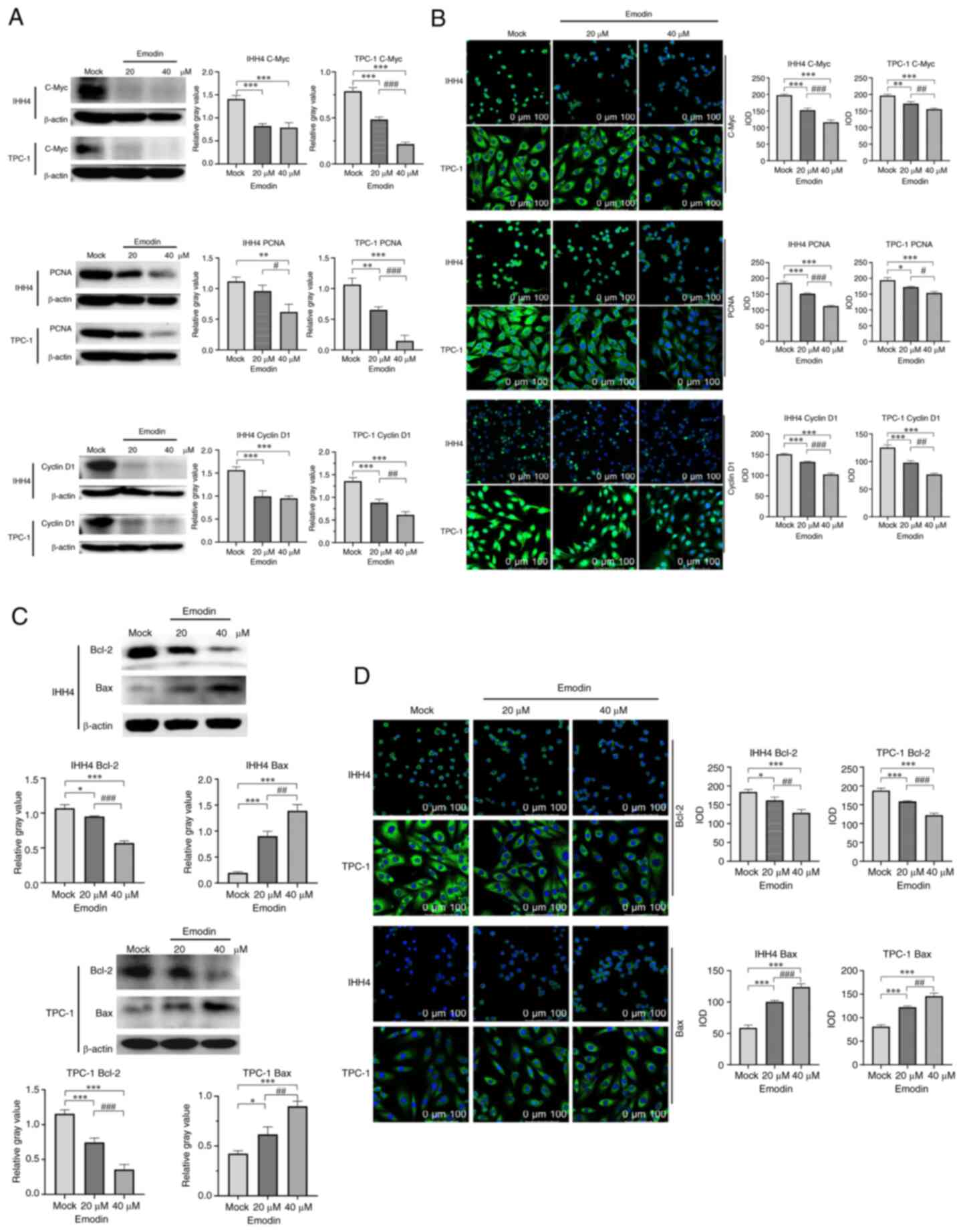
Emodin treatment of thyroid cancer
cell lines suppresses proliferative factors and promotes apoptotic
factors
To examine the effects of emodin on cellular levels
of proteins involved in cell proliferation and apoptosis, thyroid
cancer cell lines treated with or without emodin were subjected to
western blotting and IFA. Compared with the control group, the
protein expression levels of PCNA, cyclin D1 and c-Myc in the cells
treated with emodin significantly decreased (Fig. 3A). Significantly lower fluorescence
intensities in emodin-treated IHH4 and TPC-1 cells were also
identified in PCNA, cyclin D1 and c-Myc (Fig. 3B). Furthermore, the levels of
apoptotic Bcl-2 and Bax were examined. Both western blotting and
IFA revealed a decrease and an increase in Bcl-2 and Bax levels,
respectively, in the cells treated with emodin (Fig. 3C and D).
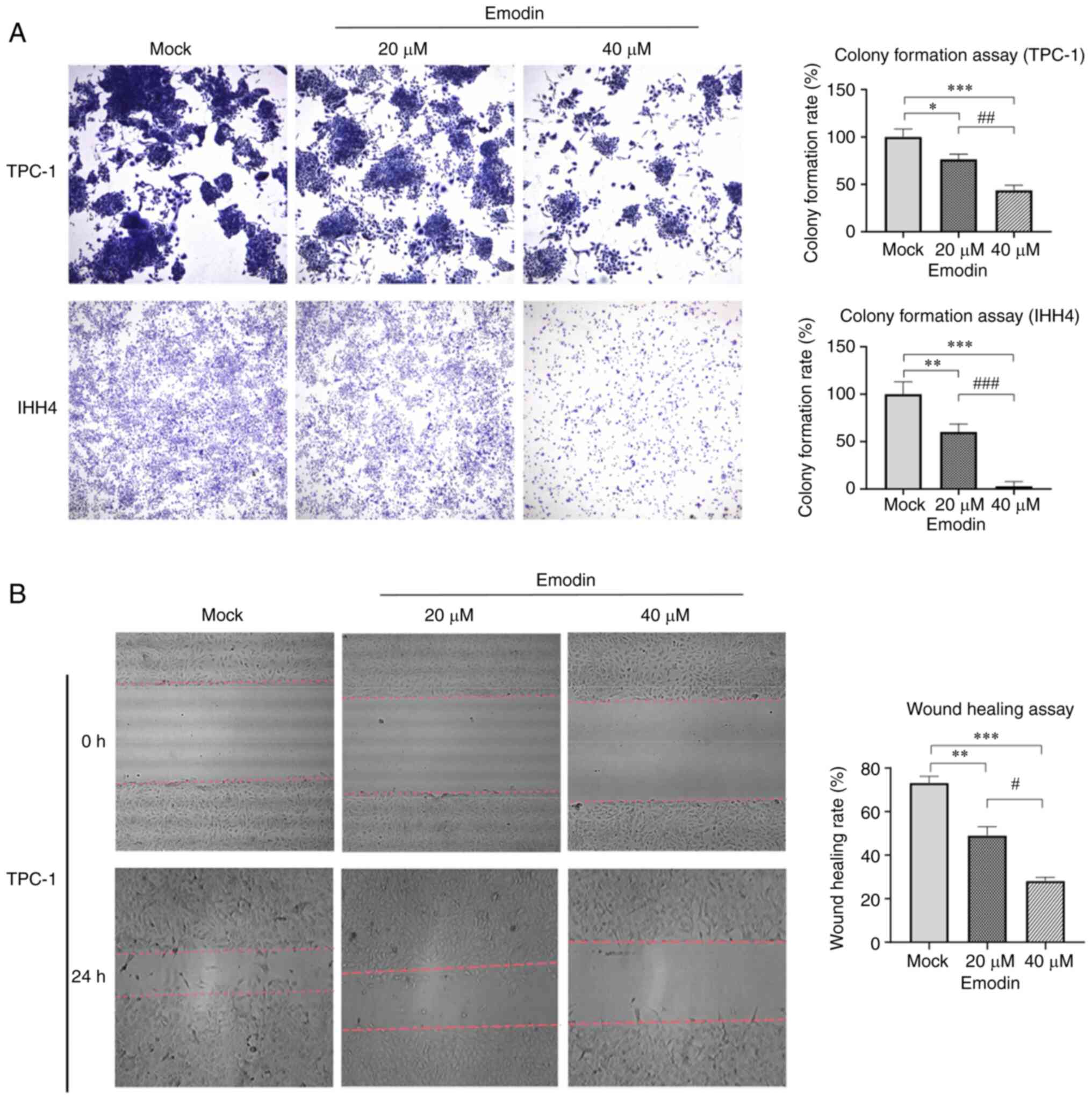
Emodin inhibits migration of thyroid
cancer cell lines in vitro
To assess the influence of emodin on tumor
characteristics such as proliferation, apoptosis, migration, and
invasion of IHH4 and TPC-1 cells (20), cells were assessed using a colony
formation assay after treatment with and without emodin for 7 days.
A lower number and smaller size of colonies were observed in cells
receiving 20 and 40 µM emodin compared with those in the mock cells
(Fig. 4A). The wound healing assay
showed that the number of TPC-1 cells treated with emodin for 24 h
was lower than that of the control group (Fig. 4B). This demonstrated that exposure
to emodin decreased colony formation and migration of thyroid
cancer cells in vitro.
Discussion
Recent studies have demonstrated that emodin
inhibits proliferation and promotes apoptosis of PTC cells by
regulating the AMP-activated protein kinase signaling pathway and
mediating VEGFR-2 expression (21,22).
However, studies of the effect of emodin on inflammatory pathways
in TC cells are relatively limited (21,23).
As a core element of the inflammatory reaction, the NF-κB signaling
pathway is involved in the pathophysiological processes of breast
cancer, colon cancer, leukemia, lung cancer and other tumors
(19). Serving as an important
transcription factor, NF-κB is widely distributed in lymphocytes,
skeletal cells, mouse mammary epithelium, fibroblasts or human
epithelial cells and other cells and controls cell stress and
survival (24–26). Dysregulation of NF-κB is associated
with malignant neoplasia, inflammation, viral infection and
autoimmune disease (rheumatoid arthritis, insulin-dependent
diabetes mellitus and multiple sclerosis) (27–29).
In line with previous studies (29,30),
aberrant upregulation of NF-κB components in thyroid cancer tissue
was assessed in the present study. Using cultured PTC cell lines,
the present study verified that emodin exposure can reverse the
upregulation of NF-κB components such as p65, p50, p-p65, p-p50 and
c-Rel, which may affect cell proliferation, apoptosis and
carcinogenicity.
The present study revealed that levels of the NF-κB
upstream components, including TLR4, MyD88, IRF3, AKT and MEK, were
downregulated in the PTC cell lines following exposure to emodin.
TLR4 is positively associated with the development of liver cancer,
breast cancer, cervical cancer and other cancers, and has an
immunosuppressive function and promotes survival of cancer cells
(31,32). There are two primary pathways of
TLR4 signaling: MyD88-dependent and -independent pathway. In the
MyD88-dependent pathway, TLR4 combines with MyD88, forming an
active TLR4/MyD88 complex. Through molecular interaction and
phosphorylation, including recruiting IL-1 and binding to TNF
receptor-associated factor 6, activating MEK and AKT, inducing
depolymerization of inhibitory factors, cellular NF-κB is
activated, resulting in phosphorylation of p65 and p50 (1,33–35).
In the MyD88-independent pathway, TLR4 directly activates IRF3,
which is associated with activation of NF-κB downstream (36). p-p65 and p-p50 can be transferred
from the cytoplasm to the nucleus and, as transcription regulators,
they participate in activation of the NF-κB pathway (34,37).
c-Rel is an important member of the NF-κB family. It usually forms
a heterodimer with p65 or p50 to participate in activation of
NF-κB. Additionally, c-Rel can activate target genes such as Bcl-XL
and enhancer of zeste 2 polycomb repressive complex 2 subunit to
promote cell proliferation (38,39).
Continuous activation of NF-κB is associated with formation,
development and metastasis of gastric cancer, colorectal cancer,
leukemia and other tumors (40–42).
The present data indicated that emodin inhibited TLR4 signaling via
both MyD88-dependent and -independent pathways, and thus it was
hypothesized that the antitumor activity of emodin depends on the
inhibition of NF-κB via downregulation of the TLR4 signaling
pathway.
The present study revealed downregulation of
proliferative factors, including c-Myc, cyclin D1 and PCNA, as well
downregulation of anti-apoptotic Bcl-2, and upregulation of the
pro-apoptotic factor Bax in PTC cell lines exposed to emodin. One
of the key biological functions of NF-κB is to control and
regulation of expression and activities of a series of cell cycle
factors such as cyclins A, H and p21 (26). A number of cell cycle proteins are
targets of NF-κB, including c-Myc, c-Rel, IRF4 and cyclins D1, D2
and D3. The anti-apoptotic Bcl-2 and Bax are also targets of NF-κB.
Knockdown of TLR4 in cervical cancer cells markedly increases the
apoptosis rate, while TLR4 upregulates Bcl-2 via the NF-κB
signaling pathway and promotes the proliferation of cancer cells
(43). Therefore, it was
hypothesized that emodin-induced changes in cell cycle and
apoptotic proteins are largely due to its inhibitory effect on
NF-κB.
The preliminary in vitro data of the
carcinoma-associated characteristics of PTC cell lines following
treatment with emodin highlighted the potential use of emodin to
reverse tumorgenicity of PTC. The therapeutic potential of emodin
in different types of malignant tumors has been examined repeatedly
(8–11). Although the majority of patients
with PTC have a good prognosis, endocrine and radionuclide therapy
are sometimes needed besides surgical operation (44,45).
The present study provided molecular evidence of the antitumor
potential of emodin.
Acknowledgements
Not applicable.
Funding
The present study was supported by National Key R&D Program
of China (grant no. 2020YFE0205700), State Key Laboratory of
Infectious Disease Development Grant (grant nos. 2019 SKLID501,
2019 SKLID307 and 2021SKLID101).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL, YZW, YW, and WWZ performed CCK-8, western blot,
IFA, colony formation and wound healing assay. WW collected samples
and performed IHC. XL and YPW conducted statistical analysis. XPD
and QS obtained financial support and designed the study. XL, YPW,
XPD and QS wrote the manuscript. All authors have read and approved
the final manuscript. LX and WYZ confirm the authenticity of all
the raw data.
Ethics approval and consent to
participate
Use of the stored surgical removed samples was
approved by Research Ethics Committee of the National Institute for
Viral Disease Control and Prevention (approval no. IVDC2021-12,
Beijing, China). Patients provided written informed consent for use
of clinical material.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lloyd RV, Osamura RY, Klöppel G and Rosai
J: WHO classification of tumours of endocrine organs. 4th edition.
IARC Publications; Lyon: 2017
|
|
3
|
Saini S, Tulla K, Maker AV, Burman KD and
Prabhakar BS: Therapeutic advances in anaplastic thyroid cancer: A
current perspective. Mol Cancer. 17:1542018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fagin JA and Wells SA Jr: Biologic and
clinical perspectives on thyroid cancer. N Engl J Med.
375:1054–1067. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boumahdi S and de Sauvage FJ: The great
escape: Tumour cell plasticity in resistance to targeted therapy.
Nat Rev Drug Discov. 19:39–56. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zeng P, Shi Y, Wang XM, Lin L, Du YJ, Tang
N, Wang Q, Fang YY, Wang JZ, Zhou XW, et al: Emodin rescued
hyperhomocysteinemia-induced dementia and Alzheimer's disease-like
features in rats. Int J Neuropsychopharmacol. 22:57–70. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang X, Wang J, Shi X, Pan C, Liu H, Dong
Y, Dong R, Mang J and Xu Z: Proteomic analyses identify a potential
mechanism by which extracellular vesicles aggravate ischemic
stroke. Life Sci. 231:1165272019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fu JM, Zhou J, Shi J, Xie JS, Huang L, Yip
AY, Loo WT, Chow LW and Ng EL: Emodin affects ERCC1 expression in
breast cancer cells. J Transl Med. 10 (Suppl 1):S72012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Y, Yu H, Zhang J, Ge X, Gao J, Zhang
Y and Lou G: Anti-tumor effect of emodin on gynecological cancer
cells. Cell Oncol (Dordr). 38:353–363. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou RS, Wang XW, Sun QF, Ye ZJ, Liu JW,
Zhou DH and Tang Y: Anticancer effects of emodin on HepG2 cell:
Evidence from bioinformatic analysis. Biomed Res Int.
2019:30658182019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang Q, Leong SA, Chan KP, Yuan XL and Ng
TK: Complex effect of continuous curcumin exposure on human bone
marrow-derived mesenchymal stem cell regenerative properties
through matrix metalloproteinase regulation. Basic Clin Pharmacol
Toxicol. 128:141–153. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stompor-Gorący M: The health benefits of
emodin, a natural anthraquinone derived from rhubarb-a summary
update. Int J Mol Sci. 22:95222021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Q, Chen WW, Sun X, Qian D, Tang DD,
Zhang LL, Li MY, Wang LY, Wu CJ and Peng W: The versatile emodin: A
natural easily acquired anthraquinone possesses promising
anticancer properties against a variety of cancers. Int J Biol Sci.
18:3498–3527. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen F, Li M and Zhu X: Propofol
suppresses proliferation and migration of papillary thyroid cancer
cells by down-regulation of lncRNA ANRIL. Exp Mol Pathol.
107:68–76. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Le F, Luo P, Ouyang Q and Zhong X: LncRNA
WT1-AS downregulates survivin by upregulating miR-203 in papillary
thyroid carcinoma. Cancer Manag Res. 12:443–449. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang N, Duan H, Zhang C, Zhou Y and Gao R:
The LINC01186 suppresses cell proliferation and invasion ability in
papillary thyroid carcinoma. Oncol Lett. 16:5639–5644.
2018.PubMed/NCBI
|
|
17
|
Qiu XX, Sun J, Zhao M, Chu X, An J and Li
W: Inhibitory effects of emodin on the biological activity of human
papillary thyroid cancer cells and related mechanisms. Acta Acad
Med Xuzhou. 41:367–372. 2021.(In Chinese).
|
|
18
|
Rajendran V and Jain MV: In vitro
tumorigenic assay: Colony forming assay for cancer stem cells.
Methods Mol Biol. 1692:89–95. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pramanik KC, Makena MR, Bhowmick K and
Pandey MK: Advancement of NF-κB signaling pathway: A novel target
in pancreatic cancer. Int J Mol Sci. 19:38902018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li W, Wang D, Li M and Li B: Emodin
inhibits the proliferation of papillary thyroid carcinoma by
activating AMPK. Exp Ther Med. 22:10752021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang W, Peng Y and Teng Y: Inhibitory
effects of emodin alone and its combination with gemcitabine on
human thyroid cancer cell K1. Chin J Tradit Med Sci Technol.
28:733–738. 2021.(In Chinese).
|
|
23
|
Shi GH and Zhou L: Emodin suppresses
angiogenesis and metastasis in anaplastic thyroid cancer by
affecting TRAF6-mediated pathways in vivo and in
vitro. Mol Med Rep. 18:5191–5197. 2018.PubMed/NCBI
|
|
24
|
Yamamoto Y and Gaynor RB: Role of the
NF-kB pathway in the pathogenesis of human disease states. Curr Mol
Med. 1:287–296. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zinatizadeh MR, Schock B, Chalbatani GM,
Zarandi PK, Jalali SA and Miri SR: The nuclear factor kappa B
(NF-kB) signaling in cancer development and immune diseases. Genes
Dis. 8:287–297. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ledoux AC and Perkins ND: NF-κB and the
cell cycle. Biochem Soc Trans. 42:76–81. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bacher S and Schmitz ML: The NF-kappaB
pathway as a potential target for autoimmune disease therapy. Curr
Pharm Des. 10:2827–2837. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Geng Xue CW and Lin Xiaoyue: Mechanism of
NF-κB signaling pathway regulating lung cancer and research
progress in intervention of traditional Chinese medicine. Chin J
Exp Tradit Med Formulae. 1–11. 2023.
|
|
29
|
Sun J X, Yy and Li JH:
Expression&clinical significance of NF-κB in papillary thyroid
carcinoma(PTC). J Microbiol. 35:58–61. 2015.
|
|
30
|
Faria M, Matos P, Pereira T, Cabrera R,
Cardoso BA, Bugalho MJ and Silva AL: RAC1b overexpression
stimulates proliferation and NF-kB-mediated anti-apoptotic
signaling in thyroid cancer cells. PLoS One. 12:e01726892017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu Y, Li T, Xu Y, Xu E, Zhou M, Wang B
and Shen J: Effects of TLR4 gene silencing on the proliferation and
apotosis of hepatocarcinoma HEPG2 cells. Oncol Lett. 11:3054–3060.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang H and Zhang S: The expression of
Foxp3 and TLR4 in cervical cancer: Association with immune escape
and clinical pathology. Arch Gynecol Obstet. 295:705–712. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Roy A, Srivastava M, Saqib U, Liu D,
Faisal SM, Sugathan S, Bishnoi S and Baig MS: Potential therapeutic
targets for inflammation in toll-like receptor 4 (TLR4)-mediated
signaling pathways. Int Immunopharmacol. 40:79–89. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhou J, Deng Y, Li F, Yin C, Shi J and
Gong Q: Icariside II attenuates lipopolysaccharide-induced
neuroinflammation through inhibiting TLR4/MyD88/NF-κB pathway in
rats. Biomed Pharmacother. 111:315–324. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yin H, Pu N, Chen Q, Zhang J, Zhao G, Xu
X, Wang D, Kuang T, Jin D, Lou W and Wu W: Gut-derived
lipopolysaccharide remodels tumoral microenvironment and synergizes
with PD-L1 checkpoint blockade via TLR4/MyD88/AKT/NF-κB pathway in
pancreatic cancer. Cell Death Dis. 12:10332021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mitra A, Ahuja A, Rahmawati L, Kim HG, Woo
BY, Hong YD, Hossain MA, Zhang Z, Kim SY, Lee J, et al: Caragana
rosea Turcz methanol extract inhibits lipopolysaccharide-induced
inflammatory responses by suppressing the TLR4/NF-κB/IRF3 signaling
pathways. Molecules. 26:66602021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen X, Zhi X, Yin Z, Li X, Qin L, Qiu Z
and Su J: 18β-Glycyrrhetinic acid inhibits osteoclastogenesis in
vivo and in vitro by blocking RANKL-mediated RANK-TRAF6
interactions and NF-κB and MAPK signaling pathways. Front
Pharmacol. 9:6472018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li T, Li X, Zamani A, Wang W, Lee CN, Li
M, Luo G, Eiler E, Sun H, Ghosh S, et al: c-Rel is a myeloid
checkpoint for cancer immunotherapy. Nat Cancer. 1:507–517. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hunter JE, Leslie J and Perkins ND: c-Rel
and its many roles in cancer: An old story with new twists. Br J
Cancer. 114:1–6. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang S, Liu Z, Wang L and Zhang X:
NF-kappaB signaling pathway, inflammation and colorectal cancer.
Cell Mol Immunol. 6:327–334. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xia Y, Shen S and Verma IM: NF-κB, an
active player in human cancers. Cancer Immunol Res. 2:823–830.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Taniguchi K and Karin M: NF-κB,
inflammation, immunity and cancer: Coming of age. Nat Rev Immunol.
18:309–324. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yan W, XU F, Liu A, Cai J and Wang S:
Effect of Toll like-receptor 4 on proliferation of cervical cancer
cells by NF-κB signaling pathway. Chin J Pathophysiol. 301–307.
2015.
|
|
44
|
Limaiem F, Rehman A, Anastasopoulou C and
Mazzoni T: Papillary thyroid carcinoma. StatPearls [Internet]
Treasure Island (FL): StatPearls Publishing; 2023
|
|
45
|
National Health Commission of the People's
Republic of China Medical Administration and Medical
Administration, . Thyroid Cancer Diagnosis and Treatment Guidelines
(2022 Edition). Chin J Pract Surg. 42:1343–1357+1363. 2022.(In
Chinese).
|