Introduction
Malignant melanoma (MM) is a malignant tumor
originating from melanocytes, which accounts for 1–3% of all
malignant tumors, and usually arises from the skin or mucus
membranes (1). MM is very
aggressive and can metastasise in an early phase of the disease. MM
generally presents as a primary neoplasm of the skin but may also
arise in other organs and tissues, such as the respiratory tract,
oral cavity, liver, ovaries, oesophagus, larynx, cervix, vagina and
gallbladder. Most MM cases of the respiratory system are metastatic
at the time of diagnosis. The typical therapy for MM is surgical
excision, immunotherapy such as use of interleukin 2, gene therapy
and biochemotherapy. Primary malignant melanoma of the lung (PMML)
is extremely rare, accounting for 0.01% of all lung tumors
(2). PMML is characterized by high
malignancy, a high recurrence rate and a poor prognosis, its
clinical manifestation and imaging features are not specific, and
it does not differ from the more usual primary bronchogenic
carcinoma. In addition, it cannot be discriminated from other forms
of primary melanoma according to its histology and
immunohistochemistry. Jensen and Egedorf suggested six criteria to
specifically diagnose PMML: i) No previously removed skin tumors;
ii) no previously removed ocular tumors; iii) a solitary lung
tumor; iv) tumor morphology compatible with a primary tumor; v) no
other organ involvement; and vi) autopsy without primary MM
demonstrated elsewhere, especially not in the skin or eyes
(3). Like other MM, the usual
treatment for PMML is an aggressive surgical approach, combined
with radiation therapy, chemotherapy and immunotherapy (4,5). The
present study, reports the case of a 72-year-old PMML patient with
an endobronchial pigmented mass on bronchoscopy.
Case report
A 72-year-old non-smoking female presented to Taihe
Hospital (Shiyan, China) after experiencing a 2-week history of
cough and expectoration. The patient denied a prior history of
skin, ear or ocular lesions. Physical examination showed percussive
dullness of the right upper lung and decreased breath sounds; no
nevus, hemorrhoids or pigmentation was observed on the skin, eyes,
perianal or external genitals. Laboratory tests reported that the
patient's carcinoembryonic antigen, cytokeratin 19 fragment,
neuron-specific enolase and squamous cell carcinoma antigen levels
were 1.79 µg/l (normal, 0–5.0 µg/l), 1.31 ng/ml (normal, 0–3.3
ng/ml), 10.8 ng/ml (normal, 0–16.3 ng/ml) and 0.58 ng/ml (normal,
0–2.7 ng/ml), respectively. Chest X-ray indicated an irregular mass
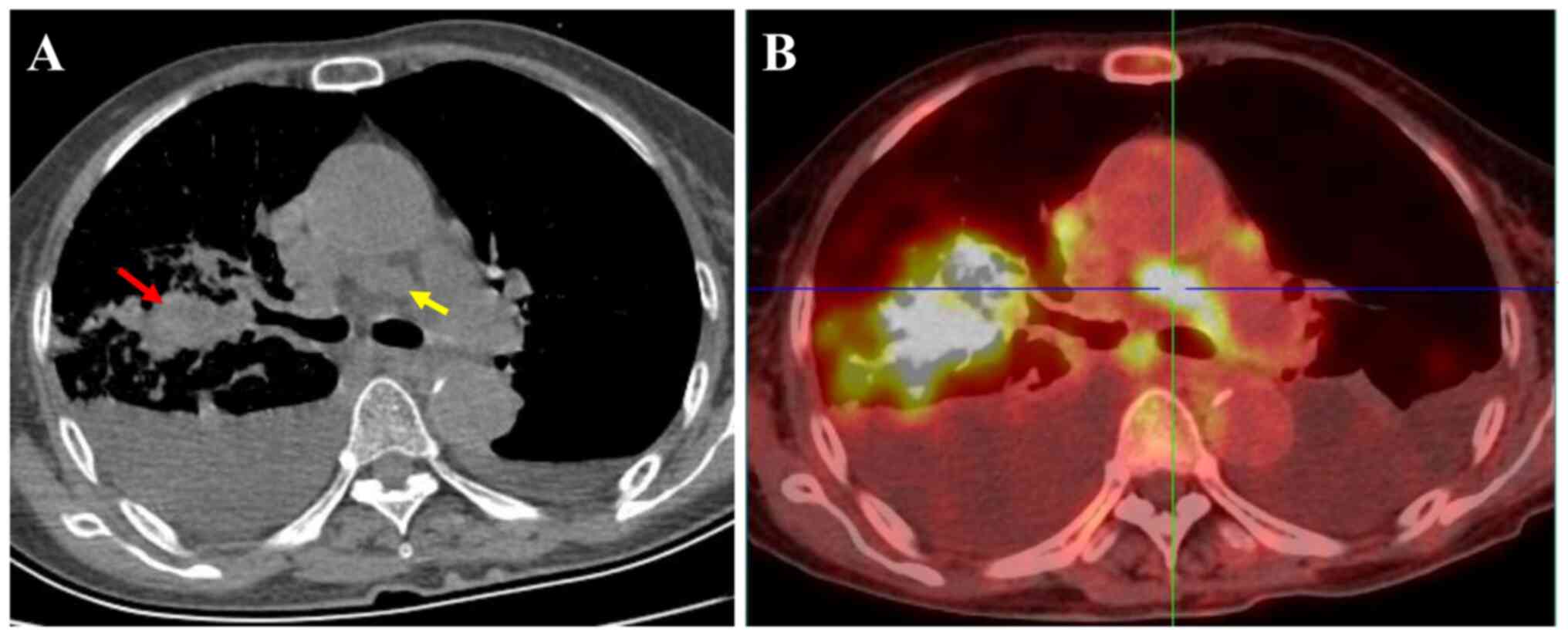
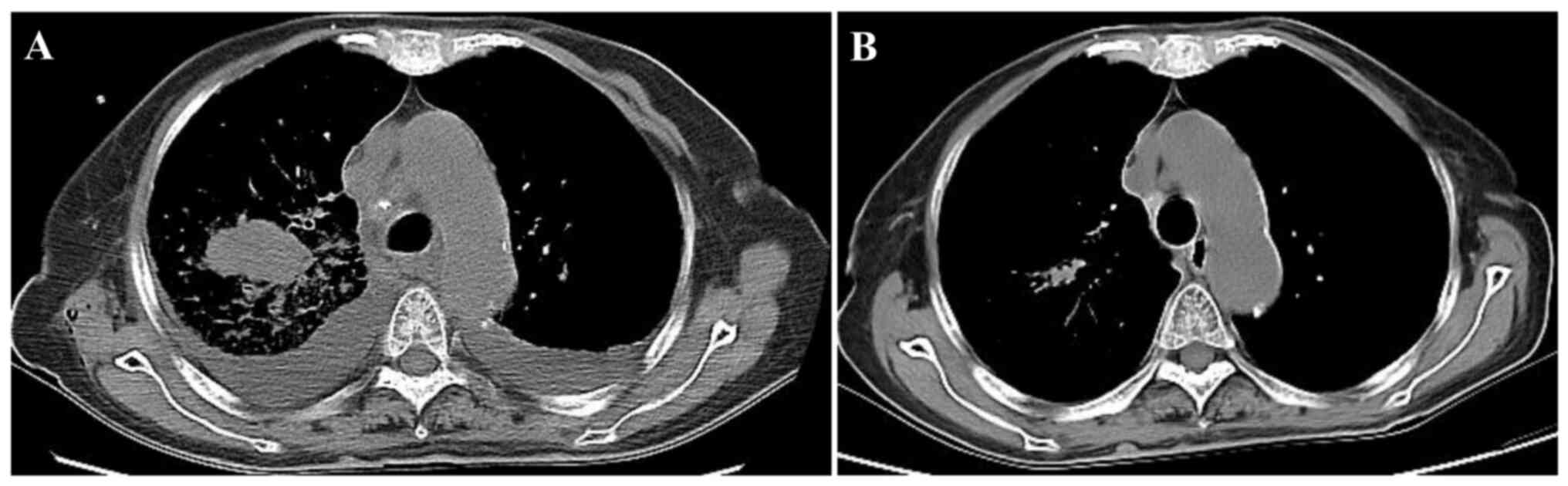
in the upper lobe of the right lung with a rough margin. Chest CT
showed a solid mass with a size of 2.1×3.9×3.0 cm in the upper lobe
of the right lung, with a CT value of 56 HU. Multiple enlarged
lymph nodes with a short diameter of 1.4 cm were observed in the
mediastinum and bilateral pleural effusion was found (Fig. 1A). Thoracentesis cytology using
hematoxylin and eosin staining showed that lymphocytes and
mesothelial cells were predominant and there was no evidence of
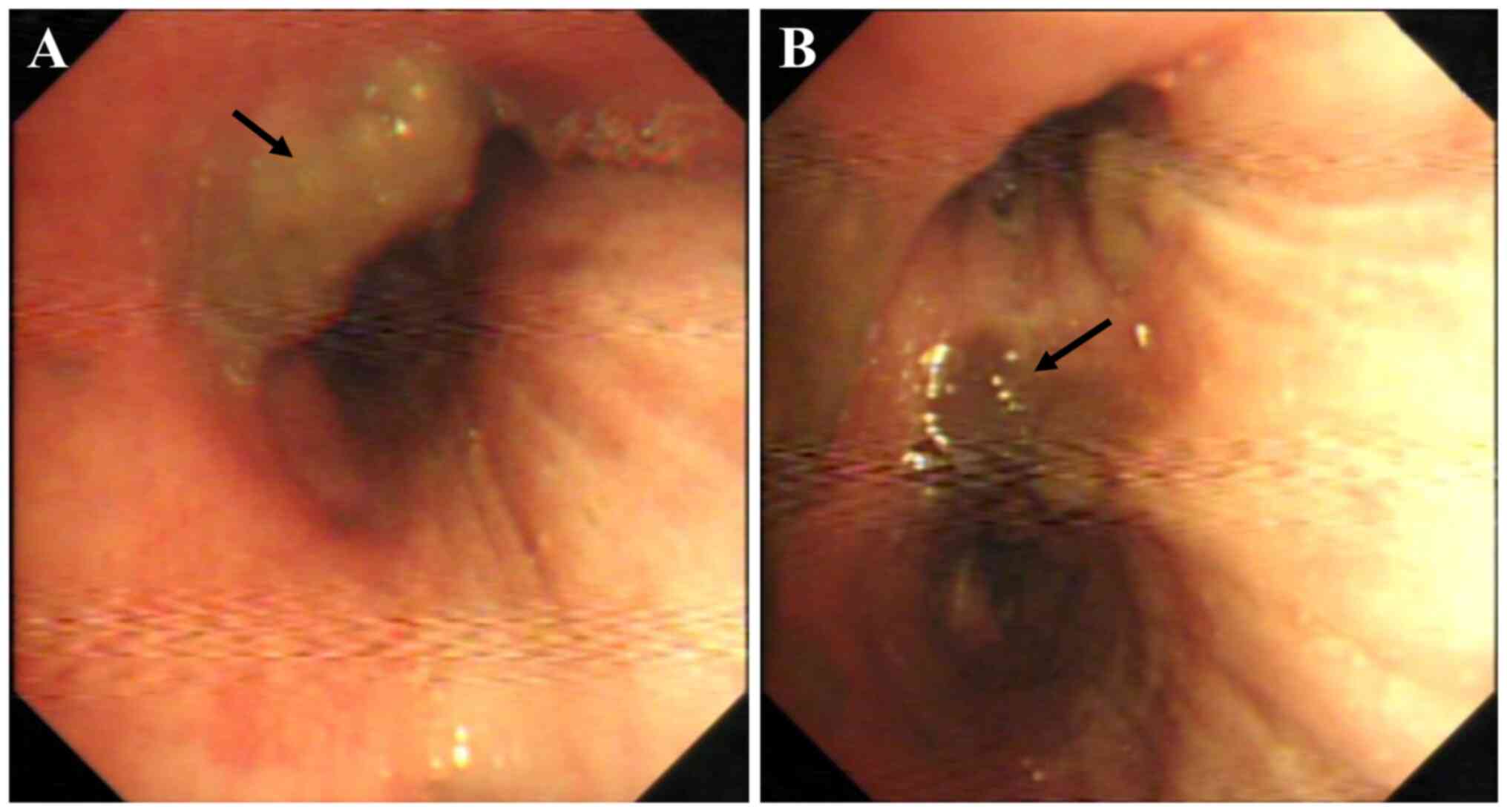
malignancy. Bronchoscopy showed an endobronchial mass in the right
main bronchus (Fig. 2A), and the
local mucosa in the distal bronchus of the right upper lobe was
rough, hypertrophic and gray-black (Fig. 2B). Transbronchial forceps biopsy of
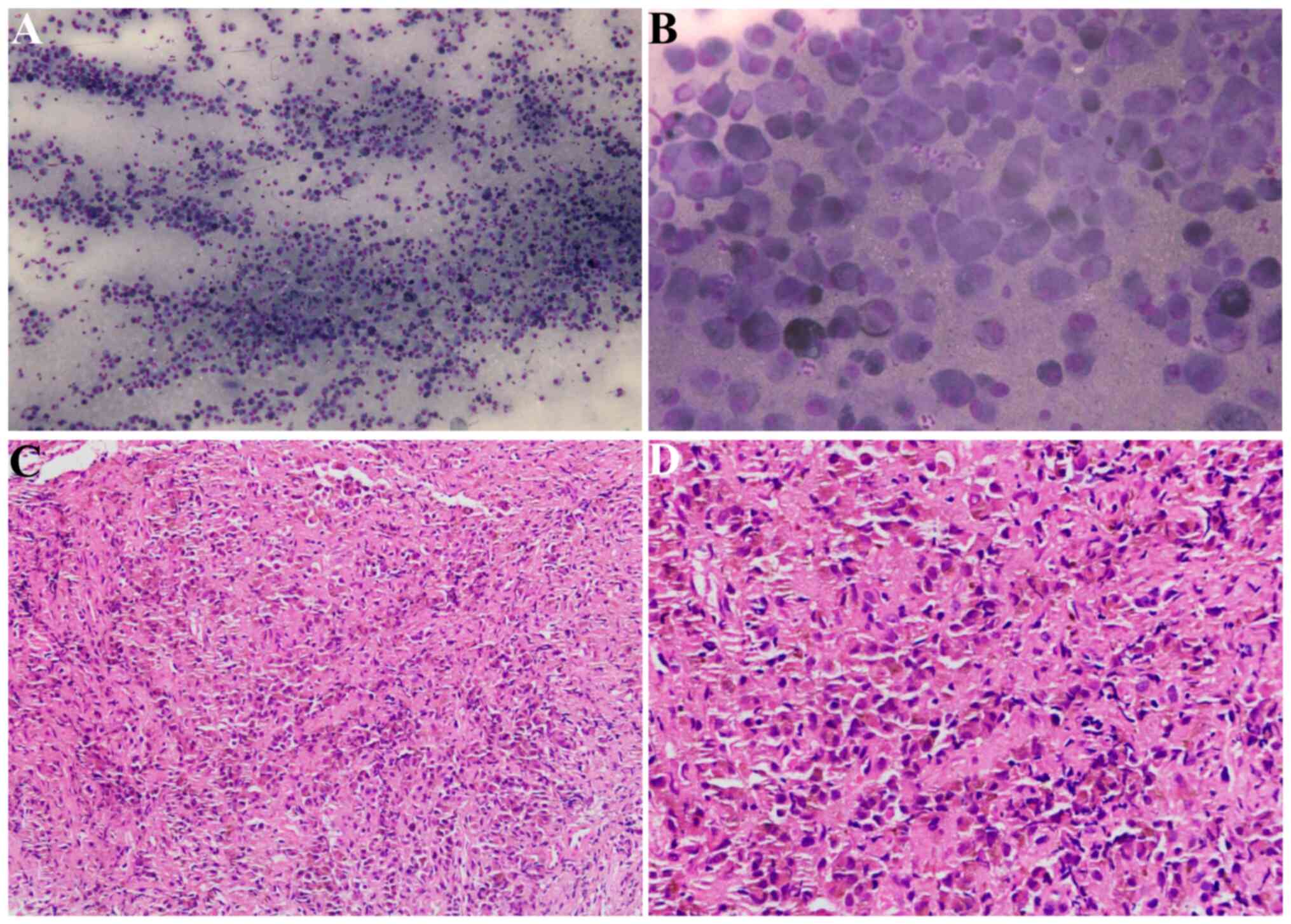
the mass was performed and rapid on-site evaluation (ROSE)
identified this as MM (Fig. 3A and
B). Based on the preliminary ROSE diagnosis, endobronchial
ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) was
performed directly for staging of the lung cancer during the
procedure.
Biopsy samples were fixed in 10% neutral buffered
formalin at room temperature for 4–6 h, dehydrated using an
increasing alcohol series, embedded in paraffin and sectioned into
3-µm slices. Hematoxylin and eosin staining was performed at room
temperature for 40 min and sections imaged using a CX31 light
microscope (Olympus Corporation). Imaging demonstrated that the
tumor cells were distributed in sheets and grew infiltratively; the
tumor cells were accompanied by a small number of inflammatory
cells in the interstitium. The tumor cells showed epithelioid or
histiocytoid changes, with abundant cytoplasm and round, oval or
irregular nuclei. Certain cells showed nucleoli or intracellular
pigment particles (Fig. 3C and
D).
Immunohistochemistry was performed using the
aforementioned biopsy sections. Sections were heated at 65°C for
120 min and then dewaxed by incubation with xylene for 3 min (six
times). Sections were rehydrated using a decreasing alcohol series
and antigen repair was performed using EDTA repair solution (pH
9.0; Fuzhou Maixin Biotech Co., Ltd.) by heating in a pressure
cooker to boiling and then being kept warm for 20 min. Sections
were incubated with 3% H2O2 for 10 min and
then rinsed with PBS for 3 min to block endogenous peroxidase
activity. Sections were incubated with ready-to-use primary
antibodies against HMB45 (cat. no. MAB-0098; Fuzhou Maixin Biotech
Co., Ltd.), SOX-10 (cat. no. MAB-0726; Fuzhou Maixin Biotech Co.,
Ltd.), S-100 (cat. no. Kit-0007; Fuzhou Maixin Biotech Co., Ltd.),
Melan-A (cat. no. JY-0083; Dako; Agilent Technologies, Inc.) and
Ki-67 (cat. no. JY-0222; Dako; Agilent Technologies, Inc.) for 1 h
at room temperature. Washing was performed using PBS. Sections were
then incubated with EnVision Detection Systems Peroxidase/DAB,
Rabbit/Mouse (cat. no. K5007; ready-to-use; Dako; Agilent
Technologies, Inc.) secondary antibodies for 30 min at room
temperature. DAB from the aforementioned secondary staining kit was
added for detection. Sections were counterstained with hematoxylin
for 2 min at room temperature and imaged using a CX31 light
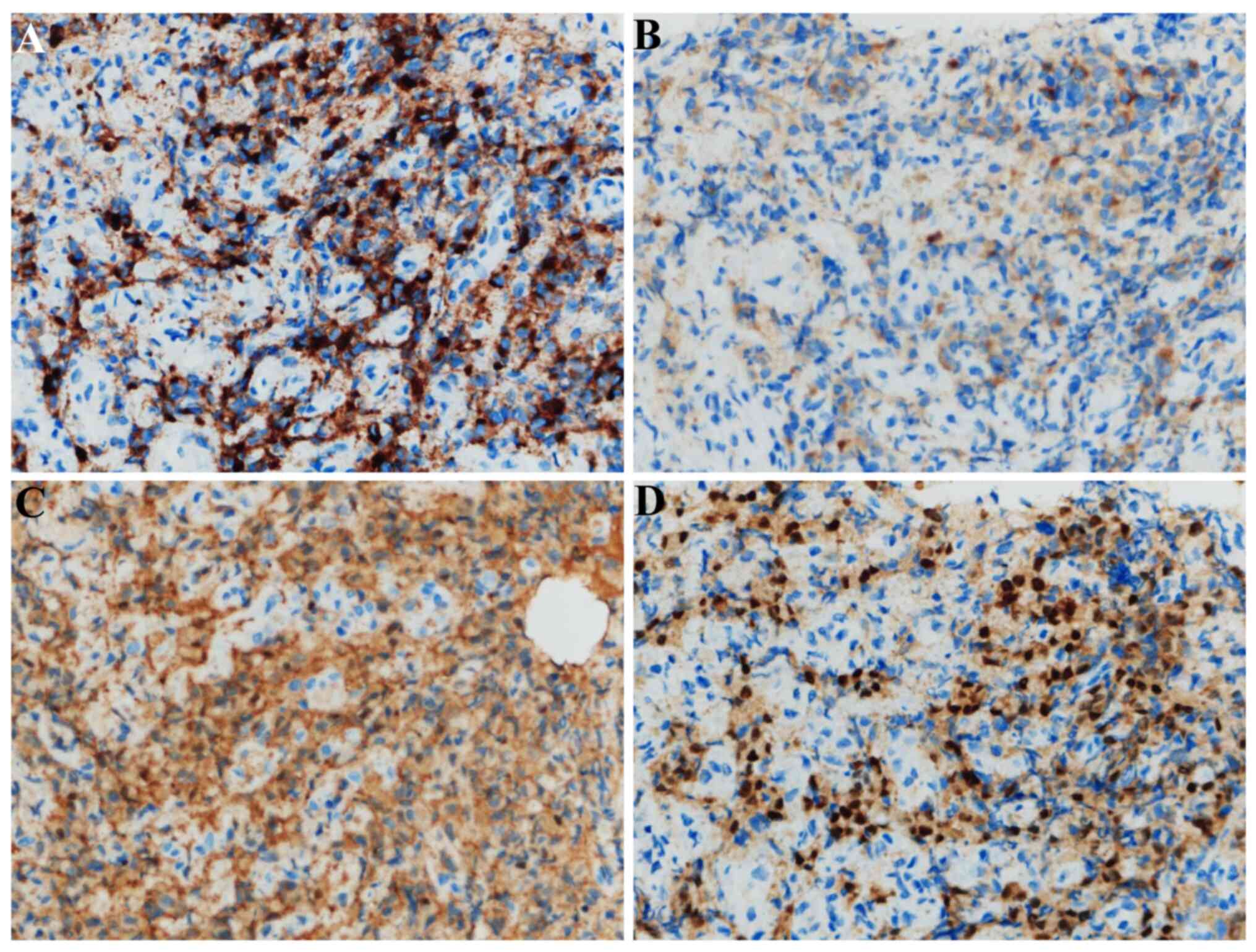
microscope (Olympus Corporation). Immunostaining revealed melanin
pigmentation and malignant cells that were positive for HMB-45,
Melan-A, SOX-10, S-100 and Ki-67 (>20%) and negative for
CKAE1/AE3 (cat. no. JY-0047), CK5/6 (cat. no. MAB-0744), TTF-1
(cat. no. MAB-0677), CD-45 (cat. no. Kit-0024) and synaptophysin
(cat. no. MAB-0742) (all ready-to-use; Fuzhou Maixin Biotech Co.,
Ltd.) (Fig. 4). Whole-body
18F-FDG PET-CT revealed a density anomaly in the mass in
the right upper lung complicated by elevated glucose metabolism
with a maximum standardized uptake value (SUVmax) of 24.7 and
elevated glucose metabolism of the hilar/mediastinum lymph nodes
with an SUVmax of 15.9 (Fig. 1B).
There were multiple metastatic lesions in the kidney, liver and
bones. On the 10th day following admission, genetic molecular
testing for BRAF or NRAS mutations using PCR-Sanger
sequencing was performed by the Clinical Molecular Diagnostic
Center of Taihe Hospital, Hubei University of Medicine, and a
BRAF V600E mutation was reported (Table I). Dabrafenib (150 mg orally twice
daily) plus trametinib (2 mg orally once daily) was subsequently
administered, and following 2 months of therapy, a chest
contrast-enhanced CT scan revealed that the malignant melanoma mass
in the right lung had markedly reduced in size (Fig. 5). The patient was followed up, 15
months to date, and remained in good health with no evidence of
recurrence. However, close follow-up of the patient is ongoing.
 | Table I.Results of genetic testing. |
Table I.
Results of genetic testing.
| Genetic testing
target | Items | Result |
|---|
| BRAF | V600E | Mutation |
| NRAS | Q61R, G12V and
G12D | No mutation |
Discussion
Melanoma usually occurs on the skin, mucous
membranes and eyeball but rarely outside cutaneous areas (6). In cases of MM in the lung, most (87%)
are metastatic MM from cutaneous lesions, and PMML is extremely
rare, accounting for only 0.01% of lung tumors (2). Therefore, prior to the diagnosis of
primary melanoma of the lung, it must be demonstrated that there is
no primary lesion at more common primary sites (6). As with the present case, it has been
previously reported that PMML may present as a bronchial mass and,
a literature review reported that from 13 patients diagnosed with
PMML by bronchoscopy, 8 had tumors detected on examination. In
addition, 16 of 20 patients were found to have endobronchial tumor
spread during surgery or autopsy (7).
There have been no significant differences reported
in PMML incidence by sex and the mean age at diagnosis reported in
the literature was 51 years (45–71 years) (2). Clinical symptoms are nonspecific,
characterized by local symptoms such as cough, sputum and chest
pain, as well as systemic symptoms such as weight loss, night
sweats and fever. In the present study, chest CT of the patient
showed an irregular lobulated mass in the right upper lobe of the
lung, which was not distinguishable from images of lung cancer.
Histologically, some of the tumor cells were arranged like acini,
some were arranged like clumps, and the cells were epithelioid with
obvious atypia, abundant cytoplasm and vacuolated nuclei, usually
with obvious nucleoli. Mitotic figures were common and melanin
granules were usually seen in the tumor cells (2). Immunohistochemical staining was
positive for HMB45, S-100, vimentin and Melan-A. HMB45 is a
specific antigen for MM and positivity for HMB45 can be used to
confirm the diagnosis of MM (8,9).
However, a subset of MM cases are negative for HMB45, mainly in
metastatic settings, and the marker is not entirely specific
(10). Melan-A and vimentin are
highly sensitive but less specific than HMB45 (11). To date, to the best of our
knowledge, this case is the only one reported which used real-time
diagnosis by ROSE. The cytomorphology of malignant melanomas
presents as dispersed or solid aggregates of highly pleomorphic
cells with large nucleoli that usually contain melanin pigment
(12). Ronchi et al
(13) stated that when present in
the appropriate morphological background, for example,
morphological features of malignant melanoma metastasis on direct
smears are shown, including large epithelioid cells with
round-to-oval nuclei and abundant, sometimes microvacuolized
cytoplasm; discohesive plasmocytoid cells with round nuclei and
dense cytoplasm, well defined borders, and evident nucleoli;
numerous spindle-shaped cells with oval-to-fusiform nuclei and
evident cytoplasmatic projections; a small cell component with
inconspicuous nucleoli and scarce cytoplasm mixed with a larger
cellular component; and malignant multinucleated cells mixed with
an epithelioid and plasmocytoid cell population, melanin
pigmentation is an important cytomorphologic indicator for the
diagnosis of MM. In addition, MMs with an epithelioid
cytomorphology represent >70% of all cases and are characterized
by the presence of cells with a polygonal shape,
moderate-to-abundant granular or clear cytoplasm, indistinct
cytoplasmatic borders and mildly or moderately hyperchromatic large
nuclei with granular and clumped chromatin (4,10)
Therefore, the diagnosis of PMML was established. Due to the rarity
of this disease, it is easily missed by clinicians, radiologists
and pathologists. In addition, melanin granules in the cytoplasm
may not be identified in the pathological examination performed
using the microscope, so it is easily misdiagnosed as small cell
lung cancer, poorly differentiated squamous cell carcinoma or
poorly differentiated adenocarcinoma. PMML needs to be
differentiated from other conditions, such as pulmonary malignant
lymphoma, undifferentiated carcinoma, stromal sarcoma and plasma
cell sarcoma. It has been previously suggested that in clinical
practice, immunohistochemical detection should be routinely
performed for patients with rapid tumor progression and a lack of
typical characteristics to prevent a missed diagnosis or
misdiagnosis (4).
The optimal treatment for patients with PMML remains
to be determined. For patients whose lesions are confined to the
lung, lobectomy or pneumonectomy with lymph node dissection remains
the first choice of treatment for PMML patients (14). In cases where surgery is not
possible or not desired by the patient, chemotherapy, radiation and
immunotherapy may be considered. The conventional preferred
chemotherapy agent is dacarbazine, which is usually used in
combination with immunotherapies such as interleukin-2 or
interferon (15). Robert et
al (16) performed randomized
trials and reported that first-line treatment with dabrafenib plus
trametinib led to long-term benefits in ~1/3 of patients who had
unresectable or metastatic melanoma with a BRAF V600E or
V600K mutation. Moreover, in two independent phase 3 trials
(COMBI-d and COMBI-v) (17,18), treatment with the BRAF inhibitor
dabrafenib (150 mg twice daily) plus the MEK inhibitor trametinib
(2 mg once daily) improved overall survival in patients with
unresectable or metastatic melanoma with BRAF V600E or V600K
mutations (72% overall survival rate at 12 months). As in the
aforementioned randomized trial, after 2 months of treatment, the
mass in the upper lobe of the right lung and the mediastinal lymph
node lesions in the present patient had almost disappeared, thus
dabrafenib plus trametinib demonstrated therapeutic efficacy.
PMML is extremely rare and is easily misdiagnosed as
lung cancer due to its nonspecific clinical manifestations and
imaging features. The diagnosis of PMML remains challenging due to
its morphologic and immunophenotypic variability. Targeted therapy
is a good option for patients with advanced PMML with BRAF
V600E mutations.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the
current study are not publicly available to protect the patient's
privacy but are available from the corresponding author on
reasonable request.
Authors' contributions
HW and TR executed the conception or design of the
written study. HW and JC drafted the manuscript and performed the
acquisition, analysis or interpretation of data for the study. MW
and TR made contributions to the interpretation of the data for the
work, and revised the manuscript critically for important
intellectual content. DL and SL collected pathological data from
the patient. XW and YL assisted with updating the patient follow-up
information and the literature search. TR and HW confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The publication of the study was approved by Taihe
Hospital Ethics Committee and performed in accordance with the
principles of Good Clinical Practice following the Tri-Council
guidelines.
Patient consent for publication
Written informed consent was obtained from the
patient for anonymized information to be published in this
article.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kim CI, Hwang SM, Park EB, Won CH and Lee
JH: Computer-aided diagnosis algorithm for classification of
malignant melanoma using deep neural networks. Sensors (Basel).
21:55512021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wilson RW and Moran CA: Primary melanoma
of the lung: A clinicopathologic and immunohistochemical study of
eight cases. Am J Surg Pathol. 21:1196–1202. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jensen OA and Egedorf J: Primary malignant
melanoma of the lung. Scand J Respir Dis. 48:127–135.
1967.PubMed/NCBI
|
|
4
|
Pan XD, Zhang B, Guo LC, Gu DM, Mao YQ, Li
J, Xie Y and Wang L: Primary malignant melanoma of the lung in the
elderly: Case report and literature review. Chin Med J (Engl).
123:1815–1817. 2010.PubMed/NCBI
|
|
5
|
Dountsis A, Zisis C, Karagianni E and
Dahabreh J: Primary malignant melanoma of the lung: A case report.
World J Surg Oncol. 1:262003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Allen AC and Spitz S: Malignant melanoma;
a clinicopathological analysis of the criteria for diagnosis and
prognosis. Cancer. 6:1–45. 1953. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ost D, Joseph C, Sogoloff H and Menezes G:
Primary pulmonary melanoma: Case report and literature review. Mayo
Clin Proc. 74:62–66. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Allen MS Jr and Drash EC: Primary melanoma
of the lung. Cancer. 21:154–159. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Alghanem AA, Mehan J and Hassan AA:
Primary malignant melanoma of the lung. J Surg Oncol. 34:109–112.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Boulaiz H, Prados J, Melguizo C, Marchal
JA, Carrillo E, Peran M, Rodríguez-Serrano F, Martínez-Amat A, Caba
O, Hita F, et al: Tumour malignancy loss and cell differentiation
are associated with induction of gef gene in human melanoma cells.
Br J Dermatol. 159:370–378. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Achilles E and Schröder S: Positive
cytokeratin results in malignant melanoma. Pitfall in differential
immunohistologic diagnosis of occult neoplasms. Pathologe.
15:235–241. 1994.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Geijer M and Domanski HA: Image-guided
fine-needle aspiration cytology. Atlas of fine needle aspiration
cytology. Domanski HA: Springer International Publishing; pp.
pp43–55, Cham. 2019, View Article : Google Scholar
|
|
13
|
Ronchi A, Montella M, Zito Marino F,
Argenziano G, Moscarella E, Brancaccio G, Ferraro G, Nicoletti GF,
Troiani T, Franco R and Cozzolino I: Cytologic diagnosis of
metastatic melanoma by fna: A practical review. Cancer Cytopathol.
130:18–29. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Peng J, Han F, Yang T, Sun J, Guan W and
Guo X: Primary malignant melanoma of the lung: A case report and
literature review. Medicine (Baltimore). 96:e87722017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gong L, Liu XY, Zhang WD, Zhu SJ, Yao L,
Han XJ, Lan M, Li YH and Zhang W: Primary pulmonary malignant
melanoma: A clinicopathologic study of two cases. Diagn Pathol.
7:1232012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Robert C, Grob JJ, Stroyakovskiy D,
Karaszewska B, Hauschild A, Levchenko E, Chiarion Sileni V,
Schachter J, Garbe C, Bondarenko I, et al: Five-year outcomes with
dabrafenib plus trametinib in metastatic melanoma. N Engl J Med.
381:626–636. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Long GV, Stroyakovskiy D, Gogas H,
Levchenko E, de Braud F, Larkin J, Garbe C, Jouary T, Hauschild A,
Grob JJ, et al: Combined braf and mek inhibition versus braf
inhibition alone in melanoma. N Engl J Med. 371:1877–1888. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Robert C, Karaszewska B, Schachter J,
Rutkowski P, Mackiewicz A, Stroiakovski D, Lichinitser M, Dummer R,
Grange F, Mortier L, et al: Improved overall survival in melanoma
with combined dabrafenib and trametinib. N Engl J Med. 372:30–39.
2015. View Article : Google Scholar : PubMed/NCBI
|