Introduction
Non-small cell lung cancer (NSCLC) is one of the
most common types of cancer worldwide (1,2). Over
the past decades, according to cancer registration in China, the
prevalence and mortality of lung cancer have exhibited an
increasing trend, which has led to an immense social burden
(3). Current treatments for NSCLC
include surgical resection, chemotherapy, radiotherapy and
immunotherapy (4). However, >30%
of patients with NSCLC are diagnosed at an advanced stage; thus,
the treatment choices for these patients are limited and the
therapeutic efficacy is not favorable (5,6).
Hence, the exploration of effective therapeutic strategies with
which to promote the management of patients with advanced-stage
NSCLC is urgently required.
Apatinib was the first oral vascular endothelial
growth factor receptor 2 (VEGFR2) tyrosine kinase inhibitor (TKI)
approved in China, which was originally used to treat
gastrointestinal tumors (7–9). Over the past decades, apatinib has
also brought hope for patients with advanced-stage NSCLC; its
application provides a favorable treatment response and survival
with tolerable adverse events (10–12).
Of note, it has also been reported that apatinib plus chemotherapy
is associated with favorable progression-free survival (PFS) with
tolerable adverse events among patients with advanced-stage NSCLC
(11,13–29).
Even though a previous meta-analysis compared the treatment
response and safety between apatinib plus chemotherapy and
chemotherapy alone among patients with advanced-stage NSCLC, no PFS
or overall survival (OS) data were analyzed in that study (30).
Thus, in order to provide more substantial evidence
supporting the use of apatinib in patients with advanced-stage
NSCLC, the present meta-analysis synthesized the data of 18 studies
and aimed to compare the efficacy and safety between apatinib plus
chemotherapy and chemotherapy among patients with advanced-stage
NSCLC.
Data and methods
Study search
A systematic literature search was carried out
through the medical databases of PubMed (https://pubmed.ncbi.nlm.nih.gov/), China National
Knowledge Infrastructure (CNKI; http://www.cnki.net/), EMBASE (https://www.embase.com/), Chongqing VIP Information
(CQVIP; http://www.cqvip.com/), Cochrane
(https://www.cochranelibrary.com/) and
Wanfang (https://www.wanfangdata.com.cn/) up to February 2023,
to screen the studies that compared the efficacy and safety of
apatinib plus chemotherapy with chemotherapy in patients with
advanced-stage non-squamous NSCLC. The following medical subject
headings and keywords were used for searching: ‘non-small cell lung
cancer’, ‘NSCLC’, ‘carcinoma of the lung’, ‘lung cancer’, ‘lung
neoplasm’, ‘apatinib’, ‘YN968D1’, ‘apatinib mesylate’,
‘chemotherapy’, ‘chemo’, ‘docetaxel’, ‘pemetrexed’, ‘gemcitabine’,
‘paclitaxel’, ‘vinorelbine’, ‘cisplatin’ and ‘carboplatin’. The
reference lists of the retrieved articles were also screened by
(SQ, XH and ZL).
Study selection
A total of two independent investigators (SQ and XH)
screened all of the relevant studies and any disagreements were
resolved via discussion between them or consultation with a third
investigator (ZL). The inclusion criteria for study screening were
as follows: i) Patients with advanced-stage NSCLC; ii) studies
comparing the efficacy and safety of apatinib plus chemotherapy and
chemotherapy; iii) studies covering at least one outcome of this
analysis, which involved the objective response rate (ORR), disease
control rate (DCR), PFS, OS and adverse events. PFS was defined as
the duration from treatment initiation to the occurrence of
progressive disease, intolerable adverse reactions or all
cause-death. The exclusion criteria were as follows: i) Systematic
reviews, meta-analyses, case studies or animal studies; ii)
duplicated studies; iii) the language of studies was not English or
Chinese.
Data extraction and quality
evaluation
The data of all studies were separately extracted by
two researchers (SQ and XH). In the case of any disagreement,
discussion between the readers or consultation with a third reader
was conducted. The information extracted in this analysis was as
follows: The first author's name, year of publication, study type,
sample size, the age of the patients, treatment regimen, treatment
line and outcomes. Patients who were treated with apatinib plus
chemotherapy were assigned to the ‘apatinib plus chemo’ group,
while those who were treated with chemotherapy alone were placed in
the ‘chemo’ group. The quality of the included randomized
controlled trials (RCTs) was assessed through a modified version of
the Cochrane Collaboration tool (31), while the quality of non-RCTs was
evaluated using the Newcastle-Ottawa scale criteria (32).
Statistical analysis
Statistical analyses were carried out using Stata
(v.14.0; StataCorp). The relative risk (RR) with 95% confidence
intervals (CI) were used to evaluate outcomes. For the assessment
of heterogeneity, I2≤50.0% and P≥0.05 indicated that the
heterogeneity was insignificant, and the fixed-effects model was
applied; otherwise, the random-effects model was utilized. Begg's
and Egger's tests were used for analyzing publication bias and a
value of P<0.05 was considered to indicate a statistically
significant difference. Sensitivity analysis (studies were excluded
one by one) was used to assess the robustness and reliability of
the results.
Results
Study selection process
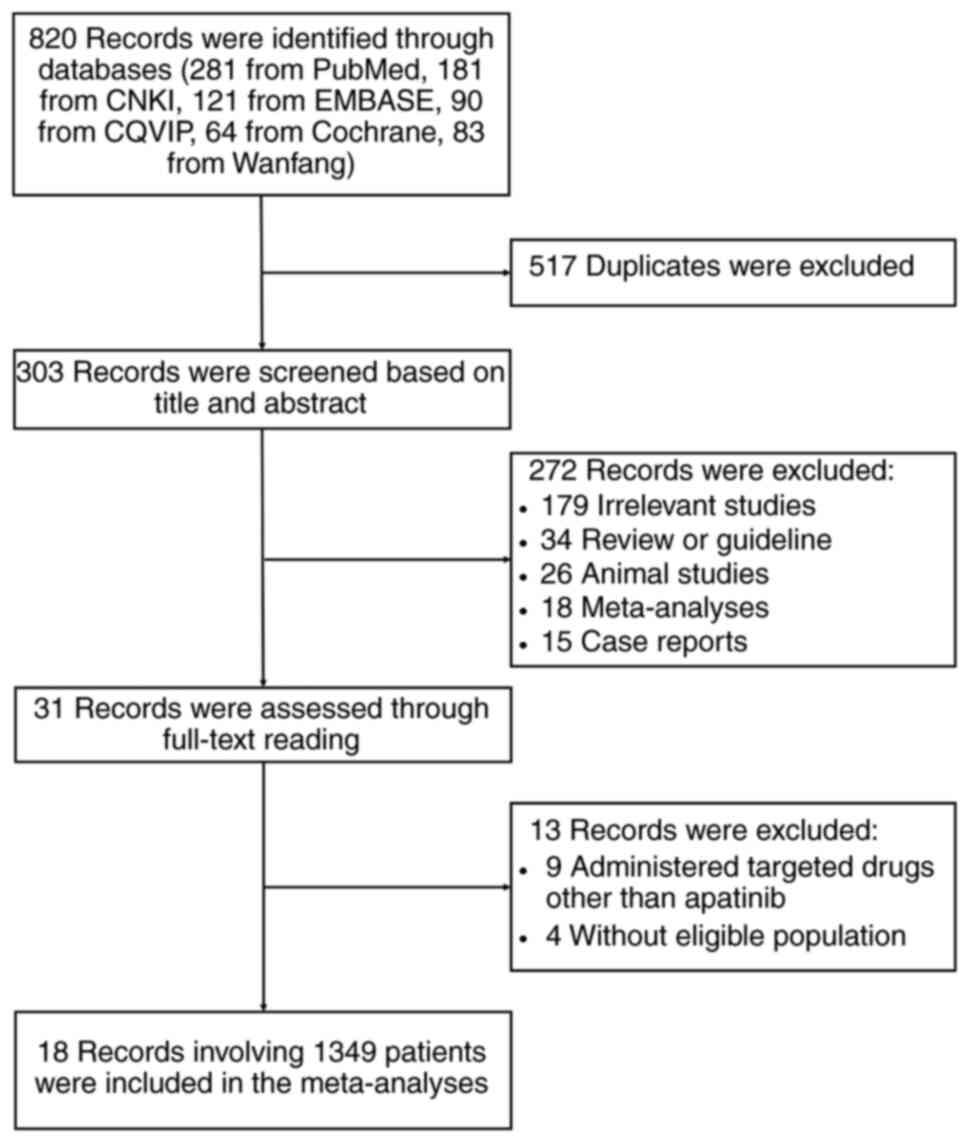
A total of 820 records were identified through the
databases (281 from PubMed, 181 from CNKI, 121 from EMBASE, 90 from
CQVIP, 64 from Cochrane and 83 from Wanfang), among which 517
duplicates were excluded. Subsequently, 303 records were screened
based on title and abstract and 272 of these were excluded
(including 179 irrelevant studies, 34 reviews or guidelines, 26
animal studies, 18 meta-analyses and 15 case reports).
Subsequently, 31 records were assessed through full-text reading,
of which 13 records were excluded (including 9 that administered
targeted drugs other than apatinib and 4 without an eligible
population). Finally, 18 studies involving 1,349 patients were
included in the meta-analysis (Fig.
1).
Features of the included studies
A total of 18 studies comprising 677 patients with
advanced-stage NSCLC receiving apatinib plus chemotherapy and 672
patients with advanced-stage NSCLC receiving chemotherapy alone
were included in the meta-analysis. The ORR, DCR, PFS and adverse
events were regarded as study outcomes to evaluate the efficacy and
safety of apatinib plus chemotherapy and chemotherapy alone.
Further detailed information is presented in Table I. The two groups showed no
significant difference in age. Gender distributions of the pooled
cohorts were 412 males vs. 265 females in the apatinib plus chemo
group; and 397 males vs. 275 females in the chemo group. There was
no significant difference in gender distribution between the two
groups either. Additional features of the included studies
(including sample size and EGFR mutation) were presented in
Table SI.
 | Table I.Features of included studies. |
Table I.
Features of included studies.
|
|
| Sample size | Age, years | Treatment
regimen |
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| Author, year | Study type | Apatinib plus
chemo | Chemo | Apatinib plus
chemo | Chemo | Apatinib plus
chemo | Chemo | Treatment line | Outcomes | (Refs.) |
|---|
| Guo, 2017 | RCT | 19 | 20 | 61.0
(33.0–72.0)a | 58.0
(36.0–75.0)a | Apatinib 500 mg/d;
docetaxel 60 mg/m2 | Docetaxel 60
mg/m2 | II | ORR, DCR, PFS,
adverse events | (13) |
| Chen, 2017 | RCT | 42 | 42 |
61.3±2.6b |
61.2±2.5b | Apatinib 850 mg/d;
paclitaxel 135–145 mg/m2; cisplatin 25
mg/m2 | Paclitaxel 135–145
mg/m2; cisplatin 25 mg/m2 | NA | ORR, DCR, adverse
events | (14) |
| Liu, 2018 | RCT | 40 | 40 |
50.5±7.1b |
52.7±7.1b | Apatinib 500 mg/d;
docetaxel 60 mg/m2 | Docetaxel 60
mg/m2 | II | ORR, DCR, adverse
events | (15) |
| Shang, 2019 | RCT | 31 | 31 |
56.1±6.2b |
54.3±6.0b | Apatinib 250–500
mg/d; docetaxel 75 mg/m2 | Docetaxel 75
mg/m2 | II | ORR, DCR, adverse
events | (16) |
| Luo, 2019 | Observational
study | 32 | 36 |
56.2±5.7b |
55.6±5.8b | Apatinib 500 mg/d;
docetaxel; pemetrexed; gemcitabine; paclitaxel | Docetaxel;
pemetrexed; gemcitabine; paclitaxel | II, III | ORR, DCR, PFS,
adverse events | (17) |
| Hu, 2020 | Observational
study | 19 | 20 |
47.0–75.0c |
48.0–75.0c | Apatinib 500 mg/d;
docetaxel 60 mg/m2 | Docetaxel 60
mg/m2 | NA | ORR, DCR, adverse
events | (11) |
| Yu, 2020 | RCT | 22 | 11 | 58.5
(31.0–73.0)a | NA | Apatinib 500 mg/d;
docetaxel 75 mg/m2; pemetrexed 500 mg/m2 | Docetaxel 75
mg/m2; pemetrexed 500 mg/m2 | II | ORR, DCR, PFS,
adverse events | (18) |
| Guo, 2020 | RCT | 50 | 50 |
61.6±7.2b |
62.9±6.5b | Apatinib 500 mg/d;
pemetrexed; gemcitabine; docetaxel; paclitaxel | Pemetrexed;
gemcitabine; docetaxel; paclitaxel | I | Adverse events | (19) |
| Xie, 2020 | RCT | 38 | 38 |
52.3±7.3b |
53.4±7.3b | Apatinib 850 mg/d;
cisplatin 25 mg/m2; paclitaxel 175 mg/m2 | Cisplatin 25
mg/m2; paclitaxel 175 mg/m2 | NA | ORR, DCR, adverse
events | (20) |
| Li, 2020 | RCT | 60 | 60 |
50.9±6.0a |
51.3±5.8a | Apatinib 850 mg/d;
paclitaxel 175 mg/m2 | Paclitaxel 175
mg/m2 | NA | ORR, DCR | (21) |
| Cui, 2021 | RCT | 40 | 40 |
43.0–75.0c |
41.0–74.0c | Apatinib 500 mg/d;
paclitaxel 75–145 mg/m2; cisplatin 25
mg/m2 | Paclitaxel 75–145
mg/m2; cisplatin 25 mg/m2 | NA | ORR, DCR, adverse
events | (22) |
| Chen, 2021 | RCT | 48 | 50 | NA | NA | Apatinib 500 mg/d;
docetaxel 75 mg/m2 | Docetaxel 75
mg/m2 | II | ORR, DCR, PFS,
adverse events | (23) |
| Huang, 2021 | RCT | 40 | 40 |
67.1±3.2b |
67.1±3.2b | Apatinib 250 mg/d;
cisplatin 25 mg/m2; paclitaxel 135 mg/m2 | Cisplatin 25
mg/m2; paclitaxel 135 mg/m2 | NA | ORR, DCR, adverse
events | (24) |
| Liu, 2021 | Observational
study | 43 | 43 | NA | NA | Apatinib 500 mg/d;
paclitaxel 175 mg/m2 | Paclitaxel 175
mg/m2 | III | ORR, DCR, adverse
events | (25) |
| Guo, 2021 | Observational
study | 30 | 30 |
62.5±8.3b |
62.3±8.2b | Apatinib 850 mg/d;
cisplatin 20 mg/m2; paclitaxel 150 mg/m2 | Cisplatin 20
mg/m2; paclitaxel 150 mg/m2 | NA | ORR, DCR, adverse
events | (26) |
| Song, 2021 | RCT | 34 | 34 |
63.0±5.1b |
63.0±5.0b | Apatinib 500 mg/d;
vinorelbine 25 mg/m2; cisplatin 25 mg/m2;
gemcitabine 250 mg/m2 | Vinorelbine 25
mg/m2; cisplatin 25 mg/m2; gemcitabine 250
mg/m | NA | ORR, DCR, PFS,
adverse events | (27) |
| Chen, 2022 | RCT | 47 | 45 |
51.5±8.3b |
53.9±7.2b | Apatinib 250 mg/d;
paclitaxel 135 mg/m2; cisplatin 20 mg/m2 | Paclitaxel 135
mg/m2; cisplatin 20 mg/m2 | NA | ORR, DCR, adverse
events | (28) |
| Lu, 2022 | Observational
study | 42 | 42 |
63.9±6.4b |
63.9±6.4b | Apatinib 850 mg/d;
paclitaxel 175 mg/m2 | Paclitaxel 175
mg/m2 | NA | ORR, DCR, adverse
events | (29) |
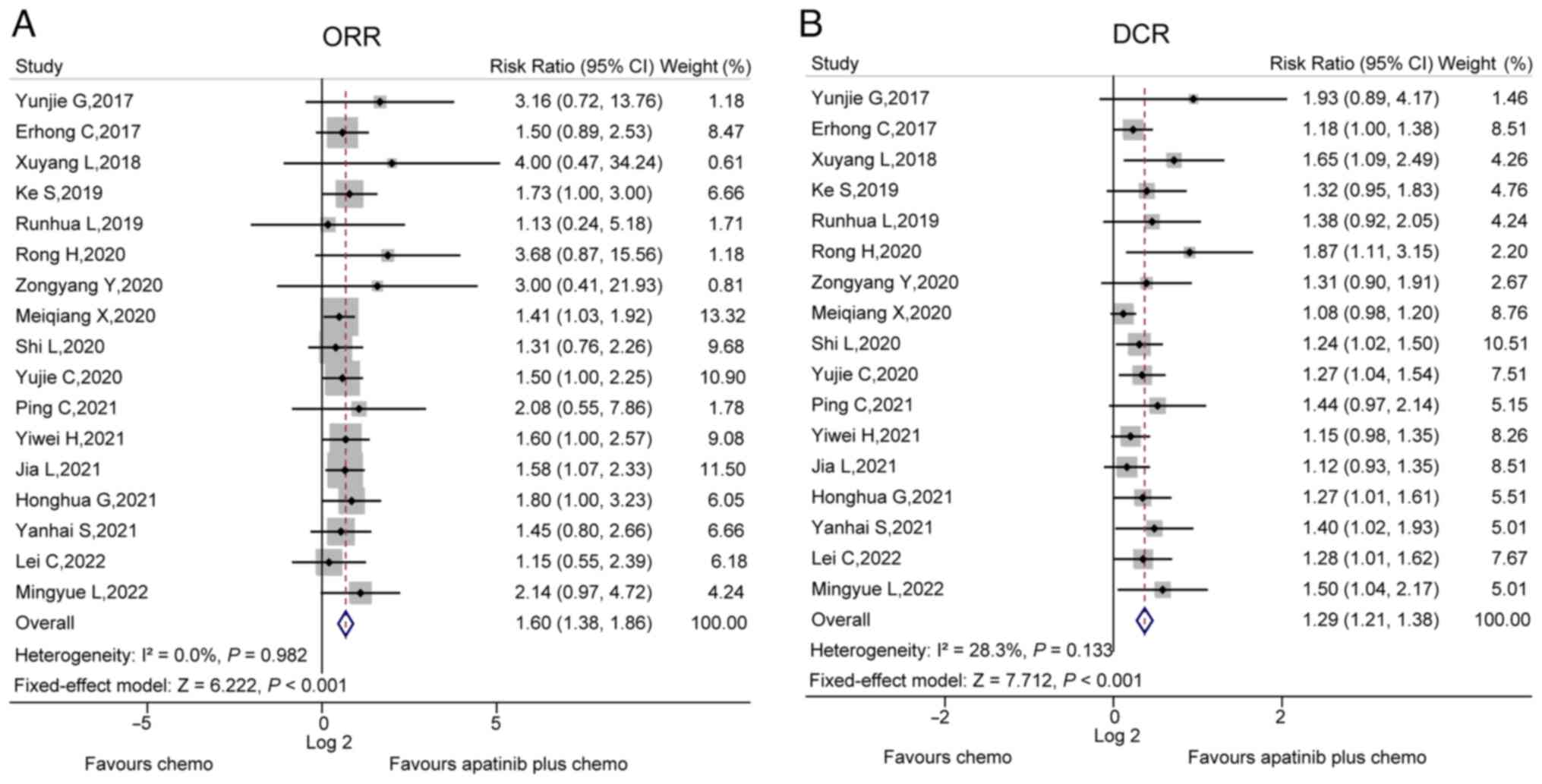
ORR and DCR
In total, 17 studies compared the ORR between the
apatinib plus chemo group and chemo group. There was no
heterogeneity among these studies (I2=0.0%, P=0.982).
After the fixed-effects model was applied, it was found that the
ORR was increased in the apatinib plus chemo group compared to the
chemo group (RR, 1.60; 95% CI, 1.38–1.86; P<0.001; Fig. 2A). Furthermore, these 17 studies
also compared the DCR between the apatinib plus chemo group and
chemo group, and no heterogeneity existed between them
(I2=28.3%, P=0.133.). Application of the fixed-effects
model then revealed that the ORR was elevated in the apatinib plus
chemo group compared to the chemo group (RR, 1.29; 95% CI,
1.21–1.38; P<0.001; Fig.
2B).
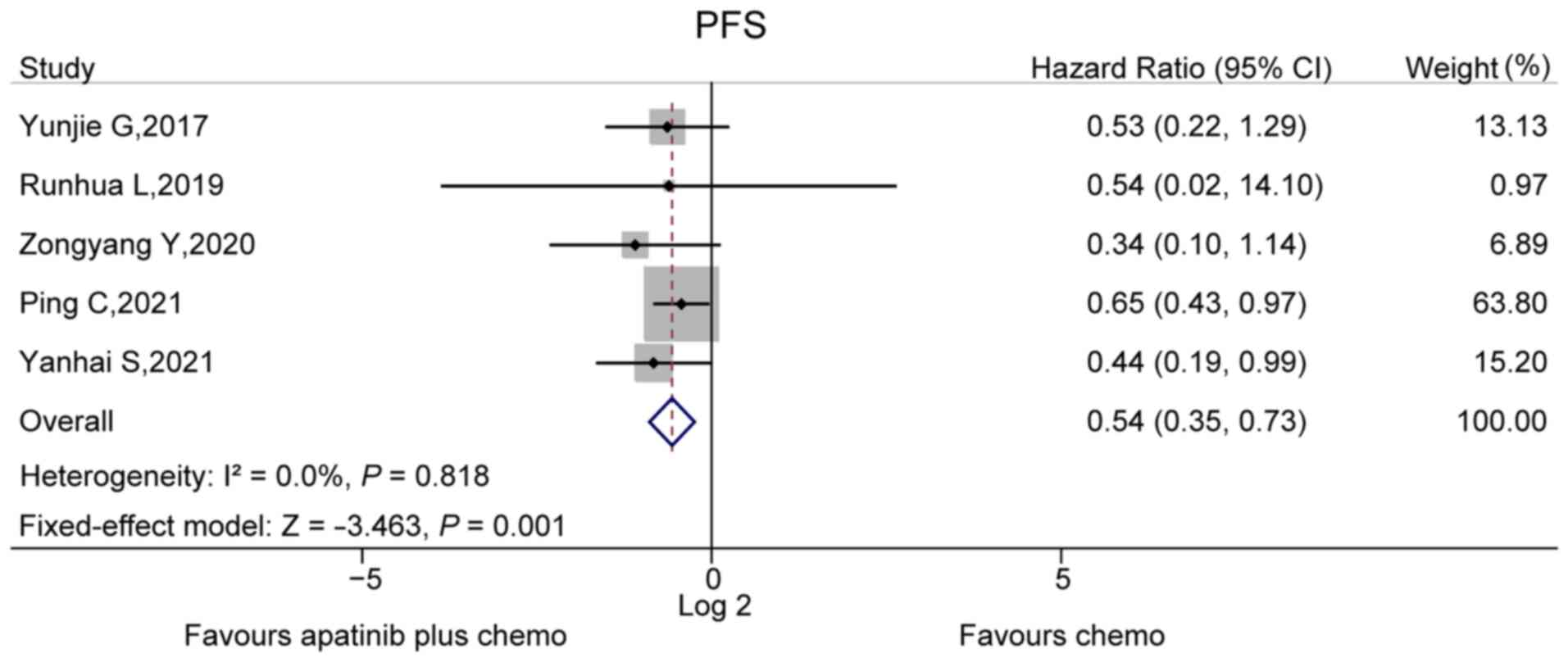
PFS
A total of five studies compared PFS between the
apatinib plus chemo group and chemo group. No heterogeneity was
found among these five studies (I2=0.0%, P=0.818). The
fixed-effects model then revealed that the PFS was prolonged in the
apatinib plus chemo group compared to the chemo group (hazard
ratio, 0.54; 95% CI, 0.35–0.73; P=0.001; Fig. 3). However, no OS data were reported
in the analyzed studies.
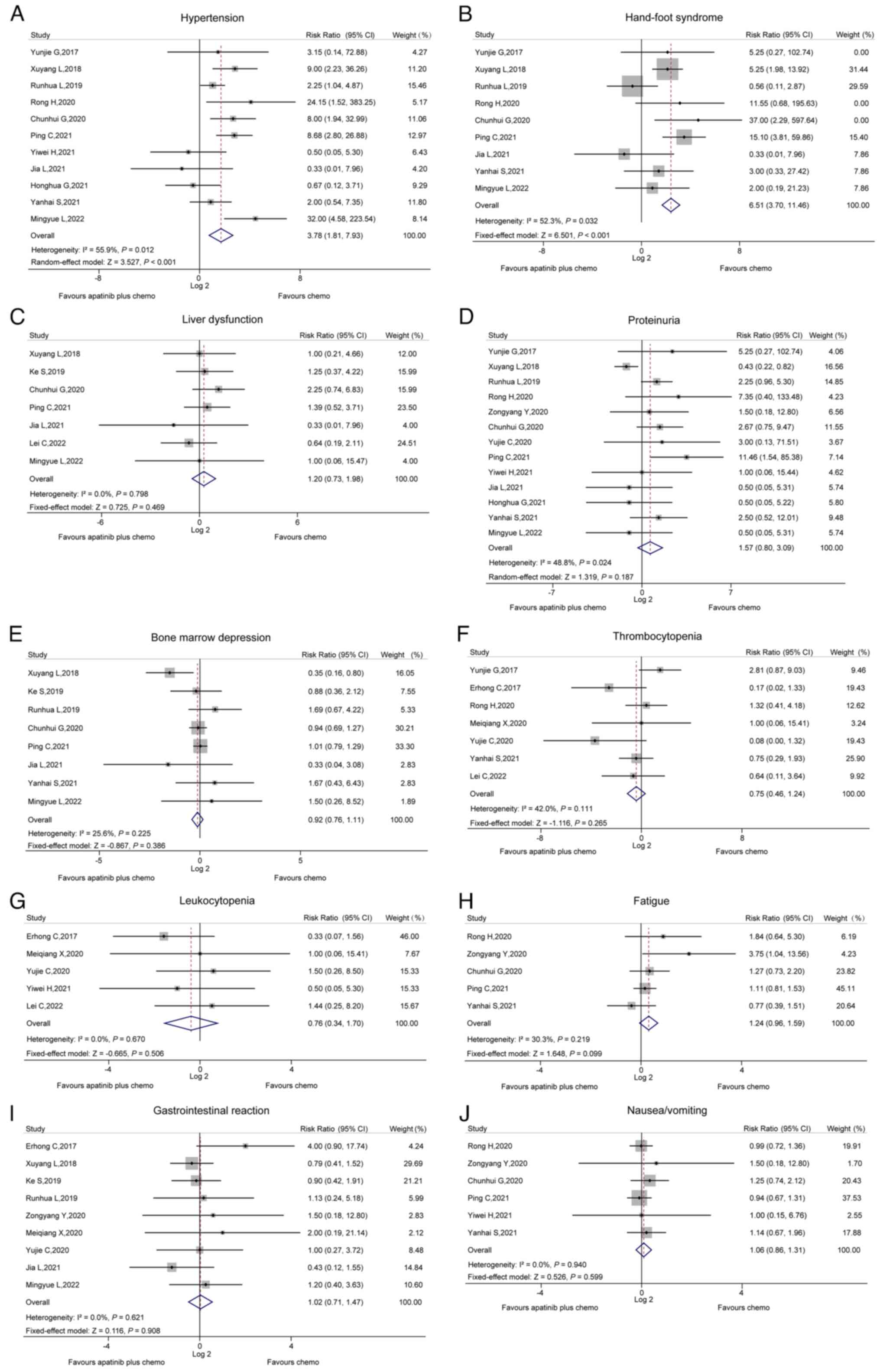
Adverse events
The incidence of hypertension between the two groups
was compared in 11 studies, and heterogeneity was found among these
(I2=55.9%, P=0.012). The random-effects model revealed
that the incidence of hypertension was increased in the apatinib
plus chemo group compared to the chemo group (RR, 3.78; 95% CI,
1.81–7.93; P<0.001; Fig.
4A).
A total of nine studies compared the incidence of
hand-foot syndrome between the two groups; the pooled analysis of
these studies revealed that the incidence of hand-foot syndrome was
increased in the apatinib plus chemo group in comparison to the
chemo group (RR, 6.51; 95% CI, 3.70–11.46; P<0.001) and
heterogeneity existed among these studies (I2=52.3%,
P=0.032; Fig. 4B).
However, no differences in the incidence of liver
dysfunction (Fig. 4C), proteinuria
(Fig. 4D), bone marrow depression
(Fig. 4E), thrombocytopenia
(Fig. 4F), leukopenia (Fig. 4G), fatigue (Fig. 4H), gastrointestinal reactions
(Fig. 4I) or nausea/vomiting
(Fig. 4J) were found between the
two groups (all P>0.05).
Publication bias
Both Begg's and Egger's tests revealed that no
publication bias existed regarding the ORR, DCR, PFS, hypertension,
hand-foot syndrome, liver dysfunction, proteinuria, bone marrow
depression, thrombocytopenia, leukocytopenia, fatigue and
nausea/vomiting (all P>0.050). However, publication bias was
found for gastrointestinal reactions based on Begg's test
(P=0.048), but not Egger's test (P=0.183) (Table II).
 | Table II.Publication bias. |
Table II.
Publication bias.
| Outcome | Studies included,
n | P-value (Begg's
test) | P-value (Egger's
test) |
|---|
| ORR | 17 | 1.000 | 0.729 |
| DCR | 17 | 0.466 | 0.215 |
| PFS | 5 | 0.806 | 0.200 |
| Hypertension | 11 | 0.876 | 0.970 |
| Hand-foot
syndrome | 9 | 0.602 | 0.713 |
| Liver
dysfunction | 7 | 0.230 | 0.261 |
| Proteinuria | 13 | 0.951 | 0.139 |
| Bone marrow
depression | 8 | 0.902 | 0.758 |
|
Thrombocytopenia | 7 | 0.368 | 0.179 |
| Leukocytopenia | 5 | 1.000 | 0.812 |
| Fatigue | 5 | 0.221 | 0.299 |
| Gastrointestinal
reaction | 9 | 0.048 | 0.183 |
|
Nausea/vomiting | 6 | 0.707 | 0.277 |
Assessment of risk of bias and
sensitivity analysis
Application of the Cochrane Collaboration's tool
revealed that the overall risk of bias in the included RCTs was
relatively low. Specifically, all included RCTs were evaluated as
low risk for sequence generation, completeness of outcome data and
being free of selective reporting. However, concealment of
allocation and blinded adjudication were unclear among all RCTs.
Regarding the risk of other biases, four studies were rated as high
risk and nine studies as unclear (Table III). Furthermore, the risk of bias
of the five non-RCTs was assessed using the Newcastle-Ottawa Scale
criteria, which indicated that the total score of these studies
ranged from 7 to 8, suggesting a relatively low risk of bias
(Table IV).
 | Table III.Assessment of the risk of bias in
randomized controlled trials by Cochrane Collaboration's tool. |
Table III.
Assessment of the risk of bias in
randomized controlled trials by Cochrane Collaboration's tool.
| Author, year | Sequence
generation | Concealment of
allocation | Blinded
adjudication | Completeness of
outcome data | Free of selective
reporting | Free of other
bias | (Refs.) |
|---|
| Guo, 2017 | Low risk | Unclear | Unclear | Low risk | Low risk | Unclear | (13) |
| Chen, 2017 | Low risk | Unclear | Unclear | Low risk | Low risk | Unclear | (14) |
| Liu, 2018 | Low risk | Unclear | Unclear | Low risk | Low risk | Unclear | (15) |
| Shang, 2019 | Low risk | Unclear | Unclear | Low risk | Low risk | Unclear | (16) |
| Yu, 2020 | Low risk | Unclear | Unclear | Low risk | Low risk | Unclear | (18) |
| Guo, 2020 | Low risk | Unclear | Unclear | Low risk | Low risk | Unclear | (19) |
| Xie, 2020 | Low risk | Unclear | Unclear | Low risk | Low risk | Unclear | (20) |
| Li, 2020 | Low risk | Unclear | Unclear | Low risk | Low risk | High risk | (21) |
| Cui, 2021 | Low risk | Unclear | Unclear | Low risk | Low risk | Unclear | (22) |
| Chen, 2021 | Low risk | Unclear | Unclear | Low risk | Low risk | High risk | (23) |
| Huang, 2021 | Low risk | Unclear | Unclear | Low risk | Low risk | High risk | (24) |
| Song, 2021 | Low risk | Unclear | Unclear | Low risk | Low risk | High risk | (27) |
| Chen, 2022 | Low risk | Unclear | Unclear | Low risk | Low risk | Unclear | (28) |
 | Table IV.Assessment of the risk of bias in
non-randomized controlled trials by the Newcastle-Ottawa Scale
criteria. |
Table IV.
Assessment of the risk of bias in
non-randomized controlled trials by the Newcastle-Ottawa Scale
criteria.
| Author, year | Selection | Comparability | Outcome | Total score | (Refs.) |
|---|
| Luo, 2019 | 4 | 1 | 3 | 8 | (17) |
| Hu, 2020 | 4 | 2 | 2 | 8 | (11) |
| Liu, 2021 | 4 | 1 | 2 | 7 | (25) |
| Guo, 2021 | 4 | 2 | 2 | 8 | (26) |
| Lu, 2022 | 4 | 2 | 2 | 8 | (29) |
Furthermore, sensitivity analysis was conducted for
all outcomes by omitting each study, one at a time. The results
indicated that no single study substantially altered the RR of most
outcomes. However, the RR of proteinuria, thrombocytopenia and
fatigue were affected by omitting the studies by Liu and Zheng
(15), Guo and Jing (13) and Song et al (27), respectively (Table SII).
Discussion
The high morbidity and mortality associated with
advanced-stage NSCLC have brought an immense burden globally,
particularly in China (2,5,6). Over
the past decade, anti-angiogenetic treatments have seen notable
progress in the treatment of advanced-stage NSCLC, which enhances
the treatment response and prolongs patient survival with tolerable
adverse events (33). Previously,
bevacizumab was the main angiogenesis inhibitor for patients with
advanced NSCLC (34). Apatinib is a
representative novel anti-angiogenetic drug approved in China,
whose application in NSCLC has been reported in previous studies
(9,11,30).
In order to provide more sufficient evidence supporting the
application of apatinib in advanced-stage NSCLC, the present
meta-analysis reviewed 18 studies (including 13 RCTs and 5
observational studies) and found that apatinib plus chemotherapy
enhanced the ORR and DCR compared to chemotherapy alone among
patients with advanced-stage NSCLC. The potential explanation for
this may be as follows: i) Apatinib, as a VEGFR2 TKI, was able to
suppress VEGF/VEGFR2 and MAPK pathways, which consequently
inhibited tumor activity (35). ii)
The synergistic antitumor effects of apatinib and chemotherapy may
increase the treatment efficacy among patients with advanced-stage
NSCLC (36). iii) Apatinib has been
found to enhance the sensitivity of chemotherapeutic drugs through
the AKT/β-catenin pathway (37).
Therefore, apatinib plus chemotherapy can enhance the treatment
response compared to chemotherapy alone among patients with
advanced-stage NSCLC.
According to the guidelines from the National
Comprehensive Cancer Network, platinum-based chemotherapy is
recommended as a first-line treatment for patients with
advanced-stage NSCLC (1). However,
according to previous studies, the survival of patients with
advanced-stage NSCLC following chemotherapy remains unsatisfactory
(38,39). Several clinical trials have
indicated that, in comparison to chemotherapy alone, apatinib plus
chemotherapy prolongs the survival of patients with advanced-stage
NSCLC (13,17,21,23,27).
However, to date, at least to the best of our knowledge, there is
no study available that comprehensively compares the survival of
patients with advanced-stage NSCLC receiving these treatment
methods. In the present systematic meta-analysis, the pooled data
of the five studies demonstrated that PFS of patients with
advanced-stage NSCLC was longer in the group receiving apatinib
plus chemotherapy compared to chemotherapy alone. The possible
reason for this may be that the favorable treatment response of
apatinib plus chemotherapy contributed to satisfactory survival
outcomes among patients with advanced-stage NSCLC.
Previous studies have demonstrated that common
apatinib-related adverse events are mild hypertension, hand-foot
syndrome and fatigue (40,41). In addition, the main toxic effects
associated with chemotherapy are leukopenia, neutropenia,
thrombocytopenia and anemia (42).
Of note, previous studies have demonstrated that apatinib plus
chemotherapy does not increase the numbers of severe adverse events
compared to chemotherapy alone when used in the treatment of
patients with advanced-stage NSCLC (11,13–29).
However, a comprehensive analysis of the safety of apatinib plus
chemotherapy in patients with advanced-stage NSCLC is still
warranted. Herein, the pooled analysis revealed that apatinib plus
chemotherapy only increased the risk of developing hypertension and
hand-foot syndrome; however, no other adverse events in comparison
to chemotherapy alone were found among the patients with
advanced-stage NSCLC; this finding was consistent with that of a
previous related meta-analysis (30). The potential reasons for this may be
the following: i) Apatinib inhibited the VEGFR-2-mediated signaling
pathway and the latter has an essential role in maintaining
vascular tone and blood pressure regulation. As a result, apatinib
may cause the incidence of hypertension (43). ii) The apatinib-induced keratinocyte
apoptosis, the persistent existence of subclinical trauma and
impaired vascular function may have resulted in the development of
hand-foot syndrome (44).
The purpose of the current study was to compare the
efficacy and safety of apatinib plus chemotherapy vs. chemotherapy
alone for patients with advanced NSCLC, since the benefit of
bevacizumab plus chemotherapy in these patients was already
suggested in other meta-analyses (45,46).
However, the present study has certain limitations, which should be
mentioned: i) The present study was limited by the fact that OS
data were not reported in the previous studies and a meta-analysis
on this aspect was thus difficult to conduct. Further studies are
required to focus on the OS benefit from the use of apatinib plus
chemotherapy in patients with advanced-stage NSCLC. ii) Sensitivity
analysis revealed that omitting the studies by Liu and Zheng
(15), Guo and Jing (13) and Song et al (27) affected the pooled analysis finding
of proteinuria, thrombocytopenia and fatigue, respectively; thus,
more updated studies are required to validate this finding. iii)
There were four studies with a high risk of other biases, which may
have interfered with the results. iv) As the original studies
provided limited relevant information, it was not possible to
perform subgroup analyses; hence, it was difficult to demonstrate
what type of patient group benefited from the combination therapy.
v) Previous treatment may have influenced the outcome, while most
studies did not present this information in detail; thus, it was
difficult to analyze.
In conclusion, the present meta-analysis
demonstrated that the use of apatinib plus chemotherapy enhanced
the treatment response and PFS. However, it increased the risk of
developing hypertension and hand-foot syndrome compared with
chemotherapy alone among patients with advanced-stage NSCLC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SQ and XH designed the study. ZL supervised the
study. SQ and XH conceived the study. SQ, XH and ZL participated in
data collection. SQ and XH performed the data analysis and wrote
the manuscript. SQ, XH and ZL contributed to the interpretation of
the results and revised the manuscript. SQ and XH confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ettinger DS, Wood DE, Aisner DL, Akerley
W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac LR, D'Amico
TA, et al: Non-small cell lung cancer, version 3.2022, NCCN
clinical practice guidelines in oncology. J Natl Compr Canc Netw.
20:497–530. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen P, Liu Y, Wen Y and Zhou C: Non-small
cell lung cancer in China. Cancer Commun (Lond). 42:937–970. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wei W, Zeng H, Zheng R, Zhang S, An L,
Chen R, Wang S, Sun K, Matsuda T, Bray F and He J: Cancer
registration in China and its role in cancer prevention and
control. Lancet Oncol. 21:e342–e349. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alexander M, Kim SY and Cheng H: Update
2020: Management of non-small cell lung cancer. Lung. 198:897–907.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen R, Manochakian R, James L, Azzouqa
AG, Shi H, Zhang Y, Zhao Y, Zhou K and Lou Y: Emerging therapeutic
agents for advanced non-small cell lung cancer. J Hematol Oncol.
13:582020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Melosky B, Wheatley-Price P, Juergens RA,
Sacher A, Leighl NB, Tsao MS, Cheema P, Snow S, Liu G, Card PB and
Chu Q: The rapidly evolving landscape of novel targeted therapies
in advanced non-small cell lung cancer. Lung Cancer. 160:136–151.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang X, Zhong D, Zhang J, Du N, Ren Y, Gao
J, Liu L, Yu J, Li X, Ma L, et al: Safety and effectiveness of
apatinib in elderly patients with metastatic gastric cancer: A
sub-analysis from the large-scale, prospective observational study
of apatinib for gastric cancer treatment in a real-world clinical
setting (AHEAD-G202). J Gastrointest Oncol. 13:1679–1689. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tang Z, Wang Y, Yu Y, Cui Y, Liang L, Xu
C, Shen Z, Shen K, Wang X, Liu T and Sun Y: Neoadjuvant apatinib
combined with oxaliplatin and capecitabine in patients with locally
advanced adenocarcinoma of stomach or gastroesophageal junction: A
single-arm, open-label, phase 2 trial. BMC Med. 20:1072022.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fathi Maroufi N, Rashidi MR, Vahedian V,
Akbarzadeh M, Fattahi A and Nouri M: Therapeutic potentials of
apatinib in cancer treatment: Possible mechanisms and clinical
relevance. Life Sci. 241:1171062020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao J, Yu H, Han T, Wang W, Tong W and
Zhu X: A study on the efficacy of recombinant human endostatin
combined with apatinib mesylate in patients with middle and
advanced stage non-small cell lung cancer. J BUON. 24:2267–2272.
2019.PubMed/NCBI
|
|
11
|
Hu R, Li T, Hui K, Chen Z, Wang N, Wu X,
Ge L and Zhou L: Apatinib sensitizes chemoresistant NSCLC cells to
doxetaxel via regulating autophagy and enhances the therapeutic
efficacy in advanced and refractory/recurrent NSCLC. Mol Med Rep.
22:3935–3943. 2020.PubMed/NCBI
|
|
12
|
Xu J, Liu X, Yang S, Zhang X and Shi Y:
Clinical response to apatinib monotherapy in advanced non-small
cell lung cancer. Asia Pac J Clin Oncol. 14:264–269. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guo Y and Jing X: Efficacy and safety of
docetaxel plus apatinib as a second-line treatment for advanced
nonsquamous non-small cell lung cancer. Chin J Clin Oncol.
44:544–546. 2017.
|
|
14
|
Chen E, Chen Z, Chen M and Che M: Clinical
effect evaluation of apatinib mesylate tablets in the treatment of
advanced non-small cell lung cancer. China Mod Med. 24:91–93.
2017.
|
|
15
|
Liu X and Zheng J: Curative effect of
second-line chemotherapy of apatinib combined with docetaxel on
advanced non-squamous non-small-cell lung cancer. Pract J Card
Cereb Pneumal Vasc Dis. 26:104–107. 2018.
|
|
16
|
Shang K, Hu R and Hu J: Effects of
apatinib combined with docetaxel on CEA, SCC and CA125 in
second-line treatment of patients with advanced non-small cell lung
cancer. Pract J Cancer. 34:1172–1175. 2019.
|
|
17
|
Luo R and Li B: Clinical effect of
apatinib combined with chemotherapy on patients with advanced
non-small cell lung cancer after failure of first-line treatment.
Oncol Prog. 17:1809–1812. 2019.
|
|
18
|
Yu Z, Cai X, Xu Z, He Z, Lai J, Wang W,
Zhang J, Kong W, Huang X, Chen Y, et al: Apatinib plus chemotherapy
as a second-line treatment in unresectable non-small cell lung
carcinoma: A randomized, controlled, multicenter clinical trial.
Oncologist. 25:e1640–e1649. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guo C, Dong J, Yin Y, Cui J, Gao Y and
Zhang J: The efficacy and safety of apatinib combined with
chemotherapy in the first-line treatment of driver-negative
advanced non-small cell lung cancer. Syst Med. 5:10–12. 2020.
|
|
20
|
Xie MQ, Wen B, Xu HX and Song YR: Efficacy
of apatinib combined with cisplatin and paclitaxel in the treatment
of driver gene wild-type advanced non-squamous non-small cell lung
cancer. J Basic Clin Oncol. 33:198–200. 2020.
|
|
21
|
Li S: Effect of Apatinib mesylate tablets
combined with paclitaxel injection on the efficacy of patients with
non-small cell lung cancer. Contemp Med. 26:175–177. 2020.
|
|
22
|
Cui YJ, Liu J, Liu MM and Zhang HZ:
Observation on the clinical effect of apatinib combined with
chemotherapy in the treatment of advanced non-small cell lung
cancer. Pak J Med Sci. 37:1036–1041. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen P, Chen W and Zhang J: Efficacy,
safety and prognosis of apatinib plus docetaxel compared with
docetaxel in the second line treatment in advanced non-squamous
non-small cell lung cancer. J Mod Oncol. 29:1513–1519. 2021.
|
|
24
|
Huang Y, Lin Q, You X, Cao X and Li W:
Clinical effects of apatinib in combination with cisplatin and
paclitaxel chemotherapy for advanced non-small cell lung cancer. J
Math Med. 34:1669–1671. 2021.
|
|
25
|
Liu J, Pan X and Zheng G: Efficacy of
apatinib mesylate in combination with paclitaxel in advanced
non-small cell lung cancer and the effect on serum tumor markers.
Shanxi Med J. 50:784–786. 2021.
|
|
26
|
Guo H: Clinical effect of apatinib
mesylate tablets combined with chemotherapy for advanced non-small
cell lung cancer. Med Forum. 25:3204–3206. 2021.
|
|
27
|
Song Y, Zhao Q and Lv J: Effect of
apatinib combined with chemotherapy on multiple tumor markers in
patients with advanced non-small cell lung cancer and its
short-term efficacy. Int J Pathol Clin Med. 41:2824–2829. 2021.
|
|
28
|
Chen L, Zhou L, Jin X, Ma S and Jiang X:
Efficacy analysis of apatinib combined with paclitaxel and
cisplatin in the treatment of non-small cell lung cancer. World J
Complex Med. 8:187–190. 1982022.
|
|
29
|
Lu M: Effects of apatinib combined with
paclitaxel in treatment of elderly patients with advanced non-small
cell lung cancer. Med J Chin People's Health. 34:28–31. 2022.
|
|
30
|
Li Z, Liu Z, Wu Y, Li H, Sun Z, Han C,
Zhang X and Zhang J: Efficacy and safety of apatinib alone or
apatinib plus paclitaxel/docetaxel versus paclitaxel/docetaxel in
the treatment of advanced non-small cell lung cancer: A
meta-analysis. Thorac Cancer. 12:2838–2848. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Higgins JP, Altman DG, Gøtzsche PC, Jüni
P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, et
al: The cochrane collaboration's tool for assessing risk of bias in
randomised trials. BMJ. 343:d59282011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wells GA, Shea B, O'Connell D, Peterson J,
Welch V, Losos M and Tugwell P: The Newcastle-Ottawa Scale (NOS)
for assessing the quality of nonrandomised studies in
meta-analyses. 2000.
|
|
33
|
Pang LL, Gan JD, Huang YH, Liao J, Zhuang
WT, Ali WA, Hong SD, Zhang L and Fang WF: Role of antiangiogenic
agents in first-line treatment for advanced NSCLC in the era of
immunotherapy. BMC Cancer. 23:722023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Manzo A, Montanino A, Carillio G, Costanzo
R, Sandomenico C, Normanno N, Piccirillo MC, Daniele G, Perrone F,
Rocco G and Morabito A: Angiogenesis inhibitors in NSCLC. Int J Mol
Sci. 18:20212017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang H: Apatinib for molecular targeted
therapy in tumor. Drug Des Devel Ther. 9:6075–6081. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Feng J and Qin S: The synergistic effects
of Apatinib combined with cytotoxic chemotherapeutic agents on
gastric cancer cells and in a fluorescence imaging gastric cancer
xenograft model. Onco Targets Ther. 11:3047–3057. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wei B, Wang Y, Wang J, Cai X, Xu L, Wu J,
Wang Y, Liu W, Gu Y, Guo W and Xu Q: Apatinib suppresses tumor
progression and enhances cisplatin sensitivity in esophageal cancer
via the Akt/β-catenin pathway. Cancer Cell Int. 20:1982020.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shi J, Yang H, Lv T, Pan H and Yang B:
Efficacy of platinum drugs in the treatment of elderly patients
with advanced non-small cell lung cancer and their effects on
prognosis and survival. J BUON. 25:1737–1744. 2020.PubMed/NCBI
|
|
39
|
Ferrara R, Imbimbo M, Malouf R,
Paget-Bailly S, Calais F, Marchal C and Westeel V: Single or
combined immune checkpoint inhibitors compared to first-line
platinum-based chemotherapy with or without bevacizumab for people
with advanced non-small cell lung cancer. Cochrane Database Syst
Rev. 4:CD0132572021.PubMed/NCBI
|
|
40
|
Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y,
Liu W, Tong J, Liu Y, Xu R, et al: Randomized, double-blind,
placebo-controlled phase III trial of apatinib in patients with
chemotherapy-refractory advanced or metastatic adenocarcinoma of
the stomach or gastroesophageal junction. J Clin Oncol.
34:1448–1454. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Geng R, Song L, Li J and Zhao L: The
safety of apatinib for the treatment of gastric cancer. Expert Opin
Drug Saf. 17:1145–1150. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Knezevic CE and Clarke W: Cancer
chemotherapy: The case for therapeutic drug monitoring. Ther Drug
Monit. 42:6–19. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li C, Ma L, Wang Q, Shao X, Guo L, Chen J,
Wang W and Yu J: Rho kinase inhibition ameliorates vascular
remodeling and blood pressure elevations in a rat model of
apatinib-induced hypertension. J Hypertens. 40:675–684. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xia H, Zhou C, Luo Z, Zhang P, Zhu L and
Gong Z: Apatinib-induced hand-foot skin reaction in chinese
patients with liver cancer. Front Oncol. 11:6243692021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Botrel TE, Clark O, Clark L, Paladini L,
Faleiros E and Pegoretti B: Efficacy of bevacizumab (Bev) plus
chemotherapy (CT) compared to CT alone in previously untreated
locally advanced or metastatic non-small cell lung cancer (NSCLC):
Systematic review and meta-analysis. Lung Cancer. 74:89–97. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang S, Mao XD, Wang HT, Cai F and Xu J:
Efficacy and safety of bevacizumab plus erlotinib versus
bevacizumab or erlotinib alone in the treatment of non-small-cell
lung cancer: A systematic review and meta-analysis. BMJ Open.
6:e0117142016. View Article : Google Scholar : PubMed/NCBI
|