Introduction
Endometrial cancer is the most common gynecological
cancer in developed countries. An estimated 65,950 new cases of
endometrial cancer have been diagnosed in 2022 in USA, with 12.550
estimated deaths (1). The majority
of women are diagnosed in an early stage, resulting in a 5-year
overall survival of 81.2% (2).
Endometrial cancer has been grouped into two main
clinicopathological and molecular types: Type I comprises
endometrioid adenocarcinoma and is the more frequent (80–90%) and
Type II includes non-endometrioid subtypes such as serous, clear
cell, undifferentiated carcinomas, and carcinosarcoma (10–20%)
(3).
Obesity is one of the main risk factors for the
development of Type I endometrial cancer and its precursor, the
atypical hyperplasia (4). In fact,
60% of these women are obese (5).
Renehan et al, found that a 5 kg/m2 increase in
BMI is related to an increased risk of 59% of having endometrial
cancer (6). Also, women with
obesity at diagnosis have a higher risk of death than those without
obesity (7–9). Overweight and obesity are related to
diabetes mellitus, hypertension, dyslipidemia, metabolic syndrome,
osteoarthritis, and obstructive sleep apnea (10). Cardiovascular co-morbidities are the
main cause of death among endometrial cancer survivors secondary to
obesity (11).
It has been shown that the incidence of endometrial
cancer in obese women can be reduced by weight loss (12,13),
while it has been observed that medical history of bariatric
surgery is associated with reduced risk for endometrial cancer
(14,15). Also, there may be further benefits
to weight loss in this context, such us improved metabolic and
cardiovascular health in women known to be at high risk of
cardiovascular events (16). The
AHA/ACC/TOS Guideline for the Management of Overweight and Obesity
in Adults (17) states that a 5%
weight loss produces clinically significant improvements in some
cardiovascular risk factors such as diabetes, lipid profile and
hypertension.
Survival benefits of weight loss following
endometrial cancer treatment are sparse and they are not well
established in the literature. There is evidence that many women do
not lose weight after surgical treatment for endometrial cancer
(18), and postoperative body
weight loss may have a better survival compared with body weight
gain (l8). So, identifying percentage of weight loss and whether
this weight loss may have scientific impact is an essential
consideration in the management of women with endometrial
cancer.
Main objective of the present study was to evaluate
the percentage of overweight and obese women who lose weight after
one year of treatment and to examine its potential impact on cancer
outcomes.
Materials and methods
Study design
A single-center retrospective cohort study which
included 526 overweight and obese women was performed in Insular
University Hospital of Las Palmas. An electronic search was
performed from our electronic database in order to retrieve
endometrial cancer patients meeting inclusion criteria. Our
Department consistently records prospectively all elements of
cancer patients treated in our Department by a dedicated senior
data administrator, while all elements are verified on a monthly
basis. The study was approved by the Ethical Committee of
University of Las Palmas de Gran Canaria (CEIm Las Palmas, approval
no. 2022-414-1) in October 2022.
Inclusion criteria for the present analysis
concerned early-stage, type-I (endometrioid) endometrial cancer
patients that were overweight or obese who have BMI ≥25
kg/m2 and ≥18 years old and treated between 2007 and
2019. Apparent early-stage (stage I–II) disease was assessed
preoperatively by vaginal ultrasound or pelvic magnetic resonance
imaging and/or intraoperatively by revision of the surgical
specimen by the pathologist. Cases with final histology different
from endometrioid (serous, clear cells, carcinosarcoma,
undifferentiated, mixed carcinoma), FIGO (The International
Federation of Gynecology and Obstetrics) stage III–IV and BMI
<25 kg/m2 were excluded. Women with incomplete
medical reports, with synchronous cancers or treated by
radiotherapy, chemotherapy or hormonal therapy at first intention
were also not included in the present analysis.
All patients underwent total hysterectomy and
unilateral or bilateral adnexectomy by minimally invasive surgery
(laparoscopy, robot, or vaginal) or laparotomy. Assessment of lymph
nodes was variable over the years and included sentinel pelvic
lymph node, pelvic or/and para-aortic lymphadenectomy. Treatment of
patients was based on current ESGO guidelines. Furthermore, in our
institution, as a standard of our treatment policy, all obese and
overweight women are advised to lose weight and a healthy life.
Also, the cardiovascular and carcinogenic risks of obesity are
explained to them.
Cohort selection and study
variables
Epidemiological, histopathological and survival
outcomes of patients were retrieved and analyzed from patient
records. Specifically, we set in the center of our analysis the
following data: age (years), height (cm), weight (kg) at diagnosis,
clinical tumor stage (FIGO), type of surgery, tumor grade (Grade
1–3), depth of myometrial invasion (<=50%, >50%)
lymphovascular invasion (present or absent), type of surgery
(laparoscopy, robot or laparotomy), pelvic and para-aortic
lymphadenectomy performance. Survival or not (death) as well as
weight in kg at the end of 1st year of follow-up were recorded.
Finally, data of adjuvant treatment (vaginal brachytherapy,
external beam radiation, or combination of both) were also
evaluated.
Study outcomes
Main objective of the present study was to determine
weight loss for overweight and obese early-stage, type-I
endometrial cancer patients at the end of 1st year of follow-up
after treatment as well as to assess the impact of this body weight
change on survival outcomes. Impact of ≥5% weight loss on
endometrial cancer survival was also set in the scope of our
analysis. A 5% of weight loss was chosen because he AHA/ACC/TOS
Guideline for the Management of Overweight and Obesity in Adults
(15) states that a 5% weight loss
produces clinically significant improvements in some cardiovascular
risk factors, so we wanted to evaluate whether this cut-off point
also impacts on survival.
Statistical analysis
Data were summarized by mean ± standard deviation
for all continuous variables if they followed a normal
distribution. Categorical variables were reported as absolute
number and percentage. To compare continuous variables, Wilcoxon
test was used, as they did not have a normal distribution. Survival
was assessed using Kaplan-Meier curves, while log-rank tests were
used to compare the curves. Logistic regression was used to assess
potential association between weight loss and survival. Univariate
and multivariate Cox regression analyses were performed to
determine the association between various factors and survival
outcomes. Factors included in the multivariate model were FIGO (The
International Federation of Gynecology and Obstetrics) stage, age,
grade, lymphovascular invasion and body weight change at 12 months.
All statistical tests were two-tailed and P<0.05 was considered
to indicate a statistically significant difference. Statistical
analysis was conducted using SPSS and JASP.
Results
Patient characteristics
All included patients underwent total hysterectomy
with bilateral adnexectomy. Surgery was performed by laparoscopy or
robot, laparotomy and vaginal approach in 441 cases (83.84%), 45
cases (8.56%) and 40 cases (7.60%), respectively. The most common
FIGO stage was IA (412 patients, 78.33%), followed by stage IB
(17.30%) and stage II (4.37%). Most of the tumors were classified
as grade 1 (76.81%) and grade 2 (18.25%). Lymphovascular invasion
was present in 80 women (15.21%). The majority of women (74.71%),
did not receive any adjuvant treatment. Pelvic and para-aortic
lymphadenectomy was performed in 78 (14.83%) and 22 (4.18%) women,
respectively. Main epidemiological and histopathological outcomes
of patients are reported in Table
I.
 | Table I.Epidemiological and histopathological
characteristics of patients. |
Table I.
Epidemiological and histopathological
characteristics of patients.
| Characteristic | Patients (n=526) |
|---|
| Mean age, years
(SD) | 63.12 (10.81) |
| BMI, n (%) |
|
|
Overweight | 152 (28.9) |
|
Obese | 374 (71.10) |
| FIGO stage, n
(%) |
|
| IA | 412 (78.33) |
| IB | 91 (17.30) |
| II | 23 (4.37) |
| Grade, n (%) |
|
| 1 | 404 (76.81) |
| 2 | 96 (18.25) |
| 3 | 26 (4.94) |
| Lymphovascular
invasion, n (%) |
|
| No | 446 (84.79) |
| Yes | 80 (15.21) |
| Pelvic
lymphadenectomy, n (%) |
|
| Yes | 78 (14.83) |
| No | 448 (85.17) |
| Para-aortic
lymphadenectomy, n (%) |
|
| Yes | 22 (4.18) |
| No | 504 (95.82) |
| Type of surgery, n
(%) |
|
|
Laparoscopy | 441 (83.84) |
|
Vaginal | 40 (7.60) |
|
Laparotomy | 45 (8.56) |
| Adjuvant treatment,
n (%) |
|
| No | 393 (74.71) |
|
Radiotherapy alone | 1 (0.19) |
|
Brachytherapy alone | 25 (4.75) |
|
Radiotherapy +
Brachytherapy | 107 (20.34) |
| Patterns of
recurrence, n (%) |
|
| Vaginal
vault | 13 (30.95) |
|
Peritoneal carcinomatosis | 9 (21.43) |
|
Metastatic lymph nodes | 5 (11.90) |
|
Visceral metastases | 14 (33.33) |
| Port
site metastases | 1 (2.38) |
| Death, n (%) |
|
| No | 449 (85.36) |
|
Yes | 77 (14.64) |
Body weight change
A total of 526 women were studied of which 152
(28.9%) were overweight (BMI ≥25 and <30) and 374 (71.10%) were
obese (BMI ≥30). Initial body weight was 85.73±17.42 kg (median: 83
kg, min: 56 kg, max: 160.20 kg) which corresponds to an initial BMI
of 34.35±6.62 (kg/m2) [median: 33.22 (kg/m2),
min: 25, (kg/m2) max: 63.56 (kg/m2)]. One
year after treatment, body weight was 84.52±16.16 kg (median: 81.00
kg, min: 53.00 kg, max: 151.00 kg). In total, one year after
treatment 271 (52.1%) women lost weight, 207 (39.8%) women gained
weight and 42 (8.1%) women presented a stable weight. Significant
differences were found in body weight change (P≤0.001).
The median follow-up was 76.17 months during which
time 77 (17.15%) women died. Regarding the survivor group, body
weight at initial diagnosis was 86.4±17.9 kg (BMI 34.22±6.69
kg/m2) vs. 84.6±16.4 kg one year after treatment, which
corresponded to a significant mean weight loss of 1.47 kg
(P<0.001). However, in the group of non-survivors, body weight
at initial diagnosis was 84.7±15.7 kg (BMI 35.24±6.72
kg/m2) vs. 84±14.6 kg one year after treatment, which
demonstrated a non-significant mean weight loss of 0.63 kg
(P=0.180) (Table II). Former
results shown in Table II were
calculated based on Wilcoxon test.
 | Table II.Weight change in the survivor and
death group. |
Table II.
Weight change in the survivor and
death group.
| Group | Initial weight,
kg | Weight at 12
months, kg | Weight change at 12
months, kg | P-value |
|---|
| Survivor group
(n=520) | 86.1±17.9 | 84.6±16.4 | −1.47±6.73 | <0.001 |
| Non-survivor group
(n= 77) | 84.7±15.7 | 84±14.6 | −0.63±4.97 | 0.180 |
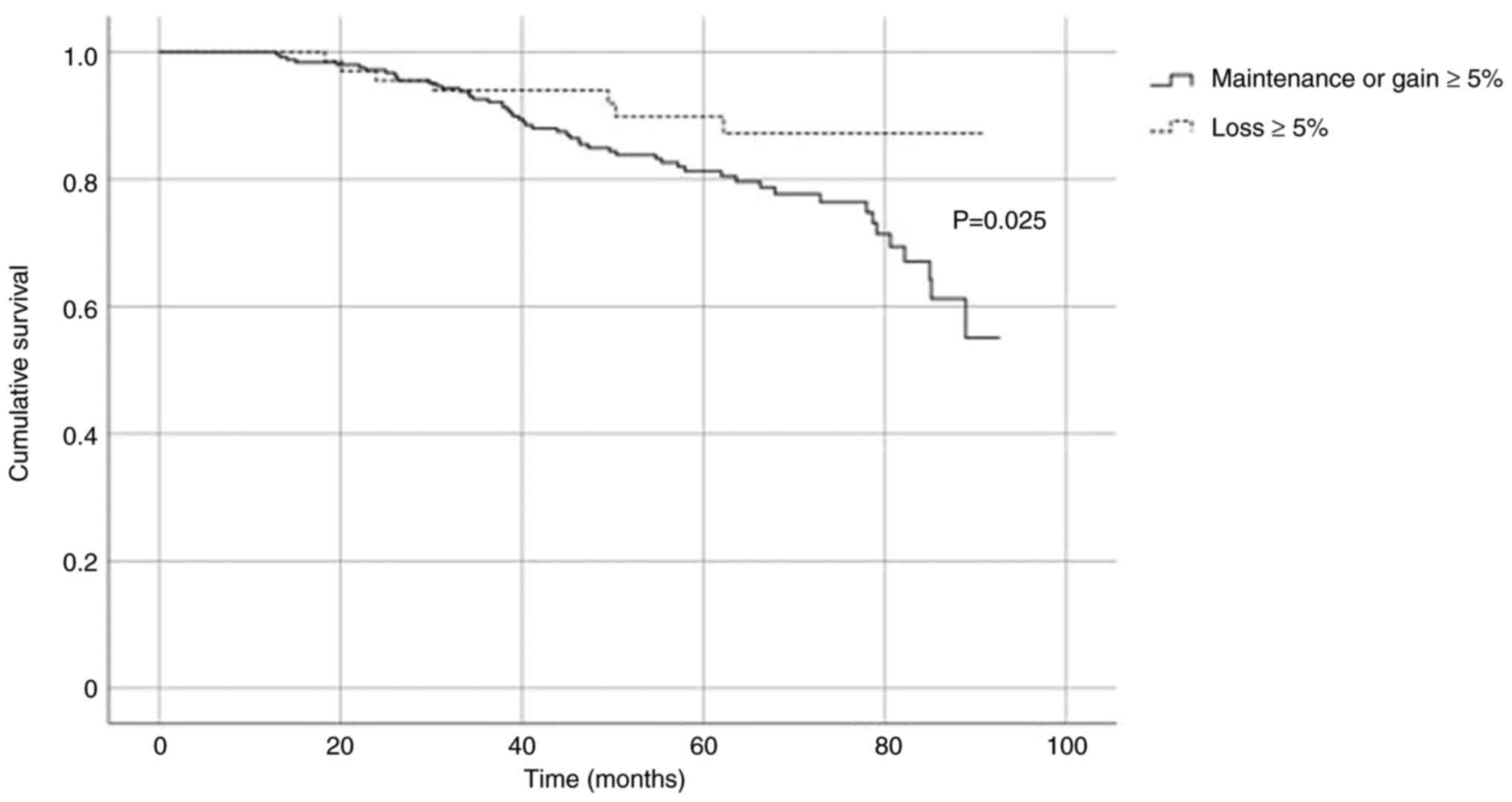
Five percent weight loss
One hundred and five (20.2%) women lost 5% or more
of their total body weight by 12 months, while another 415 (79.8%)
women maintained or gained more than 5% of their initial body
weight. When comparing between those who maintain or gain ≥5%
weight and those who lose ≥5% weight, there were no significant
differences taking into account at the whole cohort and time
(P=0.218; Log-Rank test). However, when adjusting for the period
between 32 and 98 months, survival was significantly higher in
favor of those losing more than 5% of their initial body weight
(P=0.025; Log-Rank test). Fig. 1
presents the relative survival curves within two groups.
Univariate and multivariate analysis
for overall survival and recurrence
Univariate and multivariate analysis included FIGO
stage, age, grade, lymphovascular invasion, adjuvant treatment and
body weight change at 12 months.
Body weight change at 12 months was not indicated to
be a factor significantly affecting overall survival. Adjusted
hazard ratio was 1.01 (95% CI 0.97–1.05, P=0.723).
Parameters indicated to significantly affect overall
survival were age (HR 1.11, 95% CI 1.08–1.14, P<0.001), FIGO
stage (stage IB: HR 1.96, 95% CI 1.16–3.32, P=0.013; stage II: HR
2.87 95% CI 1.35–6.10, P=0.006) and adjuvant treatment
(Brachytherapy alone: HR 3.24, 95% CI 1.45–7.20, P=0.004,
Radiotherapy + Brachytherapy: HR 1.73, 95% CI 1.04–2.89, P=0.036).
However, in the multivariate analysis, only age was independently
associated with overall survival (HR 1.12, 95% CI 1.09–1.15,
P<0.001). FIGO stage II was marginally not significant predictor
of overall survival (stage II: HR 3.07, 95% CI 0.95–9.95, P=0.006).
Furthermore, brachytherapy alone or with radiotherapy were not
indicated as significant covariates based on multivariate analysis.
(Table III). Finally, kind of
complementary therapy was not significantly associated with weight
loss based on a univariate regression model (P=0.34).
 | Table III.Univariate and multivariate Cox
regression analysis for overall survival. |
Table III.
Univariate and multivariate Cox
regression analysis for overall survival.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age | 1.11
(1.08–1.14) | <0.001 | 1.12
(1.09–1.15) | <0.001 |
| Grade | 1.23
(0.68–2.22) | 0.485 |
|
|
| Lymphovascular
invasion | 1.45
(0.81–2.59) | 0.214 |
|
|
| FIGO stage | 2.87
(1.35–6.10) | 0.006 | 3.07
(0.95–9.95) | 0.061 |
| Weight change at 12
months | 1.01
(0.97–1.05) | 0.723 |
|
|
| Adjuvant
treatment |
|
|
|
|
|
Radiotherapy alone | 0.00
(0.00-inf) | 0.996 | 0.00
(0.00-inf) | 0.995 |
|
Brachytherapy alone | 3.24
(1.45–7.20) | 0.004 | 2.63
(0.86–8.03) | 0.090 |
|
Radiotherapy +
Brachytherapy | 1.73
(1.04–2.89) | 0.036 | 1.16
(0.41–3.24) | 0.779 |
Relative remarks were also made regarding risk for
recurrence. Specifically, weight change has not been indicated as
significant predictor for recurrence (HR 1.01, 95% CI 0.97–1.07,
P=0.640). In contrary, age, grade, LVSI and FIGO stage were
demonstrated as significant parameters affecting possibility of
recurrence. Univariate and multivariate models for possibility of
death and recurrence have been mentioned in Tables III and IV.
 | Table IV.Univariate and multivariate Cox
regression analysis for recurrence. |
Table IV.
Univariate and multivariate Cox
regression analysis for recurrence.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age | 1.06
(1.03–1.09) | 0.001 | 1.06
(1.02–1.09) | <0.001 |
| Grade | 4.03
(1.38–10.35) | 0.006 | 3.6
(1.11–10.67) | 0.022 |
| Lymphovascular
invasion | 4.03
(2.09–7.60) | <0.001 | 2.2
(1.02–4.87) | 0.0045 |
| FIGO stage | 8.26
(3.08–20.98) | <0.001 | 8.05
(2.57–24.19) | <0.001 |
| Weight change at 12
months | 1.01
(0.97–1.07) | 0.640 |
|
|
| Adjuvant
treatment |
|
|
|
|
|
Radiotherapy alone | 0.00
(0.00-inf) | 0.996 | 0.00
(0.00-inf) | 0.995 |
|
Brachytherapy alone | 4.35
(1.45–7.48) | 0.013 | 2.68
(0.89–4.13) | 0.090 |
|
Radiotherapy +
Brachytherapy | 2.23
(1.04–2.89) | 0.036 | 1.24
(0.41–3.28) | 0.779 |
Discussion
Our study mainly indicated that, even if greater
weight loss is observed in endometrial cancer patients finally
surviving from disease, no significant impact on survival outcomes
is observed based on multivariate analysis. Furthermore, weight
loss ≥5% was also not indicated to affect significantly survival
parameters, except from the interim interval of 32 and 98
months.
To our knowledge, there is only one study which
analyses changes in body weight cancer patients who are finally
surviving from disease concluded that these weight changes had
repercussions on survival outcomes (18). Regarding breast cancer survivors,
one study concluded that BMI gain between 0.5 and 2.0
kg/m2 (RR 1.35; 95% CI, 0.93–1.95) or more than 2.0
kg/m2 (RR, 1.64; 95% CI, 1.07–2.51) was related to
higher rates of death (19).
However, Goodwin et al (20), randomized 171 breast cancer women to
a telephone-based weight loss lifestyle intervention vs. 167 to an
education-only arm. They observed that lifestyle intervention arm
had higher weight loss compared to education-only arm (−5.3% vs.
−0.6% at 6 months, −5.5% vs. −0.6% at 12 months, and −3.7% vs.
−0.4% at 24 months) (P<0.001). Also, they did not find
significant differences regarding disease free survival between
both groups (HR 0.71, 95% CI: 0.41–1.24, P=0.23). Moreover,
increased BMI was not significantly associated with higher risk of
colon cancer recurrence or death (P=0.54) in stage III colon cancer
patients during and 6 months after adjuvant chemotherapy (21). It appears that data published in
literature on different types of cancers reflect conflicting
results in terms of oncological outcomes.
Literature had shown that endometrial cancer
patients with obesity have reduced quality of life and increased
risk of morbidity (22–25). There have been clinical trials which
have analyzed the impact of weight loss programs vs. usual
physician care on obese endometrial cancer women. Bell et al
(26) found a remarkable BMI
reduction at 6 months and 12 months in endometrial cancer women
with obesity that followed a behavioral weight loss program. In the
behavioral weight loss program group, 80.0% patients lost greater
than 5% of initial weight compared to 28.6% individuals in the
control group. These findings differ from the results of Zamorano
et al (27) which did not
find differences in weight loss after personalized
text-message-based intervention among endometrial cancer survivors
with obesity. At 6 months, 9.2% of women randomized into the
text-message-based intervention had lost at least 5% of their body
weight vs. 11.2% of women randomized into enhanced usual care arm.
On the other hand, McCarroll et al (28) randomized 75 early-stage endometrial
cancer women to a lifestyle intervention group that was offered a
nutrition, exercise, and behavioral modification counseling or a
usual care group. These authors found that there was a significant
BMI loss for the intervention group vs. the control group at the
6-and 12-time points (P<0.001 and P=0.008, respectively). Those
randomized prospective studies had a short follow-up period of six
and 12 months, however they did not analyze oncological outcomes.
Moreover, evidence clearly highlights that dietary interventions
are beneficial for patients with gynecological cancer as they may
improve quality of life and also optimize treatment results on a
level of multidisciplinary approach (29–32).
Another important conclusion of our study is that no correlation
between weight loss and the final outcome may be attributed to
complementary treatment. Firstly, no chemotherapy was administered
in our patients' group, as they were all early-stage disease,
thereafter no detrimental effect of chemotherapy toxicity might
have affected weight loss. Furthermore, no significant correlation
was observed between weight loss and radiotherapy administration.
Therefore, weight loss in endometrial cancer patients of our study
has not been affected from complementary treatments.
Limitations of this study may be considered its
retrospective nature, and its relatively not so large sample size,
which did not allow us to find significant differences due to the
low rate of recurrences in endometrial cancer. Potential variations
on the surgical staging and adjuvant treatment over the years may
have partially affected our results. Lack of basal nutritional data
as well as exact dietary and lifestyle interventions could be also
considered as limitations. Moreover, we have not included a
normal-weight control group. However, our objective was to examine
whether body weight loss had an impact on survival on obese
patients, while normal-weight endometrial cancer are not
consistently advised to lose weight because of lack of benefit on
such a strategy. Despite potential limitations, though, this is one
amongst few studies including long-term survival data from a
trustworthy electronic registry which powered the results of the
study. Furthermore, all different types of minimally invasive
approaches and laparotomy surgery were included, while only women
with histology confirmation in the final surgical specimen were
included to decrease the risk of selection bias. Thereafter, our
findings may considerably contribute in the debate of impact of
weight loss on endometrial cancer survival outcomes and potentially
trigger further research in a field with high clinical impact.
In conclusion, our study indicated that weight loss
did not significantly affect prognosis in early-stage, type-I,
overweight and obese endometrial cancer patients. Further
prospective cohorts should rather be performed in order to further
study potential impact of weight loss on endometrial cancer
prognosis and eventually the impact of such a policy.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BNS was involved in collecting and verifying raw
data, conceptualization, methodology, formal analysis,
investigation, writing the original draft and visualization. JVS
was involved in formal analysis, verification of the repeated
results, reviewing and editing. BNS and JVS confirm the
authenticity of all the raw data. OA was involved in methodology,
investigation and writing the original draft. SP and CMS were
involved in statistical analysis, reviewing and editing. ML was
responsible for statistical analysis, identification and collection
of relative references, reviewing and editing. DG was involved in
methodology, resources, reviewing and editing. AR was responsible
for analysis and interpretation of data and writing the original
draft. AMM significantly contributed to the conception and design
of the study, and was responsible for reviewing and editing,
validation of data analysis and supervision. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethical Committee of
University of Las Palmas de Gran Canaria (CEIm Las Palmas, approval
no. 2022-414-1) in October 2022. All patients provided written
informed consent for participation.
Patient consent for publication
All patients provided written informed consent for
data analysis and potential publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer Statistics, 2022. CA Cancer J Clin. 72:7–33. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Uterine Cancer-Cancer Stat Facts, .
Available from:. https://seer.cancer.gov/statfacts/html/corp.htmlOctober
29–2022
|
|
3
|
American College of Obstetricians and
Gynecologists, . ACOG practice bulletin, clinical management
guidelines for obstetrician-gynecologists, number 65, August 2005:
Management of endometrial cancer. Obstet Gynecol. 106:413–425.
2005.PubMed/NCBI
|
|
4
|
Morice P, Leary A, Creutzberg C,
Abu-Rustum N and Darai E: Endometrial cancer. Lancet.
387:1094–1108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Duong LM, Wilson RJ, Ajani UA, Singh SD
and Eheman CR: Trends in endometrial cancer incidence rates in the
United States, 1999–2006. J Womens Health (Larchmt). 20:1157–1163.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Renehan AG, Tyson M, Egger M, Heller RF
and Zwahlen M: Body-mass index and incidence of cancer: A
systematic review and meta-analysis of prospective observational
studies. Lancet. 371:569–578. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Reeves KW, Carter GC, Rodabough RJ, Lane
D, McNeeley SG, Stefanick ML and Paskett ED: Obesity in relation to
endometrial cancer risk and disease characteristics in the Women's
Health Initiative. Gynecol Oncol. 121:376–382. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Calle EE, Rodriguez C, Walker-Thurmond K
and Thun MJ: Overweight, obesity, and mortality from cancer in a
prospectively studied cohort of U.S. adults. N Engl J Med.
348:1625–1638. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Arem H, Chlebowski R, Stefanick ML,
Anderson G, Wactawski-Wende J, Sims S, Gunter MJ and Irwin ML: Body
mass index, physical activity, and survival after endometrial
cancer diagnosis: Results from the Women's Health Initiative.
Gynecol Oncol. 128:181–186. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
American College of Cardiology/American
Heart Association Task Force on Practice Guidelines and Obesity
Expert Panel 2013, . Expert panel report: Guidelines (2013) for the
management of overweight and obesity in adults. Obesity (Silver
Spring). 22 (Suppl 2):S41–S410. 2014.PubMed/NCBI
|
|
11
|
Ward KK, Shah NR, Saenz CC, Mchale MT,
Alvarez EA and Plaxe SC: Cardiovascular disease is the leading
cause of death among endometrial cancer patients. Gynecol Oncol.
126:176–179. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Trentham-Dietz A, Nichols HB, Hampton JM
and Newcomb PA: Weight change and risk of endometrial cancer. Int J
Epidemiol. 35:151–158. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Luo J, Hendryx M, Manson JE, Figueiredo
JC, LeBlanc ES, Barrington W, Rohan TE, Howard BV, Reding K, Ho GY,
et al: Intentional weight loss and obesity-related cancer risk.
JNCI Cancer Spectr. 3:pkz0542019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ward KK, Roncancio AM, Shah NR, Davis MA,
Saenz CC, McHale MT and Plaxe SC: Bariatric surgery decreases the
risk of uterine malignancy. Gynecol Oncol. 133:63–66. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Anveden Å, Taube M, Peltonen M, Jacobson
P, Andersson-Assarsson JC, Sjöholm K, Svensson PA and Carlsson LMS:
Long-term incidence of female-specific cancer after bariatric
surgery or usual care in the Swedish Obese Subjects Study. Gynecol
Oncol. 145:224–229. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mackintosh ML and Crosbie EJ:
Obesity-driven endometrial cancer: Is weight loss the answer? BJOG.
120:791–794. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jensen MD, Ryan DH, Apovian CM, Ard JD,
Comuzzie AG, Donato KA, Hu FB, Hubbard VS, Jakicic JM, Kushner RF,
et al: AHA/ACC/TOS guideline for the management of overweight and
obesity in adults: A report of the American College of
Cardiology/American Heart Association Task Force on Practice
Guidelines and The Obesity Society. J Am Coll Cardiol. 63((25 Pt
B)): 2985–3023. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Matsuo K, Moeini A, Cahoon SS, Machida H,
Ciccone MA, Grubbs BH and Muderspach LI: Weight change pattern and
survival outcome of women with endometrial cancer. Ann Surg Oncol.
23:2988–2997. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kroenke CH, Chen WY, Rosner B and Holmes
MD: Weight, weight gain, and survival after breast cancer
diagnosis. J Clin Oncol. 23:1370–1378. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Goodwin PJ, Segal RJ, Vallis M, Ligibel
JA, Pond GR, Robidoux A, Findlay B, Gralow JR, Mukherjee SD, Levine
M and Pritchard KI: The LISA randomized trial of a weight loss
intervention in postmenopausal breast cancer. NPJ Breast Cancer.
6:62020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Meyerhardt JA, Niedzwiecki D, Hollis D,
Saltz LB, Mayer RJ, Nelson H, Whittom R, Hantel A, Thomas J and
Fuchs CS: Cancer and Leukemia Group B 89803: Impact of body mass
index and weight change after treatment on cancer recurrence and
survival in patients with stage III colon cancer: Findings from
Cancer and Leukemia Group B 89803. J Clin Oncol. 26:4109–4115.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fader AN, Frasure HE, Gil KM, Berger NA
and von Gruenigen VE: Quality of life in endometrial cancer
survivors: What does obesity have to do with it? Obstet Gynecol
Int. 2011:3086092011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Smits A, Lopes A, Das N, Bekkers R and
Galaal K: The impact of BMI on quality of life in obese endometrial
cancer survivors: Does size matter? Gynecol Oncol. 132:137–141.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
von Gruenigen VE, Waggoner SE, Frasure HE,
Kavanagh MB, Janata JW, Rose PG, Courneya KS and Lerner E:
Lifestyle challenges in endometrial cancer survivorship. Obstet
Gynecol. 117:93–100. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nock NL, Dimitropoulos A, Zanotti KM,
Waggoner S, Nagel C, Golubic M, Michener CM, Kirwan JP and Alberts
J: Sleep, quality of life, and depression in endometrial cancer
survivors with obesity seeking weight loss. Support Care Cancer.
28:2311–2319. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bell M, Reed V, Wernisch J, Papini NM and
Herrmann SD: Effectiveness of profile by Sanford behavioral weight
loss program for weight loss following endometrial cancer
treatment. Gynecol Oncol Rep. 38:1008972021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zamorano AS, Wilson EM, Liu J, Leon A,
Kuroki LM, Thaker PH, McCourt CK, Fuh KC, Powell MA, Mutch DG, et
al: Text-message-based behavioral weight loss for endometrial
cancer survivors with obesity: A randomized controlled trial.
Gynecol Oncol. 162:770–777. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
McCarroll ML, Armbruster S, Frasure HE,
Gothard MD, Gil KM, Kavanagh MB, Waggoner S and von Gruenigen VE:
Self-efficacy, quality of life, and weight loss in overweight/obese
endometrial cancer survivors (SUCCEED): A randomized controlled
trial. Gynecol Oncol. 132:397–402. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Montagnese C, Porciello G, Vitale S,
Palumbo E, Crispo A, Grimaldi M, Calabrese I, Pica R, Prete M,
Falzone L, et al: Quality of life in women diagnosed with breast
cancer after a 12-month treatment of lifestyle modifications.
Nutrients. 13:1362020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Thomson CA, Crane TE, Miller A, Gold MA,
Powell M, Bixel K, Van Le L, DiSilvestro P, Ratner E, Lele S, et
al: Lifestyle intervention in ovarian cancer enhanced survival
(LIVES) study (NRG/GOG0225): Recruitment, retention and baseline
characteristics of a randomized trial of diet and physical activity
in ovarian cancer survivors. Gynecol Oncol. 170:11–18. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Falzone L, Scandurra G, Lombardo V,
Gattuso G, Lavoro A, Distefano AB, Scibilia G and Scollo P: A:
Multidisciplinary approach remains the best strategy to improve and
strengthen the management of ovarian cancer (Review). Int J Oncol.
59:532021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Smits A, Lopes A, Das N, Bekkers R,
Massuger L and Galaal K: The effect of lifestyle interventions on
the quality of life of gynaecological cancer survivors: A
systematic review and meta-analysis. Gynecol Oncol. 139:546–552.
2015. View Article : Google Scholar : PubMed/NCBI
|