Introduction
Calcium homeostasis-related proteins have been
identified as crucial driving factors that regulate ATP synthesis
and influence tumorigenesis and progression, and are closely
associated with the proliferation, differentiation and metastasis
of cancer cells (including cervical cancer) (1). Resveratrol specifically kills cancer
cells by an increase in the Ca2+ coupling between the
endoplasmic reticulum and mitochondria (2). Calcium/calmodulin-dependent protein
kinase kinase 2 (CaMKK2) is a calcium-dependent protein kinase and
its activation can promote glycolysis, mitochondrial respiration
and fatty acid metabolism, which increases the viability of tumor
cells (3). The regulation of CaMKK2
can vary with prostate cancer, and in a previous study CaMKK2 was
reported to be a direct target of the androgen receptor in prostate
cancer cells (4). CaMKK2 inhibitors
can reduce the proliferation and metastatic ability of lung cancer
cells in vivo (5). Targeting
CaMKK2 expression can improve the survival rate of patients with
liver cancer and inhibit the tumorigenicity of liver cancer cells
in in vivo models (6). By
mediating calmodulin activation, CaMKK2 promotes the accumulation
of calcium ions in cancer cells to activate EGFR and decrease the
survival of these cells (7). The
mitochondrial sodium/calcium exchanger protein (NCLX) is a
sodium-calcium ion exchange protein involved in the proliferation,
differentiation and metastasis of cancer cells by maintaining
intracellular calcium ion balance (8). A previous study demonstrated that
knocking down NCLX significantly reduced mitochondrial ATP
generation and inhibited the proliferation and tumor growth of
colorectal cancer cells (9). In
human colorectal tumors and spontaneous colorectal cancer mouse
models, downregulation of NCLX leads to mitochondrial calcium
overload, mitochondrial depolarization, reduced expression of cell
cycle genes and inhibition of the growth of the xenograft tumor
(8). CGP37157, a benzodiazepine
derivative, promotes mitochondrial damage and induces cell
apoptosis by inhibiting NCLX expression in neuronal cells (10). CaMKK2 and NCLX both serve crucial
roles in maintaining calcium homeostasis. However, the regulatory
roles of CaMKK2 and NCLX in liver cancer cells are not clear.
The mechanism of energy metabolism regulation in
liver cancer cells is complex, but ATP synthase is essential for
energy metabolism (11,12). The expression level of ATP synthase
is a key indicator reflecting the energy metabolism state of liver
cancer cells (13). ATP synthase
(ATP1A1 and ATP5H subunits) is involved in cell proliferation,
division and the metastasis of liver cancer cells (13,14).
High expression levels of ATP synthase subunit d, mitochondrial
(ATP5H) are linked to poor prognosis of patients with ovarian
cancer and are involved in cisplatin resistance, cell experiments
have shown that dihydroartemisinin (DHA) treatment can reduce the
side effects of cisplatin and reverse cisplatin resistance
(15). Nevertheless, elevated NRF2
may mediate DHA resistance in head and neck squamous cell carcinoma
(16). Epigenetic loss of the ATP
synthase subunit ATP5H triggers a core metabolic reprogramming
pathway, leading to reactive oxygen species (ROS) accumulation and
elevated hypoxia induced factor-1α, thereby promoting multiple drug
resistance in tumor cells (17).
Bufalin inhibits tumorigenesis in liver cancer cells by regulating
sodium/potassium-transporting ATPase subunit α-1 (ATP1A1)/carbonic
anhydrase 2 signaling (18). ATP1A1
has been associated with the tamoxifen resistance of breast cancer
through screening of super-enhancer-associated proteins (19). Homologous ATP1A1 binding between
ATP1A1-overexpressing tumor cells and fibroblasts was found in
pancreatic ductal adenocarcinoma cells (20). Activin A secreted by fibroblasts
induces EMT of tumor cells and activation of myofibroblasts
(20). Disrupting ion homeostasis
in cancer cells can synergize with MAPK pathway inhibitors to
promote melanoma regression in vivo (21).
DHA, a derivative of artemisinin, has shown
potential antitumor effects in cancer therapy (22). DHA can induce ferroptosis of
cervical cancer cells by regulating the production of ROS and
accumulation of malondialdehyde, and when paired with doxorubicin,
DHA can inhibit the proliferation and metastasis of cervical cancer
cells (23). DHA can reduce the
proliferation of breast cancer cells by inhibiting STAT3
phosphorylation, inducing apoptosis and reversing cisplatin
resistance (24). DHA exerts
antitumor effects by inhibiting glycolysis, promoting autophagy and
inducing mitochondrial ATP production (25). The present study investigated the
effects of DHA on the mitochondrial function, cytoskeleton,
proliferation, migration and invasion of liver cancer cells through
ATP probes, ROS, cytoskeleton, colony formation, EDU, cell scratch,
and Transwell experiments. By constructing CaMKK2 and NCLX siRNA
cell models, it investigated whether DHA inhibited cancer cell
proliferation, reduced ATP and ROS production, promoted
cytoskeletal recombination and inhibited the invasive phenotype of
cancer cells through the CaMKK2/NCLX axis.
Materials and methods
Cell culture and transfection
Human liver cancer cells HepG2 and HuH-7 were
purchased from the Cell Bank of Type Culture Collection of The
Chinese Academy of Sciences and were grown in Dulbecco's Modified
Eagle Medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.) with 10%
fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and
1% penicillin/streptomycin (Gibco; Thermo Fisher Scientific, Inc.)
at 37°C with 5% CO2. Short tandem repeat profiling was
used to verify the HepG2 and HuH-7 cell lines. DHA was purchased
from MedChemExpress (cat. no. HY-N0176; purity, ≥99.0%) and was
dissolved in DMSO with the final working concentration as 10 µM at
room temperature (19) and DMSO was
used as a control. Full-length CaMKK2 (Sangon Biotech Co., Ltd.)
was cloned into the pcDNA3.1(+) ZB02427 vector (Sangon Biotech Co.,
Ltd.). Sangon Biotech was the supplier of CaMKK2 and the plasmid
backbone. A total of 2.5 µg CaMKK2 overexpression (OE) plasmid was
used for transfection. Then transfection with Lipo8000 (cat. no.
C0533; Beyotime Institute of Biotechnology) at 37°C for 24 h. Empty
vector was utilized as a negative control (NC). Small interfering
(si)RNAs were purchased from Sangon Biotech Co., Ltd., and their
sequences were as follows: NCLX siRNA1 (sense,
5′-UGAGUGUGCUUUGUGUGCUGCUAAU-3′, and antisense
5′-AUUAGCAGCACACAAAGCACACUCA-3′), NCLX siRNA2 (sense,
5′-GGGAAUGGUGCACCUGACAUCUUCA-3′, and antisense,
5′-UGAAGAUGUCAGGUGCACCAUUCCC-3′), NCLX siRNA3 (sense,
5′-CCGGGUAUCUUCUAAUACCAATT-3′ and antisense,
5′-UUGGUAUUAGAAGAUACCCGGTT-3′), CaMKK2 siRNA1 (sense,
5′-GAUGAAAUUGGAAAGGGCUCCUAUG-3′ and antisense,
5′-CAUAGGAGCCCUUUCCAAUUUCAUC-3′) CaMKK2 siRNA2 (sense,
5′-CCCAUUGAGCAGGUGUACCAGGAAA-3′, and antisense,
5′-UUUCCUGGUACACCUGCUCAAUGGG-3′) CaMKK2 siRNA3 (sense,
5′-CAAUACCUACUAUGCAAUGAATT-3′ and antisense,
5′-UUCAUUGCAUAGUAGGUAUUGTT-3′) and siRNA-NC (sense,
5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense,
5′-ACGUGACACGUUCGGAGAATT-3′). For all plasmid and siRNA Sequence
transfections, HepG2 and HuH-7 cells were seeded in 6-well plates
(2×105 cells/well) and incubated at 37°C and 5%
CO2 for 24 h. Then, 100 pmol siRNA was added per well
and Lipo8000 (cat. no. C0533; Beyotime Institute of Biotechnology)
used for transfection at room temperature for 24 h. Cells were
collected for subsequent western blotting assays to confirm the
success of the transfections. After successful transfection the
cells were incubated at 37°C and 5% CO2 for 24 h prior
to subsequent experiments.
EdU-594 staining
The BeyoClick™ EdU-594 cell proliferation assay kit
with Alexa Flour 594 (cat. no. C0078S; Beyotime Institute of
Biotechnology) was used according to the manufacturer's
instructions. HepG2 and HuH-7 cells (4×104 cells/well)
were seeded in 96-well plates and incubated at 37°C in a 5%
CO2 atmosphere. The cells were treated with 10 µM EDU
solution for 2 h at 37°C. Then, Click reaction solution was added
and the cells were incubated for 30 min at room temperature, then
washed three times with PBS. Cells were stained with DAPI (10
µg/ml) for 10 min at room temperature, and were subsequently imaged
using a fluorescence microscope (Leica Microsystems GmbH). Data
were analyzed by Image-Pro Plus Software 6.0 (Media Cybernetics,
Inc.).
Transwell migration assay
HepG2 and HuH-7 cells were starved without serum for
24 h in 6-well plates at 37°C. Cells were collected, resuspended in
200 µl serum-free DMEM to 1×105 cells/ml and added to
24-well upper chambers (Corning, Inc.). A total of 600 µl DMEM with
10% FBS was added to the lower chambers. After incubating for 48 h
at 37°C, cells were fixed with 4% paraformaldehyde for 10 min at
room temperature and stained using 0.1% crystal violet for 20 min
at room temperature. Cells that did not pass through the Transwell
membrane were wiped away with a cotton swab following the staining.
Matrigel inserts were used (coated in the chambers at 37°C for 5
h); the subsequent steps were identical to the migration assay. The
remaining cells were imaged using a light microscope (Olympus
Corporation). The invaded cells were counted via Image-Pro Plus
Software 6.0 (Media Cybernetics, USA).
Wound healing assay
HepG2 and HuH-7 cells were seeded in 6-well plates
(5×105 cells/well) and cultured at 37°C with 5%
CO2 until cell confluence was >90%. A single scratch
was made in the cell monolayer using a 200 µl pipette tip. After
washing with PBS three times, DMEM with 1% FBS was added to each
well (26) and cells were imaged at
0 and 48 h using a light microscope. The migration rate was
calculated using the following formula: Migration rate (%)=(S0-S48
h)/S0 h ×100. S0 h represents the distance of the scratch at 0 and
S48 h represents the distance at 48 h (27). Wound healing was analyzed using the
Image-Pro Plus Software 6.0 (Media Cybernetics, USA).
Colony formation assay
HepG2 and HuH-7 cells were seeded in 3.5 cm plates
at ~200 cells/well in DMEM with 10% FBS. The groups treated with
CaMKK2-OE, NCLX knockout and DHA were incubated at 37°C for 24 h.
Then, the treated cells were cultured for ~15 days until visible
colonies (>50 cells) formed. The supernatant was discarded and
cells were washed twice with PBS. Subsequently, cells were fixed
with 4% paraformaldehyde for 15 min at room temperature, stained
with 0. 1% crystal violet for 20 min at room temperature, washed
twice with PBS and air-dried. A light microscope (Olympus
Corporation) was used to image cells and the colonies counted
manually.
Western blotting
HepG2 and HuH-7 cells were lysed with RIPA lysis
buffer (Nanjing KeyGen Biotech Co., Ltd.) on ice for 25 min. Total
cellular protein concentration was determined using a BCA protein
assay kit (Leagene Biotechnology). SDS-PAGE loading buffer (5X;
Beijing Zoman Biotechnology Co., Ltd.) was used to denature the
proteins at 100°C for 10 min. 30 ng proteins were then separated by
9% SDS-PAGE and transferred onto PVDF membranes. The PVDF membranes
were incubated with 5% skimmed milk dissolved in TBS-1% Tween 20
(TBST) at room temperature for 1 h. After blocking, membranes were
incubated with primary antibodies overnight at 4°C. The primary
antibodies and corresponding dilutions used were as follows:
Anti-ATP1A1 (1:5,000; cat. no. 14418-1-AP; Wuhan Sanying
Biotechnology), anti-ATP5H (1:500; cat. no. 17589-1-AP; Wuhan
Sanying Biotechnology), anti-NCLX (1:1,000; cat. no. 21430-1-AP;
Wuhan Sanying Biotechnology), anti-CaMKK2 (1:500; cat. no.
11549-1-AP; Wuhan Sanying Biotechnology) and anti-β-actin (1:2,000;
cat. no. 20536-1-AP; Wuhan Sanying Biotechnology). The membranes
were then washed with TBST three times and incubated with
anti-rabbit horseradish peroxidase-conjugated secondary antibodies
(1:5,000; cat. no. ZB-2301; OriGene Technologies, Inc.) at room
temperature for 1 h. The membranes were visualized using an ECL kit
(cat. no. A38554; Thermo Fisher Scientific, Inc.) and analyzed with
Image-Pro Plus Software 6.0 (Media Cybernetics, Inc.).
Intracellular detection of ROS
levels
ROS levels were detected using ROS Assay Kit (cat.
no. S0033, Beyotime Institute of Biotechnology) according to
manufacturer's instructions. Briefly, HepG2 and HuH-7 cells were
seeded in 6-well plates (1×105 cells/well) and incubated
with 2′,7′-dichlorofluorescein diacetate (10 µM) at 37°C for 20
min. A fluorescence microscope was then used to image cells. The
level of intracellular ROS was expressed as the fluorescence
intensity. Data analysis were performed using Image-Pro Plus
Software 6.0 (Media Cybernetics, Inc.).
Detection of apoptosis by YO-PRO-1/PI
staining
The Apoptosis and Necrosis Detection Kit with
YO-PRO-1 and PI (cat. no. C1075; Beyotime Institute of
Biotechnology) was used according to the manufacturer's
instructions. YP1/PI working solution was added to HepG2 and HuH-7
cells (5×105 cells/well) were seeded in a 6-well plate,
then cells were incubated at 37°C for 20 min in the dark. After
incubation, cells were imaged using a fluorescence microscope.
Apoptotic and necrotic cells exhibited green and red fluorescence,
with overlapping orange yellow fluorescence. The percentage of
apoptotic and necrotic cells was manually counted and
calculated.
Reverse transcription-quantitative PCR
(RT-qPCR)
TRIzol® (Thermo Fisher Scientific, Inc.)
was used for RNA extraction from HepG2 and HuH-7 cells. cDNA was
synthesized using the RevertAid First Strand cDNA Synthesis Kit
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions. qPCR was performed according to the instructions of
PowerUp™ SYBR™ Green Master Mix (Applied Biosystems; Thermo Fisher
Scientific, Inc.). Thermocycling consisted of 20 sec of initial
denaturation at 95°C, followed by 35 cycles with 20 sec at 95°C for
denaturation and 20 sec at 60°C for annealing and extension.
Gene-specific primers were synthesized by Sangon Biotech Co., Ltd.
The primer sequences used were as follows: ATP1A1 forward (F),
5′-TGCCCTGGAATGGGTGTTGCT-3′ and reverse (R),
5′-TTCTCCACCCAGCCGCCAGG-3′; ATP5H F, 5′-TAATGCGCTGAAGGTTCCCG-3′ and
R, 5′-GAGAGACACCCACTCAGCAC-3′; CaMKK2 F, 5′-ATGGGCACATCAAGATCGCT-3′
and R, 5′-CATCCAAGGCCTTCCCAGAG-3′; NCLX F,
5′-GCCTTCTCTGACCCGCACAC-3′ and R, 5′-CCTCTCCGTTGCCGTTGGTAG-3′; and
GAPDH F, 5′-TCAAGATCATCAGCAATGCC-3′ and R,
5′-CGATACCAAAGTTGTCATGGA-3′. Relative gene expression levels were
normalized using the 2−ΔΔCq method (28). GAPDH was used as the reference
gene.
F-actin microfilament staining
Imaging of the microfilaments of HepG2 and HuH-7
cells was carried out according to the instruction of Actin-Tracker
Green-488 (Beyotime Institute of Biotechnology). HepG2 and HuH-7
cells (5×105 cells/well) were seeded in a 6-well plate.
They were fixed with 3.7% formaldehyde in PBS at room temperature
for 20 min. Then, the cells were incubated with Actin-Tracker
Green-488 solution labeled with phalloidin in the dark for 30 min
and observed with a fluorescence microscope. Finally, DAPI (10
µg/ml) staining solution was used to re-stain the nuclei for 5 min
at room temperature. All staining was quantified using Image-Pro
Plus Software 6.0 (Media Cybernetics, Inc.). Phalloidin-stained
areas were expressed as percentage of whole areas per microscopic
field (29). The fluorescence
intensities were measured with Image-Pro Plus Software 6.0 (Media
Cybernetics, Inc.).
Detection of ATP content
ATP fluorescent probe (pCMV-AT1.03) was purchased
from (Beyotime Institute of Biotechnology). PCMV-AT1.03 is a
plasmid tool used to express AT1.031 protein as an ATP fluorescence
probe in cells. Following transfection, the AT1.031 protein is
primarily found in the cytoplasm where it can detect changes in ATP
content and produce fluorescence when it binds to ATP. HepG2 and
HuH-7 cells (2×105 cells/well) were seeded into 12-well
plates and cultured at 37° with 5% CO2 for 24 h.
Subsequently, 1 µg of ATP fluorescent probe was added was added per
well and Lipo8000 (cat. no. C0533; Beyotime Institute of
Biotechnology) used for transfection for 24 h at 37°C. After cells
were transfected with CaMKK2-OE and NCLX siRNA and/or DHA for 24 h
at 37°C, cells were imaged using a fluorescence microscope. The
image was analyzed with Image-Pro Plus Software 6.0 (Media
Cybernetics, USA).
Statistical analysis
SPSS software (version 17.0; SPSS, Inc.) was used
for data analysis, and the data are presented as the mean ±
standard deviation. Differences between groups were compared with
one-way analysis of variance followed by Bonferroni post hoc test.
Data were visualized using GraphPad Prism software (version 8.0;
Dotmatics). P<0.05 was considered to indicate a statistically
significant difference.
Results
Effects of DHA treatment, CaMKK2-OE
and NCLX knockdown on the proliferation and apoptosis of liver
cancer cells
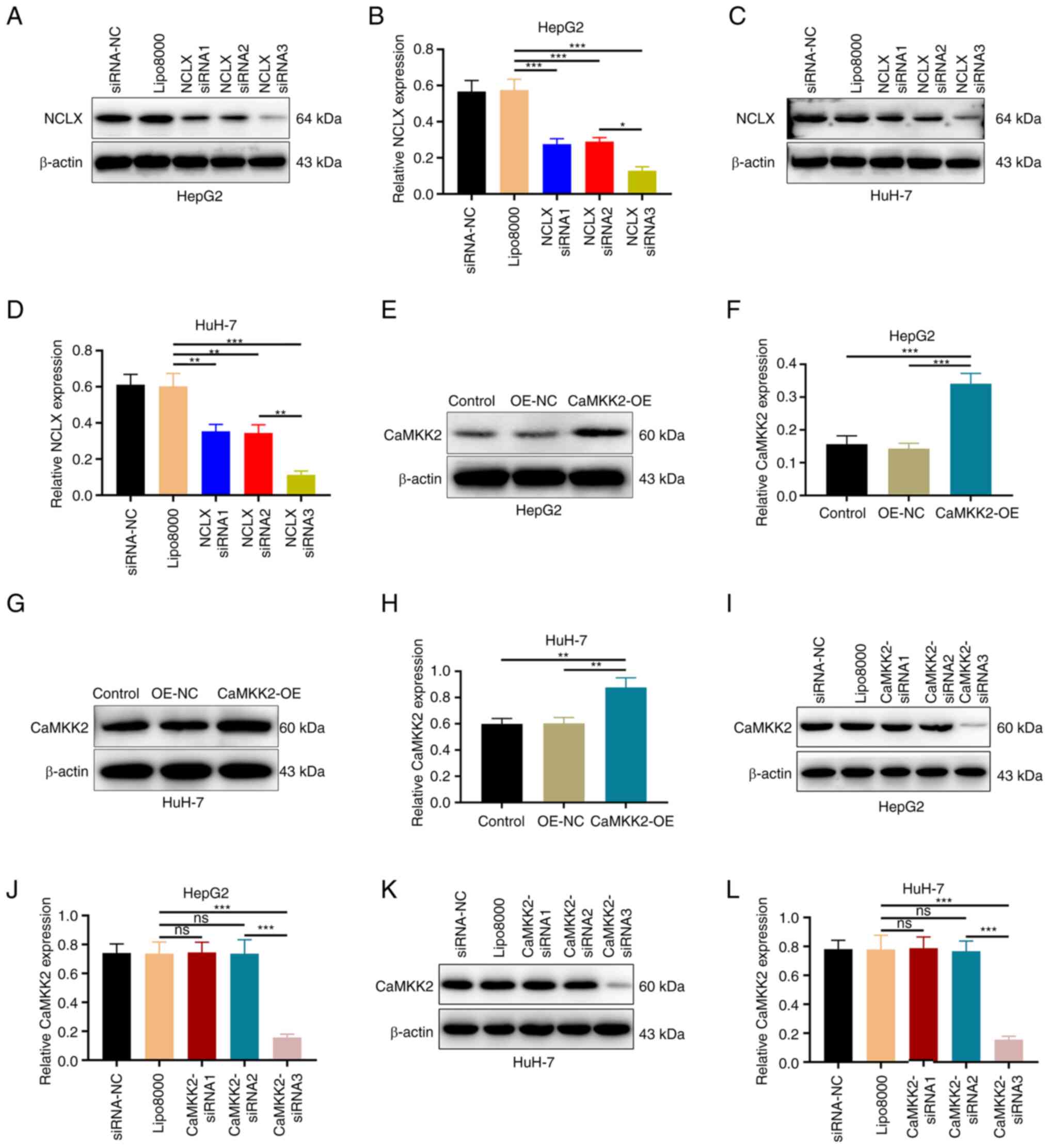
NCLX and CaMKK2 siRNA-mediated knockdown and
CaMKK2-OE in HepG2 and HuH-7 cells were verified through western
blotting (Fig. 1). The transfection
effect of NCLX siRNA3 was more significant, so this was chosen for
the following experiments (Fig.
1A-D). The overexpression of CaMKK2 was significant (Fig. 1E-H). The transfection effect of
CaMKK2 siRNA3 was more significant, which was chosen for follow-up
experiments (Fig. 1I-L).
Colony formation assay and EdU staining were used to
analyze the proliferation rate of these cells. These assays
demonstrated that CaMKK2-OE significantly increased cell
proliferation compared with the OE-NC group. DHA treatment and NCLX
knockdown significantly inhibited proliferation compared with
CaMKK2-OE group (Figs. 2A-D and
S1). In addition, analysis of
apoptosis and necrosis demonstrated that following DHA treatment or
NCLX knockdown in CaMKK2-OE group significantly increased the
number of apoptotic and necrotic liver cancer cells (Fig. 2E-G). NCLX siRNA alone and DHA alone
also significantly inhibited proliferation and increased the number
of apoptosis and necrosis of cancer cells.
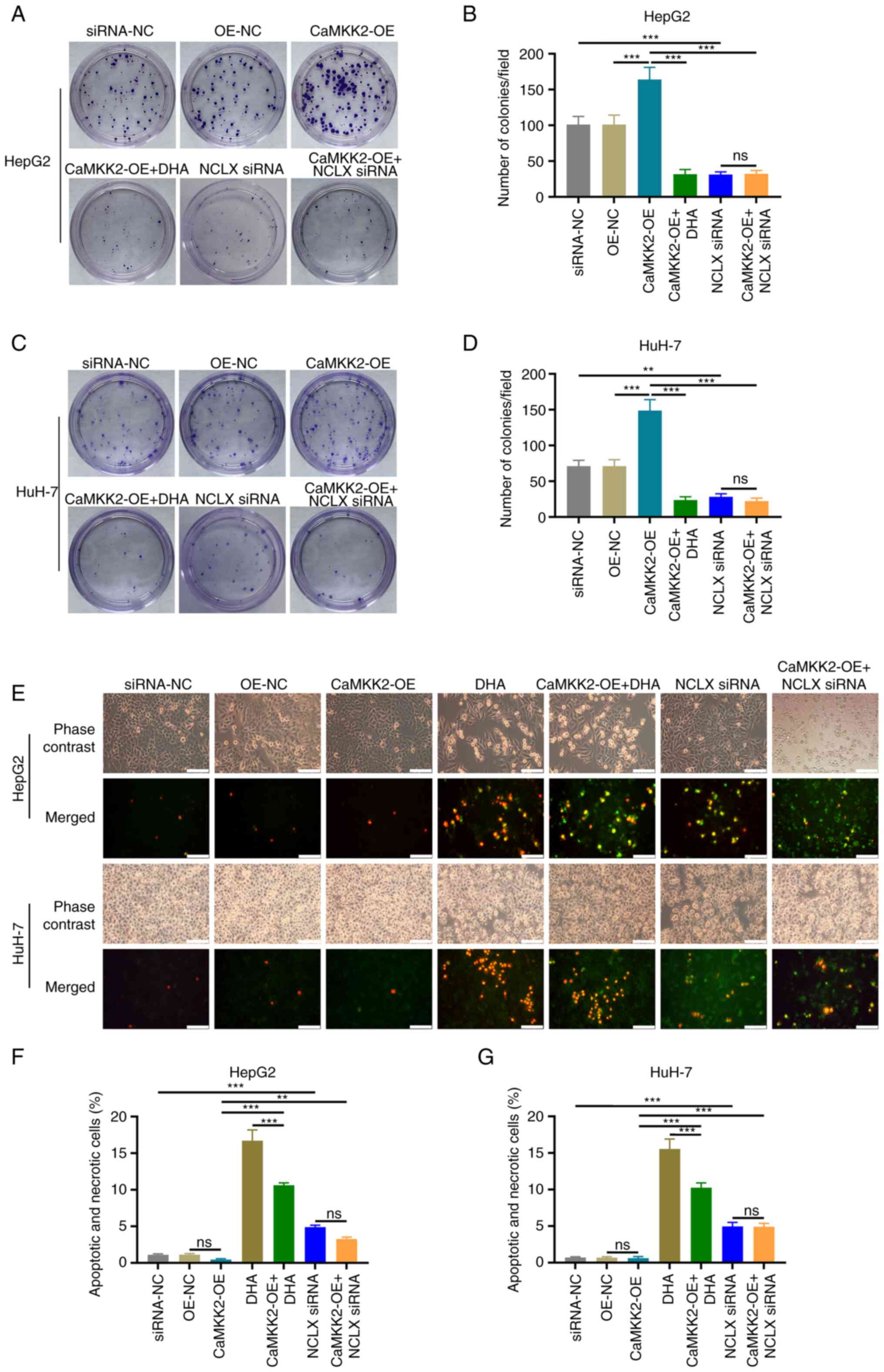 | Figure 2.Effects of DHA, CaMKK2 and NCLX on
the proliferation and apoptosis of liver cancer cells. (A) Colony
formation assay and (B) number of HepG2 colonies formed with cells
treated with DHA, CaMKK2-OE and NCLX siRNA. (C) Colony formation
assay and (D) number of HuH-7 colonies formed with cells treated
with DHA, CaMKK2-OE and NCLX siRNA. Transfected (E) HepG2 and HuH-7
cells stained with YO-PRO-1 and PI dye and treated with DHA,
CaMKK2-OE and NCLX siRNA. Apoptosis and necrosis rate of (F) HepG2
and (G) HuH-7 cells treated with DHA, CaMKK2-OE and NCLX siRNA. All
data are presented as the mean ± standard deviation (n=3). Data
were analyzed using one-way analysis of variance followed by
Bonferroni post hoc test. Scale bar, 50 µm **P<0.01;
***P<0.001. ns, not significant; NCLX, mitochondrial
sodium/calcium exchanger protein; DHA, dihydroartemisinin; CaMKK2,
calcium/calmodulin-dependent protein kinase kinase 2; siRNA, small
interfering RNA; OE, overexpression; NC, negative control. |
Effects of DHA treatment, CaMKK2-OE
and NCLX knockdown on liver cancer cell migration and invasion
Transwell assays were used to detect the invasive
capacity of liver cancer cells. The number of invasive cells
increased significantly in the CaMKK2-OE group compared with OE-NC
(Fig. 3A-D). DHA treatment and NCLX
knockdown in CaMKK2-OE cells significantly reduced the number of
invasive cells compared with the CaMKK2-OE group. Additionally NCLX
siRNA alone also reduced the number of migration and invasion
cells.
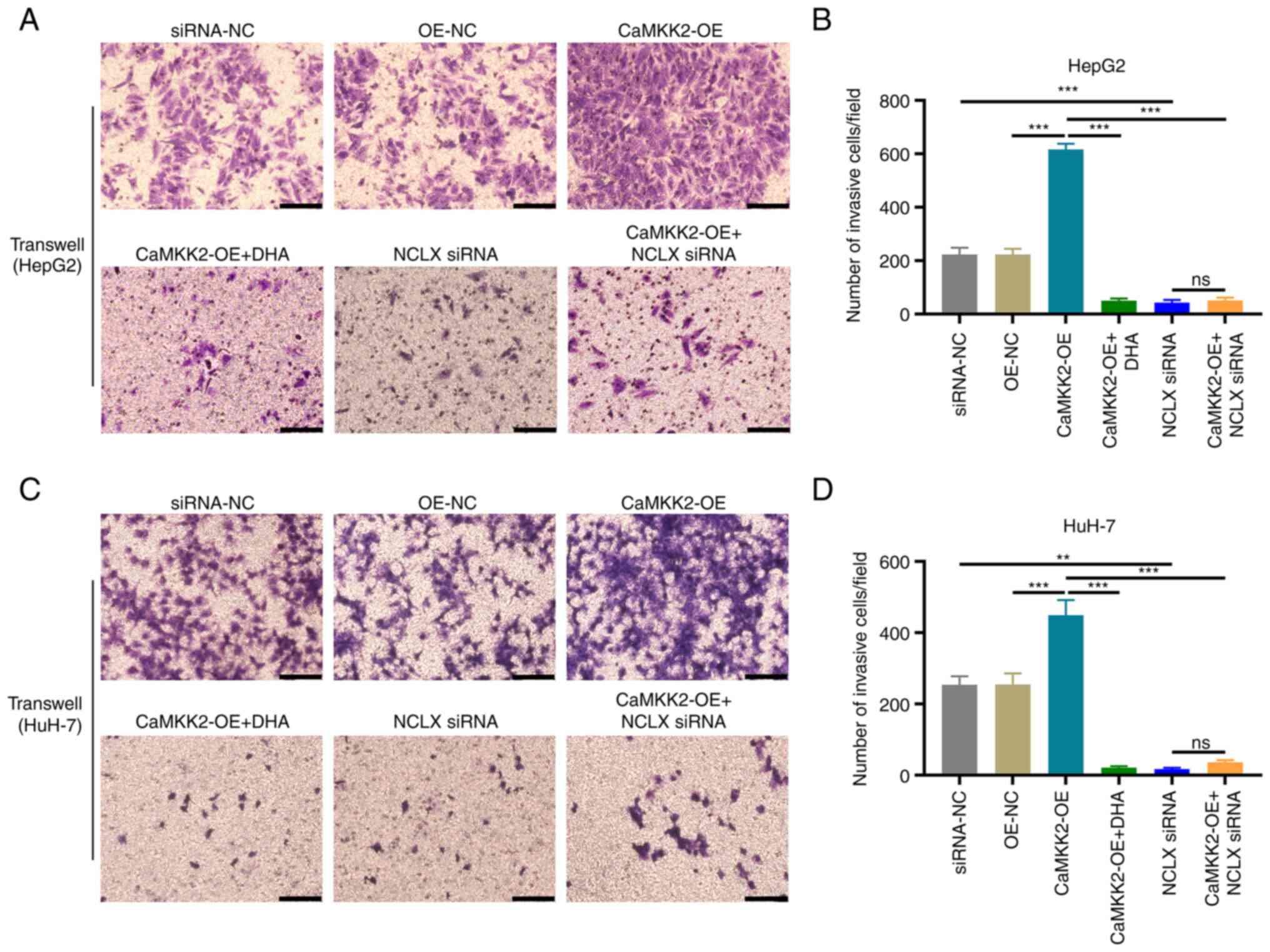 | Figure 3.Effect of DHA treatment, CaMKK2-OE
and NCLX siRNA on the invasive capacity of liver cancer cells. (A)
Transwell assay of HepG2 cells treated with DHA, CaMKK2-OE and/or
NCLX siRNA and (B) number of invasive cells per field. (C)
Transwell assay of HuH-7 cells treated with DHA, CaMKK2-OE and/or
NCLX siRNA and (D) number of invasive cells per field. Scale bar,
50 µm. All data are presented as the mean ± standard deviation
(n=3). Data were analyzed using one-way analysis of variance
followed by Bonferroni post hoc test. **P<0.01; ***P<0.001.
NCLX, mitochondrial sodium/calcium exchanger protein; DHA,
dihydroartemisinin; CaMKK2, calcium/calmodulin-dependent protein
kinase kinase 2; siRNA, small interfering RNA; OE, overexpression;
ns, not significant; NC, negative control. |
The migratory ability of liver cancer cells was
detected via wound healing assays. These assays demonstrated that
CaMKK2-OE significantly increased the migration of liver cancer
cells compared with OE-NC (Fig.
4A-D). After DHA treatment and NCLX knockdown in CaMKK2-OE
cells, the wound healing rate of the cells was significantly
decreased and the migrating distance was reduced compared with the
CaMKK2-OE group. Although CaMKK2-OE promoted the migration and
invasion of liver cancer cells, this effect could be inhibited by
DHA treatment and NCLX siRNA. Therefore, NCLX may be a critical
regulator in CaMKK2-mediated liver cancer cell metastasis and
CaMKK2 may be an effective target of DHA.
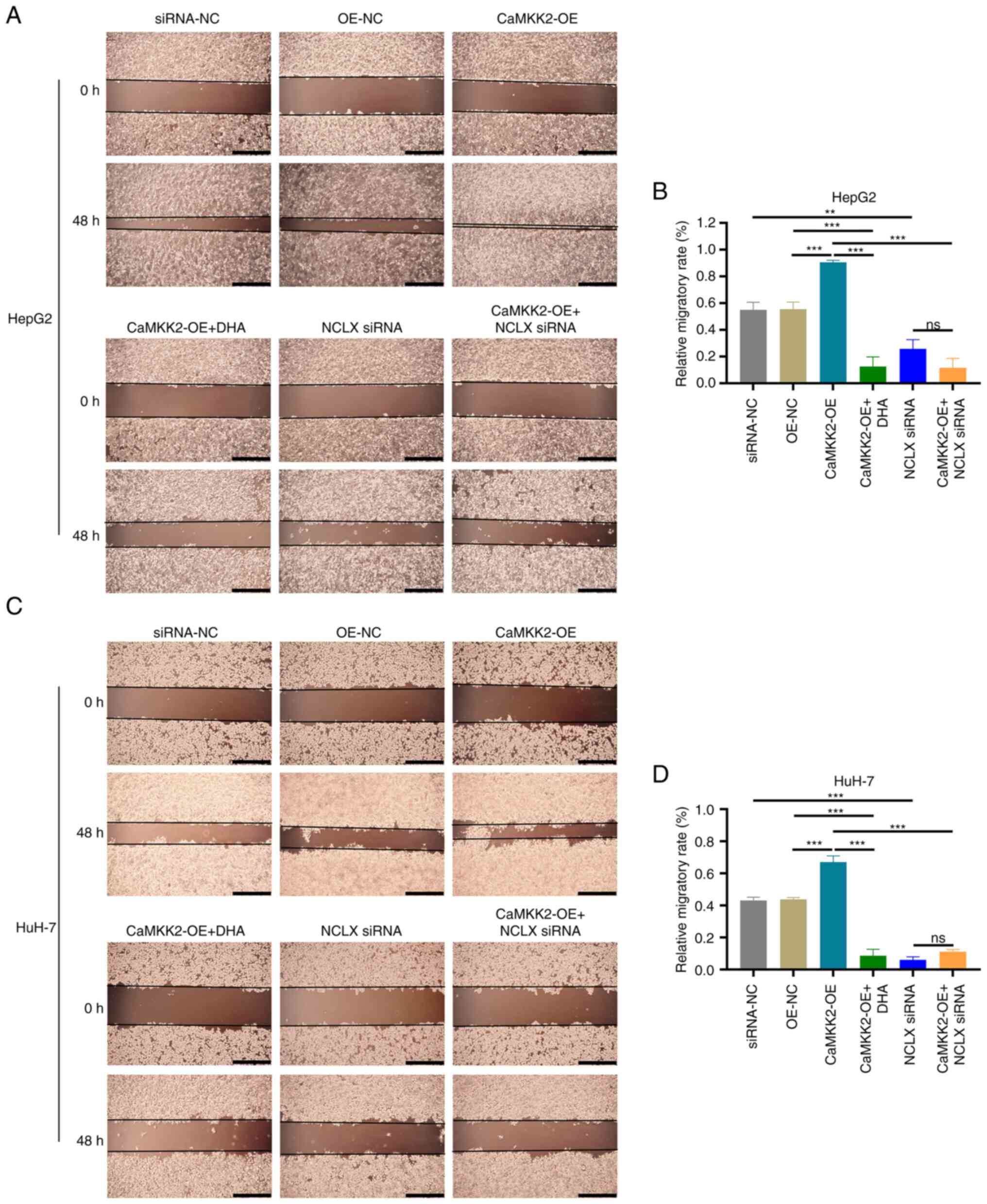 | Figure 4.Effects of DHA treatment, CaMKK2-OE
and NCLX siRNA on the migratory capacity of liver cancer cells. (A)
Wound healing assay of HepG2 cells treated with DHA, CaMKK2-OE
and/or NCLX siRNA and (B) migrated distance after 48 h. (C) Wound
healing assay of HuH-7 cells treated with DHA, CaMKK2-OE and/or
NCLX siRNA and (D) migrated distance after 48 h. Scale bar, 200 µm.
All data are presented as the mean ± standard deviation (n=3). Data
were analyzed using one-way analysis of variance followed by
Bonferroni post hoc test. **P<0.01; ***P<0.001. NCLX,
mitochondrial sodium/calcium exchanger protein; DHA,
dihydroartemisinin; CaMKK2, calcium/calmodulin-dependent protein
kinase 2; siRNA, small interfering RNA; ns, not significant; NC,
negative control. |
Effects of different treatments on the
formation of ATP and ROS in liver cancer cells
The effects of different treatment groups on the
production of ATP and ROS in cancer cells after 24 h of
intervention were analyzed. The results of ATP fluorescence probe
experiments showed that the fluorescence intensity of CaMKK2-OE
group was significantly increased compared with OE-NC, while DHA
treatment or NCLX knockout resulted in a significant decrease in
CaMKK2-OE group (Fig. 5A-D). In the
CaMKK2-OE group, intracellular ROS levels were significantly higher
compared with the OE-NC group (Fig.
5E-H). However, after DHA treatment or NCLX knockout, the ROS
level in the CaMKK2-OE treatment group was significantly reduced.
Therefore, DHA treatment and NCLX siRNA reduced the production of
ATP and ROS, indicating that DHA may play an anti-cancer role by
reducing the energy metabolism of cancer cells. In addition, it was
also demonstrated that NCLX siRNA could block CaMKK2-regulated ATP
and ROS production.
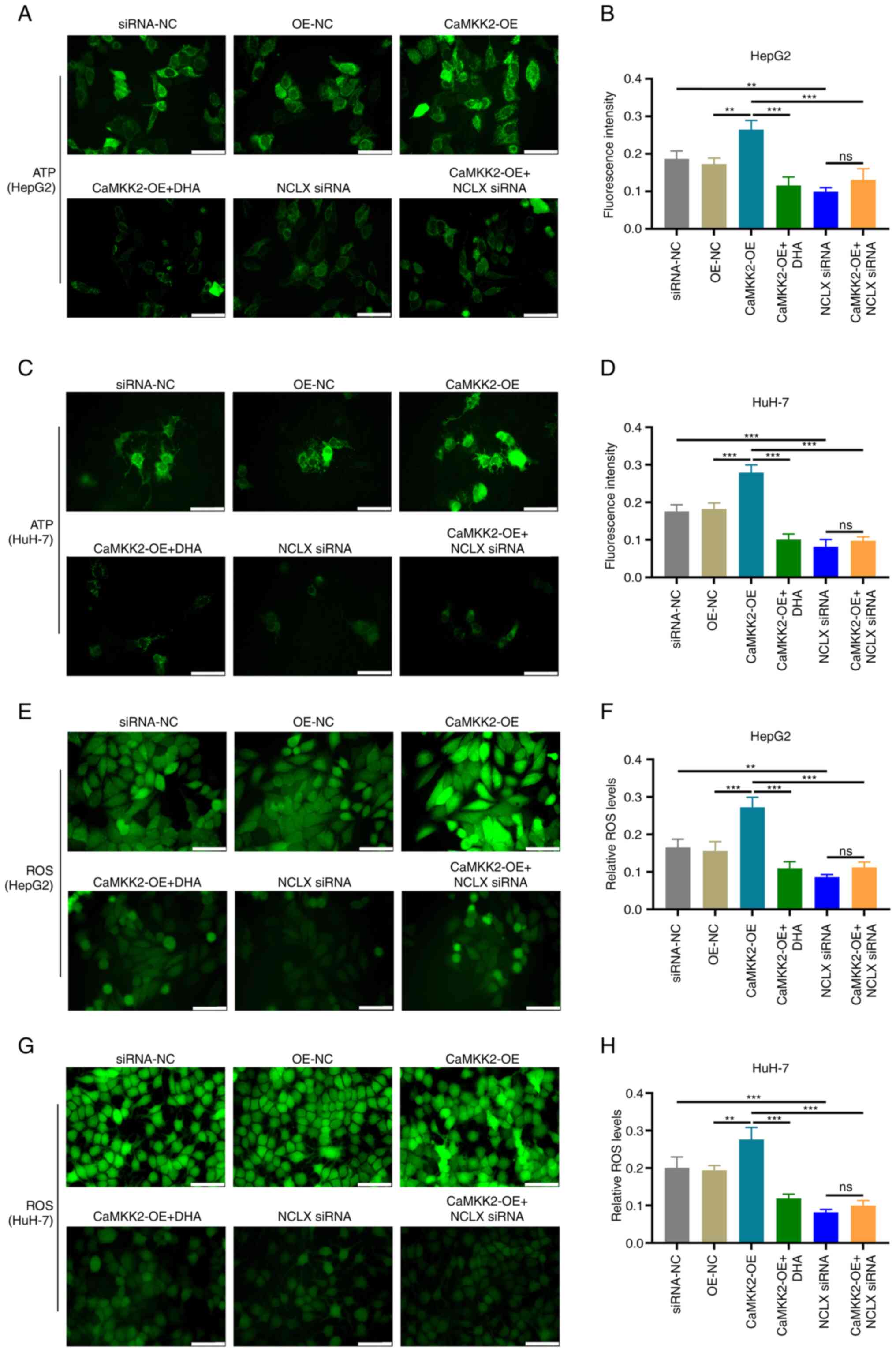 | Figure 5.Effects of DHA treatment, CaMKK2-OE
and NCLX siRNA on the production of ATP and ROS in liver cancer
cells. (A) ATP levels and (B) fluorescence intensity of HepG2 cells
treated with DHA, CaMKK2-OE and/or NCLX siRNA. (C) ATP levels and
(D) fluorescence intensity of HuH-7 cells treated with DHA,
CaMKK2-OE and/or NCLX siRNA. (E) Intracellular ROS levels and (F)
fluorescence intensity of HepG2 cells treated with DHA, CaMKK2-OE
and/or NCLX siRNA. (G) Intracellular ROS levels and (H)
fluorescence intensity of HuH-7 cells treated with DHA, CaMKK2-OE
and/or NCLX siRNA. Scale bar, 20 µm. All data are presented as the
mean ± standard deviation (n=3). Data were analyzed using one-way
analysis of variance followed by Bonferroni post hoc test.
**P<0.01; ***P<0.001. NCLX, mitochondrial sodium/calcium
exchanger protein; DHA, dihydroartemisinin; CaMKK2,
calcium/calmodulin-dependent protein kinase kinase 2; siRNA, small
interfering RNA; OE, overexpression; ns, not significant; NC,
negative control; ROS, reactive oxygen species. |
Liver cancer cell cytoskeletal
remodeling
Changes in cancer cell microfilaments were observed
in the CaMKK2-OE group. Compared with the OE-NC group, the
fluorescence intensity of the microfilaments in the CaMKK2-OE group
was significantly enhanced, and the microfilaments were increased
and stretched. However, knockdown of NCLX or DHA treatment
significantly reduced the fluorescence intensity of microfilaments,
resulting in microfilament shortening and decomposition. Similar
results were obtained after knockdown of NCLX or DHA treatment in
the CaMKK2-OE group (Fig.
6A-D).
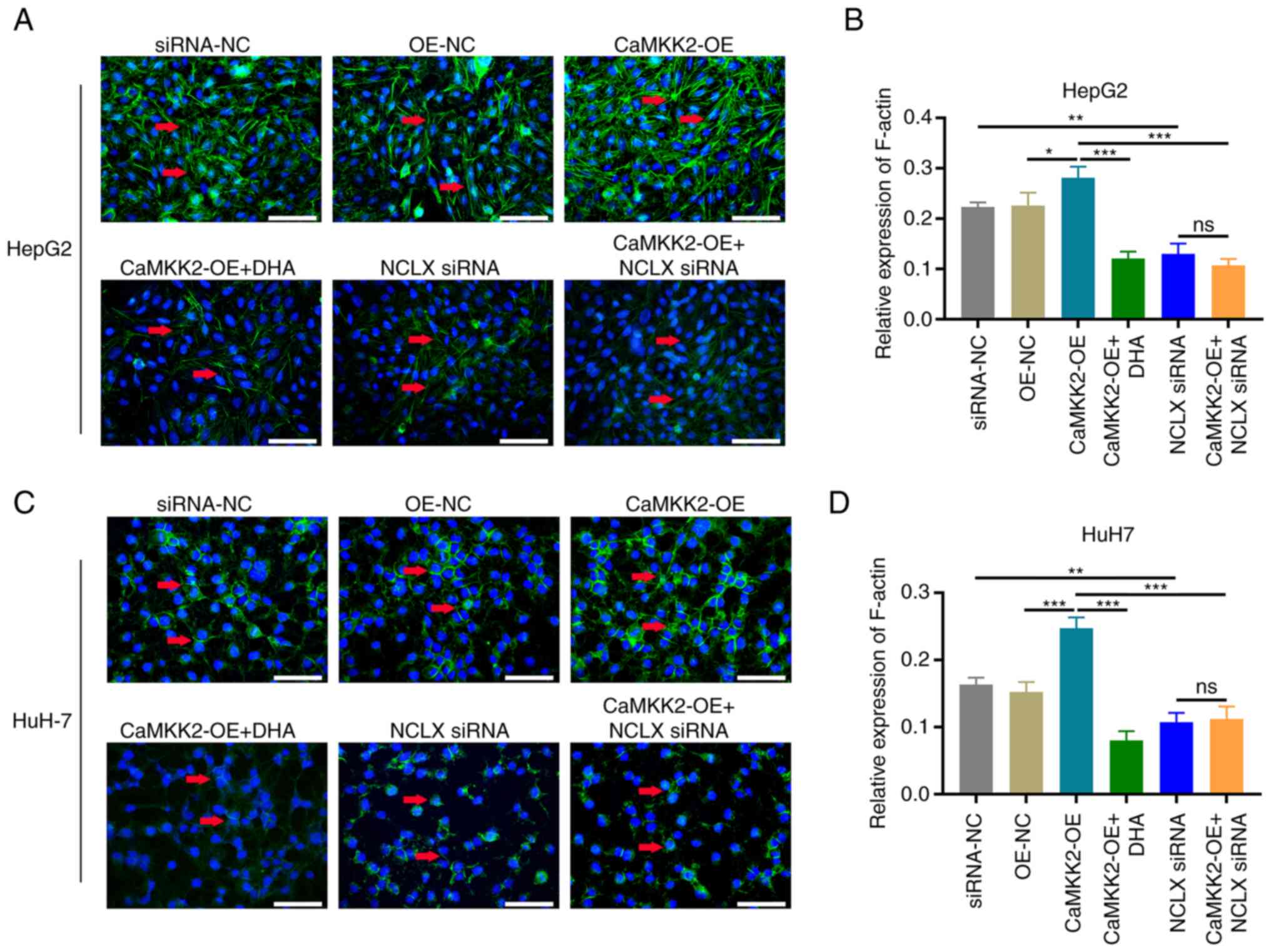 | Figure 6.Effects of DHA treatment, CaMKK2-OE
and NCLX siRNA on the actin cytoskeleton. (A) Phalloidin staining
and (B) relative expression of F-actin in HepG2 cells treated with
DHA, CaMKK2-OE and/or NCLX siRNA. (C) Phalloidin staining and (D)
relative expression of F-actin in HuH-7 cells treated with DHA,
CaMKK2-OE and/or NCLX siRNA. All data are presented as the mean ±
standard deviation (n=3). Data were analyzed using one-way analysis
of variance followed by Bonferroni post hoc test. Scale bar, 20 µm
*P<0.05; **P<0.01; ***P<0.001. NCLX, mitochondrial
sodium/calcium exchanger protein; DHA, dihydroartemisinin; CaMKK2,
calcium/calmodulin-dependent protein kinase kinase 2; OE,
overexpression; ns, not significant; NC, negative control. |
Effects of DHA treatment, CaMKK2-OE
and NCLX knockdown on ATP synthase expression in liver cancer
cells
The present study showed that compared with the
OE-NC group, the mRNA and protein expression levels of CaMKK2,
NCLX, ATP1A1 and ATP5H were upregulated in the CaMKK2 OE group.
Compared with the CaMKK2-OE group, DHA treatment reduced the
expression levels of CaMKK2, NCLX, ATP1A1 and ATP5H, and reversed
the effect of CaMKK2-OE induction (Figs. 7A-H and 8A-J). After knocking down NCLX in CaMKK2
OE group, the expression level of CaMKK2 protein was significantly
increased compared with OE-NC, while NCLX, ATP1A1 and ATP5H
expression significantly decreased (Fig. 9A-J). The above results showed that
knockdown of NCLX had no effect on the expression of CaMKK2. On the
contrary, knockdown of CaMKK2 significantly reduced the expression
of NCLX, ATP1A1 and ATP5H. Therefore, NCLX may be a downstream gene
of CaMKK2, which mediates the regulation of CaMKK2-induced ATP
synthase (ATP1A1 and ATP5H subunits) in the energy metabolism
pathway. Based on the above experimental results, DHA can target
the CaMKK2/NCLX gene, inhibit the production of ATP synthase and
destroy the energy metabolism pathway and thus may play an
anti-cancer role.
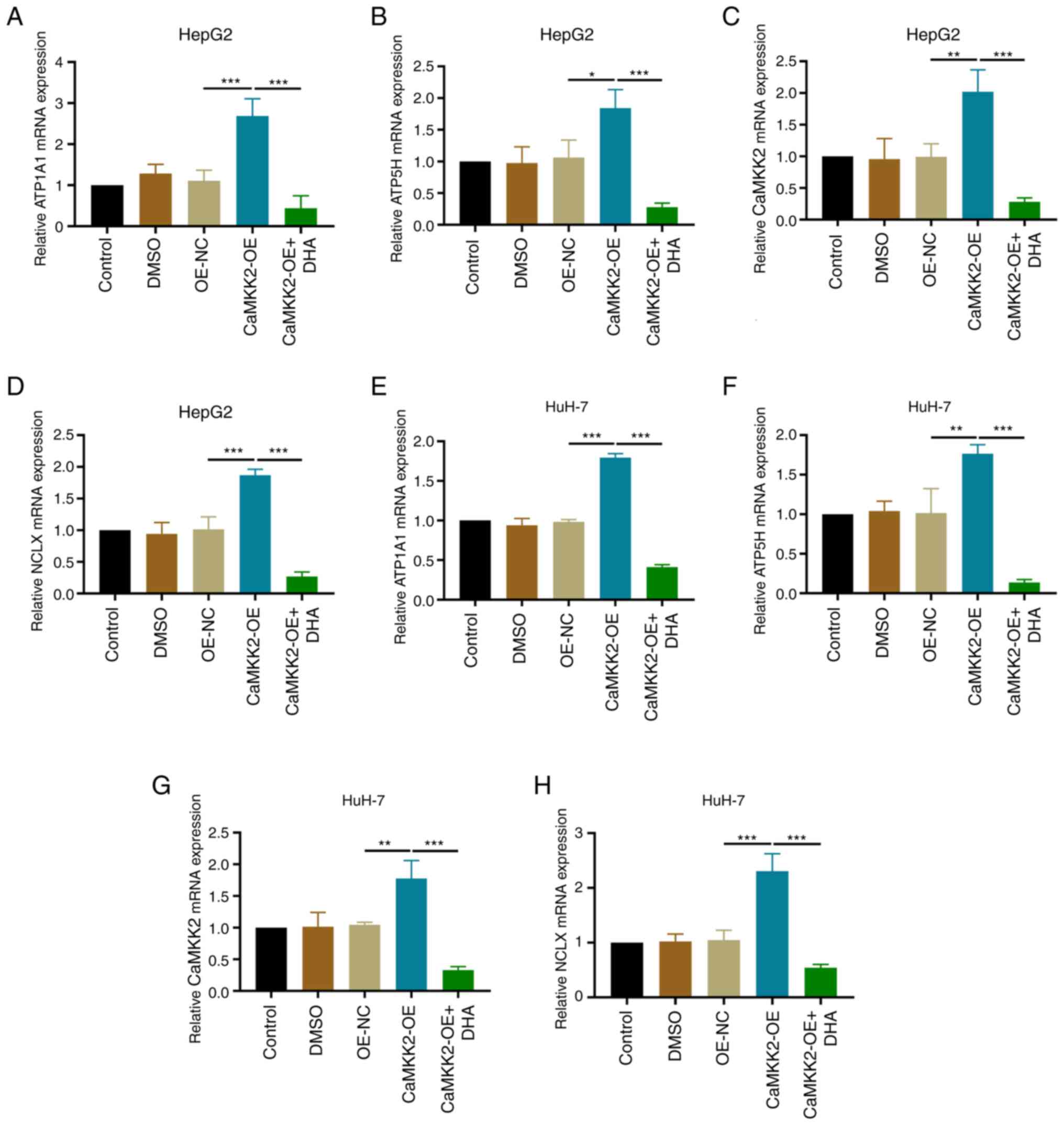 | Figure 7.Effects of DHA treatment and
CaMKK2-OE on ATP synthase expression. Relative mRNA expression
levels of (A) ATP1A1, (B) ATP5H, (C) CaMKK2 and (D) NCLX in HepG2
cells transfected with CaMKK2-OE and treated with DHA were detected
by RT-qPCR. Relative mRNA expression levels of (E) ATP1A1, (F)
ATP5H, (G) CaMKK2 and (H) NCLX in HuH-7 cells transfected with
CaMKK2-OE and treated with DHA were detected by RT-qPCR. All data
are presented as the mean ± standard deviation (n=3). Data were
analyzed using one-way analysis of variance followed by Bonferroni
post hoc test. *P<0.05; **P<0.01; ***P<0.001. NCLX,
mitochondrial sodium/calcium exchanger protein; DHA,
dihydroartemisinin; CaMKK2, calcium/calmodulin-dependent protein
kinase kinase 2; OE, overexpression; RT-qPCR, reverse
transcription-quantitative PCR; NC, negative control; ATP1A1,
sodium/potassium-transporting ATPase subunit α-1; ATP5H, ATP
synthase subunit d, mitochondrial. |
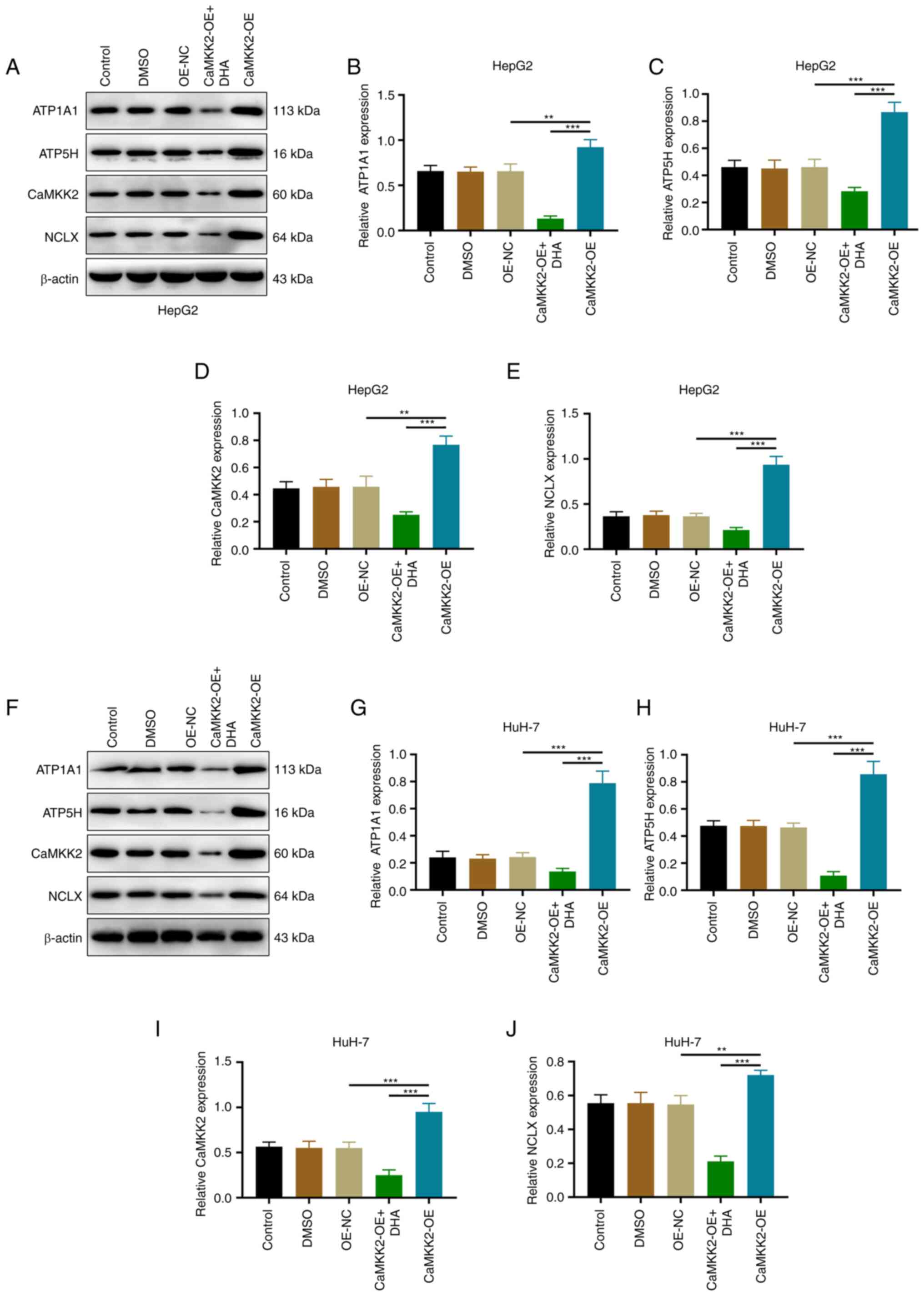 | Figure 8.Effects of DHA treatment and
CaMKK2-OE on ATP synthase expression. (A) Western blotting and
protein expression levels of (B) ATP1A1, (C) ATP5H, (D) CaMKK2 and
(E) NCLX in HepG2 cells transfected with CaMKK2-OE and treated with
DHA. (F) Western blotting and protein expression levels of (G)
ATP1A1, (H) ATP5H, (I) CaMKK2 and (J) NCLX in HuH-7 cells
transfected with CaMKK2-OE and treated with DHA. All data are
presented as the mean ± standard deviation (n=3). Data were
analyzed using one-way analysis of variance followed by Bonferroni
post hoc test. **P<0.01; ***P<0.001. NCLX, mitochondrial
sodium/calcium exchanger protein; DHA, dihydroartemisinin; CaMKK2,
calcium/calmodulin-dependent protein kinase kinase 2; OE,
overexpression; NC, negative control; ATP1A1,
sodium/potassium-transporting ATPase subunit α-1; ATP5H, ATP
synthase subunit d, mitochondrial. |
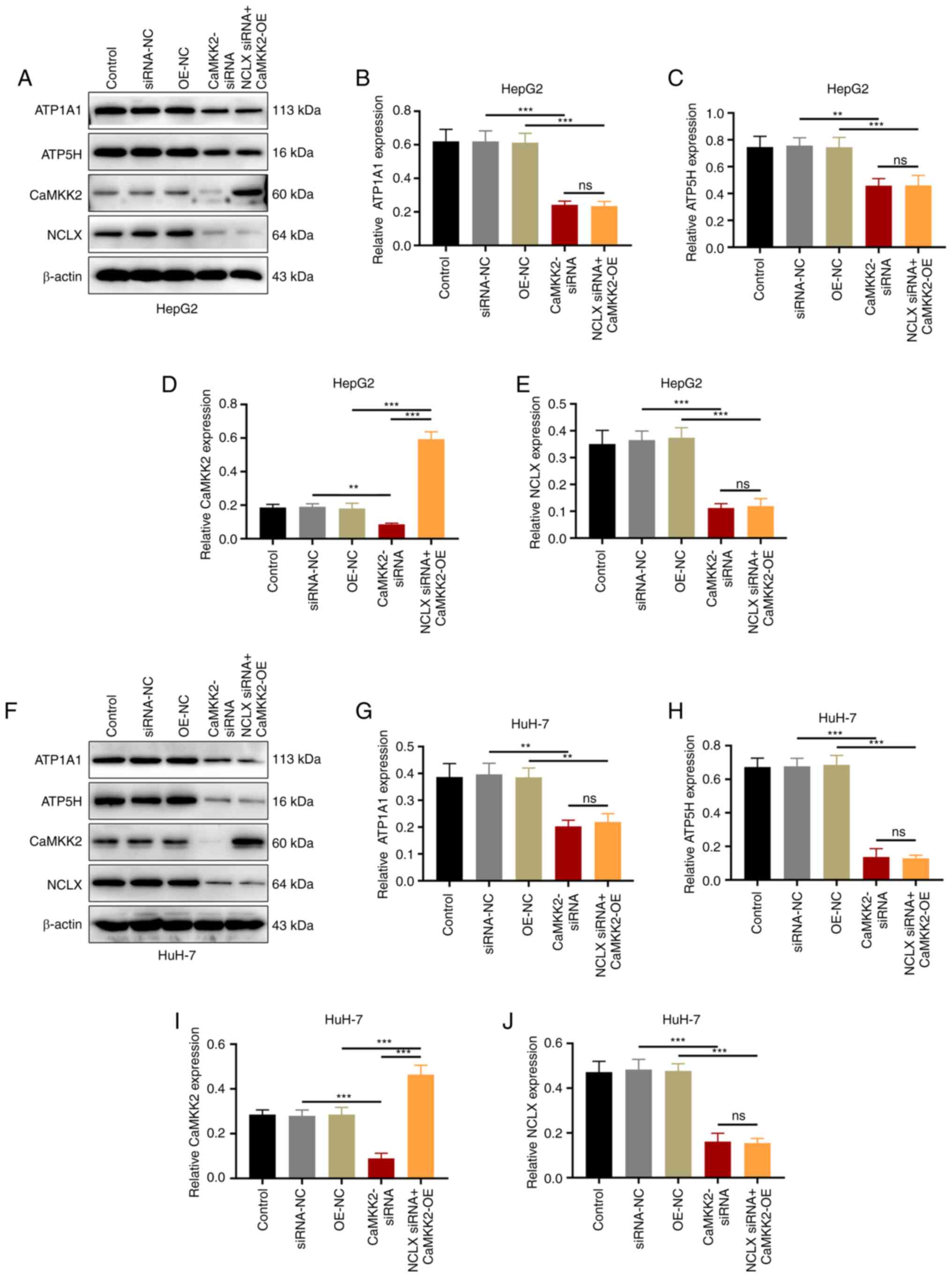 | Figure 9.Effects of DHA treatment, CaMKK2
siRNA, CaMKK2-OE and NCLX siRNA on ATP synthase expression. (A)
Western blotting and protein expression levels of (B) ATP1A1, (C)
ATP5H, (D) CaMKK2 and (E) NCLX in HepG2 cells transfected with
CaMKK2 siRNA, CaMKK2-OE and NCLX siRNA and treated with DHA. (F)
Western blotting and protein expression levels of (G) ATP1A1, (H)
ATP5H, (I) CaMKK2 and (J) NCLX in HuH-7 cells transfected with
CaMKK2 siRNA, CaMKK2-OE and NCLX siRNA and treated with DHA. All
data are presented as the mean ± standard deviation (n=3). Data
were analyzed using one-way analysis of variance followed by
Bonferroni post hoc test. **P<0.01, ***P<0.001. NCLX,
mitochondrial sodium/calcium exchanger protein; DHA,
dihydroartemisinin; CaMKK2, calcium/calmodulin-dependent protein
kinase kinase 2; siRNA, small interfering RNA; OE, overexpression;
ns, not significant; NC, negative control; ATP1A1,
sodium/potassium-transporting ATPase subunit α-1; ATP5H, ATP
synthase subunit d, mitochondrial. |
Discussion
Targeting energy metabolism pathways in tumors is an
effective strategy for inhibiting metastasis (30). Energy metabolism pathways in cancer
cells serve a crucial role in sustaining their biological behavior
and promoting metastasis (31).
Mitochondrial calcium-regulating proteins affect cellular energy
metabolism by activating oxidative metabolism, mitochondrial
respiration and ATP synthesis (32,33).
CaMKK2 and NCLX serve important roles in physiological or
pathological processes, such as maintaining cellular energy
homeostasis and cell proliferation (34,35).
In the study of gastric cancer, the application of small molecule
inhibitors of calcium-regulated protein can significantly inhibit
the peritoneal metastasis of gastric cancer cells (36). The drugs that regulate the release
of calcium ions have synergistic anti-proliferative effects in
combination with gemcitabine, oxaliplatin and adriamycin (37). Therefore, the development of drugs
targeting calcium-regulating proteins has become a focus of cancer
treatment (38). DHA is considered
an efficient anticancer agent, but its molecular mechanism of
action is not clear (24). DHA can
inhibit the proliferation activity of glioma U87 and U251 cells,
increase ROS level and promote apoptosis (39). The present study demonstrated that
DHA can significantly inhibit the expression of CaMKK2 and NCLX,
suppress the proliferative activity of liver cancer cells, reduce
the activity of ATP synthase, restructure the cytoskeleton and
inhibit the migration and invasion of liver cancer cells.
Therefore, the present study demonstrated that the anticancer
mechanism of DHA is mediated by the CaMKK2/NCLX signaling pathway,
thereby inhibiting ATP synthase expression, which aims to provide a
potential novel future treatment for liver cancer.
Mitochondrial calcium regulatory proteins are
critical factors that determine cell function, shape and survival
(40). In both cancer and
non-cancer research, CaMKK2 has been reported to serve a key role
in cell proliferation, and particularly in cancer cells it promotes
cell proliferation, migration and invasion (41). CaMKK2 is overexpressed in various
types of cancer, including prostate, breast, liver, ovarian and
gastric cancers, contributing to their progression (41,42).
In liver cancer, CaMKK2 expression is significantly upregulated and
negatively correlated with the survival of patients with liver
cancer (6). Knocking down CaMKK2
significantly inhibits proliferation of liver cancer cells and
tumorigenicity in mouse models (6).
Similarly, elevated levels of CaMKK2 have been implicated in
promoting metastasis of gastric cancer cells, while downregulation
of CaMKK2 significantly reduces cell proliferation and decreases
gastric cancer cell migration and invasion (43). CaMKK2 serves as a valuable clinical
biomarker and has the potential to be used as a therapeutic target
for advanced prostate cancer (4).
Furthermore, CaMKK2 promotes tumor development by regulating
cellular and systemic metabolism (1). In the present study, it was
demonstrated that CaMKK2-OE significantly increased proliferation
and colony formation in liver cancer cells, as well as promoting
migration and invasion. These results are consistent with
previously reported experimental results in gastric cancer cells
(44). However, the present study
further demonstrated that DHA significantly inhibited the effects
induced by CaMKK2-OE. Therefore, CaMKK2 may be a target of the
anticancer effect of DHA.
ATP is produced in mitochondria, which are the
center of cellular energy metabolism. The Ca2+ overload
in the mitochondrial matrix can induce an increase in ROS
generation, trigger mitochondrial permeability transition pore
opening and cytochrome c release and lead to cell apoptosis
(45). DHA regulates ROS levels in
liver cancer cells via multiple mechanisms (46). For example, DHA can increase the
activity of antioxidant enzymes, such as superoxide dismutase and
glutathione peroxidase, in liver cancer cells, thereby reducing ROS
generation (47). DHA can also
inhibit the activity of NADPH oxidase, thereby reducing the
production of ROS (48). In
addition, DHA can induce cell cycle arrest of liver cancer cells at
the G0/G1 phase, ROS generation and
mitochondrial membrane potential loss, thereby leading to apoptosis
(49). The present study
demonstrated that CaMKK2-OE can significantly upregulate ATP
formation and promote ROS generation in liver cancer cells, whereas
knocking down NCLX expression could block these regulatory effects
of CaMKK2.
The concentration of ROS in tumor cells is typically
100X higher than that of healthy cells (50). It has been previously suggested that
increasing ROS levels may hinder tumor growth by inducing cell
cycle arrest (51). ROS and
ROS-dependent lipid peroxidation products (including prostaglandins
and active aldehydes) activate apoptosis through mitochondrial or
endoplasmic reticulum stress-dependent pathways (52). By contrast, it has also been
reported that ROS, as a signal molecule that induces the
proliferation of cancer cells, is involved in the tumorigenic
phenotype of cancer cells. ROS can activate EGFR, leading to the
activation of the Ras/MAPK pathway involved in cell proliferation
(53). In the present study,
CaMKK2-OE promoted ROS increase, while DHA treatment significantly
inhibited ROS production, thereby exhibiting antitumor activity. In
summary, there are different views on the role of ROS in cancer
progression, possibly because it changes at different stages of
disease development. It is undeniable that ROS may be one of the
monitoring indicators during cancer treatment.
Among mitochondrial calcium regulatory proteins,
NCLX exerts a precancerous effect by regulating sodium-calcium
exchange (54). Upregulating NCLX
facilitates the release of mitochondrial Ca2+ and
enhances cisplatin resistance in cancer cells (55). It has been found that epinephrine
can stimulate NCLX-null BAT-induced Ca2+ overload,
trigger the opening of mitochondrial permeability transition pore
(mPTP), resulting in significant mitochondrial swelling and cell
death (56). Furthermore,
increasing NCLX levels can protect sensory neurons from cell damage
caused by neurite degeneration and calcium accumulation (57). In the present study, knocking down
NCLX significantly inhibited the proliferation, migration and
invasion of liver cancer cells while reducing ATP and ROS
production. The results of the present study showed that NCLX plays
a key role in the proliferation, metastasis and mitochondrial
function of liver cancer cells, which may be related to the
regulation of calcium ions or may be affected by calcium
regulation-related proteins. Knockout of NCLX inhibited the
induction of ATP and ROS by CaMKK2-OE in cancer cells. It is worth
noting that it did not affect the expression level of CaMKK2,
similarly to the effect of NCLX siRNA alone. However, there was no
significant difference between NCLX siRNA alone and NCLX siRNA +
CaMKK2 OE group, indicating that NCLX had no effect on the
expression of CaMKK2 in the process of blocking the regulation of
CaMKK2, which further proved that NCLX was likely to be a
downstream regulator of CaMKK2. Of course, other factors or genes
playing a role in this process cannot be ruled out. Notably, the
present study also demonstrated a significant reduction in the
expression levels of CaMKK2 and NCLX with DHA treatment. In
addition, DHA reduced the formation of ATP and ROS induced by
CaMKK2-OE in cancer cells, further indicating that DHA inhibited
the invasion and migration of liver cancer cells through this
effect. Therefore, DHA may serve a role in inhibiting the
metastatic phenotype of liver cancer cells through the CaMKK2/NCLX
signaling pathway. It cannot be ignored that under physiological
conditions, mitochondrial Ca2+ maintains Ca2+
homeostasis and ATP production through mitochondrial
Ca2+ uniporter and NCLX to avoid cell death caused by
too little Ca2+ or Ca2+ overload (58).
The failure of liver cancer treatment is
multifaceted, especially for highly invasive cells, which
undoubtedly increases the difficulty of treatment due to their
complex energy metabolism pathways. Therefore, finding natural
compounds for key proteins in the energy metabolism pathway may be
a boon for the treatment of cancer patients (59,60).
It has been reported that natural products have a synergistic
effect on cancer immunotherapy (cancer vaccines, immune checkpoint
inhibitors and adoptive immunotherapy). In particular, saponins,
polysaccharides and flavonoids can exert a strong anti-tumor immune
effect by reversing the tumor immunosuppressive microenvironment
and combining with cancer immunotherapy (61). For example, DHA has previously been
reported to exert significant anticancer effects, as it was shown
to induce apoptosis, reduce angiogenesis and decrease drug
resistance of breast cancer cells (MDA-MB-231 and MDA-MB-436)
(62). The present study
demonstrated that DHA inhibited the production of ATP synthase
(ATP1A1 and ATP5H subunits) in liver cancer cells, which was
upregulated through the CaMKK2/NCLX pathway. AMPK is dependent on
CaMKK2 (63) and the results of the
present study showed that CaMKK2 can also mediate the anti-cancer
effect of DHA through the NCLX pathway, enriching the CaMKK2 signal
and can also act through the NCLX pathway mediating the anticancer
effect of DHA. Tumor mitochondria are directly involved in the
formation of the immune microenvironment (64). Tumor cell mitochondria inhibit or
stimulate tumor growth and migration by releasing mitochondrial
DNA, ATP, cytochrome c or formyl peptide to the
extracellular matrix, thereby activating immune cells, which leads
to proinflammatory and immunosuppressive reactions (65). DHA may affect the immune
microenvironment by regulating mitochondrial function, which may
also improve the therapeutic effect of immunosuppressive agents.
Further studies are required in the future to investigate this
hypothesis. Mutations in mitochondrial calcium-related proteins
have different effects on the cytoskeleton (for instance, GDAP1
loss of function inhibits the mitochondrial pyruvate dehydrogenase
complex by altering the actin cytoskeleton). By observing changes
in the fluorescence intensity of microfilaments, which reflect
their stability, it was demonstrated that different stress response
patterns of liver cancer cells could be induced by CaMKK2-OE,
knockdown of NCLX or DHA treatment. This has potential implications
for elucidating the molecular mechanisms by which DHA targets
mitochondrial calcium regulatory proteins to inhibit liver cancer
metastasis.
In conclusion, the present study demonstrated that
DHA may inhibit liver cancer cell proliferation, ROS production and
metastatic phenotype by reducing ATP synthase production through
the CaMKK2/NCLX signaling pathway. The present study highlighted a
potential future treatment for liver cancer using a natural drug
derivative, which targeted calcium homeostasis to disrupt the
metabolism of cancer cells. It is essential to further explore the
effectiveness of DHA as an anticancer drug to enhance its utility
in the treatment of liver cancer.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by the High Level Talents Project of
Hainan Natural Science Foundation of 2022 (grant no. 822RC830) and
the Hainan Health Industry Scientific Research Project of 2021
(grant no. 21A200072).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JC and CX designed the experiments. CX and YiW
performed the experiments and collected data. YoW, CX and JC
discussed the results and strategy. YoW analyzed and interpreted
the data, YiW and JC supervised, directed and managed the study. JC
and YoW confirm the authenticity of all the raw data. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Stewart LM, Gerner L, Rettel M, Stein F,
Burrows JF, Mills IG and Evergren E: CaMKK2 facilitates
Golgi-associated vesicle trafficking to sustain cancer cell
proliferation. Cell Death Dis. 12:10402021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Raturi A, Gutiérrez T, Ortiz-Sandoval C,
Ruangkittisakul A, Herrera-Cruz MS, Rockley JP, Gesson K, Ourdev D,
Lou PH, Lucchinetti E, et al: TMX1 determines cancer cell
metabolism as a thiol-based modulator of ER-mitochondria Ca2+ flux.
J Cell Biol. 214:433–444. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Williams JN and Sankar U: CaMKK2 signaling
in metabolism and skeletal disease: A new axis with therapeutic
potential. Curr Osteoporos Rep. 17:169–177. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pulliam TL, Goli P, Awad D, Lin C,
Wilkenfeld SR and Frigo DE: Regulation and role of CAMKK2 in
prostate cancer. Nat Rev Urol. 19:367–380. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Han JH, Kim YK, Kim H, Lee J, Oh MJ, Kim
SB, Kim M, Kim KH, Yoon HJ, Lee MS, et al: Snail acetylation by
autophagy-derived acetyl-coenzyme A promotes invasion and
metastasis of KRAS-LKB1 co-mutated lung cancer cells. Cancer Commun
(Lond). 42:716–749. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin F, Marcelo KL, Rajapakshe K, Coarfa C,
Dean A, Wilganowski N, Robinson H, Sevick E, Bissig KD, Goldie LC,
et al: The camKK2/camKIV relay is an essential regulator of hepatic
cancer. Hepatology. 62:505–520. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dai S, Venturini E, Yadav S, Lin X, Clapp
D, Steckiewicz M, Gocher-Demske AM, Hardie DG and Edelman AM:
Calcium/calmodulin-dependent protein kinase kinase 2 mediates
pleiotropic effects of epidermal growth factor in cancer cells.
Biochim Biophys Acta Mol Cell Res. 1869:1192522022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pathak T, Gueguinou M, Walter V,
Delierneux C, Johnson MT, Zhang X, Xin P, Yoast RE, Emrich SM,
Yochum GS, et al: Dichotomous role of the human mitochondrial
Na(+)/Ca2(+)/Li(+) exchanger NCLX in colorectal cancer growth and
metastasis. eLife. 9:e596862020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guéguinou M, Ibrahim S, Bourgeais J,
Robert A, Pathak T, Zhang X, Crottès D, Dupuy J, Ternant D, Monbet
V, et al: Curcumin and NCLX inhibitors share anti-tumoral
mechanisms in microsatellite-instability-driven colorectal cancer.
Cell Mol Life Sci. 79:2842022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ruiz A, Alberdi E and Matute C: CGP37157,
an inhibitor of the mitochondrial Na+/Ca2+ exchanger, protects
neurons from excitotoxicity by blocking voltage-gated Ca2+
channels. Cell Death Dis. 5:e11562014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ni Z, He J, Wu Y, Hu C, Dai X, Yan X, Li
B, Li X, Xiong H, Li Y, et al: AKT-mediated phosphorylation of
ATG4B impairs mitochondrial activity and enhances the Warburg
effect in hepatocellular carcinoma cells. Autophagy. 14:685–701.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ge Q, Jia D, Cen D, Qi Y, Shi C, Li J,
Sang L, Yang L, He J, Lin A, et al: Micropeptide ASAP encoded by
LINC00467 promotes colorectal cancer progression by directly
modulating ATP synthase activity. J Clin Invest. 131:e1529112021.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang W, Wang L, Zhang Y and Li H:
Circ-ATP5H Induces Hepatitis B Virus replication and expression by
regulating miR-138-5p/TNFAIP3 axis. Cancer Manag Res.
12:11031–11040. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Feng XY, Zhao W, Yao Z, Wei NY, Shi AH and
Chen WH: Downregulation of ATP1A1 Expression by Panax notoginseng
(Burk.) F.H. Chen Saponins: A potential mechanism of antitumor
effects in HepG2 cells and in vivo. Front Pharmacol. 12:7203682021.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Althurwi SI, Yu JQ, Beale P and Huq F:
Sequenced combinations of cisplatin and selected phytochemicals
towards overcoming drug resistance in ovarian tumour models. Int J
Mol Sci. 21:75002020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu T, Yang Y, Chen Z, Wang J, Wang X,
Zheng Y, Wang C, Wang Y, Zhu Z, Ding X, et al: TNFAIP2 confers
cisplatin resistance in head and neck squamous cell carcinoma via
KEAP1/NRF2 signaling. J Exp Clin Cancer Res. 42:1902023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Song KH, Kim JH, Lee YH, Bae HC, Lee HJ,
Woo SR, Oh SJ, Lee KM, Yee C, Kim BW, et al: Mitochondrial
reprogramming via ATP5H loss promotes multimodal cancer therapy
resistance. J Clin Invest. 128:4098–4114. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang CJ, Zhang CY, Zhao YK, Wang D,
Zhuang L, Qian L, Xie L, Zhu Y and Meng ZQ: Bufalin inhibits
tumorigenesis and SREBP-1-mediated lipogenesis in hepatocellular
carcinoma via modulating the ATP1A1/CA2 axis. Am J Chin Med.
51:461–485. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou W, Chen MM, Liu HL, Si ZL, Wu WH,
Jiang H, Wang LX, Vaziri ND, An XF, Su K, et al: Dihydroartemisinin
suppresses renal fibrosis in mice by inhibiting
DNA-methyltransferase 1 and increasing Klotho. Acta Pharmacol Sin.
43:2609–2623. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen YI, Chang CC, Hsu MF, Jeng YM, Tien
YW, Chang MC, Chang YT, Hu CM and Lee WH: Homophilic ATP1A1 binding
induces activin A secretion to promote EMT of tumor cells and
myofibroblast activation. Nat Commun. 13:29452022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Eskiocak U, Ramesh V, Gill JG, Zhao Z,
Yuan SW, Wang M, Vandergriff T, Shackleton M, Quintana E, Johnson
TM, et al: Synergistic effects of ion transporter and MAP kinase
pathway inhibitors in melanoma. Nat Commun. 7:123362016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Peng J, Wang Q, Zhou J, Zhao S, Di P and
Chen Y, Tao L, Du Q, Shen X and Chen Y: Targeted lipid
nanoparticles encapsulating dihydroartemisinin and chloroquine
phosphate for suppressing the proliferation and liver metastasis of
colorectal cancer. Front Pharmacol. 12:7207772021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shi H, Xiong L, Yan G, Du S, Liu J and Shi
Y: Susceptibility of cervical cancer to dihydroartemisinin-induced
ferritinophagy-dependent ferroptosis. Front Mol Biosci.
10:11560622023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang J, Li Y, Wang JG, Feng JY, Huang GD
and Luo CG: Dihydroartemisinin affects STAT3/DDA1 signaling pathway
and reverses breast cancer resistance to cisplatin. Am J Chin Med.
51:445–459. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu S, Li Z, Li H and Liao K:
Dihydroartemisinin reduces irradiation-induced mitophagy and
radioresistance in lung cancer A549 cells via CIRBP Inhibition.
Life (Basel). 12:11292022.PubMed/NCBI
|
|
26
|
Chen X, Cui Y and Ma Y: Long non-coding
RNA BLACAT1 expedites osteosarcoma cell proliferation, migration
and invasion via up-regulating SOX12 through miR-608. J Bone Oncol.
25:1003142020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang X, Zheng D, Wang C and Chen W:
Knockdown of circ_0005615 enhances the radiosensitivity of
colorectal cancer by regulating the miR-665/NOTCH1 axis. Open Med
(Wars). 18:202306782023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak K and Schmittgen T: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu Y, Wang R, Huang R, Rutz B, Ciotkowska
A, Tamalunas A, Hu S, Trieb M, Waidelich R, Strittmatter F, et al:
Inhibition of growth and contraction in human prostate stromal
cells by silencing of NUAK1 and −2, and by the presumed NUAK
inhibitors HTH01-015 and WZ4003. Front Pharmacol. 14:11054272023.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zheng Y, Liu P, Wang N, Wang S, Yang B, Li
M, Chen J, Situ H, Xie M, Lin Y, et al: Betulinic acid suppresses
breast cancer metastasis by targeting GRP78-mediated glycolysis and
ER stress apoptotic pathway. Oxid Med Cell Longev.
2019:87816902019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pavlova NN, Zhu J and Thompson CB: The
hallmarks of cancer metabolism: Still emerging. Cell Metab.
34:355–377. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bonora M, Patergnani S, Rimessi A, De
Marchi E, Suski JM, Bononi A, Giorgi C, Marchi S, Missiroli S,
Poletti F, et al: ATP synthesis and storage. Purinergic Signal.
8:343–357. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Brookes PS, Yoon Y, Robotham JL, Anders MW
and Sheu SS: Calcium, ATP and ROS: A mitochondrial love-hate
triangle. Am J Physiol Cell Physiol. 287:C817–C833. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ponneri Babuharisankar A, Kuo CL, Chou HY,
Tangeda V, Fan CC, Chen CH, Kao YH and Lee AY: Mitochondrial
Lon-induced mitophagy benefits hypoxic resistance via
Ca2+-dependent FUNDC1 phosphorylation at the
ER-mitochondria interface. Cell Death Dis. 14:1992023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wells C, Liang Y, Pulliam TL, Lin C, Awad
D, Eduful B, O'Byrne S, Hossain MA, Catta-Preta CMC, Ramos PZ, et
al: SGC-CAMKK2-1: A chemical probe for CAMKK2. Cells. 12:2872023.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang X, Cheng G, Miao Y, Qiu F, Bai L, Gao
Z, Huang Y, Dong L, Niu X, Wang X, et al: Piezo type
mechanosensitive ion channel component 1 facilitates gastric cancer
omentum metastasis. J Cell Mol Med. 25:2238–2253. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sarkar A, Novohradsky V, Maji M, Babu T,
Markova L, Kostrhunova H, Kasparkova J, Gandin V, Brabec V and
Gibson D: Multitargeting prodrugs that release oxaliplatin,
doxorubicin and gemcitabine are potent inhibitors of tumor growth
and effective inducers of immunogenic cell death. Angew Chem Int Ed
Engl. 62:e2023107742023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Singh J, Meena A and Luqman S: New
frontiers in the design and discovery of therapeutics that target
calcium ion signaling: A novel approach in the fight against
cancer. Expert Opin Drug Discov. 1:1–14. 2023. View Article : Google Scholar
|
|
39
|
Que Z, Zhou Z, Liu S, Zheng W and Lei B:
Dihydroartemisinin inhibits EMT of glioma via gene BASP1 in
extrachromosomal DNA. Biochem Biophys Res Commun. 675:130–138.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fan M, Zhang J, Tsai CW, Orlando BJ,
Rodriguez M, Xu Y, Liao M, Tsai MF and Feng L: Structure and
mechanism of the mitochondrial Ca2+ uniporter
holocomplex. Nature. 582:129–133. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kennedy G, Gibson O, T O'Hare D, Mills IG
and Evergren E: The role of CaMKK2 in Golgi-associated vesicle
trafficking. Biochem Soc Trans. 51:331–342. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Stork BA, Dean A, Ortiz AR, Saha P,
Putluri N, Planas-Silva MD, Mahmud I, Rajapakshe K, Coarfa C, Knapp
S, et al: Calcium/calmodulin-dependent protein kinase kinase 2
regulates hepatic fuel metabolism. Mol Metab. 62:1015132022.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Najar M, Arefian M, Sidransky D, Gowda H,
Prasad T, Modi P and Chatterjee A: Tyrosine phosphorylation
profiling revealed the signaling network characteristics of CAMKK2
in gastric adenocarcinoma. Front Genet. 13:8547642022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Najar MA, Aravind A, Dagamajalu S,
Sidransky D, Ashktorab H, Smoot DT, Gowda H, Prasad TSK, Modi PK
and Chatterjee A: Hyperactivation of MEK/ERK pathway by
Ca2+/calmodulin-dependent protein kinase kinase 2
promotes cellular proliferation by activating cyclin-dependent
kinases and minichromosome maintenance protein in gastric cancer
cells. Mol Carcinog. 60:769–783. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Waseem M and Wang BD: Promising strategy
of mPTP modulation in cancer therapy: An emerging progress and
future insight. Int J Mol Sci. 24:55642023. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Su Y, Zhao D, Jin C, Li Z, Sun S, Xia S,
Zhang Y, Zhang Z, Zhang F, Xu X, et al: Dihydroartemisinin induces
ferroptosis in HCC by promoting the formation of PEBP1/15-LO. Oxid
Med Cell Longev. 2021:34567252021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Fu F, Wang W, Wu L, Wang W, Huang Z, Huang
Y, Wu C and Pan X: Inhalable Biomineralized liposomes for cyclic
Ca2+-Burst-centered endoplasmic reticulum stress
enhanced lung cancer ferroptosis therapy. ACS Nano. 17:5486–5502.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Timm KN, Hu DE, Williams M, Wright AJ,
Kettunen MI, Kennedy BWC, Larkin TJ, Dzien P, Marco-Rius I,
Bohndiek SE, et al: Assessing oxidative stress in tumors by
measuring the rate of hyperpolarized [1-13C]Dehydroascorbic acid
reduction using 13C magnetic resonance spectroscopy. J Biol Chem.
292:1737–1748. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hsu YF, Kung FL, Huang TE, Deng YN, Guh
JH, Marchetti P, Marchesi E, Perrone D, Navacchia ML and Hsu LC:
Anticancer activity and molecular mechanisms of an ursodeoxycholic
acid methyl Ester-dihydroartemisinin hybrid via a triazole linkage
in hepatocellular carcinoma Cells. Molecules. 28:23582023.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Szatrowski TP and Nathan CF: Production of
large amounts of hydrogen peroxide by human tumor cells. Cancer
Res. 51:794–798. 1991.PubMed/NCBI
|
|
51
|
Jia F, Liu Y, Dou X, Du C, Mao T and Liu
X: Liensinine inhibits osteosarcoma growth by ROS-mediated
suppression of the JAK2/STAT3 signaling pathway. Oxid Med Cell
Longev. 2022:82456142022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wójcik P, Žarković N, Gęgotek A and
Skrzydlewska E: Involvement of metabolic lipid mediators in the
regulation of apoptosis. Biomolecules. 10:4022020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Han H, Lim JW and Kim H: Lycopene inhibits
activation of epidermal growth factor receptor and expression of
cyclooxygenase-2 in gastric cancer cells. Nutrients. 11:21132019.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Katoshevski T, Bar L, Tikochinsky E, Harel
S, Ben-Kasus Nissim T, Bogeski I, Hershfinkel M, Attali B and
Sekler I: CKII control of axonal plasticity is mediated by
mitochondrial Ca2+ via mitochondrial NCLX. Cells.
11:39902022. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Tangeda V, Lo YK, Babuharisankar AP, Chou
HY, Kuo CL, Kao YH, Lee AY and Chang JY: Lon upregulation
contributes to cisplatin resistance by triggering NCLX-mediated
mitochondrial Ca2+ release in cancer cells. Cell Death
Dis. 13:2412022. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Assali EA, Jones AE, Veliova M, Acín-Pérez
R, Taha M, Miller N, Shum M, Oliveira MF, Las G, Liesa M, et al:
NCLX prevents cell death during adrenergic activation of the brown
adipose tissue. Nat Commun. 11:33472020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Britti E, Delaspre F, Tamarit J and Ros J:
Calpain-inhibitors protect frataxin-deficient dorsal root ganglia
neurons from loss of mitochondrial Na+/Ca2+
exchanger, NCLX, and apoptosis. Neurochem Res. 46:108–119. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lee SH, Duron HE and Chaudhuri D: Beyond
the TCA cycle: New insights into mitochondrial calcium regulation
of oxidative phosphorylation. Biochem Soc Trans. 51:1661–1673.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhao H, Yan G, Zheng L, Zhou Y, Sheng H,
Wu L, Zhang Q, Lei J, Zhang J, Xin R, et al: STIM1 is a metabolic
checkpoint regulating the invasion and metastasis of hepatocellular
carcinoma. Theranostics. 10:6483–6499. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Liu M, Jin L, Sun S, Liu P, Feng X, Cheng
Z, Liu W, Guan K, Shi Y, Yuan H, et al: Metabolic reprogramming by
PCK1 promotes TCA cataplerosis, oxidative stress and apoptosis in
liver cancer cells and suppresses hepatocellular carcinoma.
Oncogene. 37:1637–1653. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Dong S, Guo X, Han F, He Z and Wang Y:
Emerging role of natural products in cancer immunotherapy. Acta
Pharm Sin B. 12:1163–1185. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Augimeri G, Fiorillo M, Morelli C, Panza
S, Giordano C, Barone I, Catalano S, Sisci D, Andò S and Bonofiglio
D: The Omega-3 docosahexaenoyl ethanolamide reduces CCL5 secretion
in triple negative breast cancer cells affecting tumor progression
and macrophage recruitment. Cancers (Basel). 15:8192023. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Tompkins E, Mimic B, Penn RB and Pera T:
The biased M3 mAChR ligand PD 102807 mediates qualitatively
distinct signaling to regulate airway smooth muscle phenotype. J
Biol Chem. 299:1052092023. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Du F, Yang L, Liu J, Wang J, Fan L,
Duangmano S, Liu H, Liu M, Wang J, Zhong X, et al: The role of
mitochondria in the resistance of melanoma to PD-1 inhibitors. J
Transl Med. 21:3452023. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Sahinbegovic H, Jelinek T, Hrdinka M, Bago
JR, Turi M, Sevcikova T, Kurtovic-Kozaric A, Hajek R and Simicek M:
Intercellular mitochondrial transfer in the tumor microenvironment.
Cancers (Basel). 12:17872020. View Article : Google Scholar : PubMed/NCBI
|