Introduction
In recent years, the incidence of oncological
patients has risen in line with the increase in the elderly
population. Advances in early diagnosis and the progress of new
treatments for various types of cancer, such as immunotherapy or
targeted molecular therapies, and the development of support
treatment have improved prognosis and increased survival in these
patients, achieving an acceptable quality of life. As a result, the
number of cancer patients requiring ICU admission is rising, be it
for management of tumour-related complications or due to the side
effects of cancer treatment or medical conditions independent of
the cancer itself (1–6). It is widely accepted that admitting
oncological patients to the ICU is usually futile and costly in
terms of recovery (both short and long term), with a worse
prognosis and a higher mortality rate than critically ill
non-cancer patients; therefore, the indication of their admission
to the ICU has been questioned. However, recent studies have
reported that the current prognosis for the critically ill cancer
patients has significantly improved (6–9).
Therefore, in view of the increase in life expectancy among these
patients, studies investigating clinical factors predicting the
short-term prognosis of cancer patients with critical complications
are needed in order to guide the admission criteria and design new
management strategies in the ICU (10–14).
In addition, most of the studies of prognosis in critical care
units have included both patients with solid tumours and patients
with haematological malignancies, and this heterogeneity in terms
of the nature and curability of the neoplasm has limited the
validity of the results (6,12,15–19).
For this reason, we carried out an analysis of patients with solid
tumours admitted to a university hospital ICU in Spain, in order to
determine their characteristics and outcomes and to identify the
risk factors associated with in-hospital mortality. Unlike other
studies, we also included a 12-month follow-up period after
hospital discharge to assess the survival and functional status of
these patients.
Patients and methods
Study design
A retrospective observational study conducted in the
18-bed ICU at the University Hospital del Mar in Barcelona, Spain,
between January 2016 and June 2018.
Adult patients (≥18 years old) admitted to the ICU
due to acute illness with the diagnosis of active solid tumour
(defined as cancer diagnosis in the five years prior to ICU
admission). Patients with haematological malignancy were excluded.
To assess in-hospital mortality, in patients with multiple ICU
admissions only the last admission was recorded.
The following information was recorded: Demographic
data, comorbidities, body mass index (BMI), the healthcare service
of origin and health functional status, using the Eastern
Cooperative Oncology Group (ECOG) scale to quantify patients'
general well-being. Variables related to tumour status were also
recorded, namely site of the primary malignancy, local or
metastatic extension, disease status at the time of ICU admission
by radiological assessment two months prior to admission
[non-progressive or progressive disease, no evidence of relapse or
recent diagnosis (during admission)] and antineoplastic therapy
received. The reason for ICU admission and the treatment received
in the ICU were also recorded [vasoactive support, ventilatory
support (including invasive mechanical ventilation and non-invasive
ventilatory support such as non-invasive mechanical ventilation or
high-flow nasal cannula), renal replacement therapy, blood product
transfusion, parenteral nutrition and tracheostomy, as well as the
need for surgery (scheduled or urgent)] during hospital admission.
The severity of the underlying disease and the number of organ
failures were calculated on the first day of admission according to
the SOFA and Acute Physiology and Chronic Health Evaluation II
(APACHE II) scores. At the time of admission, laboratory data and
vital signs (mean blood pressure, heart rate, temperature,
respiratory rate, gasometric data, lactate, hemogram, sodium,
C-reactive protein, procalcitonin, creatinine, calcium, albumin,
bilirubin, glucose, neutrophils and prothrombin time) were
collected. Furthermore, the sequential SOFA was also recorded at 48
h and on days 5, 10 and 14. Both ICU and hospital length of stay,
ICU and in-hospital mortality rate, as well as the cause of death
[septic, respiratory, haemorrhagic, cardiac, brain death or
limitation of therapeutic effort (LTE)] were recorded. The LTE
decision was made in accordance with the ICU protocol, which was
indicated after verifying that the patient's clinical situation was
irreversible or terminal, always by consensus of the intensive care
team and with the participation of the patient and relatives.
Finally, the oncology team followed up the surviving patients after
hospital discharge and collected treatment data and functional
status after two months. Further follow-up controls were carried
out at 6 and 12 months to be able to assess patients' survival and
functional status.
The study was conducted in accordance with the
ethical principles of the Declaration of Helsinki, and was approved
by the Ethics Committee of Hospital del Mar (approval no.
2022/10570).
Statistical analysis
Continuous variables were described through means
and standard deviations, and comparisons between groups were
assessed through the unpaired Student's t-test. Levene's test was
used to check group homoscedasticity. Qualitative variables were
described as frequencies (number and percentage) and comparisons
were assessed through χ2 or Fisher exact test, as
appropriate. The McNemar test was used to check changes in ECOG
score in the follow-up after hospital discharge in comparison with
the score at ICU admission, and during the follow-up after hospital
discharge. Univariate and multivariate Cox regression was used in
the analysis of the variables related to in-hospital mortality.
Results of these analyses were expressed by means of hazard ratios.
The variables associated with higher risk of mortality (P<0.05)
in the univariate analysis were used in the multivariate analysis,
controlling for collinearity (linear relations between explanatory
covariates) and the clinical significance of these covariates. The
proportional hazard assumption, checked by examining Schoenfeld
residuals (for the model overall and variable by variable), was not
violated. A post-hoc power analysis was performed for in-hospital
mortality and taking as main factor of interest the SOFA score at
admission. Regarding this variable, a threshold of 7 (approximately
the mean in the non-survivor group) was used to perform a Kaplan
Meier survival curve comparing both groups (above and below
threshold). Log-rank test was used to check differences between
survival curves.
STATA version 15.1 (StataCorp, College Station, TX,
USA) was used for statistical analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
Characteristics of the study
population
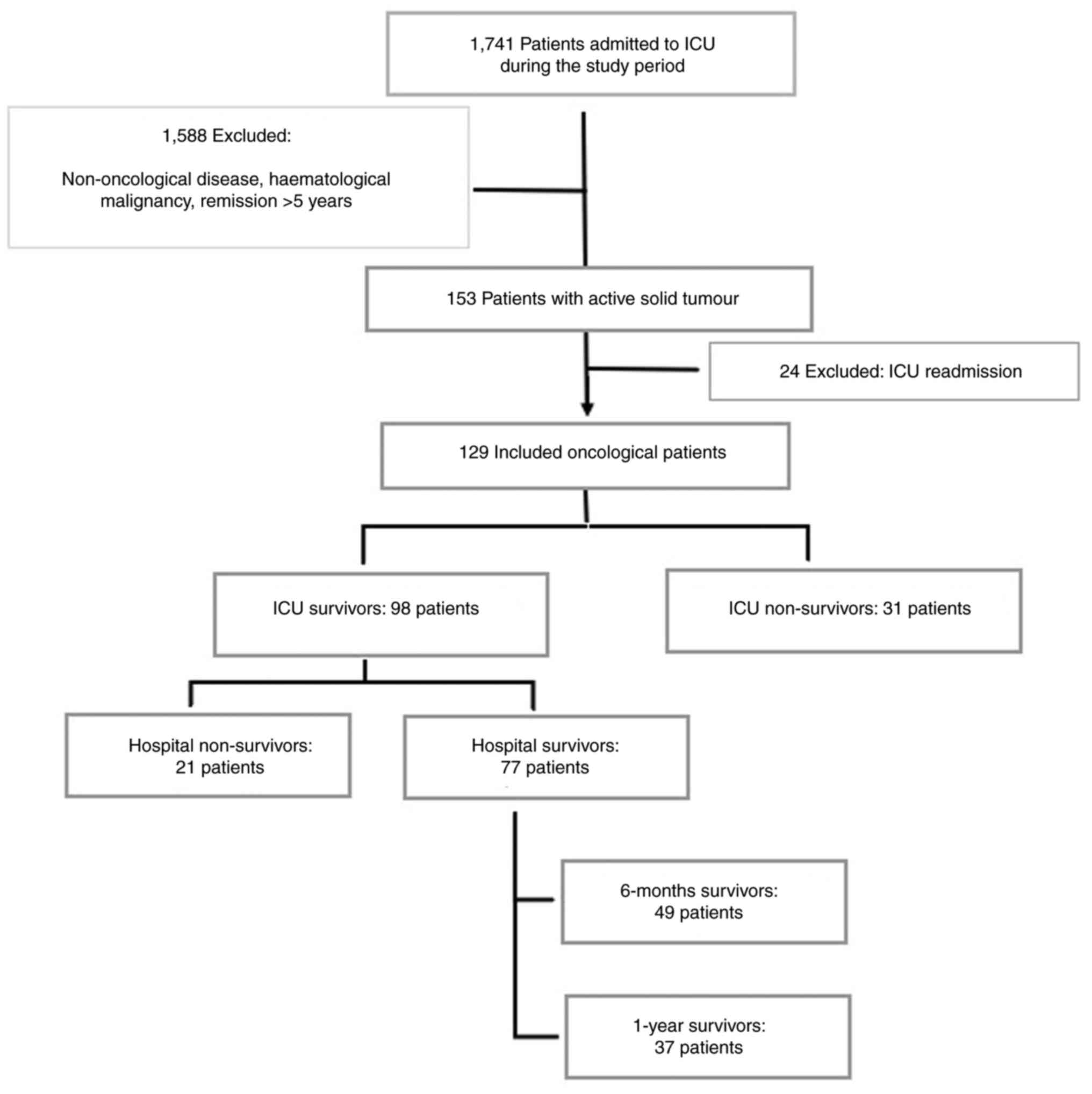
A total of 1,741 patients were recruited during the
study period, of whom 129 (7.4%) had an active solid tumour at ICU
admission. Patients with haematological malignancy were excluded.
Fig. 1 shows the flowchart of the
study. The main patient characteristics and malignancy-related data
are summarized in Table I.
 | Table I.Characteristics of ICU patients with
solid tumours. |
Table I.
Characteristics of ICU patients with
solid tumours.
| Variable | Value |
|---|
| Number | 129 |
| Male sex, n
(%) | 102 (79.07) |
| Mean age, years
(SD) | 66.97 (10.29) |
| Mean BMI,
kg/m2 (SD) | 26.33 (5.40) |
| Comorbidity, n
(%) |
|
| High
blood pressure | 72 (55.81) |
|
Cardiovascular
diseasea | 46 (35.66) |
|
COPD | 41 (31.78) |
|
Diabetes mellitus | 32 (24.81) |
| Chronic
kidney failure | 13 (10.08) |
|
Immunosuppressedb | 9 (6.98) |
| Healthcare service
of origin, n (%) |
|
|
Emergency service | 60 (46.51) |
|
Hospital ward | 56 (43.41) |
|
Othersc | 13 (10.08) |
| ECOG prior to ICU
admission, n (%) |
|
|
0-1 | 57 (44.19) |
| ≥2 | 26 (20.16) |
| NR | 46 (35.66) |
| Type of tumour, n
(%) |
|
|
Lung | 38 (29.46) |
|
Gastrointestinal | 37 (28.68) |
|
Genitourinary | 28 (21.71) |
|
Gynaecological and breast | 14 (10.85) |
|
ENT | 13 (10.08) |
| Central
nervous system | 2 (1.55) |
| Other
tumoursd | 3 (2.33) |
| Oncological
assessment prior to ICU admission, n (%)e |
|
|
Debut/first appearance | 57 (44.19) |
|
Progressive disease | 28 (21.71) |
| No
evidence of relapse | 26 (20.16) |
|
Non-progressive
diseasef | 15 (11.63) |
| NR | 3 (2.33) |
| Stage at diagnosis,
n (%)g |
|
| Located
disease | 74 (57.36) |
|
Metastatic disease | 46 (35.66) |
| NR | 9 (6.98) |
| Metastasis, n
(%) |
|
|
Hepatobiliary | 27 (20.93) |
|
Peritoneal | 24 (18.60) |
|
Bone | 18 (13.95) |
|
Pulmonary | 17 (13.18) |
|
Brain | 9 (6.98) |
|
Suprarenal | 9 (6.98) |
| Antineoplastic
therapy, n (%)e |
|
| No | 71 (55.04) |
|
Chemotherapy | 25 (19.38) |
|
Othersh | 14 (10.85) |
|
Chemotherapy +
Radiotherapy | 9 (6.98) |
|
Immunotherapy | 9 (6.98) |
| NR | 1 (0.78) |
| Reason for
intensive care unit admission, n (%) |
|
|
ARF | 51 (39.53) |
|
Shock | 50 (38.88) |
|
Septic | 36 (27.91) |
|
Hypovolemic and
cardiogenic | 9 (6.98) |
|
Anaphylactic | 5 (3.88) |
|
CPA | 10 (7.75) |
|
Surveillance and
monitoring | 10 (7.75) |
|
Coma | 4 (3.10) |
| Acute
kidney failure | 4 (3.10) |
| Life-supporting
therapies, n (%) |
|
|
Vasopressors | 63 (48.84) |
|
Invasive mechanical
ventilation | 45 (34.88) |
|
Non-invasive mechanical
support | 43 (33.33) |
| Blood
transfusion | 36 (27.91) |
|
Tracheostomy | 17 (13.19) |
|
Parenteral nutrition | 15 (11.63) |
| Renal
replacement therapy | 10 (7.75) |
| Surgical
intervention | 38 (29.46) |
| Median ICU stay,
days (IQR) | 5 (3–10) |
| ≤48
h | 24 (18.60) |
| >48
h | 105 (81.40) |
| Median hospital
stay, days (IQR) | 20 (11–40) |
| Mean severity
scores SD |
|
| APACHE
II | 22.99 (8.34) |
|
SOFA0 | 5.81 (3.60) |
|
SOFA48 h | 3.76 (3.40) |
|
SOFA5 D | 3.17 (2.78) |
Most patients were men (79%) and mean age was 67
years. The most frequent comorbidities at the time of admission
were hypertension (55.8%), cardiovascular diseases (35.7%), chronic
obstructive pulmonary disease (31.8%) and diabetes mellitus
(24.8%). Most patients were admitted to our medical ICU from the
emergency service (46.5%) or from a hospital ward (43.4%).
Fifty-seven patients had good functional health status according to
ECOG score (ECOG 0–1) before ICU admission. The most common reasons
for ICU admission were acute respiratory failure (ARF) (39.5%) and
shock (38.9%), especially septic shock (27.9%).
Lung tumours were the most frequent (29.5%) followed
by gastrointestinal (28.7%) and genitourinary cancer (21.7%).
Seventy-four patients (57.4%) had localized disease on ICU
admission, while 46 (35.7%) had metastasis, the most frequent being
hepatobiliary (20.9%), peritoneal (18.6%), bone (14%) and pulmonary
(13.2%). In most patients the cancer onset coincided with the time
of ICU admission (44.2%), while 21.7% of patients had progressive
and 11.6% non-progressive disease. Finally, 20.2% of patients had
no evidence of relapse during ICU admission. Fifty-seven patients
(44.1%) had received antineoplastic therapy within the two months
before ICU admission, while 71 (55%) had not received
cancer-specific treatment in this time period.
During the ICU stay most patients received
life-supporting therapies, such as vasopressors (48.8%), mechanical
ventilation (either invasive or non-invasive ventilatory support,
68.2%), renal replacement therapy (7.8%) and parenteral nutrition
(11.6%). Thirty-eight patients (29.5%) required surgery during
hospital admission (57.9% urgent and 42.1% scheduled). The
characteristics and reasons for surgery are found in Table SI.
The mean APACHE II and SOFA severity scores at ICU
admission were 23 (SD 8.3) and 5.8 (SD 3.6) respectively. The mean
APACHE II scores were higher in cancer patients than in non-cancer
patients admitted to the ICU in the same period of time [22.99 (SD
8.34) vs. 18.81 (SD 10.28), P<0.001].
The median duration of ICU stay was five days (IQR,
3–10), and more than 80% of cancer patients had an ICU stay of more
than 48 h. The median duration of hospital stay was 20 days (IQR,
11–40).
Finally, 21 patients (16.28%) were readmitted to the
ICU, 14 (10.85%) during the same hospital admission. Three cases
were readmitted to ICU up to three times.
Outcome and prognostic factors. Of the 129 patients,
52 died during their hospital stay (hospital mortality rate 40.3%),
31 of them in the ICU, with an ICU mortality rate of 24% (18 due to
LTE). Table II displays the
characteristics of the cancer survivors and non-survivors.
 | Table II.Characteristics of hospital survivor
and non-survivor cancer patients. |
Table II.
Characteristics of hospital survivor
and non-survivor cancer patients.
| Variable | Survivors
(n=77) | Non-survivors
(n=52) | HR (95% CI) | P-value |
|---|
| Male sex, n
(%) | 62 (80.52) | 40 (76.92) | 0.75
(0.39–1.43) | 0.376 |
| Mean age, years
(SD) | 66.19 (9.54) | 68.12 (11.31) | 1.00
(0.97–1.03) | 0.992 |
| Mean BMI,
kg/m2 (SD) | 25.93 (5.19) | 26.98 (5.74) | 1.04
(0.98–1.10) | 0.161 |
| Comorbidity, n
(%) |
|
|
|
|
| High
blood pressure | 41 (53.25) | 31 (59.62) | 0.97
(0.56–1.70) | 0.923 |
|
Cardiovascular disease | 27 (35.06) | 19 (36.54) | 0.93
(0.53–1.63) | 0.792 |
|
COPD | 20 (25.97) | 21 (40.38) | 1.24
(0.71–2.17) | 0.442 |
|
Diabetes mellitus | 15 (19.48) | 17 (32.69) | 1.50
(0.84–2.68) | 0.17 |
| Chronic
kidney failure | 7 (9.09) | 6 (11.54) | 0.99
(0.42–2.33) | 0.985 |
|
Immunosuppression | 2 (2.60) | 7 (13.46) | 1.72
(0.77–3.83) | 0.183 |
| ECOG prior to ICU
admission, n (%) |
|
|
|
|
|
0-1 | 34 (75.56) | 23 (60.53) | 1 |
|
| ≥2 | 11 (24.44) | 15 (39.47) | 1.06
(0.54–2.07) | 0.874 |
| Type of tumour, n
(%) |
|
|
|
|
|
Lung | 17 (22.08) | 21 (40.38) | 2.06
(1.18–3.60) | 0.011 |
|
Gastrointestinal | 21 (27.27) | 16 (30.77) | 1.04
(0.58–1.87) | 0.903 |
|
Genitourinary | 22 (28.57) | 6 (11.54) | 0.52
(0.22–1.21) | 0.13 |
|
Gynaecological and breast | 8 (10.39) | 6 (11.54) | 1.34
(0.57–3.14) | 0.502 |
|
ENT | 11 (14.29) | 2 (3.85) | 0.21
(0.05–0.87) | 0.031 |
| Central
nervous system | 0 (0) | 2 (3.85) | 1.94
(0.47–8.00) | 0.362 |
| Other
tumours | 1 (1.30) | 2 (3.85) | 1.88
(0.45–7.76) | 0.385 |
| Oncological
assessment prior to ICU admission, n (%) |
|
|
|
|
| No
evidence of relapse | 16 (21.33) | 10 (19.61) | 1 |
|
|
Non-progressive disease | 10 (13.33) | 5 (9.80) | 1.24
(0.42–3.67) | 0.7 |
|
Progressive disease | 19 (25.33) | 9 (17.65) | 0.99
(0.4–2.43) | 0.978 |
|
Debut/first appearance | 30 (40.00) | 27 (52.94) | 1.53
(0.74–3.16 | 0.252 |
| Stage at diagnosis,
n (%) |
|
|
|
|
| Located
disease | 53 (73.61) | 21 (43.75) | 1 |
|
|
Metastatic disease | 19 (26.39) | 27 (56.25) | 2.36
(1.33–4.19) | 0.003 |
| Metastasis, n
(%) |
|
|
|
|
|
Hepatobiliary | 14 (18.18) | 13 (25) | 2. 17
(1.14–4.15) | 0.019 |
|
Peritoneal | 14 (18.18) | 10 (19.23) | 2.21
(1.09–4.49) | 0.028 |
|
Bone | 9 (11.69) | 9 (17.31) | 2.03
(0.97–4.26) | 0.06 |
|
Pulmonary | 12 (15.58) | 5 (9.62) | 0.99
(0.39–2.51) | 0.982 |
|
Brain | 5 (6.49) | 4 (7.69) | 1 (0.36–2.80) | 0.994 |
|
Suprarenal | 4 (5.19) | 5 (9.62) | 2.01
(0.79–5.12) | 0.139 |
| Antineoplastic
therapy, n (%) |
|
|
|
|
| No | 41 (53.25) | 30 (58.82) | 1 |
|
|
Chemotherapy | 14 (18.18) | 11 (21.57) | 2.01
(0.98–4.12) | 0.057 |
|
Others | 10 (12.99) | 4 (7.84) | 0.51
(0.18–1.44) | 0.202 |
|
Chemotherapy +
radiotherapy | 6 (7.79) | 3 (5.88) | 0.95
(0.29–3.13) | 0.935 |
|
Immunotherapy | 6 (7.79) | 3 (5.88) | 2.13
(0.64–7.09) | 0.217 |
| Reason for ICU
admission, n (%) |
|
|
|
|
| Acute
respiratory failure | 33 (42.86) | 18 (34.62) | 0.53
(0.3–0.94) | 0.03 |
|
Shock | 32 (41.56) | 18 (34.62) | 1.08
(0.61–1.92) | 0.792 |
|
CPA | 2 (2.60) | 8 (15.38) | 2.81
(1.32–6.01) | 0.008 |
|
Surveillance and
monitoring | 6 (7.79) | 4 (7.69) | 1.53
(0.55–4.28) | 0.416 |
|
Coma | 1 (1.30) | 3 (5.77) | 1.34
(0.41–4.33) | 0.626 |
| Acute
kidney failure | 3 (3.90) | 1 (1.92) | 1.21
(0.16–8.89) | 0.85 |
| Life-supporting
therapies, n (%) |
|
|
|
|
|
Vasopressors | 29 (37.66) | 34 (65.38) | 1.91
(1.08–3.39) | 0.027 |
|
Invasive mechanical
ventilation | 18 (23.38) | 27 (51.92) | 1.67
(0.75–3.70) | 0.21 |
|
Non-invasive mechanical
support | 26 (33.77) | 17 (32.69) | 1.38
(0.59–3.21) | 0.456 |
| Blood
transfusion | 18 (23.38) | 18 (34.62) | 1.12
(0.63–1.99) | 0.689 |
|
Tracheostomy | 10 (12.99) | 7 (13.46) | 0.55
(0.24–1.22) | 0.142 |
|
Parenteral nutrition | 10 (12.99) | 5 (9.62) | 0.39
(0.15–0.98) | 0.045 |
| Renal
replacement therapy | 4 (5.19) | 6 (11.54) | 1.34
(0.57–3.16) | 0.498 |
| Surgical patients,
n (%) | 24 (31.17) | 14 (26.92) | 0.37
(0.20–0.70) | 0.002 |
| ICU stay, n
(%) |
|
|
|
|
| ≤48
h | 10 (12.99) | 14 (26.92) | 1 |
|
| >48
h | 67 (87.01) | 38 (73.08) | 0.16
(0.08–0.31) | <0.001 |
| Severity scores,
mean (SD) |
|
|
|
|
| APACHE
II | 21.16 (7.26) | 25.71 (9.13) | 1.04
(1.01–1.07) | 0.003 |
|
SOFA0 | 4.74 (2.89) | 7.40 (3.97) | 1.17
(1.09–1.26) | <0.001 |
|
SOFA48 h | 2.77 (2.58) | 5.45 (3.96) | 1.17
(1.07–1.28) | <0.001 |
|
SOFA5 D | 2 (1.82) | 5.21 (3.01) | 1.34
(1.16–1.54) | <0.001 |
| Analytic and vital
signs at ICU admission, median (IQR) |
|
|
|
|
| Mean
blood pressure, mmHg | 63 (53–80) | 61 (44–80) | 0.99
(0.98–1.00) | 0.05 |
| Heart
rate, beats/min | 110 (88–120) | 110 (80–128) | 0.99
(0.99–1.00) | 0.035 |
|
Temperature, °C | 36 (36–37) | 36 (35.8–36.5) | 0.86
(0.67–1.10) | 0.226 |
|
Respiratory rate,
breaths/min | 25 (18–31) | 26.5 (18–35) | 0.99
(0.96–1.02) | 0.513 |
|
PaO2/FiO2,
mmHg | 295 (185–380) | 205 (150–300) | 1.00
(1.00–1.00) | 0.154 |
|
Lactate, mmol/l | 2.3 (1.3–4.2) | 2.7 (1.9–5.6) | 1.07
(1.02–1.12) | 0.005 |
|
Haemoglobin level, g/dl | 9.8 (8.2–11.4) | 9.9 (8.4–11.5) | 1.06
(0.94–1.20) | 0.352 |
|
Leukocytes,
103/mm3 | 11.2
(7.7–19.5) | 14.6
(10.6–23.8) | 1.01
(0.99–1.04) | 0.303 |
|
Thrombocytes
(105/mm3) | 2.2 (1.3–3) | 2.1 (1.3–2.8) | 0.74
(0.58–0.94) | 0.013 |
|
Procalcitonin, ng/ml | 1.5 (0.3–6.3) | 1 (0.3–15) | 1.01
(1.00–1.02) | 0.127 |
|
C-reactive protein, mg/l | 12.2 (3.6–24) | 11.1
(5.8–28.3) | 1.01
(0.99–1.03) | 0.219 |
|
Creatinine, mg/dl | 1.1 (0.7–1.9) | 1.2 (0.7–1.9) | 1.06
(0.94–1.19) | 0.376 |
|
Albumin, g/dl | 3 (2.4–3.6) | 3.1 (2.5–3.3) | 1.38
(0.83–2.29) | 0.21 |
|
Calcium, mg/dl | 8.3 (7.8–8.9) | 8.2 (7.7–8.7) | 0.95
(0.72–1.25) | 0.725 |
|
Bilirubin, mg/dl | 0.4 (0.3–0.7) | 0.6 (0.4–1.3) | 1.20
(1.04–1.38) | 0.012 |
|
Glucose, mg/dl | 132 (113–170) | 154.5
(120–268) | 1.01
(1.00–1.01) | <0.001 |
|
Prothrombin time, % | 81.2 (59–97.6) | 72 (54.6–86.5) | 0.99
(0.98–1.00) | 0.008 |
| Neutropenia,
<1,000/mcl | 5 (6.49) | 5 (9.62) | 4.45
(1.69–11.72) | 0.003 |
Among the demographic and cancer-associated
variables, statistical analysis revealed that gender, age, BMI,
comorbidities, ECOG at ICU admission, oncological assessment and
antineoplastic therapy were not associated with a worse prognosis.
On the other hand, high severity-scores (APACHE II, SOFA) and
neutropenia were related to a higher mortality rate. Patients with
higher SOFA at ICU admission were more likely to die during
hospital stay (P=0.013; Fig.
S1).
The length of ICU stay was significantly shorter in
non-survivors than in survivors. Mortality was significantly
associated with metastatic disease, lung tumour and the need for
vasopressors during the ICU stay. Cardiopulmonary arrest (CPA) on
ICU admission was significantly more common in non-survivors.
Bilirubin, glucose and lactate levels were significantly associated
with mortality.
ENT tumours were significantly more frequent in
survivors. Survivors were also more likely to have acute
respiratory failure as the reason for ICU admission and to require
surgery during their hospital stay. Platelet count, mean blood
pressure, heart rate and coagulation rate were significantly higher
in survivors.
Multivariate analysis
The multivariate analysis showed that the risk
factors for hospital mortality were the severity of organ failure
at admission using the SOFA score (HR, 1.22; 95% CI, 1.07–1.39;
P=0.003), neutropenia (HR, 9.16; 95% CI, 2.33–36.04; P=0.002) and
metastatic disease at ICU admission (HR, 4.23; 95% CI, 2.05–8.70;
P=0.000), as well as the need for invasive mechanical ventilation
(HR, 4.83; 95% CI, 1.43–16.26; P=0.011). In addition, the ICU stay
[>48 h] (HR, 0.13; 95% CI, 0.06–0.29; P=0.000) and the need for
surgery during hospital stay (HR, 0.22; 95% CI, 0.09–0.50; P=0.000)
were identified as good prognostic factors (Table III).
 | Table III.Factors associated with hospital
mortality in critically ill cancer patients. Results from
multivariate analysis. |
Table III.
Factors associated with hospital
mortality in critically ill cancer patients. Results from
multivariate analysis.
| Variable | HR (95% CI) | P-value |
|---|
|
SOFA0 | 1.22
(1.07–1.39) | 0.003 |
| Neutropenia | 9.16
(2.33–36.04) | 0.002 |
| ICU stay (>48
h) | 0.13
(0.06–0.29) | <0.001 |
| Stage at diagnosis
(metastatic disease) | 4.23
(2.05–8.70) | <0.001 |
| Invasive mechanical
ventilation | 4.83
(1.43–16.26) | 0.011 |
| Surgical
intervention | 0.22
(0.09–0.50) | <0.001 |
Results of the power calculation for the
multivariate model showed a power of 91.56.
Follow-up
The destination of survivors discharged from
hospital (77 patients) was home in 70% of cases, a convalescence
unit in 16.8% and a palliative care unit in 11.5%. Forty-two
discharged patients (54.5%) were evaluated by a medical oncologist
on an outpatient basis within two months of discharge, twenty-five
of whom (59.5%) presented good functional status (ECOG score
0–1).
However, comparing the evolution of the ECOG score
between ICU admission and the first oncological visit at discharge,
we observed that of the 13 patients who presented poor functional
status at hospital discharge (ECOG ≥2), nine (32.1% of the
follow-up cohort) had presented good general condition prior to ICU
admission (ECOG 0–1). They showed a statistically significant
deterioration during their ICU stay (P=0.034; Table IV).
 | Table IV.ECOG score at the first hospital
discharge visit vs. pre-ICU admission. |
Table IV.
ECOG score at the first hospital
discharge visit vs. pre-ICU admission.
| ECOG previous to
ICU admission | ECOG at hospital
discharge (within 2 months) |
|
|---|
|
|
|---|
| 0-1 | ≥2 | Total |
P-valuea |
|---|
| 0-1 | 13 (59.09) | 9 (40.91) | 22 (100) | 0.0348 |
| ≥2 | 2 (33.33) | 4 (66.67) | 6 (100) |
|
| Total | 15 (53.57) | 13 (46.43) | 28 (100) |
|
In the post-discharge follow-up, 49 survivors
discharged from hospital (63.6%) were alive at six months, and 37
(48.1%) at one year. In relation to all the 129 patients admitted
to the ICU, this represents a one-year survival rate after
discharge of 28.7%. Three cases were lost to follow-up due to a
change of country or health region. Moreover, 28 of the 49
survivors at six months (57.2%) were monitored by the oncology
service, and 23 of these 28 (82.1%) had good functional status
(ECOG 0–1). A similar trend was observed in survivors at one year,
since 18 of the 21 patients followed by the oncology service
(85.7%) presented good functional status (Table V).
 | Table V.Description of ECOG evolution in
survivors over time. |
Table V.
Description of ECOG evolution in
survivors over time.
| ECOG
classification | Discharge
(n=77) | 6 months
(n=49) | 12 months
(n=37) |
|---|
| Oncological
follow-up | 42 (54.54) | 28 (57.15) | 21 (56.76) |
| ECOG 0–1 | 25 (59.52) | 23 (82.14) | 18 (85.71) |
| ECOG 2–3 | 17 (40.48) | 5 (17.86) | 3 (14.29) |
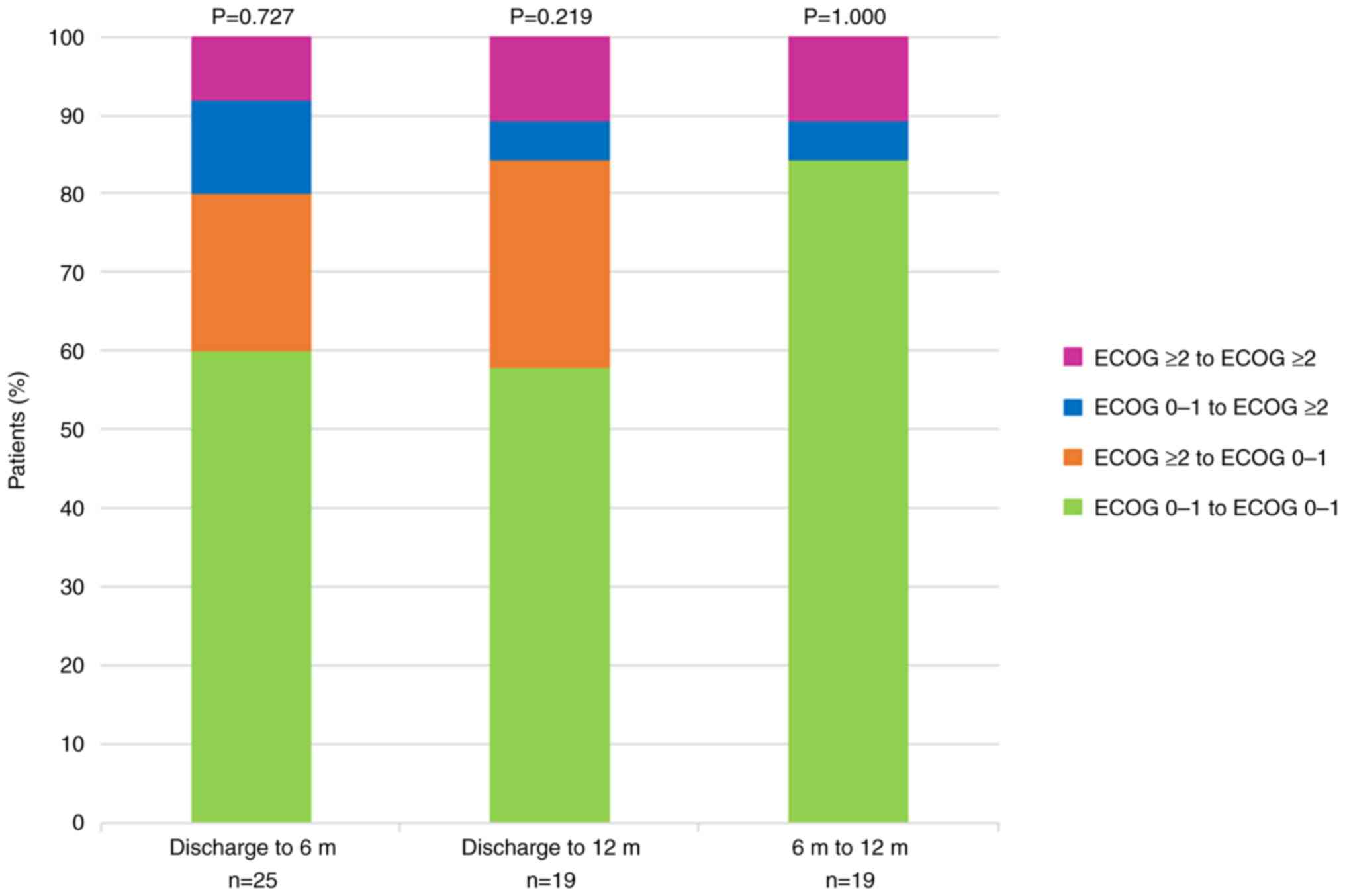
Finally, Fig. 2
shows the changes in ECOG score between different time points in
the follow-up: first oncological visit after hospital discharge,
after six months, and after 12 months. No significant changes in
functional status were observed over time.
Discussion
The main objectives of the current study were to
determine the outcome of solid cancer patients admitted to an ICU
in Spain, and to identify factors predicting in-hospital mortality.
The study also sought to analyse the evolution of these patients
inside and outside the hospital, evaluating their survival and
functional status at one year after hospital discharge.
In-hospital and ICU mortality rates were 40 and 24%
respectively. In the multivariate analysis, these rates were
negatively influenced by high SOFA score, neutropenia and
metastatic disease at ICU admission and the need for invasive
mechanical ventilation. In addition, a longer ICU stay and the need
for surgery during hospital stay were identified as protective
factors.
Our ICU mortality rate in cancer patients was 24%
and, though higher than the overall ICU mortality rate of 16.3%,
the difference was not substantial. Therefore, the diagnosis of
malignancy should not automatically contraindicate ICU admission.
Previous studies have reported ICU mortality rates in solid tumours
of between 10 and 50%. This large variation in rates between
studies makes comparisons difficult: it is due to the heterogeneity
of the cancer population, with different types of cancer and
oncologic treatments, different specific reasons for ICU admission
and differences in the implementation of end-of-life decisions
(2,10,20,21).
In the critical care setting, scoring systems for
quantifying severity of illness and organ failure such as APACHE II
or SOFA scores have proved to be valuable tools for identifying
patients at high risk for hospital mortality. So, the decision to
admit cancer patients to the ICU should be based on the severity of
the acute illness, as some authors have indicated (2,6,12,13,19,22,23).
In our study, we also found that SOFA score at ICU admission, which
identifies organ dysfunction, is one of the main prognostic factors
in critically ill patients with cancer. Previous studies have
proposed an admission and treatment modality called the ICU trial,
which consists in initial full intensive care without limitations
and mandatory daily assessments of organ failures and their
evolution during the first 3–7 days, with particular attention to
the development of multiple organ dysfunction during the ICU stay.
The ICU trial may help in making decisions in a complex situation
and in prompting early palliative and end-of-life discussions,
especially in patients who do not progress toward recovery in the
first days of ICU care, and in those in whom symptom palliation
would improve quality of life (13,18,24,25).
In our study, we observed that sequential SOFA between day 2 and
day 5 continues to be a good prognostic marker of in-hospital
mortality. It is recommended that follow-up be carried out by an
interdisciplinary team (ICU specialists, oncologists, and
palliative care specialists if appropriate). Quality of life, the
patient's wishes and the family's opinion should also be taken into
account.
As many as 80% of cancer patients are admitted to
our ICU due to shock or ARF, that is, organ failures requiring
life-supporting therapies. Lung cancer is the most common solid
tumour in our critically ill patients. ARF is one of the most
frequent reasons for ICU admission in cancer patients. There are
many possible causes of ARF, including the local effect of the
tumour, pneumonia, acute respiratory distress syndrome and
congestive heart failure. Supplemental oxygen and treatment of the
underlying disorder is the fundamental approach to ARF, but severe
cases require ventilatory support. However, despite significant
advances in ventilatory support and cancer management, numerous
studies have found invasive mechanical ventilation for more than 24
h to be associated with high mortality rates (13,18,19,22,26).
Mortality may be related to multiple factors including
complications of ventilation such as ventilator-induced lung injury
or ventilator-associated pneumonia. Moreover, the local and
systemic effects of tumour may also play a role.
Neutropenia remains a common side effect of cancer
chemotherapy and, although transient and expected, may lead to
immune dysfunction in oncological patients. Beyond specific cancer
therapies, several additional factors including lung injury,
sepsis, underlying malignancy and its stage are habitually
associated with neutropenia duration. ICU admission is frequently
required in these patients as a consequence of severe sepsis or
ARF. In the general ICU population with septic shock, neutropenia
is an independent predictor of mortality. In cancer patients in
this setting the influence of neutropenia on outcome is uncertain;
however, some recent systematic reviews and meta-analyses have
shown it to be associated with an increase in mortality in
critically ill cancer patients (27,28).
In our study, the presence of neutropenia on ICU admission was also
significantly associated with the risk of hospital death.
In most studies, the characteristics of the
underlying cancer have little impact on short-term survival, and
are not enough to rule out ICU admission (2,10,29).
Similarly, in our study, only metastatic disease emerged as a
prognostic factor for in-hospital mortality; the oncological
assessment prior to ICU admission (i.e., no evidence of relapse,
progressive disease, first appearance or non-progressive disease)
or the antineoplastic treatment received had no influence. In this
regard, it must not be forgotten that immunotherapy can improve the
prognosis of patients with metastatic disease, and indeed its use
has increased exponentially in recent years. In our study only nine
patients received immunotherapy. In addition, the heterogeneity of
the types of cancer makes the interpretation of survival data more
difficult.
In our study, an ICU stay of more than 48 h was a
protective factor. The reason for this may be that, in contrast to
other studies, we included patients with admissions of less than 24
h, and 88% of these patients died. Moreover, in 8% of all cases,
the reason for ICU admission was CPA and the chances of survival
after CPA in patients with advanced cancer are low. Consequently,
in our series, 50% died in the first 48 h of ICU admission, due to
brain death, multiorgan dysfunction or LTE.
In contrast, we found that undergoing surgery during
hospitalization was related to good prognosis. It is difficult to
extrapolate these results to other settings, given the
heterogeneous nature of the cancer patients who undergo surgery and
the differences in surgical goals; there may also be a selection
bias, since the majority of surgical patients are admitted to the
resuscitation service. More than 40% of these surgeries are
scheduled, mainly primary tumour surgeries in patients with a
recent diagnosis and treated with curative intent. In addition,
more than 50% of the surgical patients are operated upon more than
72 h prior to admission to the ICU, and so the reason for ICU
admission is medical. In our study, only one patient received
palliative surgery.
Finally, in our study, functional status prior to
ICU admission as defined by the ECOG scale was not found to be a
predictor of mortality, in contrast to other studies (11–13,17,19,30).
This is probably because most of our patients had a recent
oncological diagnosis (without previous oncological evaluation),
and the sample size for this variable was small. Nevertheless,
patients with poor ECOG tend to have worse survival results. As a
general rule, patients with good ECOG in situations in which
life-extending treatment options are available should be routinely
admitted to the ICU. Moreover, in our study we analysed the
evolution of ECOG at hospital discharge and follow-up at the
oncology department over the following 12 months, and concluded
that even though ICU stay significantly worsens the functional
capacity of cancer patients in 32.1% of cases, more than half of
the survivors (59.5%) with follow-up after discharge had good
functional status. With subsequent follow-up, we can affirm that
most patients (more than 80%) who survive and undergo oncological
follow-up at 6 and 12 months maintain good functional status (ECOG
0–1); the group with worse functional status (ECOG ≥2) had the
highest mortality. There were no significant changes in their
functional status during the follow-up period. This suggests that
patients with cancer and good ECOG should receive full intensive
care, but it should be borne in mind that ICU treatment entails a
short-term functional worsening, and so ECOG is a simple and highly
effective clinical tool for assessing patients' overall health
status.
Our study has several limitations that should be
considered. First, it is a descriptive retrospective observational
study at a single centre with a sample that is small and very
heterogeneous from the oncological point of view, a circumstance
that limits the reliability of the statistical analysis and the
extrapolation of the results to other samples. It is also difficult
to compare crude mortality in different studies due to the high
variability of the underlying oncological disease, admission
criteria, and treatment decision criteria. Furthermore, certain
oncological data such as the ECOG scale are not available in
patients admitted to the ICU with a recent cancer diagnosis who
have not been evaluated previously by oncologists. In addition, the
ICU mortality rate may suffer from a sampling bias because patients
who are repeatedly admitted to the ICU were recorded only once.
Moreover, factors such as ethnic origin and toxic habits were not
been recorded. Finally, this series is a few years old, since
during the pandemic we were unable to proceed with the study and
publish the findings.
In conclusion, only 40% of patients with cancer
requiring ICU admission died during hospitalization, and more than
half of the survivors presented good functional status at hospital
discharge. Moreover, the similar ICU mortality rate observed in
cancer patients and critically ill non-cancer patients supports a
broader policy on ICU admission in this population. The survival
rate of oncological patients admitted to the ICU one year from
hospital discharge was 28.7%. Most of the patients (85.7%) who
survived at one year and who were controlled by the oncology
service had good functional status (ECOG 0–1).
The prognosis of critically ill adult patients with
cancer is best determined by the nature and number of organ
failures with the SOFA score, rather than by the stage of the
underlying oncological malignancy. Therefore, admission should not
be limited to patients with an active tumour. Need for invasive
mechanical ventilation, neutropenia and metastatic disease were
variables related to in-hospital mortality. ICU stay (>48 h) and
the need for surgery during hospital admission were protective
variables.
Further multicentre and prospective studies should
now be conducted to determine the prognostic factors of critical
patients with solid tumours and their later post-hospital
evolution.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
The authors would like to thank Mr. Joan Nolla
(retired physician) for their dedication and commitment in helping
to design this study.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RBC and LV contributed to the study design. RBC, MFR
and GGV collected the critical patient data. LV and AR collected
the oncological and follow-up data. RBC and LV confirm the
authenticity of all the raw data. RBC was the major contributor in
writing the manuscript. RBC, LV, JRM and XD contributed to the
discussion, analysis and interpretation of the results obtained. XD
contributed to the statistical analysis. JRM supervised the
project. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the ethical principles of The Declaration of Helsinki, and was
approved by the Ethics Committee of Hospital del Mar (No. 10570).
The Clinical Research Ethics Committee understood that it was not
necessary to obtain informed consent to participate in the study
since the data were collected and analysed anonymously, and due to
the observational character of the trial.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
ORCID: Raquel Bosch-Compte. https://orcid.org/0000-0002-7861-9474.
Glossary
Abbreviations
Abbreviations:
|
ICU
|
Intensive Care Unit
|
|
SOFA
|
Sequential Organ Failure
Assessment
|
|
HR
|
hazard ratio
|
|
CI
|
confidence interval
|
|
BMI
|
body mass index
|
|
ECOG
|
Eastern Cooperative Oncology Group
|
|
APACHE II
|
Acute Physiology and Chronic Health
Evaluation II
|
|
LTE
|
limitation of therapeutic effort
|
|
ARF
|
acute respiratory failure
|
|
SD
|
standard deviation
|
|
IQR
|
interquartile range
|
|
COPD
|
chronic obstructive pulmonary
disease
|
|
NR
|
not reported
|
|
ENT
|
ear-nose-throat
|
|
CPA
|
cardiopulmonary arrest
|
|
PaO2/FiO2
|
arterial oxygen partial pressure to
inspiratory oxygen fraction
|
References
|
1
|
Henley SJ, Singh SD, King J, Wilson
RO'Neil ME and Ryerson AB: Centers for Disease Control and
Prevention (CDC): Invasive cancer incidence and survival-United
States, 2011. MMWR Morb Mortal Wkly Rep. 64:237–242.
2015.PubMed/NCBI
|
|
2
|
Azoulay E, Soares M, Darmon M, Benoit D,
Pastores S and Afessa B: Intensive care of the cancer patient:
Recent achievements and remaining challenges. Ann Intensive Care.
1:52011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Puxty K, McLoone P, Quasim T, Sloan B,
Kinsella J and Morrison DS: Risk of critical illness among patients
with solid cancers: A population-based observational study. JAMA
Oncol. 1:1078–7085. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shimabukuro-Vornhagen A, Böll B, Kochanek
M, Azoulay É and von Bergwelt-Baildon MS: Critical care of patients
with cancer. CA Cancer J Clin. 66:496–517. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Taccone FS, Artigas AA, Sprung CL, Moreno
R, Sakr Y and Vincent JL: Characteristics and outcomes of cancer
patients in European ICUs. Crit Care. 13:R152009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ñamendys-Silva SA, Plata-Menchaca EP,
Rivero-Sigarroa E and Herrera-Gómez A: Opening the doors of the
intensive care unit to cancer patients: A current perspective.
World J Crit Care Med. 4:159–162. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sauer CM, Dong J, Celi LA and Ramazzotti
D: Improved survival of cancer patients admitted to the intensive
care unit between 2002 and 2011 at a U.S. Teaching hospital. Cancer
Res Treat. 51:973–981. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Darmon M, Bourmaud A, Georges Q, Soares M,
Jeon K, Oeyen S, Rhee CK, Gruber P, Ostermann M, Hill QA, et al:
Changes in critically ill cancer patients' short-term outcome over
the last decades: Results of systematic review with meta-analysis
on individual data. Intensive Care Med. 45:977–987. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McGrath S, Chatterjee F, Whiteley C and
Ostermann M: ICU and 6-month outcome of oncology patients in the
intensive care unit. QJM. 103:397–403. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Díaz-Díaz D, Villanova Martínez M and
Palencia Herrejón E: Oncological patients admitted to an intensive
care unit. Analysis of predictors of in-hospital mortality. Med
Intensiva (Engl Ed). 42:346–353. 2018.(In English, Spanish).
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aygencel G, Turkoglu M, Turkoz Sucak G and
Benekli M: Prognostic factors in critically ill cancer patients
admitted to the intensive care unit. J Crit Care. 29:618–626. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Azoulay E, Schellongowski P, Darmon M,
Bauer PR, Benoit D, Depuydt P, Divatia JV, Lemiale V, van Vliet M,
Meert AP, et al: The intensive care medicine research agenda on
critically ill oncology and hematology patients. Intensive Care
Med. 43:1366–1382. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hourmant Y, Mailloux A, Valade S, Lemiale
V, Azoulay E and Darmon M: Impact of early ICU admission on outcome
of critically ill and critically ill cancer patients: A systematic
review and meta-analysis. J Crit Care. 61:82–88. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Soubani AO: Critical care prognosis and
outcomes in patients with cancer. Clin Chest Med. 38:333–353. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Oeyen SG, Benoit DD, Annemans L, Depuydt
PO, Van Belle SJ, Troisi RI, Noens LA, Pattyn P and Decruyenaere
JM: Long-term outcomes and quality of life in critically ill
patients with hematological or solid malignancies: A single center
study. Intensive Care Med. 39:889–898. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zampieri FG, Romano TG, Salluh JIF,
Taniguchi LU, Mendes PV, Nassar AP Jr, Costa R, Viana WN, Maia MO,
Lima MFA, et al: Trends in clinical profiles, organ support use and
outcomes of patients with cancer requiring unplanned ICU admission:
A multicenter cohort study. Intensive Care Med. 47:170–179. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lecuyer L, Chevret S, Thiery G, Darmon M,
Schlemmer B and Azoulay E: The ICU trial: A new admission policy
for cancer patients requiring mechanical ventilation. Crit Care
Med. 35:808–814. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Azevedo LCP, Caruso P, Silva UVA, Torelly
AP, Silva E, Rezende E, Netto JJ, Piras C, Lobo SMA, Knibel MF, et
al: Outcomes for patients with cancer admitted to the ICU requiring
ventilatory support: Results from a prospective multicenter study.
Chest. 146:257–266. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Carmona-Bayonas A, Gordo F, Beato C,
Castaño Pérez J, Jiménez-Fonseca P, Virizuela Echaburu J and
Garnacho-Montero J: Intensive care in cancer patients in the age of
immunotherapy and molecular therapies: Commitment of the
SEOM-SEMICYUC. Med Intensiva (Engl Ed). 42:363–369. 2018.(In
English, Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kingah P, Alzubaidi N, Yafawi JZD, Shehada
E, Alshabani K and Soubani AO: Factors associated with mortality in
patients with a solid malignancy admitted to the intensive care
unit-A prospective observational study. J Crit Care Med (Targu
Mures). 4:137–142. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pérez Pérez ML, Gonzaga López A, Balandín
Moreno B, Maximiano Alonso C, Palacios Castañeda D, Ferreres Franco
J, García Sanz J, Villanueva Fernández H, Valdivia de la Fuente M,
Ortega López A, et al: Characteristics and outcome of patients with
solid tumour requiring admission to the intensive care unit.
Usefulness of three severity score systems. Med Clin (Barc).
153:270–275. 2019.(In English, Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
23
|
van der Zee EN, Noordhuis LM, Epker JL,
van Leeuwen N, Wijnhoven BPL, Benoit DD, Bakker J and Kompanje EJO:
Assessment of mortality and performance status in critically ill
cancer patients: A retrospective cohort study. PLoS One.
16:e02527712021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Biskup E, Cai F, Vetter M and Marsch S:
Oncological patients in the intensive care unit: Prognosis,
decision-making, therapies and end-of-life care. Swiss Med Wkly.
147:w144812017.PubMed/NCBI
|
|
25
|
Prieto Del Portillo I, Polo Zarzuela M and
Pujol Varela I: Patients with cancer in the intensive monitoring
unit. New perspectives. Rev Clin Esp (Barc). 214:403–409. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Martos-Benítez FD, Gutiérrez-Noyola A,
Badal M and Dietrich NA: Risk factors and outcomes of severe acute
respiratory failure requiring invasive mechanical ventilation in
cancer patients: A retrospective cohort study. Med Intensiva (Engl
Ed). 42:354–362. 2018.(In English, Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bouteloup M, Perinel S, Bourmaud A,
Azoulay E, Mokart D and Darmon M; pour le Groupe de Recherche en
Réanimation Respiratoire du patient d'Onco-Hématologie (GRRR-OH), :
Outcomes in adult critically ill cancer patients with and without
neutropenia: A systematic review and meta-analysis of the Groupe de
Recherche en Réanimation Respiratoire du patient d'Onco-Hématologie
(GRRR-OH). Oncotarget. 8:1860–1870. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Georges Q, Azoulay E, Mokart D, Soares M,
Jeon K, Oeyen S, Rhee CK, Gruber P, Ostermann M, Hill QA, et al:
Influence of neutropenia on mortality of critically ill cancer
patients: Results of a meta-analysis on individual data. Crit Care.
22:3262018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Heo SJ, Kim G, Lee CK, Chung KS, Choi HJ,
Sohn J and Lee S: Prediction of short- and long-term survival for
advanced cancer patients after ICU admission. Support Care Cancer.
23:1647–1655. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gheerbrant H, Timsit JF, Terzi N, Ruckly
S, Laramas M, Levra MG, Jacquet E, Falque L, Moro-Sibilot D and
Toffart AC: Factors associated with survival of patients with solid
cancer alive after intensive care unit discharge between 2005 and
2013. BMC Cancer. 21:92021. View Article : Google Scholar : PubMed/NCBI
|