|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ceasovschih A, Voloc G, Sorodoc V, Vâță D,
Lupașcu CD, Preda C, Lionte C, Stoica A, Sîrbu O, Grigorescu ED, et
al: From chronic pruritus to neuroendocrine tumor: A case report.
Exp Ther Med. 23:1892022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Watanabe M, Baba H, Ishioka C, Nishimura Y
and Muto M: Recent advances in diagnosis and treatment for
malignancies of the gastrointestinal tract. Digestion. 85:95–98.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Matsuoka T and Yashiro M: Precision
medicine for gastrointestinal cancer: Recent progress and future
perspective. World J Gastrointest Oncol. 12:1–20. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nagahashi M, Shimada Y, Ichikawa H,
Kameyama H, Takabe K, Okuda S and Wakai T: Next generation
sequencing-based gene panel tests for the management of solid
tumors. Cancer Sci. 110:6–15. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bahassi el M and Stambrook PJ:
Next-generation sequencing technologies: Breaking the sound barrier
of human genetics. Mutagenesis. 29:303–310. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sicklick JK, Kato S, Okamura R,
Schwaederle M, Hahn ME, Williams CB, De P, Krie A, Piccioni DE,
Miller VA, et al: Molecular profiling of cancer patients enables
personalized combination therapy: The I-PREDICT study. Nat Med.
25:744–750. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Massard C, Michiels S, Ferté C, Le Deley
MC, Lacroix L, Hollebecque A, Verlingue L, Ileana E, Rosellini S,
Ammari S, et al: High-throughput genomics and clinical outcome in
hard-to-treat advanced cancers: Results of the MOSCATO 01 trial.
Cancer Discov. 7:586–595. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
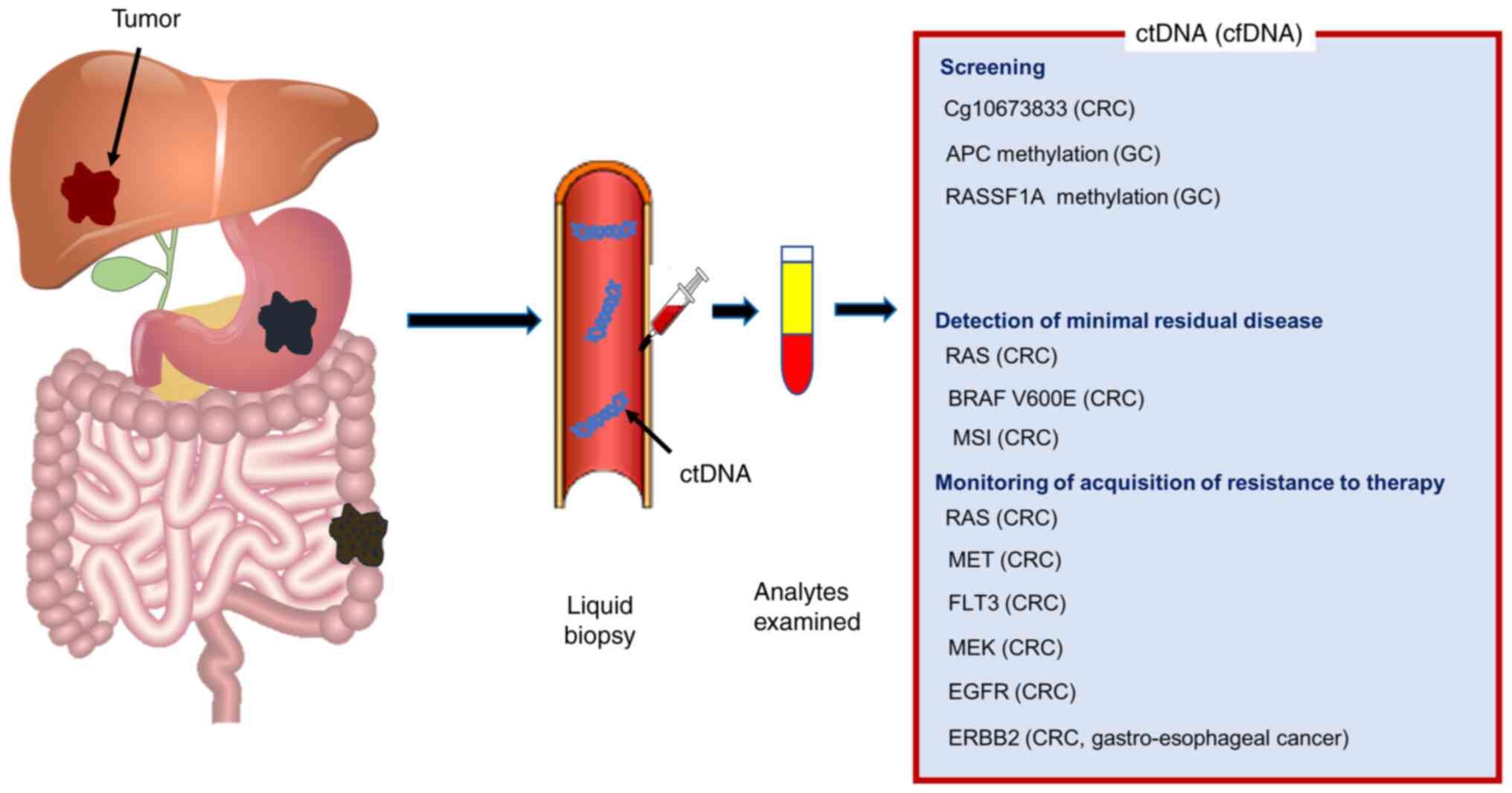
Cobain EF, Wu YM, Vats P, Chugh R, Worden
F, Smith DC, Schuetze SM, Zalupski MM, Sahai V, Alva A, et al:
Assessment of clinical benefit of integrative genomic profiling in
advanced solid tumors. JAMA Oncol. 7:525–533. 2021.PubMed/NCBI
|
|
10
|
Pishvaian MJ, Blais EM, Brody JR, Lyons E,
DeArbeloa P, Hendifar A, Mikhail S, Chung V, Sahai V, Sohal DPS, et
al: Overall survival in patients with pancreatic cancer receiving
matched therapies following molecular profiling: A retrospective
analysis of the know your tumor registry trial. Lancet Oncol.
21:508–518. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Van Cutsem E, Cervantes A, Adam R, Sobrero
A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson
A, Bodoky G, et al: ESMO consensus guidelines for the management of
patients with metastatic colorectal cancer. Ann Oncol.
27:1386–1422. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Venderbosch S, Nagtegaal ID, Maughan TS,
Smith CG, Cheadle JP, Fisher D, Kaplan R, Quirke P, Seymour MT,
Richman SD, et al: Mismatch repair status and BRAF mutation status
in metastatic colorectal cancer patients: A pooled analysis of the
CAIRO, CAIRO2, COIN, and FOCUS studies. Clin Cancer Res.
20:5322–5330. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jones JC, Renfro LA, Al-Shamsi HO, Schrock
AB, Rankin A, Zhang BY, Kasi PM, Voss JS, Leal AD, Sun J, et al:
Non-V600 BRAF mutations define a clinically distinct
molecular subtype of metastatic colorectal cancer. J Clin Oncol.
35:2624–2630. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yaeger R, Kotani D, Mondaca S, Parikh AR,
Bando H, Van Seventer EE, Taniguchi H, Zhao H, Thant CN, de
Stanchina E, et al: Response to anti-EGFR therapy in patients with
BRAF non-V600-mutant metastatic colorectal cancer. Clin Cancer Res.
25:7089–7097. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Johnson B, Loree JM, Jacome AA, Mendis S,
Syed M, Morris Ii VK, Parseghian CM, Dasari A, Pant S, Raymond VM,
et al: Atypical, non-V600 BRAF mutations as a potential mechanism
of resistance to EGFR inhibition in metastatic colorectal cancer.
JCO Precis Oncol. 3:PO.19.00102. 2019.
|
|
16
|
Corcoran RB, Ebi H, Turke AB, Coffee EM,
Nishino M, Cogdill AP, Brown RD, Della Pelle P, Dias-Santagata D,
Hung KE, et al: EGFR-mediated re-activation of MAPK signaling
contributes to insensitivity of BRAF mutant colorectal cancers to
RAF inhibition with vemurafenib. Cancer Discov. 2:227–235. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yaeger R, Cercek A, O'Reilly EM, Reidy DL,
Kemeny N, Wolinsky T, Capanu M, Gollub MJ, Rosen N, Berger MF, et
al: Pilot trial of combined BRAF and EGFR inhibition in BRAF-mutant
metastatic colorectal cancer patients. Clin Cancer Res.
21:1313–1320. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
van Geel RMJM, Tabernero J, Elez E,
Bendell JC, Spreafico A, Schuler M, Yoshino T, Delord JP, Yamada Y,
Lolkema MP, et al: A phase Ib dose-escalation study of encorafenib
and cetuximab with or without alpelisib in metastatic BRAF-mutant
colorectal cancer. Cancer Discov. 7:610–619. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kopetz S, Guthrie KA, Morris VK, Lenz HJ,
Magliocco AM, Maru D, Yan Y, Lanman R, Manyam G, Hong DS, et al:
Randomized trial of irinotecan and cetuximab with or without
vemurafenib in BRAF-mutant metastatic colorectal cancer (SWOG
S1406). J Clin Oncol. 39:285–294. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Corcoran RB, André T, Atreya CE, Schellens
JHM, Yoshino T, Bendell JC, Hollebecque A, McRee AJ, Siena S,
Middleton G, et al: Combined BRAF, EGFR, and MEK inhibition in
patients with BRAFV600E-mutant colorectal cancer. Cancer
Discov. 8:428–443. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Roviello G, D'Angelo A, Petrioli R,
Roviello F, Cianchi F, Nobili S, Mini E and Lavacchi D:
Encorafenib, binimetinib, and cetuximab in BRAF V600E-mutated
colorectal cancer. Transl Oncol. 13:1007952020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Grothey A, Fakih M and Tabernero J:
Management of BRAF-mutant metastatic colorectal cancer: A review of
treatment options and evidence-based guidelines. Ann Oncol.
32:959–967. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nagasaka M, Li Y, Sukari A, Ou SHI,
Al-Hallak MN and Azmi AS: KRAS G12C Game of Thrones, which direct
KRAS inhibitor will claim the iron throne? Cancer Treat Rev.
84:1019742020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang C and Fakih M: Targeting KRAS in
colorectal cancer. Curr Oncol Rep. 23:282021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stadler ZK, Battaglin F, Middha S,
Hechtman JF, Tran C, Cercek A, Yaeger R, Segal NH, Varghese AM,
Reidy-Lagunes DL, et al: Reliable detection of mismatch repair
deficiency in colorectal cancers using mutational load in
next-generation sequencing panels. J Clin Oncol. 34:2141–2147.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Maby P, Tougeron D, Hamieh M, Mlecnik B,
Kora H, Bindea G, Angell HK, Fredriksen T, Elie N, Fauquembergue E,
et al: Correlation between density of CD8+ T-cell infiltrate in
microsatellite unstable colorectal cancers and frameshift
mutations: A rationale for personalized immunotherapy. Cancer Res.
75:3446–3455. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Andre T, Amonkar M, Norquist JM, Shiu KK,
Kim TW, Jensen BV, Jensen LH, Punt CJA, Smith D, Garcia-Carbonero
R, et al: Health-related quality of life in patients with
microsatellite instability-high or mismatch repair deficient
metastatic colorectal cancer treated with first-line pembrolizumab
versus chemotherapy (KEYNOTE-177): An open-label, randomised, phase
3 trial. Lancet Oncol. 22:665–677. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Richman SD, Southward K, Chambers P, Cross
D, Barrett J, Hemmings G, Taylor M, Wood H, Hutchins G, Foster JM,
et al: HER2 overexpression and amplification as a potential
therapeutic target in colorectal cancer: Analysis of 3256 patients
enrolled in the QUASAR, FOCUS and PICCOLO colorectal cancer trials.
J Pathol. 238:562–570. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang G, He Y, Sun Y, Wang W, Qian X, Yu X
and Pan Y: Prevalence, prognosis and predictive status of HER2
amplification in anti-EGFR-resistant metastatic colorectal cancer.
Clin Transl Oncol. 22:813–822. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sartore-Bianchi A, Trusolino L, Martino C,
Bencardino K, Lonardi S, Bergamo F, Zagonel V, Leone F, Depetris I,
Martinelli E, et al: Dual-targeted therapy with trastuzumab and
lapatinib in treatment-refractory, KRAS codon 12/13 wild-type,
HER2-positive metastatic colorectal cancer (HERACLES): A
proof-of-concept, multicentre, open-label, phase 2 trial. Lancet
Oncol. 17:738–746. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Meric-Bernstam F, Hurwitz H, Raghav KPS,
McWilliams RR, Fakih M, VanderWalde A, Swanton C, Kurzrock R,
Burris H, Sweeney C, et al: Pertuzumab plus trastuzumab for
HER2-amplified metastatic colorectal cancer (MyPathway): An updated
report from a multicentre, open-label, phase 2a, multiple basket
study. Lancet Oncol. 20:518–530. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Siena S, Di Bartolomeo M, Raghav K,
Masuishi T, Loupakis F, Kawakami H, Yamaguchi K, Nishina T, Fakih
M, Elez E, et al: Trastuzumab deruxtecan (DS-8201) in patients with
HER2-expressing metastatic colorectal cancer (DESTINY-CRC01): A
multicentre, open-label, phase 2 trial. Lancet Oncol. 22:779–789.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hallberg B and Palmer RH: The role of the
ALK receptor in cancer biology. Ann Oncol. 27 (Suppl 3):iii4–iii15.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pietrantonio F, Di Nicolantonio F, Schrock
AB, Lee J, Tejpar S, Sartore-Bianchi A, Hechtman JF, Christiansen
J, Novara L, Tebbutt N, et al: ALK, ROS1, and NTRK rearrangements
in metastatic colorectal cancer. J Natl Cancer Inst. 109:2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ruela AL, de Figueiredo EC, de Araújo MB,
Carvalho FC and Pereira GR: Molecularly imprinted microparticles in
lipid-based formulations for sustained release of donepezil. Eur J
Pharm Sci. 93:114–122. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hosoda W, Chianchiano P, Griffin JF,
Pittman ME, Brosens LA, Noë M, Yu J, Shindo K, Suenaga M, Rezaee N,
et al: Genetic analyses of isolated high-grade pancreatic
intraepithelial neoplasia (HG-PanIN) reveal paucity of alterations
in TP53 and SMAD4. J Pathol. 242:16–23. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Waddell N, Pajic M, Patch AM, Chang DK,
Kassahn KS, Bailey P, Johns AL, Miller D, Nones K, Quek K, et al:
Whole genomes redefine the mutational landscape of pancreatic
cancer. Nature. 518:495–501. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Witkiewicz AK, McMillan EA, Balaji U, Baek
G, Lin WC, Mansour J, Mollaee M, Wagner KU, Koduru P, Yopp A, et
al: Whole-exome sequencing of pancreatic cancer defines genetic
diversity and therapeutic targets. Nat Commun. 6:67442015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sohal DPS, Kennedy EB, Cinar P, Conroy T,
Copur MS, Crane CH, Garrido-Laguna I, Lau MW, Johnson T,
Krishnamurthi S, et al: Metastatic pancreatic cancer: ASCO
guideline update. J Clin Oncol. 38:3217–3230. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Robson M, Im SA, Senkus E, Xu B, Domchek
SM, Masuda N, Delaloge S, Li W, Tung N, Armstrong A, et al:
Olaparib for metastatic breast cancer in patients with a germline
BRCA mutation. N Engl J Med. 377:523–533. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Del Campo JM, Matulonis UA, Malander S,
Provencher D, Mahner S, Follana P, Waters J, Berek JS, Woie K, Oza
AM, et al: Niraparib maintenance therapy in patients with recurrent
ovarian cancer after a partial response to the last platinum-based
chemotherapy in the ENGOT-OV16/NOVA trial. J Clin Oncol.
37:2968–2973. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Curtin NJ and Szabo C: Poly(ADP-ribose)
polymerase inhibition: Past, present and future. Nat Rev Drug
Discov. 19:711–736. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Park W, Chen J, Chou JF, Varghese AM, Yu
KH, Wong W, Capanu M, Balachandran V, McIntyre CA, El Dika I, et
al: Genomic methods identify homologous recombination deficiency in
pancreas adenocarcinoma and optimize treatment selection. Clin
Cancer Res. 26:3239–3247. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Golan T, Hammel P, Reni M, Van Cutsem E,
Macarulla T, Hall MJ, Park JO, Hochhauser D, Arnold D, Oh DY, et
al: Maintenance olaparib for germline BRCA-mutated metastatic
pancreatic cancer. N Engl J Med. 381:317–327. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hu ZI, Shia J, Stadler ZK, Varghese AM,
Capanu M, Salo-Mullen E, Lowery MA, Diaz LA Jr, Mandelker D, Yu KH,
et al: Evaluating mismatch repair deficiency in pancreatic
adenocarcinoma: Challenges and recommendations. Clin Cancer Res.
24:1326–1336. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Latham A, Srinivasan P, Kemel Y, Shia J,
Bandlamudi C, Mandelker D, Middha S, Hechtman J, Zehir A,
Dubard-Gault M, et al: Microsatellite instability is associated
with the presence of lynch syndrome pan-cancer. J Clin Oncol.
37:286–295. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): A phase 3,
open-label, randomised controlled trial. Lancet. 376:687–697. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Takegawa N, Tsurutani J, Kawakami H,
Yonesaka K, Kato R, Haratani K, Hayashi H, Takeda M, Nonagase Y,
Maenishi O and Nakagawa K: [fam-] trastuzumab deruxtecan, antitumor
activity is dependent on HER2 expression level rather than on HER2
amplification. Int J Cancer. 145:3414–3424. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Shitara K, Bang YJ, Iwasa S, Sugimoto N,
Ryu MH, Sakai D, Chung HC, Kawakami H, Yabusaki H, Lee J, et al:
Trastuzumab deruxtecan in previously treated HER2-positive gastric
cancer. N Engl J Med. 382:2419–2430. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Helsten T, Elkin S, Arthur E, Tomson BN,
Carter J and Kurzrock R: The FGFR landscape in cancer: Analysis of
4,853 tumors by next-generation sequencing. Clin Cancer Res.
22:259–267. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hur JY, Chao J, Kim K, Kim ST, Kim KM,
Klempner SJ and Lee J: High-level FGFR2 amplification is associated
with poor prognosis and lower response to chemotherapy in gastric
cancers. Pathol Res Pract. 216:1528782020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Catenacci DVT, Rasco D, Lee J, Rha SY, Lee
KW, Bang YJ, Bendell J, Enzinger P, Marina N, Xiang H, et al: Phase
I escalation and expansion study of bemarituzumab (FPA144) in
patients with advanced solid tumors and FGFR2b-selected
gastroesophageal adenocarcinoma. J Clin Oncol. 38:2418–2426. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Catenacci DV, Tesfaye A, Tejani M, Cheung
E, Eisenberg P, Scott AJ, Eng C, Hnatyszyn J, Marina N, Powers J
and Wainberg Z: Bemarituzumab with modified FOLFOX6 for advanced
FGFR2-positive gastroesophageal cancer: FIGHT Phase III study
design. Future Oncol. 15:2073–2082. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Nault JC, Mallet M, Pilati C, Calderaro J,
Bioulac-Sage P, Laurent C, Laurent A, Cherqui D, Balabaud C and
Zucman-Rossi J: High frequency of telomerase reverse-transcriptase
promoter somatic mutations in hepatocellular carcinoma and
preneoplastic lesions. Nat Commun. 4:22182013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Schulze K, Imbeaud S, Letouzé E,
Alexandrov LB, Calderaro J, Rebouissou S, Couchy G, Meiller C,
Shinde J, Soysouvanh F, et al: Exome sequencing of hepatocellular
carcinomas identifies new mutational signatures and potential
therapeutic targets. Nat Genet. 47:505–511. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Jhunjhunwala S, Jiang Z, Stawiski EW, Gnad
F, Liu J, Mayba O, Du P, Diao J, Johnson S, Wong KF, et al: Diverse
modes of genomic alteration in hepatocellular carcinoma. Genome
Biol. 15:4362014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Cancer Genome Atlas Research Network.
Electronic address, . simplewheeler@bcm.edu; Cancer
Genome Atlas Research Network: Comprehensive and integrative
genomic characterization of hepatocellular carcinoma. Cell.
169:1327–1341.e23. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Xu Z, Hu J, Cao H, Pilo MG, Cigliano A,
Shao Z, Xu M, Ribback S, Dombrowski F, Calvisi DF and Chen X: Loss
of Pten synergizes with c-Met to promote hepatocellular carcinoma
development via mTORC2 pathway. Exp Mol Med. 50:e4172018.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Jin M, Piao Z, Kim NG, Park C, Shin EC,
Park JH, Jung HJ, Kim CG and Kim H: p16 is a major inactivation
target in hepatocellular carcinoma. Cancer. 89:60–68. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kudo M, Finn RS, Qin S, Han KH, Ikeda K,
Piscaglia F, Baron A, Park JW, Han G, Jassem J, et al: Lenvatinib
versus sorafenib in first-line treatment of patients with
unresectable hepatocellular carcinoma: A randomised phase 3
non-inferiority trial. Lancet. 391:1163–1173. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Finn RS, Qin S, Ikeda M, Galle PR, Ducreux
M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, et al: Atezolizumab
plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J
Med. 382:1894–1905. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Bruix J, Qin S, Merle P, Granito A, Huang
YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, et al:
Regorafenib for patients with hepatocellular carcinoma who
progressed on sorafenib treatment (RESORCE): A randomised,
double-blind, placebo-controlled, phase 3 trial. Lancet. 389:56–66.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Abou-Alfa GK, Meyer T, Cheng AL,
El-Khoueiry AB, Rimassa L, Ryoo BY, Cicin I, Merle P, Chen Y, Park
JW, et al: Cabozantinib in patients with advanced and progressing
hepatocellular carcinoma. N Engl J Med. 379:54–63. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Valle J, Wasan H, Palmer DH, Cunningham D,
Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira
SP, et al: Cisplatin plus gemcitabine versus gemcitabine for
biliary tract cancer. N Engl J Med. 362:1273–1281. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Lamarca A, Palmer DH, Wasan HS, Ross PJ,
Ma YT, Arora A, Falk S, Gillmore R, Wadsley J, Patel K, et al:
Second-line FOLFOX chemotherapy versus active symptom control for
advanced biliary tract cancer (ABC-06): A phase 3, open-label,
randomised, controlled trial. Lancet Oncol. 22:690–701. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Javle M, Bekaii-Saab T, Jain A, Wang Y,
Kelley RK, Wang K, Kang HC, Catenacci D, Ali S, Krishnan S, et al:
Biliary cancer: Utility of next-generation sequencing for clinical
management. Cancer. 122:3838–3847. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Nakamura Y and Yoshino T: Clinical utility
of analyzing circulating tumor DNA in patients with metastatic
colorectal cancer. Oncologist. 23:1310–1318. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Nakamura Y and Shitara K: Development of
circulating tumour DNA analysis for gastrointestinal cancers. ESMO
Open. 5 (Suppl 1):e0006002020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Parikh AR, Van Seventer EE, Siravegna G,
Hartwig AV, Jaimovich A, He Y, Kanter K, Fish MG, Fosbenner KD,
Miao B, et al: Minimal residual disease detection using a
plasma-only circulating tumor DNA assay in patients with colorectal
cancer. Clin Cancer Res. 27:5586–5594. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Kwapisz D: The first liquid biopsy test
approved. Is it a new era of mutation testing for non-small cell
lung cancer? Ann Transl Med. 5:462017.PubMed/NCBI
|
|
71
|
Merker JD, Oxnard GR, Compton C, Diehn M,
Hurley P, Lazar AJ, Lindeman N, Lockwood CM, Rai AJ, Schilsky RL,
et al: Circulating tumor DNA analysis in patients with cancer:
American society of clinical oncology and college of american
pathologists joint review. J Clin Oncol. 36:1631–1641. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Luo H, Zhao Q, Wei W, Zheng L, Yi S, Li G,
Wang W, Sheng H, Pu H, Mo H, et al: Circulating tumor DNA
methylation profiles enable early diagnosis, prognosis prediction,
and screening for colorectal cancer. Sci Transl Med.
12:eaax75332020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Balgkouranidou I, Matthaios D,
Karayiannakis A, Bolanaki H, Michailidis P, Xenidis N, Amarantidis
K, Chelis L, Trypsianis G, Chatzaki E, et al: Prognostic role of
APC and RASSF1A promoter methylation status in cell free
circulating DNA of operable gastric cancer patients. Mutat Res.
778:46–51. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Ng CKY, Di Costanzo GG, Tosti N, Paradiso
V, Coto-Llerena M, Roscigno G, Perrina V, Quintavalle C, Boldanova
T, Wieland S, et al: Genetic profiling using plasma-derived
cell-free DNA in therapy-naïve hepatocellular carcinoma patients: A
pilot study. Ann Oncol. 29:1286–1291. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Kisiel JB, Dukek BA, V S R Kanipakam R,
Ghoz HM, Yab TC, Berger CK, Taylor WR, Foote PH, Giama NH,
Onyirioha K, et al: Hepatocellular carcinoma detection by plasma
methylated DNA: Discovery, phase I pilot, and phase II clinical
validation. Hepatology. 69:1180–1192. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Maleddu A, Pantaleo MA, Nannini M and
Biasco G: The role of mutational analysis of KIT and PDGFRA in
gastrointestinal stromal tumors in a clinical setting. J Transl
Med. 9:752011. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Larribère L and Martens UM: Advantages and
challenges of using ctDNA NGS to assess the presence of minimal
residual disease (MRD) in solid tumors. Cancers (Basel).
13:56982021. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Tabernero J, Lenz HJ, Siena S, Sobrero A,
Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C, et al:
Analysis of circulating DNA and protein biomarkers to predict the
clinical activity of regorafenib and assess prognosis in patients
with metastatic colorectal cancer: A retrospective, exploratory
analysis of the CORRECT trial. Lancet Oncol. 16:937–948. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Taniguchi H, Nakamura Y, Kotani D, Yukami
H, Mishima S, Sawada K, Shirasu H, Ebi H, Yamanaka T, Aleshin A, et
al: CIRCULATE-Japan: Circulating tumor DNA-guided adaptive platform
trials to refine adjuvant therapy for colorectal cancer. Cancer
Sci. 112:2915–2920. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Misale S, Yaeger R, Hobor S, Scala E,
Janakiraman M, Liska D, Valtorta E, Schiavo R, Buscarino M,
Siravegna G, et al: Emergence of KRAS mutations and acquired
resistance to anti-EGFR therapy in colorectal cancer. Nature.
486:532–536. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Siravegna G, Mussolin B, Buscarino M,
Corti G, Cassingena A, Crisafulli G, Ponzetti A, Cremolini C, Amatu
A, Lauricella C, et al: Clonal evolution and resistance to EGFR
blockade in the blood of colorectal cancer patients. Nat Med.
21:795–801. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Wang DS, Liu ZX, Lu YX, Bao H, Wu X, Zeng
ZL, Liu Z, Zhao Q, He CY, Lu JH, et al: Liquid biopsies to track
trastuzumab resistance in metastatic HER2-positive gastric cancer.
Gut. 68:1152–1161. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Martinelli E, Ciardiello D, Martini G,
Troiani T, Cardone C, Vitiello PP, Normanno N, Rachiglio AM,
Maiello E, Latiano T, et al: Implementing anti-epidermal growth
factor receptor (EGFR) therapy in metastatic colorectal cancer:
Challenges and future perspectives. Ann Oncol. 31:30–40. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Cremolini C, Rossini D, Dell'Aquila E,
Lonardi S, Conca E, Del Re M, Busico A, Pietrantonio F, Danesi R,
Aprile G, et al: Rechallenge for patients with RAS and BRAF
wild-type metastatic colorectal cancer with acquired resistance to
first-line cetuximab and irinotecan: A phase 2 single-arm clinical
trial. JAMA Oncol. 5:343–350. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Nakamura Y, Taniguchi H, Ikeda M, Bando H,
Kato K, Morizane C, Esaki T, Komatsu Y, Kawamoto Y, Takahashi N, et
al: Clinical utility of circulating tumor DNA sequencing in
advanced gastrointestinal cancer: SCRUM-Japan GI-SCREEN and GOZILA
studies. Nat Med. 26:1859–1864. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Catana A, Apostu AP and Antemie RG: Multi
gene panel testing for hereditary breast cancer-is it ready to be
used? Med Pharm Rep. 92:220–225. 2019.PubMed/NCBI
|
|
87
|
Stjepanovic N, Moreira L, Carneiro F,
Balaguer F, Cervantes A, Balmaña J and Martinelli E; ESMO
Guidelines Committee. Electronic address, : simpleclinicalguidelines@esmo.org:
Hereditary gastrointestinal cancers: ESMO clinical practice
guidelines for diagnosis, treatment and follow-up†. Ann Oncol.
30:1558–1571. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Daly MB, Pal T, Berry MP, Buys SS, Dickson
P, Domchek SM, Elkhanany A, Friedman S, Goggins M, Hutton ML, et
al: Genetic/familial high-risk assessment: Breast, ovarian, and
pancreatic, version 2.2021, NCCN clinical practice guidelines in
oncology. J Natl Compr Canc Netw. 19:77–102. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Taylor A, Brady AF, Frayling IM, Hanson H,
Tischkowitz M, Turnbull C and Side L; UK Cancer Genetics Group
(UK-CGG), : Consensus for genes to be included on cancer panel
tests offered by UK genetics services: Guidelines of the UK Cancer
Genetics Group. J Med Genet. 55:372–377. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Cerretelli G, Ager A, Arends MJ and
Frayling IM: Molecular pathology of Lynch syndrome. J Pathol.
250:518–531. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Win AK, Lindor NM, Young JP, Macrae FA,
Young GP, Williamson E, Parry S, Goldblatt J, Lipton L, Winship I,
et al: Risks of primary extracolonic cancers following colorectal
cancer in lynch syndrome. J Natl Cancer Inst. 104:1363–1372. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Syngal S, Brand RE, Church JM, Giardiello
FM, Hampel HL and Burt RW; American College of Gastroenterology, :
ACG clinical guideline: Genetic testing and management of
hereditary gastrointestinal cancer syndromes. Am J Gastroenterol.
110:223–263. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Le DT, Kim TW, Van Cutsem E, Geva R, Jäger
D, Hara H, Burge M, O'Neil B, Kavan P, Yoshino T, et al: Phase II
open-label study of pembrolizumab in treatment-refractory,
microsatellite instability-high/mismatch repair-deficient
metastatic colorectal cancer: KEYNOTE-164. J Clin Oncol. 38:11–19.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Overbeek KA, Cahen DL, Canto MI and Bruno
MJ: Surveillance for neoplasia in the pancreas. Best Pract Res Clin
Gastroenterol. 30:971–986. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Shindo K, Yu J, Suenaga M, Fesharakizadeh
S, Cho C, Macgregor-Das A, Siddiqui A, Witmer PD, Tamura K, Song
TJ, et al: Deleterious germline mutations in patients with
apparently sporadic pancreatic adenocarcinoma. J Clin Oncol.
35:3382–3390. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Chaffee KG, Oberg AL, McWilliams RR,
Majithia N, Allen BA, Kidd J, Singh N, Hartman AR, Wenstrup RJ and
Petersen GM: Prevalence of germ-line mutations in cancer genes
among pancreatic cancer patients with a positive family history.
Genet Med. 20:119–127. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Hu C, Hart SN, Polley EC, Gnanaolivu R,
Shimelis H, Lee KY, Lilyquist J, Na J, Moore R, Antwi SO, et al:
Association between inherited germline mutations in cancer
predisposition genes and risk of pancreatic cancer. JAMA.
319:2401–2409. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Bartsch DK, Gress TM and Langer P:
Familial pancreatic cancer-current knowledge. Nat Rev Gastroenterol
Hepatol. 9:445–453. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Hampel H, Bennett RL, Buchanan A, Pearlman
R and Wiesner GL: Guideline Development Group, American College of
Medical Genetics and Genomics Professional Practice and Guidelines
Committee and National Society of Genetic Counselors Practice
Guidelines Committee: A practice guideline from the American
college of medical genetics and genomics and the national society
of genetic counselors: Referral indications for cancer
predisposition assessment. Genet Med. 17:70–87. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Pilonis ND, Tischkowitz M, Fitzgerald RC
and di Pietro M: Hereditary diffuse gastric cancer: Approaches to
screening, surveillance, and treatment. Annu Rev Med. 72:263–280.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Donner I, Kiviluoto T, Ristimäki A,
Aaltonen LA and Vahteristo P: Exome sequencing reveals three novel
candidate predisposition genes for diffuse gastric cancer. Fam
Cancer. 14:241–246. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Berger MF and Mardis ER: The emerging
clinical relevance of genomics in cancer medicine. Nat Rev Clin
Oncol. 15:353–365. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Nakagawa H, Wardell CP, Furuta M,
Taniguchi H and Fujimoto A: Cancer whole-genome sequencing: Present
and future. Oncogene. 34:5943–5950. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Colomer R, Mondejar R, Romero-Laorden N,
Alfranca A, Sanchez-Madrid F and Quintela-Fandino M: When should we
order a next generation sequencing test in a patient with cancer?
EClinicalMedicine. 25:1004872020. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
ICGC/TCGA Pan-Cancer Analysis of Whole
Genomes Consortium, . Pan-cancer analysis of whole genomes. Nature.
578:82–93. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Frank MO, Koyama T, Rhrissorrakrai K,
Robine N, Utro F, Emde AK, Chen BJ, Arora K, Shah M, Geiger H, et
al: Sequencing and curation strategies for identifying candidate
glioblastoma treatments. BMC Med Genomics. 12:562019. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Takai E, Nakamura H, Chiku S, Kubo E,
Ohmoto A, Totoki Y, Shibata T, Higuchi R, Yamamoto M, Furuse J, et
al: Whole-exome Sequencing reveals new potential susceptibility
genes for Japanese familial pancreatic cancer. Ann Surg.
275:e652–e658. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Tan M, Brusgaard K, Gerdes AM, Mortensen
MB, Detlefsen S, Schaffalitzky de Muckadell OB and Joergensen MT:
Whole genome sequencing identifies rare germline variants enriched
in cancer related genes in first degree relatives of familial
pancreatic cancer patients. Clin Genet. 100:551–562. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Bala P, Singh AK, Kavadipula P, Kotapalli
V, Sabarinathan R and Bashyam MD: Exome sequencing identifies ARID2
as a novel tumor suppressor in early-onset sporadic rectal cancer.
Oncogene. 40:863–874. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Mendelaar PAJ, Smid M, van Riet J, Angus
L, Labots M, Steeghs N, Hendriks MP, Cirkel GA, van Rooijen JM, Ten
Tije AJ, et al: Whole genome sequencing of metastatic colorectal
cancer reveals prior treatment effects and specific metastasis
features. Nat Commun. 12:5742021. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Ishaque N, Abba ML, Hauser C, Patil N,
Paramasivam N, Huebschmann D, Leupold JH, Balasubramanian GP,
Kleinheinz K, Toprak UH, et al: Whole genome sequencing puts
forward hypotheses on metastasis evolution and therapy in
colorectal cancer. Nat Commun. 9:47822018. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Alves JM, Prado-López S, Tomás L, Valecha
M, Estévez-Gómez N, Alvariño P, Geisel D, Modest DP, Sauer IM,
Pratschke J, et al: Clonality and timing of relapsing colorectal
cancer metastasis revealed through whole-genome single-cell
sequencing. Cancer Lett. 543:2157672022. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Erkizan HV, Sukhadia S, Natarajan TG,
Marino G, Notario V, Lichy JH and Wadleigh RG: Exome sequencing
identifies novel somatic variants in African American esophageal
squamous cell carcinoma. Sci Rep. 11:148142021. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Rose Brannon A, Jayakumaran G, Diosdado M,
Patel J, Razumova A, Hu Y, Meng F, Haque M, Sadowska J, Murphy BJ,
et al: Enhanced specificity of clinical high-sensitivity tumor
mutation profiling in cell-free DNA via paired normal sequencing
using MSK-ACCESS. Nat Commun. 12:37702021. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Razavi P, Li BT, Brown DN, Jung B, Hubbell
E, Shen R, Abida W, Juluru K, De Bruijn I, Hou C, et al:
High-intensity sequencing reveals the sources of plasma circulating
cell-free DNA variants. Nat Med. 25:1928–1937. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Modi A, Vai S, Caramelli D and Lari M: The
illumina sequencing protocol and the novaseq 6000 system. Methods
Mol Biol. 2242:15–42. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Subramanian I, Verma S, Kumar S, Jere A
and Anamika K: Multi-omics data integration, interpretation, and
its application. Bioinform Biol Insights. 14:11779322198990512020.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Basu A, Bodycombe NE, Cheah JH, Price EV,
Liu K, Schaefer GI, Ebright RY, Stewart ML, Ito D, Wang S, et al:
An interactive resource to identify cancer genetic and lineage
dependencies targeted by small molecules. Cell. 154:1151–1161.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Zhang B, Wang J, Wang X, Zhu J, Liu Q, Shi
Z, Chambers MC, Zimmerman LJ, Shaddox KF, Kim S, et al:
Proteogenomic characterization of human colon and rectal cancer.
Nature. 513:382–387. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Li Z, Gao X, Peng X, May Chen MJ, Li Z,
Wei B, Wen X, Wei B, Dong Y, Bu Z, et al: Multi-omics
characterization of molecular features of gastric cancer correlated
with response to neoadjuvant chemotherapy. Sci Adv. 6:eaay42112020.
View Article : Google Scholar : PubMed/NCBI
|