Introduction
Immunotherapy has transformed cancer treatment in
less than a decade. It is used primarily in lung cancer and
melanomas, significantly improving overall survival for patients
suffering from these cancers (1,2).
However, for sarcoma patients, the responses to checkpoint
inhibitors have been disappointing, with an objective response rate
of only about 4% and progression-free survival of 2.4 months
(3). It is proposed that sarcomas
possess immune-evading mechanisms, which are not addressed by
existing immunotherapeutic drugs. Therefore, understanding the
connection between the immune system and sarcomas is crucial in
developing improved immunotherapy approaches for sarcoma
patients.
One of the earliest and most robust responses to
inflammatory conditions is an increased C-reactive protein (CRP)
level. CRP is an acute-phase reactant primarily synthesised in the
liver and shed into the bloodstream as an early response to acute
inflammation and, to a lesser extent, during chronic inflammation
(4,5). However, it has been shown that small
quantities of CRP are produced by other than liver cells, such as
smooth muscle cells (6,7), epithelial cells (8), fat cells (9), and even by immune-modulating
macrophages (10). Different
cytokines stimulate the production of CRP, such as IL-6, IL-1 and
tumour necrosis factor. CRP interacts with the vascular
endothelium, contributes to monocyte-endothelium adhesion,
increases reactive oxygen species, and triggers platelet
aggregation, as shown in rodents (11). Furthermore, CRP binds to
lysophosphatidylcholine on the surface of dead or dying eukaryotic
cells and bacteria, activating the complement system and an
essential player in the innate immune response (12). In vitro studies have shown
that CRP inhibits the proliferation and activation of T-cells and
is, therefore, believed to play a part in both the innate and
adaptive immune response (13).
It has been shown that conventional measurement of
CRP, where a clinical cut point of 8 mg/l has been used, is a
prognostic factor for patients with metastatic soft tissue sarcoma
(14,15) and localised soft tissue sarcoma
(16,17) and bone sarcoma (18,19).
However, conventional CRP measurements are limited by the
sensitivity to detect low values of CRP.
The high-sensitivity C-reactive protein (hs-CRP)
quantifies CRP using an assay with a very low detection level. This
allows for detecting and graduating low-grade inflammatory states
such as obesity, diabetes, and cardiovascular disease (20,21).
Hs-CRP is elevated in patients with soft tissue sarcoma (STS)
compared to patients without sarcoma, and it has been proposed that
it can be a diagnostic marker (22).
Macrophage-related biomarkers (sCD163 and SIRPα)
were shown to be prognostic for overall survival and add prognostic
values to a model containing known prognostic factors such as grade
and age (23). However, the
prognostic value and the association of hs-CRP levels and other
inflammatory parameters have not been investigated in sarcoma
patients.
This study investigates the association between
serum hs-CRP and mortality among patients with primarily localised
sarcoma.
Materials and methods
Study cohort
This prospective, non-randomised, non-interventional
explorative study investigates the prognostic value of hs-CRP in
sarcoma patients. Patients referred to the sarcoma centre of Aarhus
University Hospital, Denmark, between April 2014 and December 2020
were included in the present study. All patients signed an informed
consent form before inclusion. The inclusion criteria were age (18
years or older) and willingness to donate a blood sample. Several
patients were diagnosed with conditions other than sarcoma and
served as a control group in this study. This control cohort was
chosen because they were referred to the sarcoma centre with the
suspicion of having a sarcoma. The control groups also included
patients with desmoid tumour which is a local aggressive benign
tumour and therefore not regarded as a sarcoma. However, these
patients include patients with benign tumours such as lipomas or
unspecific tissue reactions, to mention a few. After inclusion and
following the national guidelines, patients were diagnosed and
treated by an experienced multidisciplinary sarcoma team. Most
patients were diagnosed with localised grade II and III STS and
treated with surgery with or without pre- or postoperative
radiation therapy. We have previously published results on immune
suppressive macrophages using the same patient cohort (23).
Data sources
Clinical data were obtained from the national
quality sarcoma database, which contains comprehensive clinical
information on individual sarcoma patients since 2009 in Denmark.
The patients' records were reviewed to fill in missing values.
Biomarkers other than hs-sensitive CRP were obtained
from the clinical laboratory information system (LABKA) database,
which reports all blood tests taken according to the international
nomenclature, properties, and Units (NPU) coding system. The values
selected for analysis were the biomarkers closest to the day of the
sarcoma diagnosis. LABKA results were included from 60 days before
sarcoma diagnosis until 60 days after. Monocyte count, C-reactive
protein (CRP), albumin, leucocyte count, and neutrophil count were
selected for inclusion in this study. Each biomarker was
categorised into normal or high/low according to the reference
value at Aarhus University Hospital. High monocyte count was
defined as ≥0.7×109 cells/l; low albumin levels were
defined as <36 g/l. Elevated CRP was defined as values ≥8 mg/l;
elevated leucocyte count was defined as values ≥10×109
cells/l, and high neutrophil count was defined as values
≥7×109 cells/l.
Hs-sensitive CRP measurements
Before initiating the treatment, 30 ml of peripheral
blood was collected in sodium citrate tubes and centrifuged at
2,000 or 2,500 g for ten minutes. The plasma was isolated and
stored at −80°C until measurement according to the instructions by
Danish Cancer Biobank, Bio- and GenomeBank, Denmark. Hs-sensitive
CRP was measured with a chemiluminescent immunometric assay using
the Atellica CH (Siemens, Germany). The upper limit of normal for
hs-CRP is in our institution 3.0 mg/l, which is based on a Danish
population. The hs-CRP values were used to allocate patients into
high and low hs-CRP groups based on the medium value of hs-CRP.
Prognostic profile
A predictive profile was created as described in our
previous publication (23). In
short, sCD163 and sSIRPα were divided into low or high groups based
on their median serum concentration. CRP was separated into two
categories, low or high, based on a threshold of 8 mg/l. Each
categorical variable was assigned a score of 1 or 2, while the
grade was assigned a score of 1, 2, or 3, depending on grade. The
sum of all the assigned scores made up the final profile. The
profile was then divided into three risk stratification groups:
low-risk (score 4–5), intermediate risk (score 6–7), and high-risk
(score 8–9). This profile is named profile 1 (23). In this paper, hs-CRP replaced the
normal CRP measurement named profile 2.
Statistical analysis
Clinical data and information on biomarkers were
linked by the unique 10-digit civil personal registration (CPR)
number, allowing for individual linkage between different reporting
systems.
Patient, tumour, and treatment-related variables
were reported as frequencies, percentages or continuous variables
expressed as medians with interquartile range (IQR). The variables
were compared using the chi-squared, Fisher's exact test or
Wilcoxon rank-sum test, depending on the variable in question, and
stratified by the median value of the hs-CRP in the STS patients.
When the median value of multiple groups was compared, the Kruskal
Wallis test was used with the Dunn's test for multiple comparisons
when significant results were obtained. The median values of hs-CRP
were used to categorise patients into a high and low hs-CRP
group.
The primary endpoints were recurrent disease and
overall survival (OS). Time to recurrent disease was defined as the
interval between the primary diagnosis and the first recurrent,
local or metastatic. OS was defined as the time from the date of
diagnosis until the death of any causes. Kaplan-Meier curves were
used to visualise survival and the log rank test was used for
comparison of groups. The study period ended on October 15th 2022,
and patients alive at this date were censored. Crude (univariate)
and adjusted (multivariate) analyses were performed by using the
Cox proportional hazard model. A test for proportional hazard was
used before including an additional variable in the analysis. Based
on the literature, the following variables were included in the
adjusted analysis: age, size of the primary tumour, and grade.
Tumour size and age were included as continuous variables; all
other variables were analysed as categorical. ROC curve for cut
point determination using the common criteria with the point on the
ROC curve where the sensitivity and specificity of the test are
equal (24). Akaike's information
criteria (AIC) and Harrell's concordance index determined the model
with the minimum AIC values, regarded as the best model.
Likelihood-ratio tests were used to evaluate whether the addition
of a potential prognostic profile contributed significantly to the
models' prognostic value. A Two-sided P<0.05 was considered
statistically significant. All analyses were performed using Stata
(version 17.1) software.
Results
Demographic data
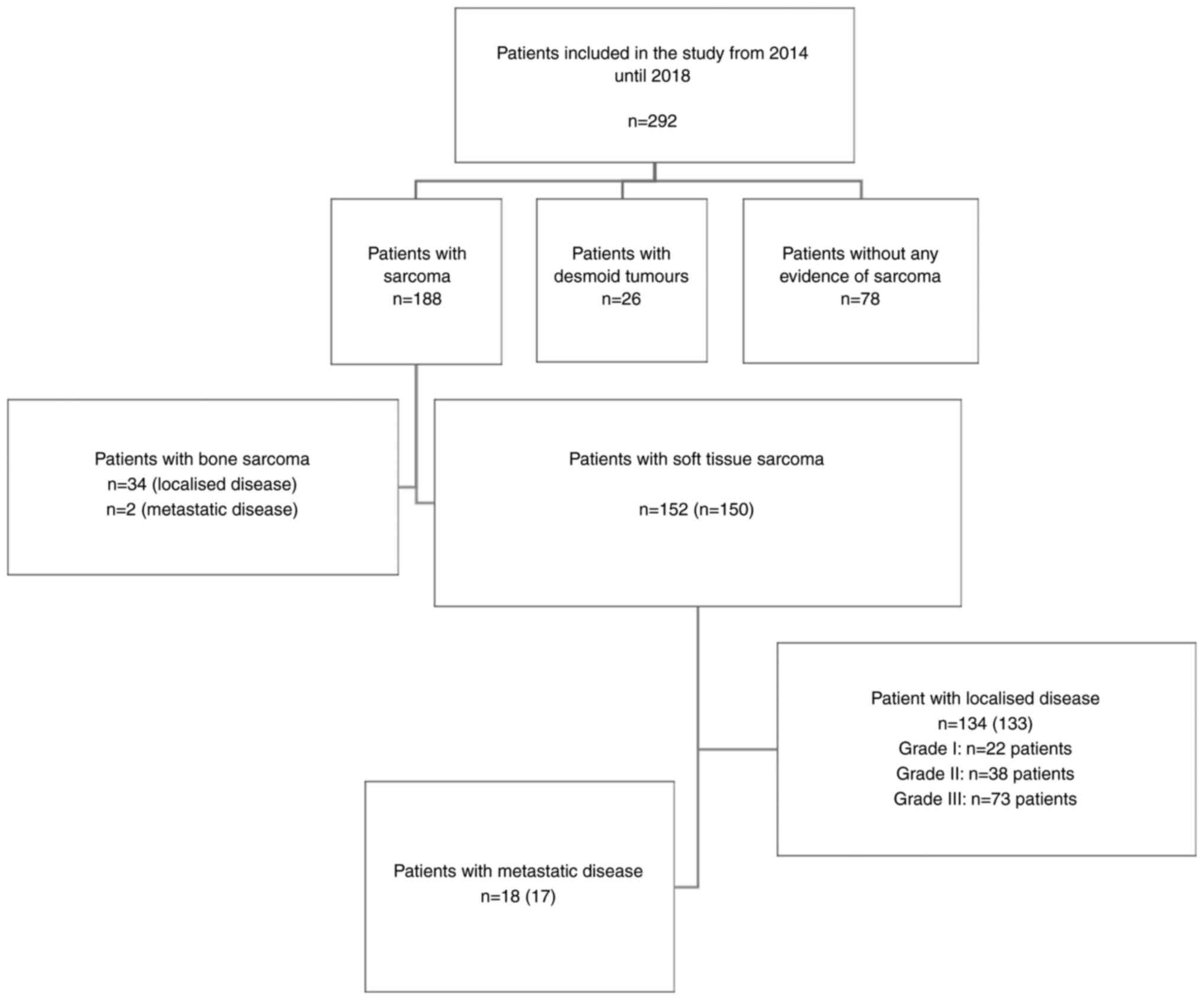
A total of 292 patients were included in this study.
The majority of patients were diagnosed with sarcoma (n=188:
STS=152, bone sarcoma=36), compared to patients with desmoid
fibromatosis (n=26) and patients diagnosed with conditions other
than sarcoma or desmoid fibromatosis and therefore defined as
control patients (n=78) (Fig.
1).
A significantly higher median hs-CRP was observed in
patients with STS or bone sarcoma compared to patients with desmoid
tumours and control patients (Table
I). For STS, grade III tumours were associated with a higher
hs-CRP level (P=0.0001), whereas patients with metastatic disease
did not have a higher hs-CRP level than those with localised
disease. The median hs-CRP values with a 95% confidence interval
(CI) are depicted for each subtype of STS, bone sarcoma, desmoid
tumours, and the control group in Fig.
2. The median hs-CRP level varies between the different
histologic subtypes, with undifferentiated pleomorphic sarcoma
having the highest levels. The difference between subtypes is
significant compared to the control group and between UPS and the
other histological groups.
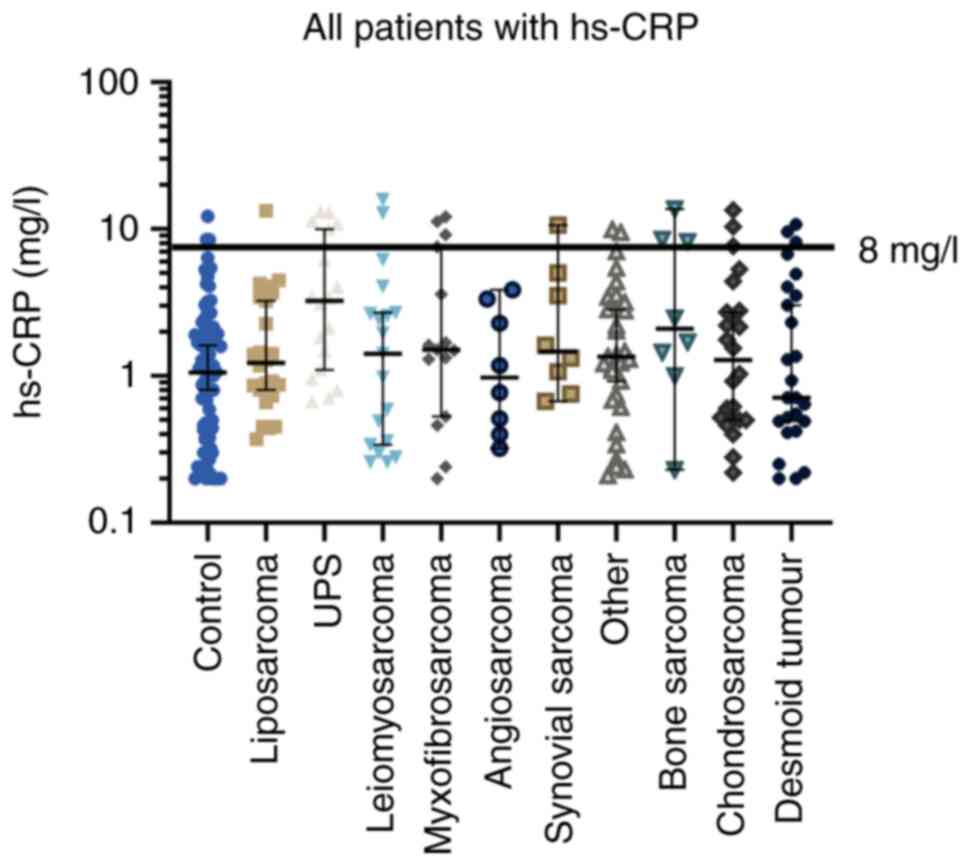 | Figure 2.Median hs-CRP value in mg/l according
to histological subtype and for control patients. The median value
is shown with a 95% confidence interval. The comparisons among
different groups were performed using the Kruskal-Wallis test with
Dunn's test as post hoc analysis. For patients with liposarcoma
(P<0.0001), UPS (P<0.0001), myxofibromsarcoma (P=0.02), other
(P=0.01) and bone sarcoma (P=0.02) a significant difference was
observed compared with control patients. For patients with UPS
compared with the other histological groups, a significant
difference was observed for liposarcoma (P=0.003), myxofibrosarcoma
(P=0.04), angiosarcoma (P=0.01), others (P=0.007), chondrosarcoma
(P=0.005) and desmoid tumours (P<0.001). Hs-CRP,
high-sensitivity C-reactive protein; UPS, undifferentiated
pleomorphic sarcoma. |
 | Table I.Median concentration of hs-CRP for
different subgroups at diagnosis. |
Table I.
Median concentration of hs-CRP for
different subgroups at diagnosis.
|
|
| CRP, mg/l |
|
|
|---|
|
|
|
|
|
|
|---|
| Clinical
variables | No. | Median | Range | Kruskal Wallis test
P-value | Dunn's test
P-value |
|---|
| Histology |
|
|
| 0.0004 |
|
|
Control | 78 | 1.09 | 0.20–8.51 |
|
|
| Desmoid
tumours | 26 | 0.68 | 0.20–23.11 |
| 0.340a |
| Bone
sarcoma | 34 | 2.16 | 0.23–20.36 |
| 0.018a |
| Soft
tissue sarcoma (localized only) | 133 | 2.19 | 0.28–71.69 |
|
<0.001a |
| Soft tissue
sarcoma |
|
|
| 0.0001 |
|
| Grade
I | 22 | 1.27 | 0.23–5.42 |
|
|
| Grade
II | 38 | 1.34 | 0.26–19.75 |
| 0.220b |
| Grade
III | 73 | 3.43 | 0.46–109.17 |
|
<0.001b; <0.001c |
| Soft tissue
sarcoma |
|
|
| 0.9244 |
|
|
Localised | 133 | 2.23 | 0.28–71.69 |
|
|
|
Metastatic | 17 | 1.53 | 0.21–38.78 |
|
|
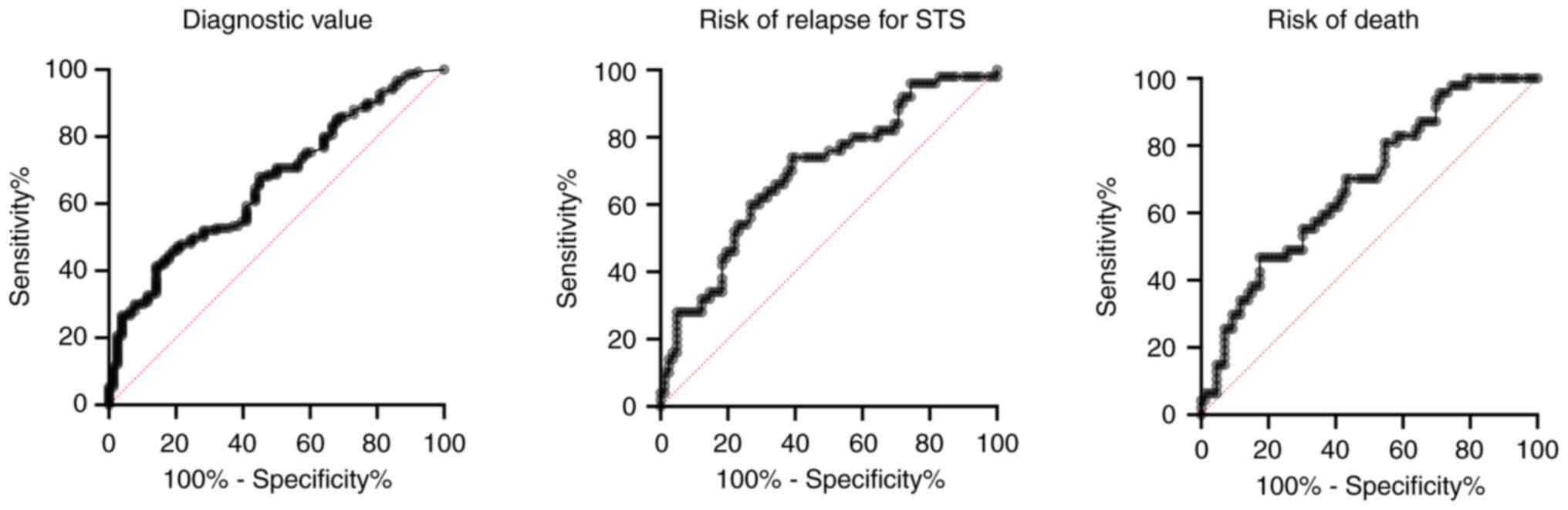
The diagnostic value of hs-CRP was evaluated by
comparing patients with localised sarcoma (bone and soft tissue
sarcoma) with patients without sarcoma disease (desmoid
fibromatosis and control patients). Fig. 3 shows the ROC curve with the
sensitivity and specificity for hs-CRP. The area under the curve is
0.66 (95% CI: 0.59–0.73), and according to the ROC curve, the
optimal cut point is 1.41 mg/l for local recurrence and 1.72 mg/l
for overall survival.
Prognostic value of hs-CRP
Only patients with localised soft tissue sarcoma
(n=133) were included in analysing the effect of hs-CRP.
Clinical and tumour characteristics for patients
with localised STS are shown in Table
II. Patients were divided into a high and a low hs-CRP group
based on the median value (2.19 mg/l). For patients with a high
level of hs-CRP, the tumours were more extensive, were of a higher
grade, and more patients experienced relapse after curative
treatment. Six patients with a high level of hs-CRP also had
increased leucocyte and neutrophil cell count; only two patients
had increased liver enzymes. For patients with a low hs-CRP, 76%
(95% CI: 64–84%) had not experienced a relapse of disease after
five years of follow-up compared to 45% (95% CI: 32–57%) of the
patient with a high hs-CRP. The 5-year overall survival for
patients with a low hs-CRP was 83% (95% CI: 72–90%) compared to 59%
(95% CI: 46–70%) for patients with a high hs-CRP.
 | Table II.Clinical, tumour and treatment
characteristics of patients with localised soft tissue sarcoma and
CRP measurements (n=133). |
Table II.
Clinical, tumour and treatment
characteristics of patients with localised soft tissue sarcoma and
CRP measurements (n=133).
|
Characteristics | Total | Low CRP | High CRP | P-value |
|---|
| Sex, n (%) |
|
|
|
|
|
Male | 59 (44) | 29 (43) | 30 (45) | 0.801 |
|
Female | 74 (56) | 38 (57) | 36 (55) |
|
| Median age, years
(p5-p95) | 67 (27–85) | 65 (25–84) | 69 (47–85) | 0.06 |
| Histological
subtype, n (%) |
|
|
|
|
|
Liposarcoma | 32 (24) | 15 (22) | 17 (26) | 0.792 |
|
UPS | 23 (17) | 8 (12) | 15 (23) |
|
|
Leiomyosarcoma | 17 (13) | 9 (13) | 8 (12) |
|
|
Myxofibrosarcoma | 17 (13) | 10 (15) | 7 (11) |
|
|
Angiosarcoma | 8 (6) | 4 (6) | 4 (6) |
|
|
Synovial sarcoma | 7 (5) | 4 (6) | 3 (5) |
|
|
Others | 29 (22) | 17 (25) | 12 (18) |
|
| Median tumour size,
cm (p5-p95) | 6 (1–23) | 5 (1–16) | 8 (1–27) | <0.01 |
| Tumour grade, n
(%) |
|
|
|
|
|
Low | 22 (17) | 16 (24) | 6 (9) | 0.002 |
|
Intermediate | 38 (29) | 23 (34) | 15 (23) |
|
|
High | 73 (55) | 28 (42) | 45 (68) |
|
| Treatment, n
(%) |
|
|
|
|
|
Surgery | 132 (99) | 67 (98) | 65 (100) | 0.496 |
|
Radiation therapy | 50 (38) | 21 (31) | 29 (44) | 0.154 |
| Treatment intent, n
(%) |
|
|
|
|
|
Curativea | 132 (99) | 67 (100) | 65 (98) | 0.312 |
| Relapse, n (%) |
|
|
|
|
|
Yes | 51 (39) | 17 (25) | 34 (52) | 0.001 |
| No | 82 (61) | 50 (75) | 32 (48) |
|
The univariate analyses are shown in Table III. Patient age, tumour size,
tumour grade, CRP level, and neutrophile count are prognostic for
recurrent disease, and patient's age, tumour size, tumour grade,
CRP level, albumin level, and neutrophile count were prognostic for
overall survival. The multivariate analysis showed that a high
hs-CRP was an independent prognostic factor for recurrent disease
with a hazard ratio of 1.90 (95% CI: 1.03–3.52) and overall
survival with a hazard ratio of 2.20 (95% CI: 1.13–4.29).
 | Table III.Univariate analyses in patients with
localised soft tissue sarcoma (n=133) using Cox regression
analysis. |
Table III.
Univariate analyses in patients with
localised soft tissue sarcoma (n=133) using Cox regression
analysis.
|
| Risk of
relapse | Overall
survival |
|---|
|
|
|
|
|---|
|
Characteristics | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
|---|
| Age, years | 1.03 | 1.01–1.05 | 0.007 | 1.06 | 1.03–1.09 | <0.001 |
| Sex |
|
|
|
|
|
|
|
Female | 1 |
|
| 1 |
|
|
|
Male | 1.21 | 0.68–2.16 | 0.513 | 1.22 | 0.67–2.21 | 0.509 |
| Size, cm | 1.04 | 1.01–1.08 | 0.018 | 1.05 | 1.01–1.08 | 0.011 |
| Grade |
|
|
|
|
|
|
| I | 1 |
|
| 1 |
|
|
| II | 1.17 | 0.35–3.90 | 0.793 | 2.96 | 0.64–13.75 | 0.165 |
|
III | 4.26 | 1.52–11.97 | 0.006 | 8.22 | 1.97–34–39 | 0.004 |
| Serum
biomarkers |
|
|
|
|
|
|
|
Monocytes count,
×109 cells/l | 1.09 | 0.55–2.16 | 0.796 | 1.60 | 0.83–3.10 | 0.159 |
| hs-CRP,
mg/l | 2.74 | 1.52–4.92 | 0.001 | 2.62 | 1.40–4.90 | 0.002 |
| CRP,
mg/l | 1.95 | 1.08–3.51 | 0.027 | 2.22 | 1.19–4.12 | 0.012 |
|
Albumin, g/l | 1.60 | 0.82–3.15 | 0.165 | 2.06 | 1.03–4.09 | 0.040 |
|
Leucocyte count,
×109 cells/l | 1.39 | 0.65–2.98 | 0.395 | 2.02 | 0.99–1.12 | 0.054 |
|
Neutrophil count,
×109 cells/l | 2.29 | 1.10–4.78 | 0.028 | 3.06 | 1.53–6.13 | 0.002 |
All patients with localised soft tissue sarcoma
treated with curative intent were included in the model selection
analysis (n=133). The c statistics showed that adding hs-CRP to
known prognostic factors such as grade, tumour size, and age
significantly improved the prognostic model. Table IV shows the c-statistics for
comparing different models. Replacing normal CRP with hs-CRP
lowered the AIC from 414 to 411 when evaluating the risk of disease
or recurrent disease. However, when evaluating the ability to
predict overall survival, AIC was unchanged (AIC=322 when CRP was
included and AIC=323 when hs-CRP was included). Moreover, a model
containing the macrophage markers sCD163 and sSIRPα significantly
improved the model, including hs-CRP adjusted for age, tumour size,
and grade (P=0.0021).
 | Table IV.Model fitting. |
Table IV.
Model fitting.
| Prognostic
models | Relapse |
| Survival |
|---|
|
|
|
|
|---|
| Model | AIC | C-index | AIC | C-index |
|---|
| Grade | 456 | 0.66 | 397 | 0.67 |
| Age | 466 | 0.61 | 392 | 0.70 |
| Tumour size | 468 | 0.63 | 409 | 0.65 |
| hs-CRP | 461 | 0.62 | 404 | 0.64 |
| Grade + hs-CRP | 451 | 0.79 | 394 | 0.71 |
| Grade + age +
hs-CRP | 448 | 0.73 | 373 | 0.77 |
| Grade + age +
tumour size + hs-CRP | 446 | 0.74 | 369 | 0.79 |
| Grade + age +
tumour size | 448 | 0.73 | 373 | 0.78 |
| Grade + age +
tumour size + hs-CRP + sCD163 and sSIRPα | 450 | 0.74 | 362 | 0.79 |
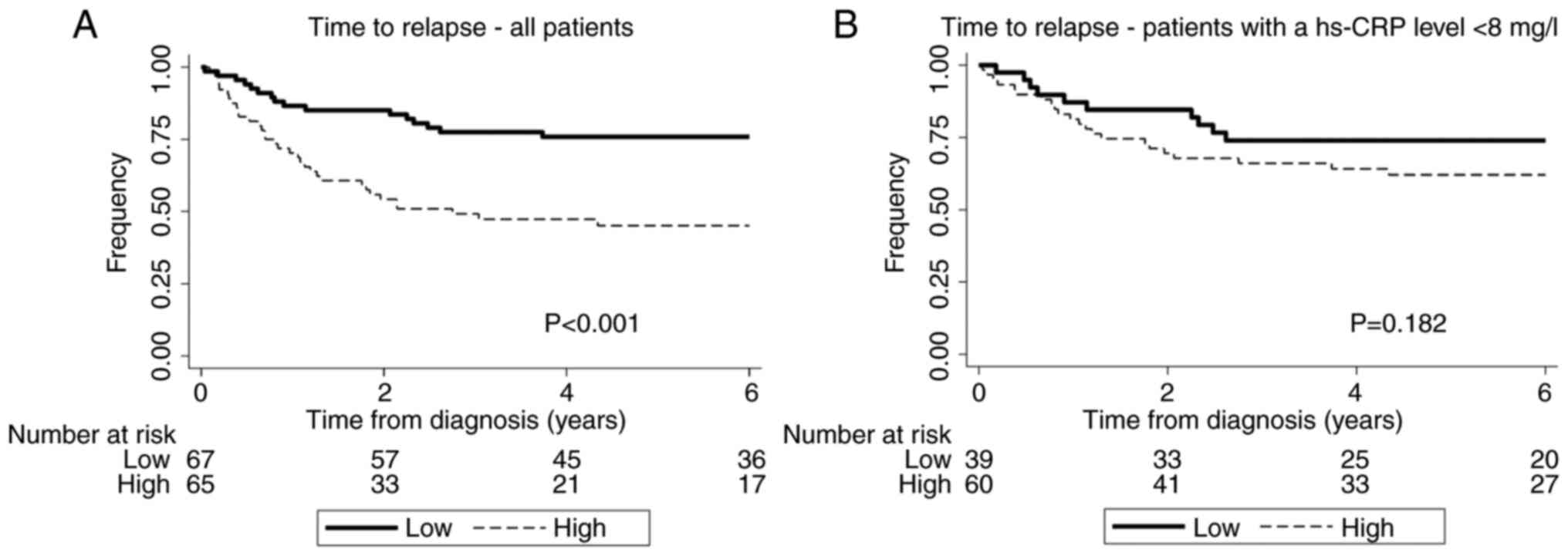
Analyses of patients with a hs hs-CRP
<8 mg/l
One hundred patients had a hs hs-CRP <8 mg/l, the
reference level defining elevated CRP levels in Denmark. In a
subgroup analysis including only patients with hs-CRP <8 mg/l,
patients were divided into a high and low group based on the median
hs-CRP value (1.32 mg/l). Age and tumour size were associated with
high levels of hs-CRP. Again, high hs-CRP was associated with an
increased risk of recurrent disease as the 5-year recurrence-free
survival for patients with a hs-CRP below the median value was 74%
(95% CI: 57–85%) compared to patients with a hs-CRP above the
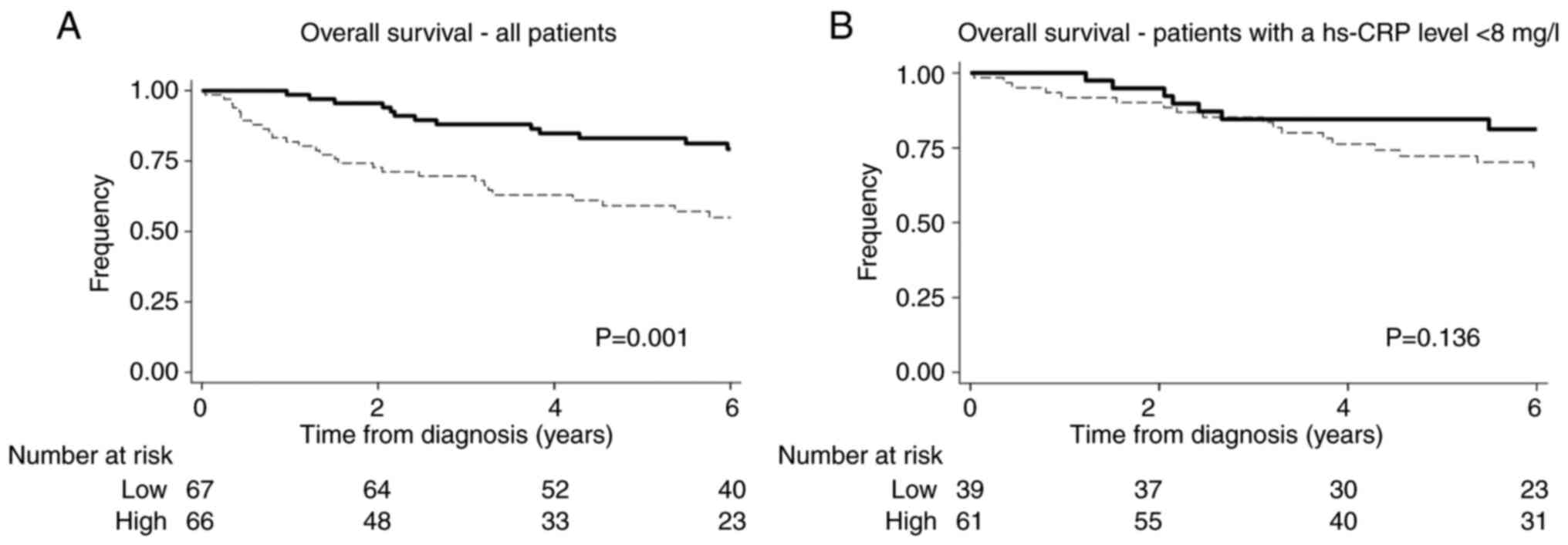
median value 62% (95% CI: 48–73%) see Fig. 4. Furthermore, a higher hs-CRP was
associated with a reduced overall survival demonstrated by the
5-year overall survival for patients with a hs-CRP below the median
value: of 85% (95% CI: 69–93%) compared to patients with a hs-CRP
above the median value: 72% (95% CI: 59–82%) see Fig. 5.
Discussion
This study investigated the association between
serum high-sensitivity C-reactive protein (hs-CRP) and
progression-free survival in patients with localised sarcoma
treated with curative intent. The results showed that hs-CRP is an
adverse prognostic factor for progression-free survival in these
patients, even after adjusting for known prognostic factors.
Several studies have investigated the relationship
between hs-CRP and the presence of cancer. In this article, the
optimal cut-off for the prognostic values of hs-CRP was determined
as the median values and not the cut-off based on the ROC curve.
The ROC curve cut-off is based on the endpoint investigated and,
therefore, this can only be applied when using a test and
validation cohort. Other studies used the median value.
A study by Lee et al (25) found that hs-CRP levels are
significantly higher in different cancer patients than in healthy
controls, with a median hs-CRP of 0.77 and 0.59 mg/l for men and
women, respectively. Nakamura et al (22) found a threshold level of 0.95 mg/l
for discriminating between sarcoma and healthy control patients.
These results are in alliance with our findings. However, the
median level in our study was higher than that reported by Lee
et al (25) and Nakamura
et al (22). This difference
could be due to the body mass index (BMI) being higher in
Caucasians than the Asian participants (25). We did not have information about BMI
in our study. Nevertheless, this is supported by Cho et al
(26), who found higher hs-CRP
among breast cancer survivors with a larger body mass index than
patients with a lower BMI.
Lee et al (25) found that a one mg/l increase in
hs-CRP was associated with increased mortality after a 17-year
follow-up, but the association was found only in women. Our study
did not show any difference in the CRP levels between sexes;
however, we observed that an increase in CRP by one mg/l led to a
2.69 increased risk of dying (median follow-up time was 6.9 years).
Similarly, in the study by Nakamura et al (22), hs-CRP levels were associated with
poor prognosis and decreased survival in patients with soft tissue
sarcoma. All these results suggest a relationship between hs-CRP
and sarcoma.
Desmoid tumours included in this study represent
locally aggressive neoplasms that do not metastasise but are
challenging to treat. As for other cancers, the tumour immune
microenvironment (TME) is essential when considering immunotherapy.
One study investigated the immune expression markers of 33 tissue
samples from patients suffering from desmoid tumours. This study
concluded that desmoid tumours have a solid immune infiltration in
the tumour margins; however, PD-L1 was not present in the tumour
cells. PD-L1 is a target for immunotherapy (27). In our study, the level of hs-CRP was
not elevated compared to the control group, indicating that the
inflammation in these patients may not play a pivotal role.
Several mechanisms have been proposed to explain the
link between CRP and cancer. One mechanism involves the role of
inflammation in promoting tumour initiation and progression
(28,29). CRP is a marker of systemic
inflammation, and chronic inflammation has been linked to several
types of cancer, including sarcoma (15,17,18).
In addition, an in vivo experiment has shown that CRP
influences the tumour microenvironment by promoting suppressive
tumour-associated macrophages (30). These macrophages promote
angiogenesis, an essential process for tumour growth and metastases
(31). Another proposed mechanism
involves the role of CRP as a marker of immune system dysfunction
(13).
The strengths of this study include its prospective
design, large sample size and the use of a highly sensitive assay
to measure hs-CRP, which allowed for the detection of low levels of
inflammation. In addition, the comprehensive clinical data and
biomarker measurements allowed for the adjustment of potential
confounders in the analysis. Patients suspected of having sarcoma
but who turned out to have a benign condition comprise the control
group in this study. It is a strength in this study as the
destining of sarcoma and non-sarcoma patients is essential.
However, there are also some limitations to this study. First, the
study evaluated soft tissue sarcoma patients as one collective
group of patients, which could blur variations between different
histological subtypes. Second, the study was not designed to
investigate the underlying mechanisms linking hs-CRP and mortality
in sarcoma patients, and further studies are needed to elucidate
these mechanisms.
However, it is known that tumour-associated
macrophages (TAM) are believed to be one of the major
immunosuppressive components in cancers. The association between
high levels of serum CRP and the number of tumour-associated
macrophages of the subtypes CD204 and CD163 has been shown for
patients with hepatocellular cancer (32). The activation of monocytes to
immunosuppressive macrophages is believed to be facilitated by CRP
along with the activation of endothelial cells, platelets and the
complement system, which are important in the innate immune
response leading to macrophage recruitment and activation (33). Fig.
6 is a proposed association between CRP and the innate immune
system.
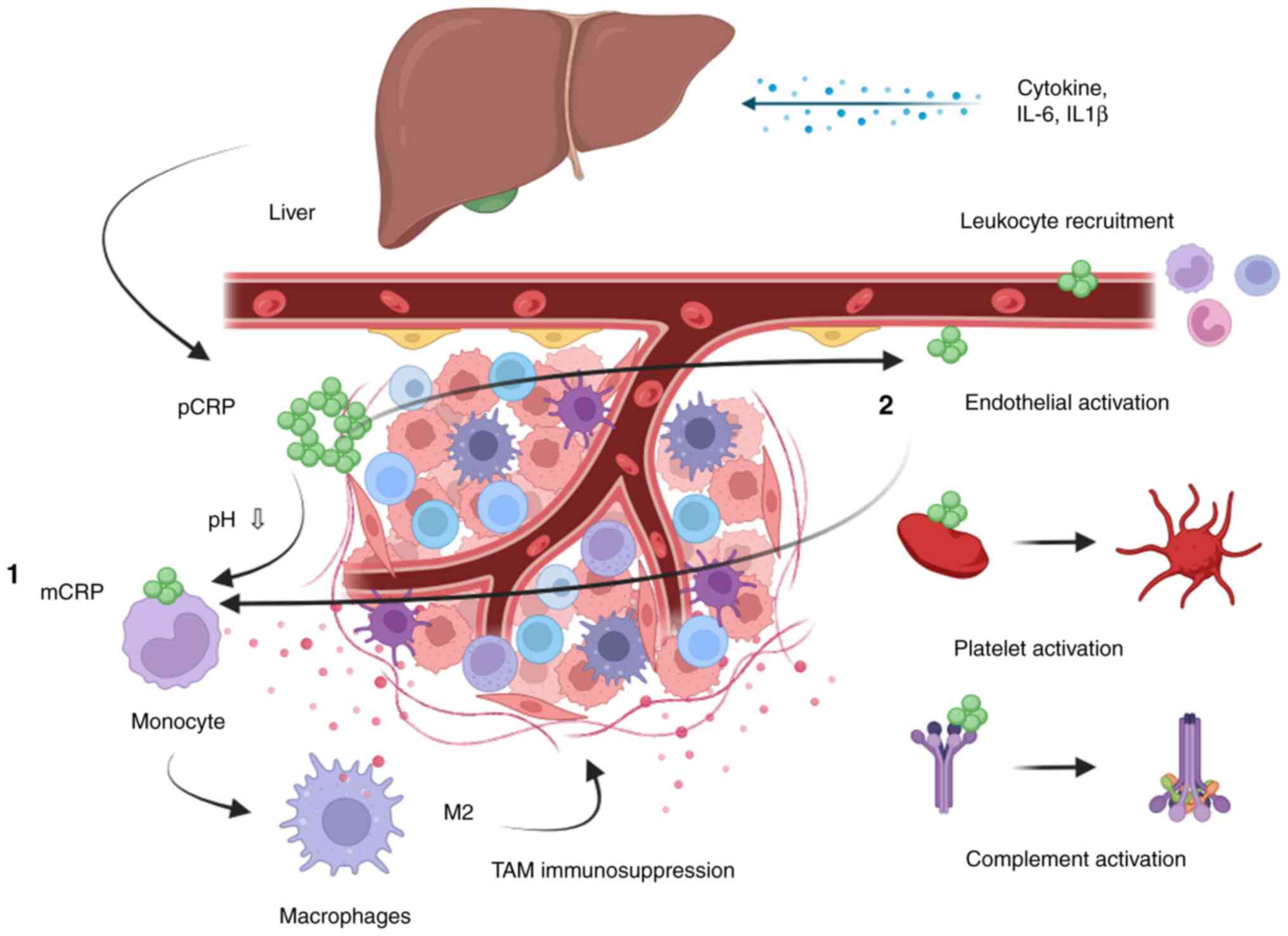 | Figure 6.Illustration of the proposed role of
CRP and the tumour microenvironment. The liver produces pCRP as a
result of inflammatory cytokines, such as IL-6 and IL1β, excreted
from cancer cells. pCRP is dissociated into mCRP on the surface of
different cells, such as monocytes (1) and endothelial cells (2), through the binding to a ligand called
phosphocholine. 1, mCRP together with other inflammatory markers
might promote the production of immune-inhibitory TAMs called
M2-directed macrophages. 2, The recruitment and activation of
M2-directed TAMs could also be due to the endothelial activation by
CRP. The endothelial activation might facilitate leukocyte
recruitment, including recruitment of monocytes to the tumour. The
recruitment of monocytes to the tumour increases and promotes the
development into TAMs (M2 directed). Furthermore, platelet and
complement activation are facilitated, which are essential in the
innate immune response. CRP, C-reactive protein; mCRP, monomeric
CRP; pCRP, pentamer of CRP; TAM, tumour-associated macrophage. |
Additionally, it is essential to note that sarcoma
is a rare and complex disease, and more research is needed to
understand the underlying mechanisms fully and develop new
treatments and therapies for sarcoma patients. Using biomarkers
such as hs-CRP and inflammation can help improve the diagnosis,
prognosis, and management of sarcoma, but it is crucial to use them
in conjunction with other diagnostic tests and imaging studies to
make a definitive diagnosis. Furthermore, integrating these
biomarkers with other omics technologies can provide a
comprehensive understanding of the disease and help identify new
diagnostic and therapeutic targets.
In conclusion, High-Sensitive C-reactive protein and
inflammation are important biomarkers where high levels of the
individual biomarker are linked to more advanced stages of sarcoma
and increased risk of mortality. These biomarkers can also
influence a patient's response to therapy; therefore, treatment
plans for sarcoma must be tailored to account for an individual
inflammatory profile. More research is needed to understand the
underlying mechanisms and develop new treatments and therapies for
sarcoma patients.
Acknowledgements
The Danish CancerBiobank (Aarhus, Denmark) is
acknowledged for handling and storing biological material.
Funding
The present study was supported by a grant from the Danish
Cancer Society (grant no. R248-Ai4683).
Availability of data and materials
The data that support the findings of this study are
available from the Danish Regions but restrictions apply to the
availability of these data, which were used under license for the
current study, and so are not publicly available. Data are however
available from the authors upon reasonable request and with
permission of Danish Ethical commission.
Authors' contributions
NAP, TBH, HJM and BSP conceived the study. NAP and
BSP wrote the original draft. NAP, TBH, HJM and BSP wrote, reviewed
and edited the manuscript. BSP and NAP confirm the authenticity of
all the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The regional Ethics Committee of Denmark (approval
no. 1-10-72-58-14; Region Midtjylland, Viborg, Denmark) and the
Danish Agency of Data Protection (approval no. 1-16-02-112-14;
Valby, Denmark) have approved the study. Before obtaining the blood
samples, written informed consent was obtained from the
patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Schwartzberg L, Korytowsky B, Penrod JR,
Zhang Y, Le TK, Batenchuk C and Krug L: Real-world clinical impact
of immune checkpoint inhibitors in patients with
advanced/metastatic non-small cell lung cancer after platinum
chemotherapy. Clin Lung Cancer. 20:287–296.e4. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li J and Gu J: Efficacy and safety of PD-1
inhibitors for treating advanced melanoma: A systematic review and
meta-analysis. Immunotherapy. 10:1293–1302. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Saerens M, Brusselaers N, Rottey S,
Decruyenaere A, Creytens D and Lapeire L: Immune checkpoint
inhibitors in treatment of soft-tissue sarcoma: A systematic review
and meta-analysis. Eur J Cancer. 152:165–182. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kolb-Bachofen V: A review on the
biological properties of C-reactive protein. Immunobiology.
183:133–145. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Clyne B and Olshaker JS: The C-reactive
protein. J Emerg Med. 17:1019–1025. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Calabró P, Willerson JT and Yeh ETH:
Inflammatory cytokines stimulated C-reactive protein production by
human coronary artery smooth muscle cells. Circulation.
108:1930–1932. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guo F, Liu JT, Wang CJ and Pang XM:
Pravastatin inhibits C-reactive protein generation induced by
fibrinogen, fibrin and FDP in isolated rat vascular smooth muscle
cells. Inflamm Res. 61:127–134. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xing XQ, Duan S, Wu XW, Gan Y, Zhao SP,
Chen P and Wu SJ: Atorvastatin reduces lipopolysaccharide-induced
expression of C-reactive protein in human lung epithelial cells.
Mol Med Rep. 4:753–757. 2011.PubMed/NCBI
|
|
9
|
Peyrin-Biroulet L, Gonzalez F, Dubuquoy L,
Rousseaux C, Dubuquoy C, Decourcelle C, Saudemont A, Tachon M,
Béclin E, Odou MF, et al: Mesenteric fat as a source of C reactive
protein and as a target for bacterial translocation in Crohn's
disease. Gut. 61:78–85. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li M, Liu JT, Pang XM, Han CJ and Mao JJ:
Epigallocatechin-3-gallate inhibits angiotensin II and
interleukin-6-induced C-reactive protein production in macrophages.
Pharmacol Rep. 64:912–918. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Banait T, Wanjari A, Danade V, Banait S
and Jain J: Role of high-sensitivity C-reactive protein (Hs-CRP) in
non-communicable diseases: A review. Cureus.
14:e302252022.PubMed/NCBI
|
|
12
|
Du Clos TW: Function of C-reactive
protein. Ann Med. 32:274–278. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoshida T, Ichikawa J, Giuroiu I, Laino
AS, Hao Y, Krogsgaard M, Vassallo M, Woods DM, Stephen Hodi F and
Weber J: C reactive protein impairs adaptive immunity in immune
cells of patients with melanoma. J Immunother Cancer.
8:e0002342020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nakamura T, Katagiri H, Shido Y, Hamada S,
Yamada K, Nagano A, Yamada S, Tsukushi S, Ishimura D, Matsumine A,
et al: Analysis of factors for predicting survival in soft-tissue
sarcoma with metastatic disease at initial presentation. Anticancer
Res. 37:3137–1341. 2017.PubMed/NCBI
|
|
15
|
Aggerholm-Pedersen N, Maretty-Kongstad K,
Keller J and Safwat A: Serum biomarkers as prognostic factors for
metastatic sarcoma. Clin Oncol (R Coll Radiol). 31:242–249. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nakamura T, Matsumine A, Matsubara T,
Asanuma K, Uchida A and Sudo A: Clinical significance of
pretreatment serum C-reactive protein level in soft tissue sarcoma.
Cancer. 118:1055–1061. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maretty-Kongstad K, Aggerholm-Pedersen N,
Keller J and Safwat A: A validated prognostic biomarker score for
adult patients with nonmetastatic soft tissue sarcomas of the trunk
and extremities. Transl Oncol. 10:942–948. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Aggerholm-Pedersen N, Maretty-Kongstad K,
Keller J, Baerentzen S and Safwat A: The prognostic value of serum
biomarkers in localized bone sarcoma. Transl Oncol. 9:322–328.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Funovics PT, Edelhauser G, Funovics MA,
Laux C, Berzaczy D, Kubista B, Kotz RI and Dominkus M:
Pre-operative serum C-reactive protein as independent prognostic
factor for survival but not infection in patients with high-grade
osteosarcoma. Int Orthop. 35:1529–1536. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kandelouei T, Abbasifard M, Imani D,
Aslani S, Razi B, Fasihi M, Shafiekhani S, Mohammadi K,
Jamialahmadi T, Reiner Ž and Sahebkar A: Effect of statins on serum
level of hs-CRP and CRP in patients with cardiovascular diseases: A
systematic review and meta-analysis of randomized controlled
trials. Mediators Inflamm. 2022:87323602022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ghule A, Kamble TK, Talwar D, Kumar S,
Acharya S, Wanjari A, Gaidhane SA and Agrawal S: Association of
serum high sensitivity C-reactive protein with pre-diabetes in
rural population: A two-year cross-sectional study. Cureus.
13:e190882021.PubMed/NCBI
|
|
22
|
Nakamura T, Matsumine A, Iino T, Matsubara
T, Asanuma K, Uchida A and Sudo A: Role of high-sensitivity
C-reactive protein in the differentiation of benign and malignant
soft tissue tumors. Anticancer Res. 34:933–936. 2014.PubMed/NCBI
|
|
23
|
Aggerholm-Pedersen N, Friis HN,
Baad-Hansen T, Møller HJ and Sandfeld-Paulsen B: Macrophage
biomarkers sCD163 and sSIRPα in serum predict mortality in sarcoma
patients. Cancers (Basel). 15:15442023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Unal I: Defining an optimal cut-point
value in ROC analysis: An alternative approach. Comput Math Methods
Med. 2017:37626512017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee SA, Kwon SO, Song M, Choi JY, Shin A,
Shu XO, Zheng W, Lee JK and Kang D: The association of serum
high-sensitivity C-reactive protein level with the risk of
site-specific cancer mortality: The health examinees (HEXA) study
cohort. Am J Epidemiol. 191:2002–2013. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cho HJ, Song S, Kim Z, Youn HJ, Cho J, Min
JW, Kim YS, Choi SW and Lee JE: Associations of body mass index and
weight change with circulating levels of high-sensitivity
C-reactive protein, proinflammatory cytokines, and adiponectin
among breast cancer survivors. Asia Pac J Clin Oncol. 19:113–125.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Siozopoulou V, Marcq E, Jacobs J,
Zwaenepoel K, Hermans C, Brauns J, Pauwels S, Huysentruyt C,
Lammens M, Somville J, et al: Desmoid tumors display a strong
immune infiltration at the tumor margins and no PD-L1-driven immune
suppression. Cancer Immunol Immunother. 68:1573–1583. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Aggarwal BB, Shishodia S, Sandur SK,
Pandey MK and Sethi G: Inflammation and cancer: how hot is the
link? Biochem Pharmacol. 72:1605–1621. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–868. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Marjon KD, Marnell LL, Mold C and Du Clos
TW: Macrophages activated by C-reactive protein through Fc gamma RI
transfer suppression of immune thrombocytopenia. J Immunol.
182:1397–1403. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fujiwara T, Healey J, Ogura K, Yoshida A,
Kondo H, Hata T, Kure M, Tazawa H, Nakata E, Kunisada T, et al:
Role of tumor-associated macrophages in sarcomas. Cancers (Basel).
13:10862021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang Y, Li Z, Huang Z, Yu X, Zheng L and
Xu J: C-reactive protein is an indicator of the immunosuppressive
microenvironment fostered by myeloid cells in hepatocellular
carcinoma. Front Oncol. 11:7748232022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
McFadyen JD, Kiefer J, Braig D,
Loseff-Silver J, Potempa LA, Eisenhardt SU and Peter K:
Dissociation of C-reactive protein localizes and amplifies
inflammation: Evidence for a direct biological role of C-reactive
protein and its conformational changes. Front Immunol. 9:13512018.
View Article : Google Scholar : PubMed/NCBI
|