Introduction
Primary liver cancer is the fourth most common
malignancy and the second cause of cancer-related death in China,
posing a severe threat to the life and health of Chinese patients.
Besides, the morbidity and mortality of primary liver cancer are
increasing on a global scale (1,2). In
pathology, liver cancers mainly include hepatocellular carcinoma
(HCC, 75–85%), intrahepatic cholangiocarcinoma (10–15%), and
combined hepatocellular-cholangiocarcinoma (3). The present study focused on HCC for
analysis. For the past few years, treatment for HCC has been
significantly advanced, and surgery remains the first choice
(4). However, the incidence of
complications after hepatectomy remains high, especially in
patients complicated by primary liver fibrosis and cirrhosis
(5). According to the Barcelona
Clinic Liver Cancer (BCLC) Staging System, transarterial
chemoembolization (TACE) is recommended as the standard therapy for
patients with advanced or unresectable tumor (hepatic compensation,
Child B) (6,7). Some clinical trials have proved the
effectiveness of the TACE as an adjuvant therapy for HCC (6,8,9). For
example, a randomized controlled trial revealed that post-operative
adjuvant TACE (PATACE) was highly effective in HCC patients but at
a high risk of recurrence, such as those with multiple tumor
nodules, macroscopic vascular invasion, or large tumor diameter
(>5 cm) (8). Multiple studies
recommended PATACE for HCC patients with multiple tumor nodules, a
tumor of a large size, or vascular invasion (10–12).
The efficacy of PATACE can be attributed to its effect to improve
the prognosis of HCC patients, as traditional imaging fails to
discover the microscopic tumor foci and concealed multiple foci in
the liver before surgery (10).
Therefore, PATACE has been extensively applied in post-operative
HCC patients who have multiple risk factors of recurrence, such as
large tumor diameter, multiple tumor nodules, microvascular
invasion (MVI) and satellite lesions (6,10).
Nevertheless, the long-term survival of this
population remains unclear (4,13).
Even though TACE and liver resection are considered the most
effective therapies, this population may likely not receive
benefits from surgery and therefore have poor survival outcomes
(14). Assessment for the hepatic
functional reserve is critical for HCC treatment, as liver
cirrhosis potentially is a major cause of post-operative death
(15) and it is also a leading
cause of 70–90% HCC cases. Liver reserve is markedly more important
in early HCC than that in advanced disease (16,17).
Different from other malignancies, the survival outcome of HCC
patients is largely dependent on the baseline liver function and
the dissemination of the primary tumor. In this context, there is
an urgent need to look for non-invasive, accurate biomarkers that
are indicative of liver function and inflammation. In the meantime,
a prognostic model based on the biomarker, liver function and tumor
stage is also in demand. Hematological parameters following
systemic inflammation have been assembled as an inflammation-based
prognostic scoring system to predict cancer survival. For example,
peripheral blood subpopulations, including lymphocytes, monocytes
and platelets (PLT), are prognostic for the outcome of varying
cancers. A previous study found that anti-PLT therapy could prevent
HCC and improve the survival of mice with chronic hepatitis B (CHB)
(18). Moreover, numerous studies
have reported the relationship between PLT and HCC diagnosis,
post-operative complications, and survival (19–24). A
previous study found that preoperative PLT decline predicted an
increased incidence of complications, liver insufficiency and death
in HCC patients after tumor resection (22). The preoperative aspartate
aminotransferase (AST)-to-PLT ratio index (APRI) has been
identified as an independent prognostic index for liver failure in
HCC patients after hepatectomy (20). However, it has been controversial
how the preoperative PLT increase affects the survival outcome of
HCC patients. Amano et al (21) reported that a PLT count lower than
105/mm3 was unfavorable for the overall
survival (OS) and disease-free survival (DFS) of HCC patients. They
also found that patients beyond the Milan criteria could benefit
from hepatectomy, and patients with a sufficiently high PLT count
enjoyed an improved survival outcome. By contrast, Hwang et
al (23) pointed out that HCC
patients with PLT increase reversely had significantly shorter
survival times. Combining the studies, it is of paramount
importance to identify the prognostic significance of PLT-related
indicators, including PLT count, PLT-to-lymphocyte ratio (PLR), and
APRI. AST, a spectrum ranging from hepatitis to fatty liver, is
commonly used to assess liver injury. Previous studies have proved
that inflammation-based prognostic factors, including PLR, Glasgow
outcome scale score, neutrophil-to-lymphocyte ratio (NLR),
lymphocyte-to-monocyte ratio (LMR), and AST/alanine
aminotransferase ratio (AST/ALT), are prognostic for the outcome of
HCC. APRI is reported as a convenient indicator that can predict
the post-operative outcome of HBV-related HCC patients (16,25–27).
It is applicable in clinical practice as a tool for fast and
reliable assessment of the severity of liver function and
cirrhosis, and it even can be used as an alternative to liver
biopsy. However, it remains unclear whether APRI remains applicable
for identifying the patients responsive to PATACE.
Clinical staging is critical for patient prognosis
and treatment selection. Motivated by this fact, multiple staging
systems have been developed all over the world for the past few
years, including TNM staging (28),
Okuda staging (29), BCLC (30), Japan-integrated staging (JIS)
(31), Cancer of the Liver Italian
Program (CLIP) (32), China liver
cancer staging (CNLC) and Hong Kong liver cancer (HKLC). Okuda
staging is the first to consider liver function as a
tumor-influencing factor, but it does not include certain important
prognostic factors such as vascular invasion, tumor number and
metastasis. JIS is an assembly of the TNM staging and Child-Pugh
scoring systems released by the Liver Cancer Study Group of Japan
(LCSGJ), while the patient's personal situation is not considered.
CLIP fails to identify patients with early-stage disease and assess
cancer-related symptoms that are essential for patient prognosis.
HKLC was developed in 2014 based on a large group of patients with
HBV-related HCC patients. It is more sensitive than the BCLC
staging system to patients requiring aggressive treatment. Despite
several studies (33,34) reporting the superior capability of
HKLC to BCLC in predicting survival outcomes, validation in
different cohorts is still required. Presently, the BCLC and
AJCC-TNM remain the most effective and reliable staging systems
(28). Since the present study
mainly focused on HCC patients in China, the CNLC staging system
was also applied here.
Nomogram is devised as a prognostic tool combining
known prognostic markers to quantify the prognostic risk as
precisely as possible. The present study attempted to discuss the
prognostic significance of the APRI for OS and DFS of HCC patients
receiving radical hepatectomy and PATACE. In addition, a potent
nomogram of high clinical prognostic value was established based on
the APRI and HCC staging systems. The present study was in
accordance with the TRIPOD reporting checklist.
Materials and methods
Patients
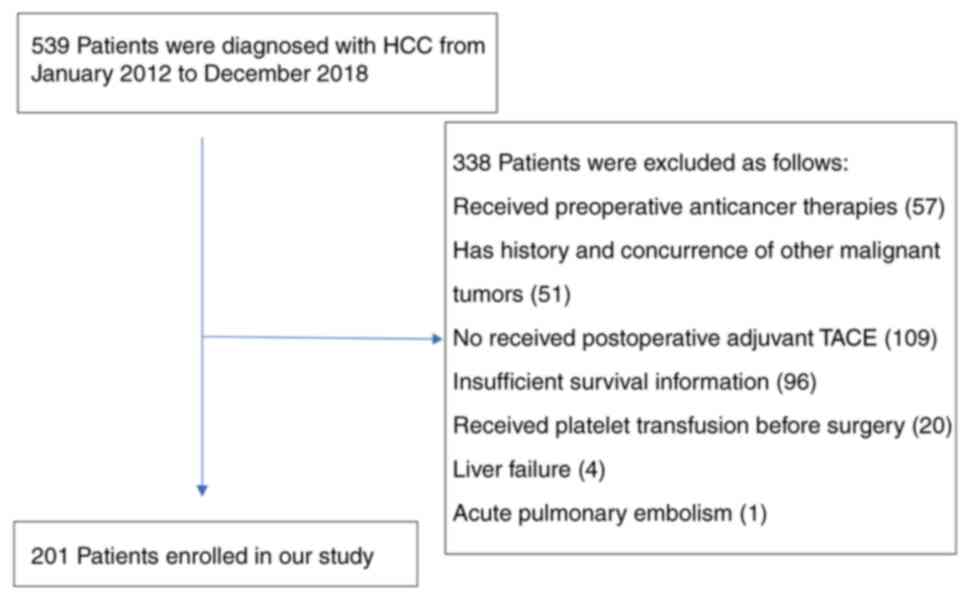
The current study retrospectively analyzed the data
from 201 HCC patients, who received radical hepatectomy in the
Second Hospital of Nanjing from January 2012 to December 2018
(Fig. 1). Data on follow-up were
collected from outpatient medical records, telephone interviews, or
WeChat conversations. Inclusion criteria were as follows: i)
pathological diagnosis of HCC; ii) presence of any of the following
risk factors, such as multiple tumor nodules, macroscopic vascular
invasion, tumor diameter >5 cm, and microsatellite lesions; iii)
no preoperative interventional, targeted, or immune therapy; iv)
without or complicated by other malignancies; v) complete tumor
resection; vi) first PATACE (lobaplatin and iodized oil) within 4–6
weeks after hepatectomy; and vii) long-term treatment with oral
antivirals.
All participants were reviewed 4 weeks after
hepatectomy, and PATACE was recommended in cases without recurrent
lesions in the liver. Recurrence was considered in the presence of
a new tumor focus within 4 weeks after radical hepatectomy, and the
cases were excluded from the present study. Patients who underwent
preoperative anti-cancer treatment, had not received a PATACE, had
a history of other malignancy or had incomplete follow-up data were
excluded from the present study. The present study was approved
(approval no. B2021-322) by the Second Hospital of Nanjing ethics
committee (Nanjing, China) and was conducted in line with the
standards of the Declaration of Helsinki (as revised in 2013). As
part of the consent process, written informed consent was provided
by all participants.
Data collection and definitions
Clinical data were collected within one week
preceding surgery, including age, sex, pathology report, serum
albumin, total bilirubin, ALT, AST, alpha-fetoprotein (AFP), PLT,
tumor parameters and globulin. Staging systems adopted the AJCC-TNM
(8th edition), BCLC and CNLC staging systems. APRI was calculated
as follows: APRI={[AST (IU/l)/upper limit of
normal]/PLT(×109/l)} ×100 (35). Any discrepancy was resolved by
discussion.
Follow-up
Follow-up data as of December 2021 were acquired. OS
was defined by a period from the beginning of PATACE to patient
death. Patients surviving the last follow-up were censored. DFS was
defined by a period from the beginning of PATACE to recurrence or
the last follow-up. Follow-up examinations included HCC markers
(AFP, AFP-L3, DCP and GP73), abdominal ultrasound, upper abdominal
contrast-enhanced CT or Gd-EOB-DTPA MRI, and liver function test.
All participants were followed up every 3–4 months within 2 years
after surgery and every 6 months after 2 years. Recurrence was
diagnosed in the context of post-operative serum AFP >20 ng/ml
and the presence of a new focus on abdominal ultrasound, upper
abdominal contrast-enhanced CT, or Gd-EOB-DTPA MRI. Patient death,
time to recurrence, imaging results and HCC markers during the
follow-up period were recorded in detail. Follow-up and outcome
assessments were fulfilled by two researchers to minimize bias.
Treatment process
Radical surgery a right subcostal ‘inverted-L’
incision was created, and it was extended to the left subcostal
region if necessary. Careful examination for peritoneal cavity was
needed to confirm the tumor extent, extrahepatic metastasis or
possible intraperitoneal dissemination. Anatomical hepatectomy was
preferred for the tumor occupying half of the liver, or in the
liver lobe or segment. For the tumor with a large size, a deep
position, or next to blood vessel, preoperative 3D CT image was
obtained to confirm the tumor border and the extent of resection.
Liver parenchyma devascularization was performed with a cavitron
ultrasonic surgical aspirator (CUSA) and bipolar
electrocoagulation, during which corresponding blood vessels were
ligated or sutured by silk or 3–0 Proline. The Pringle maneuver was
used to control hepatic portal blood flow if necessary, with the
time strictly controlled to be less than 15 min.
PATACE
After 4–6 weeks of hepatectomy, TACE was conducted
for the remaining liver when the liver function recovered. The
Seldinger technique was applied to gain vascular access in the
femoral artery (or the radial artery), and the catheter was
extended to the celiac trunk or common hepatic artery to complete
DSA. Subtraction images were acquired in the arterial, parenchymal
and venous phases. The catheter head was selectively inserted into
the right or left hepatic artery, and the emulsion mixed with 2–10
ml Lipiodol Ultra Fluide (Guerbet) and 10–20 mg Lobaplatin for
Injection (Hainan Changan International Pharmaceutical Co., Ltd.)
was injected through a microcatheter (Progreat; Terumo Corp.). A
combination of liver function and area of the body was used to
determine the doses of chemotherapeutics and iodized oil to be
given.
Statistical analysis
Statistical analysis was fulfilled with the SPSS
26.0 (IBM Corp.) and GraphPad Prism 8 (Dotmatics). The optimal
cut-off value for APRI was determined by drawing a receiver
operating characteristic (ROC) curve. Chi-square test, Fisher's
exact test, and Mann-Whitney U test was applied to analyze the
relationship between clinical pathological features and APRI.
One-way analysis of variance was used for pairwise comparisons.
Kaplan-Meier method together with the log-rank test was adopted to
explore the effect of APRI on DFS and OS. Backward stepwise Cox
regression modeling was applied to perform multivariate analysis
for the variables with P<0.1 in univariate analysis. P<0.05
demonstrated a difference with statistical significance.
Concordance index values (C-index) and calibration plots were
employed to assess the performance of nomogram using the R package
‘rms’ (v4.2.1, http://www.r-project.org/). Time-dependent ROC curves
and estimates of the area under the curve (AUC) were used to
compare the predictive value of the nomogram with OS and DFS. Using
the decision curve analysis (DCA), net benefits and threshold
probabilities were quantified for the nomogram, and the clinical
benefit was quantified.
Results
Clinicopathological characteristics of
patients and optimal cut-off value of APRI
There were more male than female patients (83.6 vs.
16.4%). Among all patients, ages ranged from 11–81 years (median,
53.6±10.9 years). A total of 96% of the cases were caused by
hepatitis B. Patients with HCV-RNA or HBV-DNA levels exceeding 500
IU/ml were 1.5 or 23.9% of the total, respectively, while 59.7% of
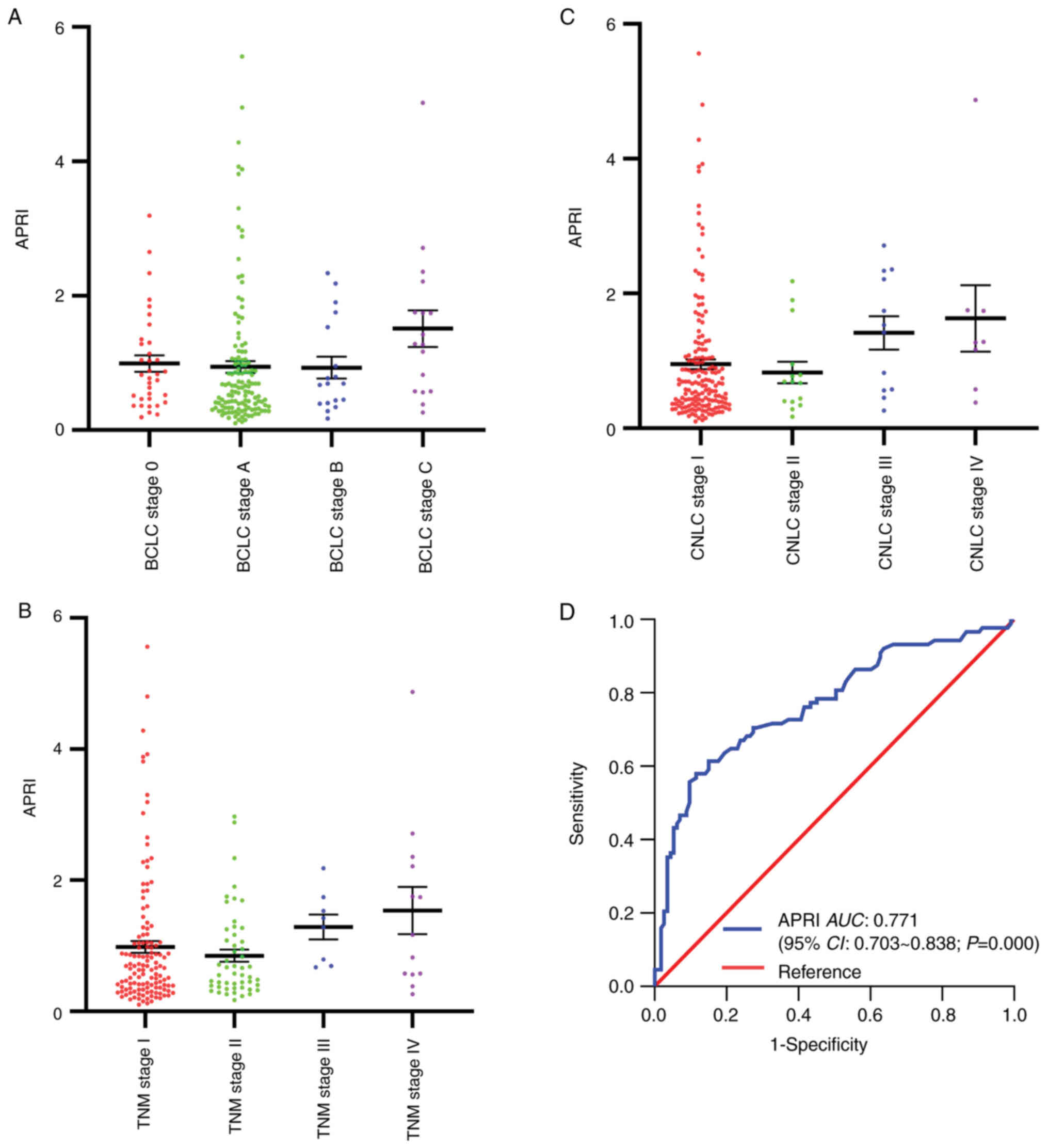
the patients developed liver cirrhosis. The number of APRI exhibits
non-significant difference among samples of different BCLC stage,
TNM and CNLC stage (Fig. 2A-C). The
time-dependent ROC curve was generated to identify the optimal
cut-off value of APRI for OS prediction (Fig. 2D). The cut-off value of APRI was
determined as 1.02, associated with an AUC of 0.771, sensitivity of
58.0%, specificity of 88.5, and 95% confidence interval (95% CI) of
0.703–0.838 (Table I).
 | Table I.Analysis of ROC curves of APRI. |
Table I.
Analysis of ROC curves of APRI.
| Variable | AUC | Optimal cutoff
value | Youden index | Sensitivity
(%) | Specificity
(%) | P-value | 95% CI |
|---|
| APRI | 0.771 | 1.02 | 0.465 | 58.0 | 88.5 | 0.000 | 0.703–0.838 |
Correlation between APRI and
clinicopathological features
According to the cut-off value, patients were
divided into the High-APRI group (APRI >1.02) and Low-APRI group
(APRI ≤1.02). Correlational analysis demonstrated that APRI was
significantly associated with AST, total bilirubin, prothrombin
time (PT), ALT, albumin, HBV-DNA, PLT, WBC, CNLC stage, BCLC stage
and AJCC-TNM stage (all P<0.05). The relationship between APRI
and clinicopathological features of HCC patients was detailed in
Table II.
 | Table II.Relations of APRI with the
clinicopathological characteristics of HCC patients. |
Table II.
Relations of APRI with the
clinicopathological characteristics of HCC patients.
|
|
| APRI |
|
|
|---|
|
|
|
|
|
|
|---|
| Variables | Number of patients,
n (%) | ≤1.02 n (%) | >1.02 n (%) | χ2 | P-value |
|---|
| Age, years |
|
|
|
| 0.748 |
|
≤40 | 23 (11.4) | 15 (0.9) | 8 (12.5) | 0.104 |
|
|
>40 | 178 (88.6) | 122 (89.1) | 56 (87.5) |
|
|
| Sex |
|
|
| 1.050 | 0.305 |
|
Male | 168 (83.6) | 112 (81.8) | 56 (87.5) |
|
|
|
Female | 33 (16.4) | 25 (18.2) | 8 (12.5) |
|
|
| Viral
hepatitis |
|
|
| - | 0.113 |
|
HBV | 193 (96.0) | 134 (97.8) | 59 (92.2) |
|
|
|
HCV | 8 (4.0) | 3 (2.2) | 5 (7.8) |
|
|
| Liver
cirrhosis |
|
|
| 0.742 | 0.389 |
|
Yes | 120 (59.7) | 79 (57.7) | 41 (64.1) |
|
|
| No | 81 (40.3) | 58 (42.3) | 23 (35.9) |
|
|
| HBV-DNA, IU/ml |
|
|
| 3.986 | 0.046 |
|
≤500 | 144 (71.6) | 106 (77.4) | 38 (59.4) |
|
|
|
>500 | 48 (23.9) | 28 (20.4) | 20 (31.3) |
|
|
| HCV-RNA, IU/ml |
|
|
| - | 0.226 |
|
≤500 | 6 (3.0) | 1 (0.7) | 5 (7.8) |
|
|
|
>500 | 3 (1.5) | 2 (1.5) | 1 (1.5) |
|
|
| AFP, ng/ml |
|
|
| 2.714 | 0.099 |
|
≥20 | 115 (57.2) | 73 (53.3) | 42 (65.6) |
|
|
|
<20 | 86 (42.8) | 64 (46.7) | 22 (34.4) |
|
|
| AFP-L3, % |
|
|
| 0.365 | 0.546 |
|
≥10 | 88 (43.8) | 58 (42.3) | 30 (46.9) |
|
|
|
<10 | 113 (56.2) | 79 (57.7) | 34 (53.1) |
|
|
| Tumor size, cm |
|
|
| 1.388 | 0.239 |
| ≤5 | 140 (69.7) | 99 (72.3) | 41 (64.1) |
|
|
|
>5 | 61 (30.3) | 38 (27.7) | 23 (35.9) |
|
|
| AST level, U/l |
|
|
| 60.208 | <0.0001 |
|
<40 | 149 (74.1) | 124 (90.5) | 25 (39.1) |
|
|
|
≥40 | 52 (25.9) | 13 (9.5) | 39 (60.9) |
|
|
| TB, µmol/l |
|
|
| 7.299 | 0.007 |
|
<34.2 | 187 (93.0) | 132 (96.4) | 55 (85.9) |
|
|
|
≥34.2 | 14 (7.0) | 5 (3.6) | 9 (14.1) |
|
|
| Prothrombin time,
sec |
|
|
| 34.280 | <0.0001 |
|
<14 | 157 (78.1) | 123 (89.8) | 34 (53.1) |
|
|
|
≥14 | 44 (21.9) | 14 (10.2) | 30 (46.9) |
|
|
| ALT level, U/l |
|
|
| 21.083 | <0.0001 |
|
<40 | 133 (66.2) | 105 (76.6) | 28 (43.8) |
|
|
|
≥40 | 68 (33.8) | 32 (23.4) | 36 (56.2) |
|
|
| Albumin, g/dl |
|
|
| 23.660 | <0.0001 |
|
<35 | 41 (20.4) | 15 (10.9) | 26 (40.6) |
|
|
|
≥35 | 160 (79.6) | 122 (89.1) | 38 (59.4) |
|
|
| PLT,
×109/l |
|
|
| 60.552 | 0.0001 |
|
<100 | 81 (40.3) | 30 (21.9) | 51 (79.7) |
|
|
|
≥100 | 120 (59.7) | 107 (78.1) | 13 (20.3) |
|
|
| WBC,
×109/l |
|
|
| 17.702 | <0.0001 |
|
<5 | 104 (51.7) | 57 (41.6) | 47 (73.4) |
|
|
| ≥5 | 97 (48.3) | 80 (58.4) | 17 (26.6) |
|
|
| Tumour capsule |
|
|
| 5.642 | 0.018 |
|
Complete | 161 (80.1) | 116 (84.7) | 45 (70.3) |
|
|
|
Incomplete | 40 (19.9) | 21 (15.3) | 19 (29.7) |
|
|
| Vascular
invasiona |
|
|
| 3.101 | 0.078 |
|
Present | 53 (26.4) | 31 (22.6) | 22 (34.4) |
|
|
|
Absent | 148 (73.6) | 106 (77.4) | 42 (65.6) |
|
|
| Nerve invasion |
|
|
| 2.641 | 0.104 |
|
Present | 16 (8.0) | 8 (5.8) | 8 (12.5) |
|
|
|
Absent | 185 (92.0) | 129 (94.2) | 56 (87.5) |
|
|
| Edmondson-Steiner
grade |
|
|
| 1.475 | 0.224 |
|
I–II | 113 (56.2) | 81 (59.1) | 32 (50.0) |
|
|
|
III–IV | 88 (43.7) | 56 (40.9) | 32 (50.0) |
|
|
| Tumor number |
|
|
| 2.641 | 0.104 |
| ≤3 | 185 (92.0) | 129 (94.2) | 56 (87.5) |
|
|
|
>3 | 16 (8.0) | 8 (5.8) | 8 (12.5) |
|
|
| BCLC stage |
|
|
| 5.466 | 0.019 |
|
0-A | 166 (82.6) | 119 (86.9) | 47 (73.4) |
|
|
|
B-C | 35 (17.4) | 18 (13.1) | 17 (26.6) |
|
|
| CNLC stage |
|
|
| 11.252 | 0.001 |
|
I–II | 181 (90.0) | 130 (94.9) | 51 (79.7) |
|
|
|
III–IV | 20 (10.0) | 7 (5.1) | 13 (20.3) |
|
|
| TNM stage |
|
|
| 6.917 | 0.009 |
|
I–II | 180 (89.6) | 128 (93.4) | 52 (81.3) |
|
|
|
III–IV | 21 (10.4) | 9 (6.6) | 12 (18.7) |
|
|
Survival analysis of APRI
The relationship between the APRI and survival
outcomes (OS and DFS) of HCC patients was then analyzed in the
High-APRI and Low-APRI groups using the Kaplan-Meier method
followed by log-rank test. The results revealed that the DFS
(P<0.0001) and OS (P<0.0001) of patients in the low-APRI
group were significantly improved than those of patients in the
High-APRI group (Fig. 3A and B).
Subsequently, stratification analysis was performed based on CNLC,
BCLC and AJCC-TNM staging systems. In the present study,
early-stage tumor was classified as CNLC I/II, BCLC 0/A, and TNM
I/II, while late-stage tumor was classified as CNLC III/IV, BCLC
B/C, and TNM III/IV. It was revealed that either in BCLC 0/A or
BCLC B/C stage, patients with a relatively low APRI had
significantly longer DFS and OS than patients with a relatively
high APRI (both P<0.05) (Fig.
3C-F). While in CNLC I/II (Fig. 3G
and H) and TNM I/II (Fig. 3K and
L) stage patients, relatively low APRI was associated with
improved DFS and OS (both P<0.05). There were no distinct
differences in DFS and OS between the High-APRI and Low-APRI groups
in CNLC III/IV (DFS, P=0.502; OS, P=0.775; Fig. 3I and J) and TNM III/IV (DFS,
P=0.184; OS, P=0.212; Fig. 3M and
N) patients.
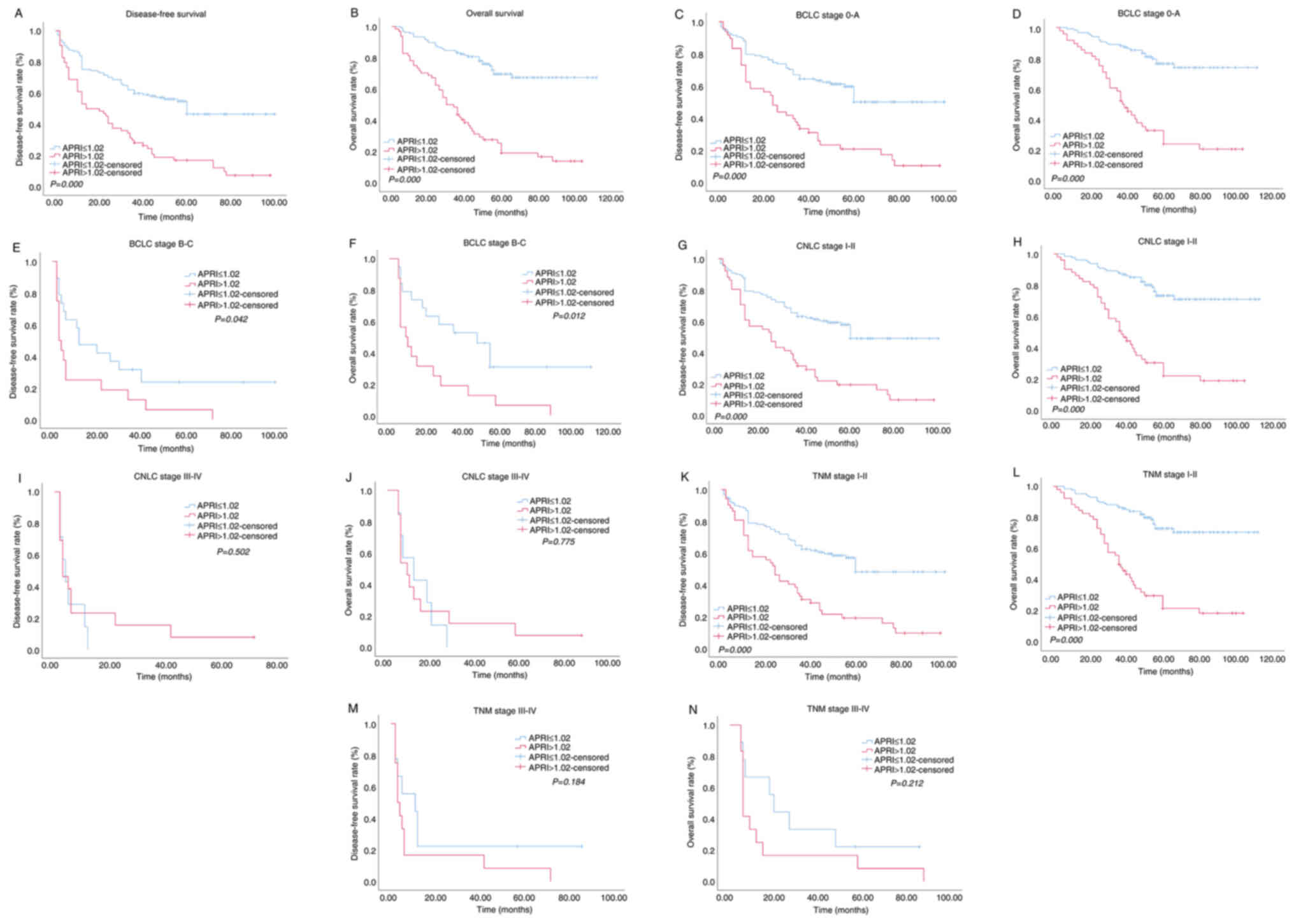 | Figure 3.Kaplan-Meier survival curves of HCC
patients in high APRI group and low APRI group after BCLC, CNLC and
TNM stage stratification. Patients were divided into two groups,
APRI ≤1.02 and >1.02, by optimal cutoff value of APRI. (A) DFS
of patients with APRI ≤1.02 was higher than those with APRI
>1.02 (P<0.0001, log-rank test). (B) OS of patients with APRI
≤1.02 was also higher than those with APRI >1.02 (P<0.0001,
log-rank test). (C) DFS curves of patients classified as BCLC 0-A
stage (P<0.0001). (D) OS curves of patients classified as BCLC
0-A stage (P<0.0001). (E). DFS curves of patients classified as
BCLC B-C stage (P=0.042). (F) OS curves of patients classified as
BCLC B-C stage (P=0.012). (G) DFS curves of patients classified as
CNLC I–II stage (P<0.0001). (H) OS curves of patients classified
as CNLC I–II stage (P<0.0001). (I) DFS curves of patients
classified as CNLC III–IV stage (P=0.502). (J) OS curves of
patients classified as CNLC III–IV stage (P=0.775). (K) DFS curves
of patients classified as TNM I–II stage (P<0.0001). (L) OS
curves of patients classified as TNM I–II stage (P<0.0001). (M)
DFS curves of patients classified as TNM III–IV stage (P=0.184).
(N) OS curves of patients classified as TNM III–IV stage (P=0.212).
HCC, hepatocellular carcinoma; APRI, preoperative aspartate
aminotransferase-to-platelet ratio index; BCLC, Barcelona Clinic
Liver Cancer; CNLC, China liver cancer staging; TNM, Tumor Node
Metastasis classification; DFS, disease-free survival; OS, overall
survival. |
Risk factors influencing the survival
of HCC patients
The uni- and multi-variate Cox regression analyses
on the prognostic significance of APRI for DFS in patients with HCC
patients are shown in Table III.
Univariate analysis demonstrated that AFP (P=0.017), PLT (P=0.022),
ALT (P=0.004), AST (P<0.0001), total bilirubin (P=0.001), PT
(P=0.010), tumor size (P=0.025), tumor number (P=0.002), tumor
capsule (P<0.0001), vascular invasion (P<0.0001), neural
invasion (P=0.005), Edmondson-Steiner grade (P=0.002), TNM stage
(P<0.0001), CNLC stage (P<0.0001), BCLC stage (P<0.0001)
and APRI (P<0.0001) were prognostic for the DFS of patients. The
variables with P<0.1 were further included in the backward
stepwise Cox regression model. The result showed that tumor capsule
(P=0.040), Edmondson-Steiner grade (P=0.038), BCLC stage
(P<0.0001) and APRI (P<0.0001) were independent prognostic
factors of DFS in HCC patients.
 | Table III.Univariate and multivariate Cox
proportional hazards regression analysis of factors for DFS of HCC
patients. |
Table III.
Univariate and multivariate Cox
proportional hazards regression analysis of factors for DFS of HCC
patients.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Sex (male vs.
female) | 1.256 | 0.761–2.072 | 0.373 |
|
|
|
| Age (≤40 vs.
>40, years) | 0.685 | 0.410–1.145 | 0.148 |
|
|
|
| Types of viral
hepatitis (HBV vs. HCV) | 1.309 | 0.610–2.811 | 0.489 |
|
|
|
| Livers cirrhosis
(Yes vs. No) | 1.092 | 0.757–1.575 | 0.639 |
|
|
|
| AFP (<20 vs.
≥20, ng/ml) | 1.574 | 1.086–2.281 | 0.017 | 1.203 | 0.799–1.812 | 0.376 |
| AFP-L3 (<10 vs.
≥10, %) | 1.336 | 0.935–1.911 | 0.112 |
|
|
|
| HBV-DNA (≤500 vs.
>500, IU/ml) | 0.895 | 0.576–1.392 | 0.623 |
|
|
|
| HCV-DNA (≤500 vs.
>500, IU/ml) | 0.707 | 0.135–3.697 | 0.681 |
|
|
|
| WBC (<5 vs. ≥5,
×109/l) | 1.097 | 0.768–1.567 | 0.610 |
|
|
|
| PLT (<100 vs.
≥100, ×109/l) | 0.660 | 0.461–0.943 | 0.022 | 0.984 | 0.607–1.594 | 0.947 |
| Albumin (<35 vs.
≥35, g/dl) | 0.824 | 0.540–1.258 | 0.370 |
|
|
|
| ALT level (<40
vs. ≥40, U/l) | 1.705 | 1.188–2.447 | 0.004 | 1.056 | 0.688–1.620 | 0.804 |
| AST level (<40
vs. ≥40, U/l) | 2.477 | 1.710–3.589 | 0.000 | 0.931 | 0.523–1.659 | 0.810 |
| TB (<34.2 vs.
≥34.2, µmol/l) | 2.604 | 1.459–4.646 | 0.001 | 1.717 | 0.931–3.166 | 0.083 |
| Prothrombin time
(≤14 vs. >14, sec) | 1.675 | 1.130–2.483 | 0.010 | 1.128 | 0.716–1.778 | 0.603 |
| Tumour size (≤5 vs.
>5, cm) | 1.541 | 1.056–2.248 | 0.025 | 1.381 | 0.928–2.056 | 0.112 |
| Tumour number (≤3
vs. >3) | 2.468 | 1.382–4.408 | 0.002 | 0.960 | 0.432–2.134 | 0.920 |
| Tumour capsule
(Complete vs. Incomplete) | 2.616 | 1.759–3.889 | 0.000 | 1.595 | 1.022–2.490 | 0.040 |
| Vascular invasion
(Present vs. Absent) | 2.170 | 1.485–3.170 | 0.000 | 0.875 | 0.512–1.496 | 0.626 |
| Nerve invasion
(Present vs. Absent) | 2.286 | 1.281–4.077 | 0.005 | 1.165 | 0.595–2.279 | 0.656 |
| Edmondson-Steiner
grade (I–II vs. III–IV) | 1.783 | 1.247–2.549 | 0.002 | 1.478 | 1.021–2.140 | 0.038 |
| BCLC stage (0-A vs.
B-C) | 3.214 | 2.122–4.869 | 0.000 | 2.310 | 1.467–3.638 | <0.001 |
| CNLC stage (I–II
vs. III–IV) | 5.466 | 3.322–8.993 | 0.000 | 1.631 | 0.731–3.641 | 0.232 |
| TNM stage (I–II vs.
III–IV) | 3.422 | 2.082–5.624 | 0.000 | 0.742 | 0.312–1.762 | 0.499 |
| APRI (≤1.02 vs.
>1.02) | 2.641 | 1.842–3.788 | 0.000 | 2.159 | 1.480–3.150 | <0.001 |
The uni- and multi-variate Cox regression analyses
on the prognostic significance of APRI for OS in HCC patients are
demonstrated in Table IV. The
result of univariate analysis revealed significant prognostic value
of AFP (P=0.015), PLT (P<0.0001), ALT (P=0.003), AST
(P<0.0001), total bilirubin (P<0.0001), PT (P=0.003), tumor
size (P=0.003), tumor number, tumor capsule, vascular invasion,
neural invasion, Edmondson-Steiner grade, TNM, CNLC and BCLC stage
and APRI (all P<0.0001). In the backward stepwise Cox regression
model, tumor size (P=0.003), Edmondson-Steiner grade (P=0.007),
BCLC stage (P<0.0001) and APRI (P<0.0001) were independent
prognostic factors of OS in HCC patients.
 | Table IV.Univariate and multivariate Cox
proportional hazards regression analysis of factors for OS of HCC
patients. |
Table IV.
Univariate and multivariate Cox
proportional hazards regression analysis of factors for OS of HCC
patients.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Sex (male vs.
female) | 0.980 | 0.562–1.708 | 0.943 |
|
|
|
| Age (≤40 vs.
>40, years) | 0.721 | 0.399–1.302 | 0.278 |
|
|
|
| Viral hepatitis
(HBV vs. HCV) | 0.913 | 0.335–2.491 | 0.859 |
|
|
|
| Livers cirrhosis
(Yes vs. No) | 0.922 | 0.604–1.408 | 0.707 |
|
|
|
| AFP (<20 vs.
≥20, ng/ml) | 1.734 | 1.111–2.705 | 0.015 | 1.171 | 0.70–1.954 | 0.546 |
| AFP-L3 (<10 vs.
≥10, %) | 1.246 | 0.819–1.894 | 0.304 |
|
|
|
| HBV-DNA (≤500 vs.
>500, IU/ml) | 0.958 | 0.575–1.597 | 0.869 |
|
|
|
| HCV-DNA (≤500 vs.
>500, IU/ml) | 0.844 | 0.152–4.685 | 0.846 |
|
|
|
| WBC (<5 vs. ≥5,
×109/l) | 0.810 | 0.531–1.233 | 0.325 |
|
|
|
| PLT (<100 vs.
≥100, ×109/l) | 0.446 | 0.292–0.681 | 0.000 | 0.855 | 0.492–1.486 | 0.579 |
| Albumin (<35 vs.
≥35, g/dl) | 0.700 | 0.432–1.136 | 0.149 |
|
|
|
| ALT level (<40
vs. ≥40, U/l) | 1.900 | 1.247–2.895 | 0.003 | 0.907 | 0.532–1.547 | 0.720 |
| AST level (<40
vs. ≥40,U/l) | 3.492 | 2.292–5.321 | 0.000 | 0.940 | 0.468–1.887 | 0.862 |
| TB (<34.2 vs.
≥34.2, µmol/l) | 3.163 | 1.675–5.974 | 0.000 | 1.857 | 0.943–3.658 | 0.073 |
| Prothrombin time
(≤14 vs. >14, sec) | 1.975 | 1.260–3.094 | 0.003 | 1.276 | 0.779–2.091 | 0.333 |
| Tumour size (≤5 vs.
>5, cm) | 1.907 | 1.244–2.926 | 0.003 | 1.982 | 1.271–3.089 | 0.003 |
| Tumour number (≤3
vs. >3) | 3.149 | 1.664–5.959 | 0.000 | 1.316 | 0.571–3.033 | 0.519 |
| Tumour capsule
(Complete vs. Incomplete) | 2.914 | 1.870–4.541 | 0.000 | 1.163 | 0.620–2.180 | 0.638 |
| Vascular invasion
(Present vs. Absent) | 3.040 | 1.983–4.660 | 0.000 | 0.947 | 0.490–1.830 | 0.872 |
| Nerve invasion
(Present vs. Absent) | 3.581 | 1.976–6.489 | 0.000 | 1.454 | 0.711–2.972 | 0.305 |
| Edmondson-Steiner
grade (I–II vs. III–IV) | 2.355 | 1.533–3.619 | 0.000 | 1.838 | 1.182–2.860 | 0.007 |
| BCLC stage (0-A vs.
B-C) | 4.198 | 2.675–6.585 | 0.000 | 3.370 | 2.102–5.403 | <0.001 |
| CNLC stage (I–II
vs. III–IV) | 8.272 | 4.915–13.923 | 0.000 | 1.918 | 0.816–4.508 | 0.135 |
| TNM stage (I–II vs.
III–IV) | 5.100 | 3.047–8.536 | 0.000 | 0.809 | 0.295–2.221 | 0.681 |
| APRI (≤1.02 vs.
>1.02) | 4.381 | 2.859–6.714 | 0.000 | 3.590 | 2.300–5.604 | <0.001 |
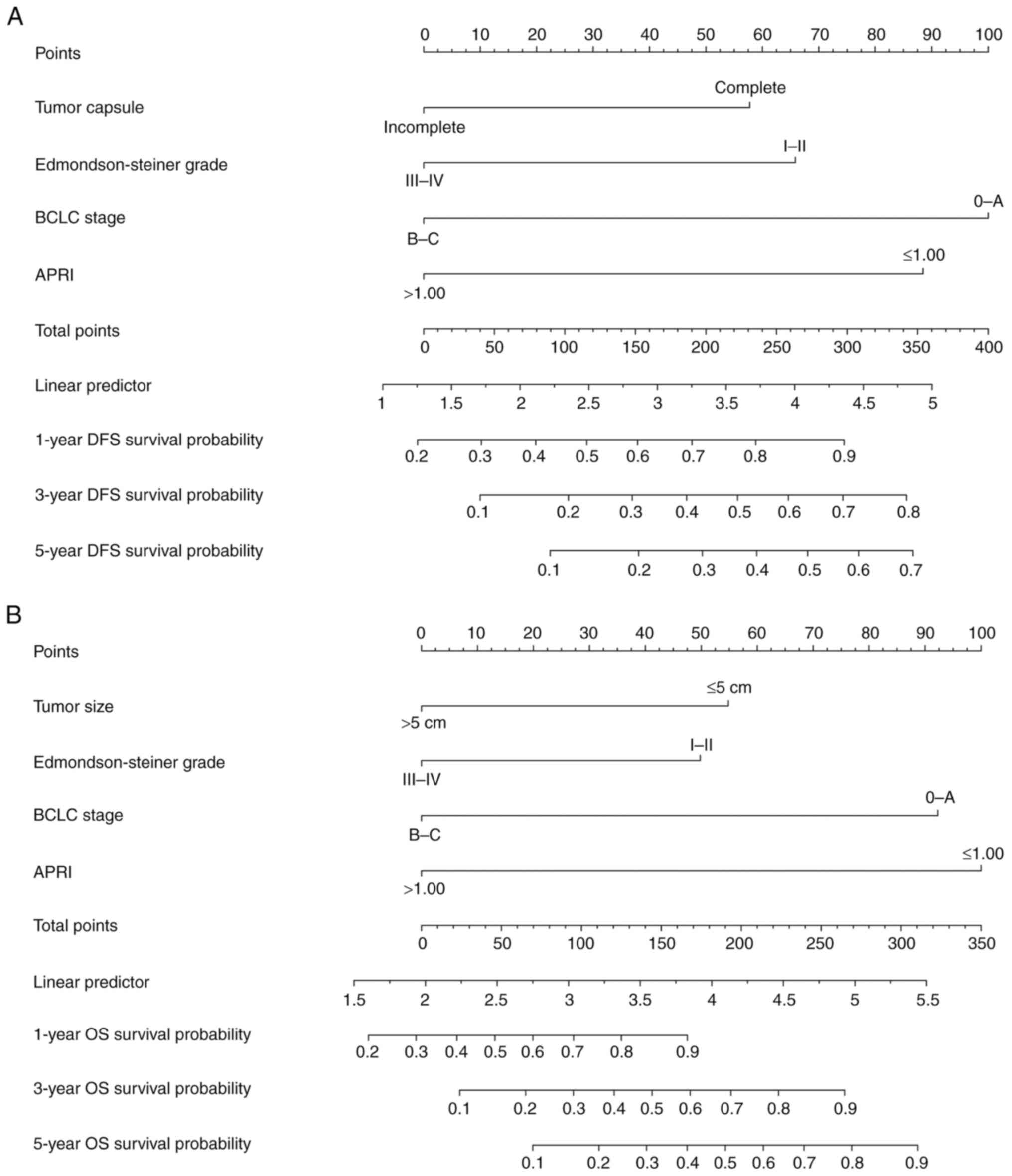
Nomogram construction and
validation
To improve stratification of patients with different
prognoses, a nomogram was constructed based on the multivariate Cox
regression analysis of the DFS and OS (Fig. 4). Scores were assigned to each risk
factor, and each included patient's grade was determined by the sum
of these scores. In this nomogram, a higher score predicts an
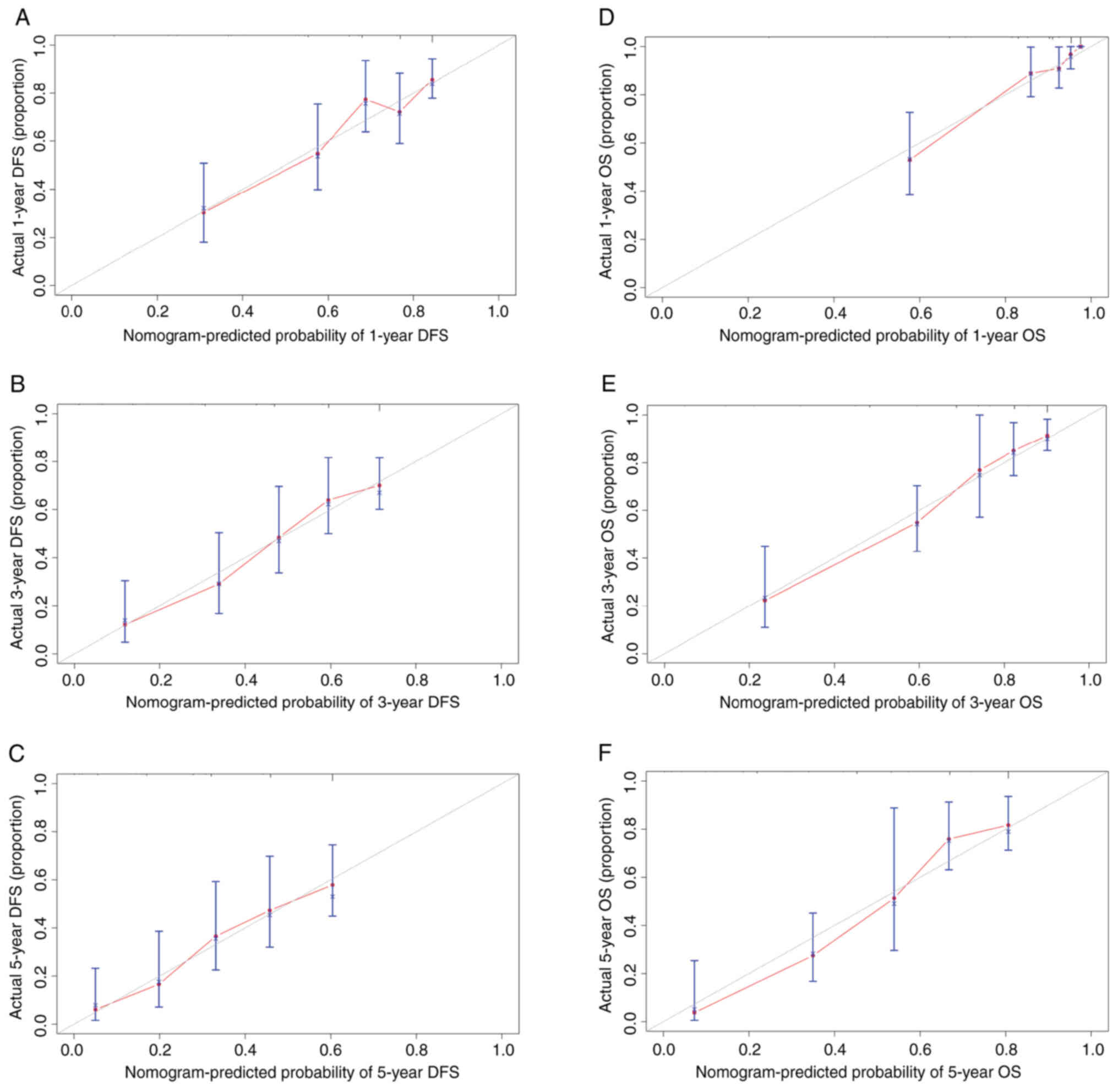
improved survival outcome. Internal validation was performed with
500 re-samplings. The calibration curves plotted with the 1-, 3-
and 5-year DFS (Fig. 5A-C,
respectively) were well matched with the idealized 45 line (the
x-axis represents the nomogram-predicted probability of DFS,
whereas the y-axis shows the actual DFS). The calibration curves
plotted with 1-, 3- and 5-year OS were well matched with the
idealized 45 line [Fig. 5D-F, (the
x-axis represents the nomogram-predicted probability of OS, whereas
the y-axis shows the actual OS). The C-index of the nomogram for
predicting DFS and OS probability were 0.700 (95% CI: 0.650–0.750)
and 0.775 (95% CI: 0.726–0.824) (Tables
V and VI). The C-index for 1-,
3- and 5-year DFS was 0.735 (95% CI: 0.636–0.834), 0.735 (95% CI:
0.659–0.810), and 0.686 (95% CI: 0.599–0.774), respectively; while
that for 1, 3, and 5-year OS was 0.864 (95% CI: 0.792–0.936), 0.812
(95% CI: 0.731–0.893), and 0.783 (95% CI: 0.704–0.862),
respectively (Table VII). The
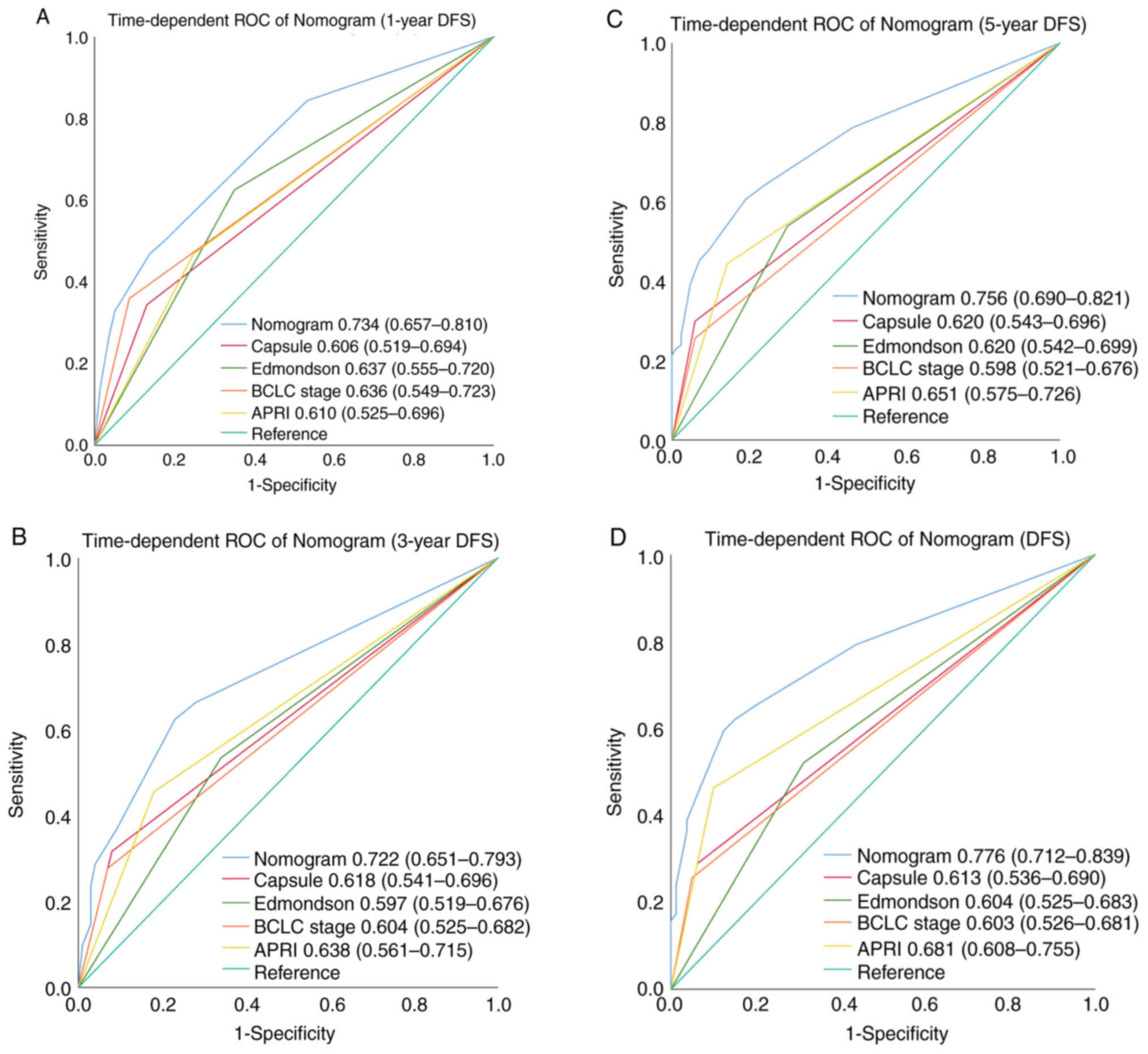
time-dependent AUC (t-AUC) for predicting the 1-, 3- and 5-year and
all DFS was 0.734 (95% CI: 0.657–0.810) (Fig. 6A), 0.722 (95% CI: 0.651–0.793)
(Fig. 6B) and 0.756 (95% CI:
0.690–0.821) (Fig. 6C), and 0.776
(95% CI: 0.712–0.839; Fig. 6D),
respectively (Table VIII). The
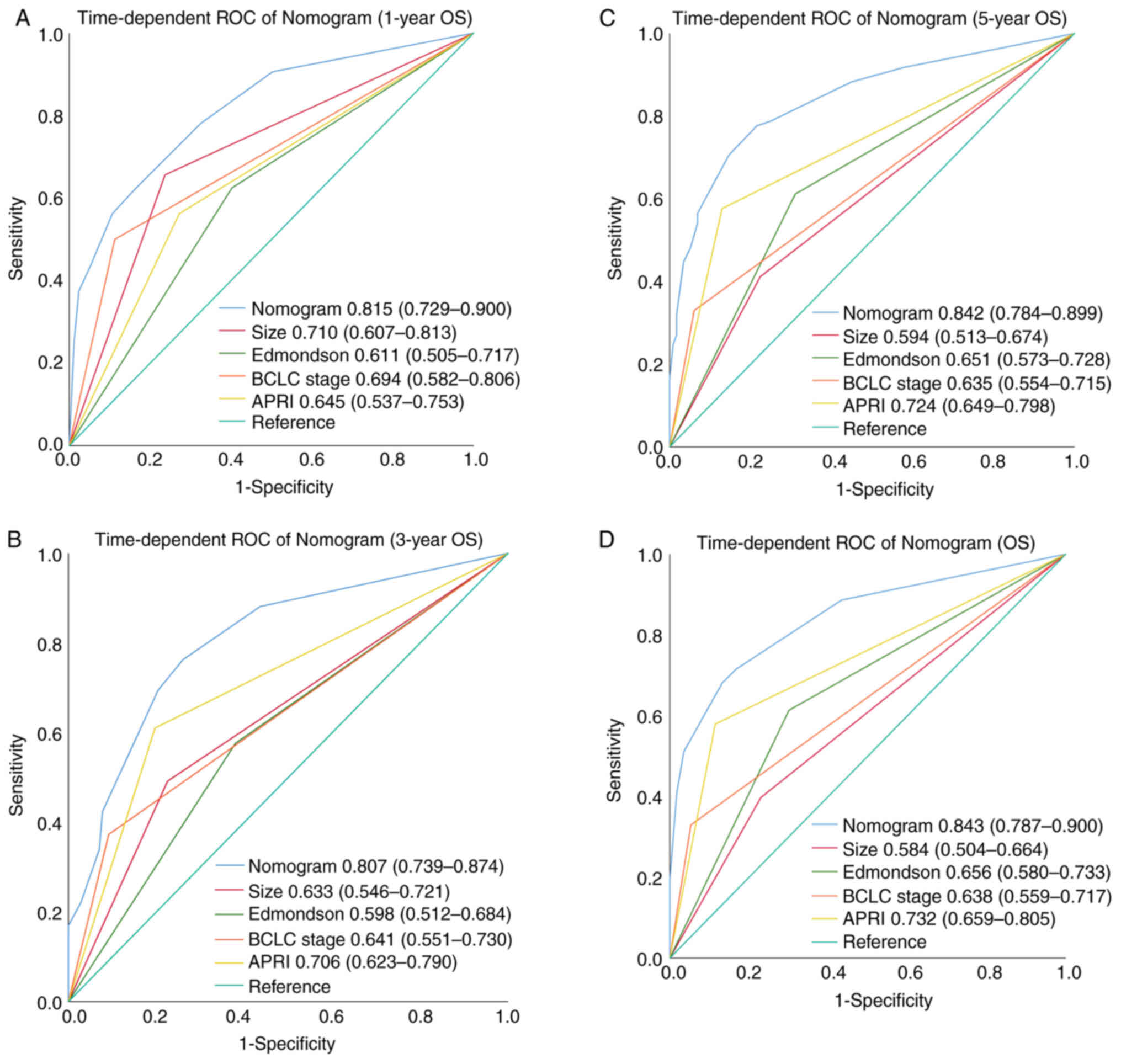
t-AUC for predicting the 1-, 3-, 5-year and all OS was 0.815 (95%
CI: 0.729–0.900) (Fig. 7A), 0.807
(95% CI: 0.739–0.874) (Fig. 7B),
0.842 (95% CI: 0.784–0.899) (Fig.
7C), and 0.843 (95%CI: 0.787–0.900) (Fig. 7D), respectively (Table VIII). The result demonstrated
high consistency between the predicted and actual outcome,
suggesting favorable performance of the nomogram.
 | Table V.C-index of nomogram and other
predictors in OS. |
Table V.
C-index of nomogram and other
predictors in OS.
|
| OS |
|---|
| Predictors | C-index | 95% CI | P-value |
|---|
| Nomogram | 0.775 | 0.726–0.824 | <0.001 |
| APRI | 0.673 | 0.623–0.723 | <0.001 |
| Tumour size | 0.604 | 0.550–0.658 | 0.003 |
| Edmondson-Steiner
grade | 0.607 | 0.553–0.661 | <0.001 |
| BCLC stage | 0.624 | 0.576–0.672 | <0.001 |
 | Table VI.C-index of nomogram and other
predictors in DFS. |
Table VI.
C-index of nomogram and other
predictors in DFS.
|
| DFS |
|---|
|
|
|
|---|
| Predictors | C-index | 95% CI | P-value |
|---|
| Nomogram | 0.700 | 0.650–0.750 | <0.001 |
| APRI | 0.607 | 0.564–0.650 | <0.001 |
| Tumour capsule | 0.592 | 0.553–0.631 | <0.001 |
| Edmondson-Steiner
grade | 0.593 | 0.547–0.639 | <0.001 |
| BCLC stage | 0.598 | 0.558–0.637 | <0.001 |
 | Table VII.The C-index of nomogram and other
predictors in 1-year, 3-year, 5-year DFS and OS. |
Table VII.
The C-index of nomogram and other
predictors in 1-year, 3-year, 5-year DFS and OS.
|
| Overall
survival |
| Disease-free
survival |
|---|
|
|
|
|
|
|---|
| Predictors | 1-year C-index (95%
CI) | P-value | 3-year C-index (95%
CI) | P-value | 5-year C-index (95%
CI) | P-value | Predictors | 1-year C-index (95%
CI) | P-value | 3-year C-index (95%
CI) | P-value | 5-year C-index (95%
CI) | P-value |
|---|
| Nomogram | 0.864 | <0.001 | 0.812 | <0.001 | 0.783 | <0.001 | Nomogram | 0.735 | <0.001 | 0.735 | <0.001 | 0.686 | <0.001 |
|
| (0.792- |
| (0.731- |
| (0.704- |
|
| (0.636- |
| (0.659- |
| (0.599- |
|
|
| 0.936) |
| 0.893) |
| 0.862) |
|
| 0.834) |
| 0.810) |
| 0.774) |
|
| APRI | 0.679 | <0.001 | 0.671 | <0.001 | 0.673 | <0.001 | APRI | 0.602 | <0.001 | 0.638 | <0.001 | 0.605 | <0.001 |
|
| (0.585- |
| (0.610- |
| (0.622- |
|
| (0.541- |
| (0.561- |
| (0.562- |
|
|
| 0.773) |
| 0.732) |
| 0.724) |
|
| 0.663) |
| 0.715) |
| 0.648) |
|
| Tumour size | 0.709 | <0.001 | 0.628 | <0.001 | 0.606 | 0.001 | Tumour | 0.597 | <0.001 | 0.595 | <0.001 | 0.592 | <0.001 |
|
| (0.618–0.799) |
| (0.567–0.689) |
| (0.551–0.661) |
| capsule | (0.540–0.649) |
| (0.556–0.636) |
| (0.553–0.631) |
|
| Edmondson- | 0.655 | 0.004 | 0.600 | 0.004 | 0.607 | <0.001 | Edmondson- | 0.625 | <0.001 | 0.595 | <0.001 | 0.595 | <0.001 |
| Steiner grade | (0.564–0.745) |
| (0.537–0.663) |
| (0.554–0.660) |
| Steiner grade | (0.566–0.683) |
| (0.546–0.644) |
| (0.548–0.642) |
|
| BCLC stage | 0.733 | <0.001 | 0.636 | <0.001 | 0.624 | <0.001 | BCLC stage | 0.626 | <0.001 | 0.600 | <0.001 | 0.598 | <0.001 |
|
| (0.639–0.827) |
| (0.577–0.695) |
| (0.575–0.673) |
|
| (0.571–0.681) |
| (0.557–0.643) |
| (0.559–0.637) |
|
 | Table VIII.The AUC of nomogram and other
predictors in 1-year, 3-year, 5-year DFS and OS. |
Table VIII.
The AUC of nomogram and other
predictors in 1-year, 3-year, 5-year DFS and OS.
|
| Overall
survival |
| Disease-free
survival |
|---|
|
|
|
|
|
|---|
| Predictors | 1-year AUC (95%
CI) | P-value | 3-year AUC (95%
CI) | P-value | 5-year AUC (95%
CI) | P-value | Predictors | 1-year AUC (95%
CI) | P-value | 3-year AUC (95%
CI) | P-value | 5-year AUC (95%
CI) | P-value |
|---|
| Nomogram | 0.815 | <0.001 | 0.807 | <0.001 | 0.842 | <0.001 | Nomogram | 0.734 | <0.001 | 0.722 | <0.001 | 0.756 | <0.001 |
|
| (0.729–0.900) |
| (0.739–0.874) |
| (0.784–0.899) |
|
| (0.657–0.810) |
| (0.651–0.793) |
| (0.690–0.821) |
|
| APRI | 0.645 | 0.009 | 0.706 | <0.001 | 0.724 | <0.001 | APRI | 0.610 | 0.002 | 0.638 | <0.001 | 0.651 | <0.001 |
|
| (0.537–0.753) |
| (0.623–0.790) |
| (0.649–0.798) |
|
| (0.525–0.696) |
| (0.561–0.715) |
| (0.575–0.726) |
|
| Tumour size | 0.710 | <0.001 | 0.633 | 0.003 | 0.594 | 0.023 | Tumour | 0.606 | 0.015 | 0.618 | 0.004 | 0.62 | 0.004 |
|
| (0.607–0.813) |
| (0.546–0.721) |
| (0.513–0.674) |
| capsule | (0.519–0.694) |
| (0.541–0.696) |
| (0.543–0.696) |
|
| Edmondson- | 0.611 | 0.046 | 0.598 | 0.029 | 0.651 | 0.040 | Edmondson- | 0.637 | 0.002 | 0.597 | 0.017 | 0.620 | 0.004 |
| Steiner grade | (0.505–0.717) |
| (0.512–0.684) |
| (0.573–0.728) |
| Steiner grade | (0.555–0.720) |
| (0.519–0.676) |
| (0.542–0.699) |
|
| BCLC stage | 0.694 | 0.001 | 0.641 | 0.002 | 0.635 | <0.001 | BCLC stage | 0.636 | 0.002 | 0.604 | 0.011 | 0.598 | 0.017 |
|
| (0.582–0.806) |
| (0.551–0.730) |
| (0.554–0.715) |
|
| (0.549–0.723) |
| (0.561–0.715) |
| (0.521–0.676) |
|
To gain insight into the performance of the
nomogram, the nomogram with other significant prognostic factors
was compared (Tables V and VI). For DFS, the C-index of nomogram
(0.700) was markedly higher than that of tumor capsule (0.592),
Edmondson-Steiner grade (0.593), APRI (0.607) and BCLC stage
(0.598); while for OS, the C-index of nomogram (0.775) was also
improved as compared with that of tumor size (0.604),
Edmondson-Steiner grade (0.607), APRI (0.673) and BCLC stage
(0.624). For DFS, the t-AUC of nomogram (0.776) was markedly higher
than that of tumor capsule (0.613), Edmondson-Steiner grade
(0.604), APRI (0.681), and BCLC stage (0.603); while for OS, the
t-AUC of nomogram (0.843) was also improved as compared with that
of tumor size (0.584), Edmondson-Steiner grade (0.656), APRI
(0.732) and BCLC stage (0.638) (Table
IX).
 | Table IX.The AUC of nomogram and other
predictors in DFS and OS. |
Table IX.
The AUC of nomogram and other
predictors in DFS and OS.
|
| Overall
survival |
| Disease-free
survival |
|---|
|
|
|
|
|
|---|
| Predictors | AUC | 95% CI | P-value | Predictors | AUC | 95% CI | P-value |
|---|
| Nomogram | 0.843 | 0.787–0.900 | <0.001 | Nomogram | 0.776 | 0.712–0.839 | <0.001 |
| APRI | 0.732 | 0.659–0.805 | <0.001 | APRI | 0.681 | 0.608–0.755 | <0.001 |
| Tumour size | 0.584 | 0.504–0.664 | 0.042 | Tumour capsule | 0.613 | 0.536–0.690 | 0.007 |
| Edmondson-Steiner
grade | 0.656 | 0.580–0.733 | 0.001 | Edmondson-Steiner
grade | 0.604 | 0.525–0.683 | 0.013 |
| BCLC stage | 0.638 | 0.559–0.717 | <0.001 | BCLC stage | 0.603 | 0.526–0.681 | 0.013 |
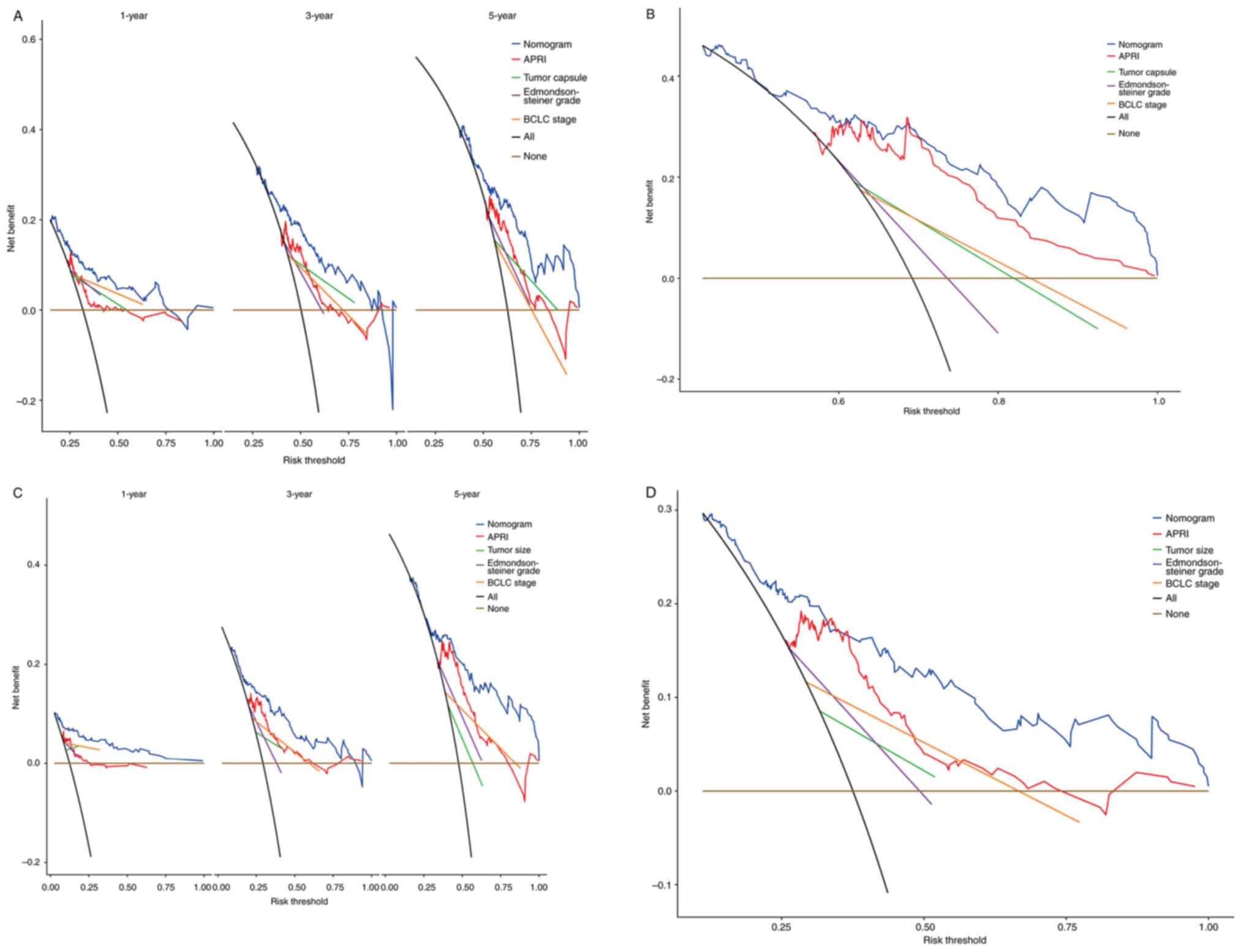
DCA is commonly used to evaluate the clinical net
benefit of a nomogram. In the present study, DCA showed that the
nomogram increased net benefits and exhibited a wider range of
threshold probabilities in predicting 1-, 3-, and 5-year OS and DFS
(Fig. 8A-D). Collectively, the
combination of APRI and HCC staging systems can improve
stratification of patients with different prognoses.
Discussion
The present study identified preoperative APRI and
BCLC staging as independent prognostic factors for OS and DFS of
HCC patients receiving PATACE. In addition, APRI was also
significantly associated with the DFS and OS of patients stratified
by BCLC, CNLC and TNM stages. It was also found that absence of
tumor capsule and tumor size >5 cm were independently prognostic
for the DFS and OS of patients, consistent with the previous
literature (36). Moreover, the
present study constructed a nomogram by combining the APRI and HCC
staging systems (CNLC, BCLC and TNM) to help quantify the
prognostic risk and then provide more prognostic information. It is
known that HCC patients are usually accompanied by liver cirrhosis
and fibrosis, which are great concerns for patients (17). Besides, hepatectomy can lead to
post-operative alterations of neuroendocrine, metabolism and immune
systems, resulting in immune dysfunction and increasing the risk of
complications after PATACE. Theoretically, factors such as
malnutrition, poor immune status and liver cirrhosis, may affect
the prognosis of HCC patients after PATACE. Previous studies proved
the prognostic value of APRI in liver malignancy (37–39),
but its significance in prognosis of HCC patients undergoing PATACE
is less studied (36). Therefore,
more independent data are in demand to validate the prognostic
value of APRI in HCC.
Growing evidence has suggested that host
inflammatory responses are predictive of the clinical outcome of
HCC patients given their significant implications for tumorigenesis
and metastasis. In addition, HCC patients receiving PATACE are at a
high risk of recurrence and post-operative alterations in the
immune system. A recent study revealed that APRI is an effective
and non-invasive indicator that can be used to assess the risk of
liver fibrosis in patients with CHB and chronic hepatitis C (CHC).
APRI was first reported by Wai et al (35) as a biochemical alternative to
predict the advanced fibrosis and liver cirrhosis in patients with
CHC. In the following years, the effect of APRI was further
explored in identifying HIV infection and differentiating liver
cirrhosis in hepatitis B cohorts. While in previous years, APRI has
been proven as capable of predicting the risk of developing HCC in
patients with liver cirrhosis and the death rate associated with
tumor resection (16,20,40).
It was also revealed that APRI can be used as a valuable prognostic
indicator for HCC patients undergoing radiofrequency ablation and
hepatectomy (26,41).
The present study applied ROC analysis to determine
the optimal cut-off value of APRI as 1.02 for OS of HCC patients,
and classified patients into the High- and Low-APRI groups. It was
found that the DFS (P<0.0001) and OS (P<0.0001) of patients
in the Low-APRI group were significantly improved than those of
patients in the High-APRI group. In the multivariate Cox regression
analysis, tumor capsule (P=0.040), Edmondson-Steiner grade
(P=0.038), BCLC stage (P<0.0001) and APRI (P<0.0001) were
identified as significantly prognostic for the DFS of patients;
while tumor size (P=0.003), Edmondson-Steiner grade (P=0.007), BCLC
stage (P<0.0001) and APRI (P<0.0001) were independent
prognostic factors for OS of patients. It was previously reported
that in patients with single HCC, APRI<0.47 was associated with
a significantly higher OS rate and a remarkably lower recurrence
rate, as compared with the control group (26). Hung et al (40) and Shen et al (26) reported a cut-off value of APRI of
0.47 and 0.62, respectively. The discrepancy may be attributed to
the different methods used to calculate APRI cut-off value and the
different etiologies of HCC. In addition, the differences in tumor
status, sample size, and patient inclusion criteria can also make
an effect. In a previous study by Hung et al (40) involving 76 HBV-mono-infected HCC
patients from 12 trials, all the tumors were <5 cm in diameter
with the median value of 2.5 cm, which is significantly smaller
than the 4.8 cm (median) in the present study. In another study
made by Shen et al (26)
included 332 HCC patients with a mean tumor size of 8.76 cm. Among
them, 25.6% had large vessels, and 20.8% were complicated by portal
vein tumor thrombosis. All these patients with such characteristics
were not considered in the present study. In spite of the different
cut-off values of APRI, a lower value of APRI generally predicts an
improved OS rate.
However, the relationship between APRI increase and
poor prognosis remains unclear. It is hypothesized that numerous
HCC patients with APRI increase have a low preoperative PLT, which
may be a result of spleen enlargement that leads to PLT destruction
or progressive liver fibrosis that leads to decreased production of
thrombopoietin. In addition, the low preoperative PLT may also
correlate to major post-operative complications, liver failure and
mortality. It has also been reported that PLT increase is
associated with the poor prognosis of nasopharyngeal carcinoma,
gastric, colorectal and endometrial cancer (23,42–44).
Similarly, there is no specific explanation for the relationship
between PLT increase and poor prognosis. The current potential
explanations are as follows: First, PLT increase can promote tumor
growth by advancing angiogenesis. As cancer patients usually
present with coagulation abnormalities leading to disturbed PLT
function, increase in PLT secrets more angiogenic factors to
stimulate tumor angiogenesis. Second, PLT can interact with tumor
cells via receptor-ligand pairs, thereby promoting tumor cell
growth and invasion (45–47). Third, PLT are actively involved in
host immune attack to tumor (48,49).
Previous studies theorized that PLT decrease the cytolytic activity
of NK cells to protect tumor cells from NK attack (48). Inflammation of the liver caused by
viral infection and alcohol consumption leads to HCC onset
(36), and AST from the
mitochondria in hepatocytes is a reliable and sensitive biomarker
of liver inflammation. Disorders in the liver result in
mitochondrial damage and subsequent release of intrahepatic AST
into the blood, suggesting heavily impaired liver function. It is
known that hepatocellular injury is tightly associated with the
occurrence of HCC (50). Moreover,
a large group of HCC patients with APRI elevation may also have an
increase in AST, indicating stress response or injury of the liver,
such as reactivation of HBV replication or progressive liver
fibrosis (51). These events are
associated with a poor survival rate in HCC patients. This also
supports the finding of the present study that patients with APRI
>1.02 had poorer survival outcomes and a higher risk of
recurrence. Therefore, APRI elevation indicates severely impaired
liver function and poor tumor prognosis.
CNLC, BCLC and AJCC-TNM (8th edition) staging
systems were found to be capable of stratifying HCC patients based
on their risk categories in the present study. BCLC and AJCC-TNM
(8th edition) staging systems are the most widely used tools
currently. Multivariate Cox regression analysis demonstrated that
the BCLC stage was an independent risk factor of prognosis in HCC
patients undergoing PATACE. The nomogram established by significant
independent prognostic factors is intuitive and can maximize the
predictive accuracy in assessment for prognosis in individuals. The
present study constructed a nomogram based on the multivariate Cox
regression analysis for DFS and OS of HCC patients. It was
identified that tumor size, tumor capsule and BCLC stage were
correlated with the prognosis of HCC patients receiving PATACE. The
multivariate analysis of the present study also identified that
APRI was superior to AST and PLT in predicting survival outcomes in
this population. Referring to literature, there was only one study
reporting the use of an APRI-based nomogram in predicting the
prognosis of HCC patients receiving radical surgery followed by
adjuvant TACE. However, the previous study did not further explore
the relationship between the APRI and HCC stage and OS of patients
(39,16,52).
Besides, the case data in that study were limited and not
sufficient for external validation, and certain bias existed in
partial data. The present study generated a nomogram that combined
tumor status, tumor stage and APRI. This nomogram can be used for
individualized survival estimation in patients with HCC patients
receiving radical surgery followed by adjuvant TACE and can also
help surgeons make appropriate post-operative decisions for
treatment and individualized monitoring.
Furthermore, there are some considerations when
building a nomogram. The number of surviving and succumbed patients
should be 10-fold greater than the number of variables used to
construct the nomogram in order to reduce the prediction error in
the predicted probability <10%. The number of deaths was 88,
which is 22-fold larger than the number of variables in the present
study. Due to the insufficient number of cases in the external
validation group, an internal validation was conducted with 500
sets of bootstrap samples with a calibration curve and well
verified the nomogram. Collectively, the present study proved the
favorable performance of the nomogram in predicting survival
outcome in these patients using the calibration plot and C-index,
which was superior to other prognostic factors.
At present, multiple prognostic indicators for
post-operative HCC patients have been identified, but there is a
paucity of indicators for patients receiving PATACE. This may be
attributed to some factors that affect the clinical outcome of
patients with TACE, such as liver function, tumor characteristics
and treatment modalities. HCC patients who received PATACE were
included in the present study as second-line treatment, in an
attempt to avoid the confounders caused by other treatments. In
addition, 1.02 as the cut-off value was used of APRI for analysis,
which is not in line with the previous studies. For example, the
cut-off value of APRI reported by Shen et al (26) and Tang et al (38) were 0.40 and 0.62, respectively,
which were obtained from the ROC curve for patients receiving TACE
or liver operations. Moreover, it was also identified that a larger
tumor size and a higher BCLC grade were predictive of poorer
survival outcomes of patients with HCC patients, consistent with
previous studies (17,53,54).
It has been established that tumor and clinical features, such as
tumor size, tumor number, BCLC stage and MVI, have implications for
survival and recurrence of HCC patients, while non-tumor
parameters, such as inflammation, viral infection and liver
fibrosis, are important in HCC recurrence.
There are certain limitations to the present study.
For example, this was a single-center retrospective study, and the
majority of participants had an HBV infection background (96%).
Therefore, a large-cohort, multi-center study with stratification
analysis based on etiology is in demand to validate the findings of
the present study. In addition, bias may not be completely avoided
given that all participants were treated in the same medical
center. Moreover, further prospective study is required to validate
the results of the present study, in other centers. A control group
may also be warranted to reinforce these results. Finally, the
present study adopted baseline APRI for analysis, but APRI values
were dynamic during follow-up. Thus, it may lead to loss of patient
data during follow-up. In conclusion to sum up, the present study
identified that APRI has certain clinical value as an independent
prognostic factor for DFS and OS of HCC patients receiving PATACE.
Combining the APRI and HCC stages, surgeons can improve stratifying
of patients into different risk categories, thereby efficiently
identifying the high-risk group for improved treatment.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Nanjing Medical
University Science and Technology Development Fund (grant no.
NNUB20210165).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
QHS, NNZ, JBH, YXY and YFZ conceptualized and
designed the study. XPY, BWS and LZ acquired, analyzed and
interpreted the data. QHS, NNZ and JBH drafted the manuscript. QHS,
YXY and YFZ critically revised the manuscript for important
intellectual content. QHS and YFZ confirm the authenticity of all
the raw data. All authors have made a significant contribution to
the present study, and have read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the Declaration of Helsinki (as revised in 2013) and was approved
(approval no. B2021-322) by the Second Hospital of Nanjing ethics
committee (Nanjing, China). All patients enrolled in this study
signed an informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhou M, Wang H, Zeng X, Yin P, Zhu J, Chen
W, Li X, Wang L, Wang L, Liu Y, et al: Mortality, morbidity, and
risk factors in China and its provinces, 1990–2017: A systematic
analysis for the Global Burden of Disease Study 2017. Lancet.
394:1145–1158. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Llovet JM, Villanueva A, Marrero JA,
Schwartz M, Meyer T, Galle PR, Lencioni R, Greten TF, Kudo M,
Mandrekar SJ, et al: Trial design and endpoints in hepatocellular
carcinoma: AASLD Consensus Conference. Hepatology. 73 (Suppl
1):S158–S191. 2021. View Article : Google Scholar
|
|
5
|
Cescon M, Vetrone G, Grazi GL, Ramacciato
G, Ercolani G, Ravaioli M, Del GM and Pinna AD: Trends in
perioperative outcome after hepatic resection: Analysis of 1500
consecutive unselected cases over 20 years. Ann Surg. 249:995–1002.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bruix J and Sherman M: Management of
hepatocellular carcinoma: An update. Hepatology. 53:1020–1022.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Forner A, Gilabert M, Bruix J and Raoul
JL: Treatment of intermediate-stage hepatocellular carcinoma. Nat
Rev Clin Oncol. 11:525–535. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tournoux C, Paoletti X and Barbare JC:
Treatment outcomes for hepatocellular carcinoma using
chemoembolization in combination with other therapies. Cancer Treat
Rev. 33:762–763. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lau WY, Lai EC, Leung TW and Yu SC:
Adjuvant intra-arterial iodine-131-labeled lipiodol for resectable
hepatocellular carcinoma: A prospective randomized trial-update on
5-year and 10-year survival. Ann Surg. 247:43–48. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang JH, Guo Z, Lu HF, Wang XB, Yang HJ,
Yang FQ, Bao SY, Zhong JH, Li LQ, Yang RR and Xiang BD: Adjuvant
transarterial chemoembolization after curative resection of
hepatocellular carcinoma: Propensity score analysis. World J
Gastroenterol. 21:4627–4634. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Paik KY and Kim EK: Pathologic response to
preoperative transarterial chemoembolization for resectable
hepatocellular carcinoma may not predict recurrence after liver
resection. Hepatobiliary Pancreat Dis Int. 15:158–164. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Z, Ren Z, Chen Y, Hu J, Yang G, Yu L,
Yang X, Huang A, Zhang X, Zhou S, et al: Adjuvant transarterial
chemoembolization for HBV-related hepatocellular carcinoma after
resection: A randomized controlled study. Clin Cancer Res.
24:2074–2081. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bruix J, Qin S, Merle P, Granito A, Huang
YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, et al:
Regorafenib for patients with hepatocellular carcinoma who
progressed on sorafenib treatment (RESORCE): A randomised,
double-blind, placebo-controlled, phase 3 trial. Lancet. 389:56–66.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tsurusaki M and Murakami T: Surgical and
Locoregional therapy of HCC: TACE. Liver Cancer. 4:165–175. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu CY, Lin JT, Ho HJ, Su CW, Lee TY, Wang
SY, Wu C and Wu JC: Association of nucleos(t)ide analogue therapy
with reduced risk of hepatocellular carcinoma in patients with
chronic hepatitis B: A nationwide cohort study. Gastroenterology.
147:143–151. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hann HW, Wan S, Lai Y, Hann RS, Myers RE,
Patel F, Zhang K, Ye Z, Wang C and Yang H: Aspartate
aminotransferase to platelet ratio index as a prospective predictor
of hepatocellular carcinoma risk in patients with chronic hepatitis
B virus infection. J Gastroenterol Hepatol. 30:131–138. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Su CW, Chau GY, Hung HH, Yeh YC, Lei HJ,
Hsia CY, Lai CR, Lin HC and Wu JC: Impact of Steatosis on prognosis
of patients with early-stage hepatocellular carcinoma after hepatic
resection. Ann Surg Oncol. 22:2253–2261. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sitia G, Aiolfi R, Di Lucia P, Mainetti M,
Fiocchi A, Mingozzi F, Esposito A, Ruggeri ZM, Chisari FV,
Iannacone M and Guidotti LG: Antiplatelet therapy prevents
hepatocellular carcinoma and improves survival in a mouse model of
chronic hepatitis B. Proc Natl Acad Sci USA. 109:E2165–E2172. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hwang SJ, Luo JC, Li CP, Chu CW, Wu JC,
Lai CR, Chiang JH, Chau GY, Lui WY, Lee CC, et al: Thrombocytosis:
A paraneoplastic syndrome in patients with hepatocellular
carcinoma. World J Gastroenterol. 10:2472–2477. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ichikawa T, Uenishi T, Takemura S, Oba K,
Ogawa M, Kodai S, Shinkawa H, Tanaka H, Yamamoto T, Tanaka S, et
al: A simple, noninvasively determined index predicting hepatic
failure following liver resection for hepatocellular carcinoma. J
Hepatobiliary Pancreat Surg. 16:42–48. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Amano H, Tashiro H, Oshita A, Kobayashi T,
Tanimoto Y, Kuroda S, Tazawa H, Itamoto T, Asahara T and Ohdan H:
Significance of platelet count in the outcomes of hepatectomized
patients with hepatocellular carcinoma exceeding the Milan
criteria. J Gastrointest Surg. 15:1173–1181. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maithel SK, Kneuertz PJ, Kooby DA,
Scoggins CR, Weber SM, Martin RC II, Mcmasters KM, Cho CS, Winslow
ER, Wood WC and Staley CA III: Importance of low preoperative
platelet count in selecting patients for resection of
hepatocellular carcinoma: A multi-institutional analysis. J Am Coll
Surg. 212:638–650. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hwang SG, Kim KM, Cheong JH, Kim HI, An
JY, Hyung WJ and Noh SH: Impact of pretreatment thrombocytosis on
blood-borne metastasis and prognosis of gastric cancer. Eur J Surg
Oncol. 38:562–567. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kurt M, Onal IK, Sayilir AY, Beyazit Y,
Oztas E, Kekilli M, Turhan N, Karaman K and Akdogan M: The role of
mean platelet volume in the diagnosis of hepatocellular carcinoma
in patients with chronic liver disease. Hepatogastroenterology.
59:1580–1582. 2012.PubMed/NCBI
|
|
25
|
Murray KF and Carithers RJ: AASLD practice
guidelines: Evaluation of the patient for liver transplantation.
Hepatology. 41:1407–1432. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shen SL, Fu SJ, Chen B, Kuang M, Li SQ,
Hua YP, Liang LJ, Guo P, Hao Y and Peng BG: Preoperative aspartate
aminotransferase to platelet ratio is an independent prognostic
factor for hepatitis B-induced hepatocellular carcinoma after
hepatic resection. Ann Surg Oncol. 21:3802–3809. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang C, Wu J, Xu J, Xu J, Xian J, Xue S
and Ye J: Association between aspartate
Aminotransferase-to-Platelet ratio index and hepatocellular
carcinoma risk in patients with chronic hepatitis: A meta-analysis
of cohort study. Dis Markers. 2019:20468252019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Amin MB, Greene FL, Edge SB, Compton CC,
Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and
Winchester DP: The Eighth edition AJCC Cancer Staging Manual:
Continuing to build a bridge from a population-based to a more
‘personalized’ approach to cancer staging. CA Cancer J Clin.
67:93–99. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Okuda K, Ohtsuki T, Obata H, Tomimatsu M,
Okazaki N, Hasegawa H, Nakajima Y and Ohnishi K: Natural history of
hepatocellular carcinoma and prognosis in relation to treatment.
Study of 850 patients. Cancer. 56:918–928. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Llovet JM, Bru C and Bruix J: Prognosis of
hepatocellular carcinoma: The BCLC staging classification. Semin
Liver Dis. 19:329–338. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kudo M, Chung H, Haji S, Osaki Y, Oka H,
Seki T, Kasugai H, Sasaki Y and Matsunaga T: Validation of a new
prognostic staging system for hepatocellular carcinoma: The JIS
score compared with the CLIP score. Hepatology. 40:1396–1405. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Llovet JM and Bruix J: Prospective
validation of the Cancer of the Liver Italian Program (CLIP) score:
A new prognostic system for patients with cirrhosis and
hepatocellular carcinoma. Hepatology. 32:679–680. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim JY, Sinn DH, Gwak GY, Choi GS, Saleh
AM, Joh JW, Cho SK, Shin SW, Carriere KC, Ahn JH, et al:
Transarterial chemoembolization versus resection for
intermediate-stage (BCLC B) hepatocellular carcinoma. Clin Mol
Hepatol. 22:250–258. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Selby LK, Tay RX, Woon WW, Low JK, Bei W,
Shelat VG, Pang TC and Junnarkar SP: Validity of the Barcelona
clinic liver cancer and Hong Kong liver cancer staging systems for
hepatocellular carcinoma in Singapore. J Hepatobiliary Pancreat
Sci. 24:143–152. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wai CT, Greenson JK, Fontana RJ,
Kalbfleisch JD, Marrero JA, Conjeevaram HS and Lok AS: A simple
noninvasive index can predict both significant fibrosis and
cirrhosis in patients with chronic hepatitis C. Hepatology.
38:518–526. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhu GQ, Wang K, Wang B, Zhou YJ, Yang Y,
Chen EB, Zhou ZJ, Zhou SL, Shi YH, Zhou J and Dai Z: Aspartate
aminotransferase-to-platelet ratio index predicts prognosis of
hepatocellular carcinoma after postoperative adjuvant transarterial
chemoembolization. Cancer Manag Res. 11:63–79. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang Y, Wan G, Li H, Gao L, Liu N, Gao P,
Liu Y, Gao X and Duan X: A prediction nomogram for hepatitis B
virus-associated hepatocellular carcinoma. Scand J Gastroenterol.
1–8. 2023.doi: 10.1080/00365521.2023.2252546 (Epub ahead of print).
View Article : Google Scholar
|
|
38
|
Tang T, Qiu JL, Li GW, Huang MP, Li Y, Li
YJ and Gu SZ: Aspartate aminotransferase-to-platelet ratio predicts
response to transarterial chemoembolisation and prognosis in
hepatocellular carcinoma patients. Clin Radiol. 73:259–265. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang X, Svn Z, Liv M, Liu M, Zhang Y and
Sun Q: Assessment of prognostic value of aspartate
Aminotransferase-to-Platelet ratio index in patients with
hepatocellular carcinoma: Meta-analysis of 28 cohort studies. Front
Med (Lausanne). 8:7562102021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hung HH, Su CW, Lai CR, Chau GY, Chan CC,
Huang YH, Huo TI, Lee PC, Kao WY, Lee SD and Wu JC: Fibrosis and
AST to platelet ratio index predict post-operative prognosis for
solitary small hepatitis B-related hepatocellular carcinoma.
Hepatol Int. 4:691–699. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang X, Xin Y, Yang Y, Chen Y, Cao XJ,
Wang Y, Fan Q, Zhou X and Li X: Aspartate
Aminotransferase-to-Platelet ratio index for predicting late
recurrence of hepatocellular carcinoma after radiofrequency
ablation. Int J Hyperthermia. 39:437–445. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gorelick C, Andikyan V, Mack M, Lee YC and
Abulafia O: Prognostic significance of preoperative thrombocytosis
in patients with endometrial carcinoma in an inner-city population.
Int J Gynecol Cancer. 19:1384–1389. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lin MS, Huang JX, Zhu J and Shen HZ:
Elevation of platelet count in patients with colorectal cancer
predicts tendency to metastases and poor prognosis.
Hepatogastroenterology. 59:1687–1690. 2012.PubMed/NCBI
|
|
44
|
Gao J, Zhang HY and Xia YF: Increased
platelet count is an indicator of metastasis in patients with
nasopharyngeal carcinoma. Tumour Biol. 34:39–45. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kim YJ, Borsig L, Varki NM and Varki A:
P-selectin deficiency attenuates tumor growth and metastasis. Proc
Natl Acad Sci USA. 95:9325–9330. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Pinedo HM, Verheul HM, D'Amato RJ and
Folkman J: Involvement of platelets in tumour angiogenesis? Lancet.
352:1775–1777. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jain S, Harris J and Ware J: Platelets:
Linking hemostasis and cancer. Arterioscler Thromb Vasc Biol.
30:2362–2367. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nieswandt B, Hafner M, Echtenacher B and
Mannel DN: Lysis of tumor cells by natural killer cells in mice is
impeded by platelets. Cancer Res. 59:1295–1300. 1999.PubMed/NCBI
|
|
49
|
Maini MK and Schurich A: Platelets harness
the immune response to drive liver cancer. Proc Natl Acad Sci USA.
109:12840–12841. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Witjes CD, Ijzermans JN, van der Eijk AA,
Hansen BE, Verhoef C and de Man RA: Quantitative HBV DNA and AST
are strong predictors for survival after HCC detection in chronic
HBV patients. Neth J Med. 69:508–513. 2011.PubMed/NCBI
|
|
51
|
Kamimoto Y, Horiuchi S, Tanase S and
Morino Y: Plasma clearance of intravenously injected aspartate
aminotransferase isozymes: Evidence for preferential uptake by
sinusoidal liver cells. Hepatology. 5:367–375. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yugawa K, Maeda T, Nagata S, Sakai A,
Edagawa M, Omine T, Kometani T, Yamaguchi S, Konishi K and
Hashimoto K: A novel combined prognostic nutritional index and
aspartate aminotransferase-to-platelet ratio index-based score can
predict the survival of patients with hepatocellular carcinoma who
undergo hepatic resection. Surg Today. 52:1096–1108. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kao W, Chiou Y, Hung H, Chou Y, Su C, Wu
J, Huo T, Huang Y, Lin H and Lee S: Risk factors for long-term
prognosis in hepatocellular carcinoma after radiofrequency ablation
therapy. Eur J Gastroenterol Hepatol. 23:12017.
|
|
54
|
Persico M, Aglitti A, Aghemo A, Rendina M,
Lleo A, Ciancio A, Di Marco V, Lampertico P, Brunetto MR, Zuin M,
et al: High efficacy of direct-acting anti-viral agents in
hepatitis C virus-infected cirrhotic patients with successfully
treated hepatocellular carcinoma. Aliment Pharmacol Ther.
47:1705–1712. 2018. View Article : Google Scholar : PubMed/NCBI
|