Introduction
α-Fetoprotein (AFP) is a 70-kDa glycoprotein that
was first identified as a fetus-specific protein in human serum in
1956 (1). Although physiologically
produced by fetal liver and yolk sac, AFP is also secreted by
several kinds of tumor cells. Hepatocellular carcinoma (HCC) and
yolk sac tumor are diseases that are associated with the aberrant
production of AFP (2), and in those
cases, AFP is clinically used as a biomarker for diagnosis,
treatment evaluation and surveillance of disease progression or
relapse. Certain gastric carcinomas (GCs) also express AFP and
patients with GC of this type have high serum AFP levels (3). Such AFP-producing GCs (AFPGCs) may be
clinically discriminated from common GCs by their hepatocyte-like
appearance in histology, high potential for venous invasion in
pathology and poor clinical prognosis (4–6).
Earlier studies have reported that patients with AFPGC are prone to
having liver metastasis (LM) synchronously or metachronously
(5–8). Thus, controlling LM is critical to
improve the survival of patients with AFPGC. However, the treatment
strategy has remained unestablished because AFPGC is a relatively
rare disease.
Chemotherapy is generally selected as the treatment
of LM from AFPGC because multiple metastatic foci are observed in
most cases and LM is considered a result of hematogenous spreading.
However, no recommendable chemotherapeutic regimen has been
established to date. On the other hand, surgery is adopted for
single or oligometastasis to the liver. Unfortunately, the outcomes
of surgical resection are unsatisfactory (9,10).
Radiotherapy, another local therapeutic option, is
rarely indicated for LM from AFPGC because of a lack of evidence of
the radiosensitivity of this tumor and a significant risk of
radiation-induced liver diseases. However, advances in irradiation
technology, including stereotactic body radiotherapy (SBRT), have
expanded the indications for radiotherapy and upgraded this therapy
into a recommendable alternative for the treatment of HCC, a
primary liver cancer (11,12). However, there is currently no
information on the efficacy of SBRT for LM from AFPGC.
The present study reported the case of a 76-year-old
male patient with AFPGC who underwent SBRT for LM after gastrectomy
and showed a complete response (CR). The treatment strategy for LM
from AFPGC was also discussed with a literature review.
Case report
A 76-year-old male patient underwent follow-up
endoscopy for a small gastric polyp at Sanjo General Hospital
(Sanjo, Japan) in February 2018. The endoscopy revealed type 1
gastric carcinoma (Japanese Classification of Gastric Carcinoma)
(13) in the upper body of the
stomach. The biopsy specimens of the tumor histologically showed
poorly differentiated adenocarcinoma, solid type. The patient
underwent total gastrectomy with D2 lymph node dissection and the
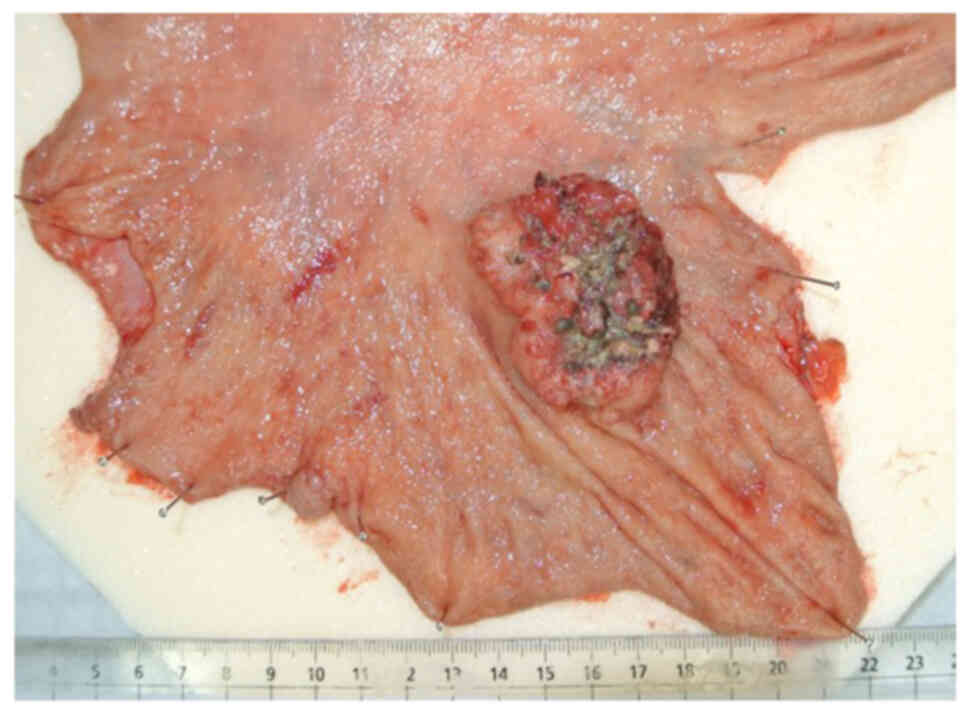
surgery ended in complete resection (Fig. 1). Due to the unique gross tumor form
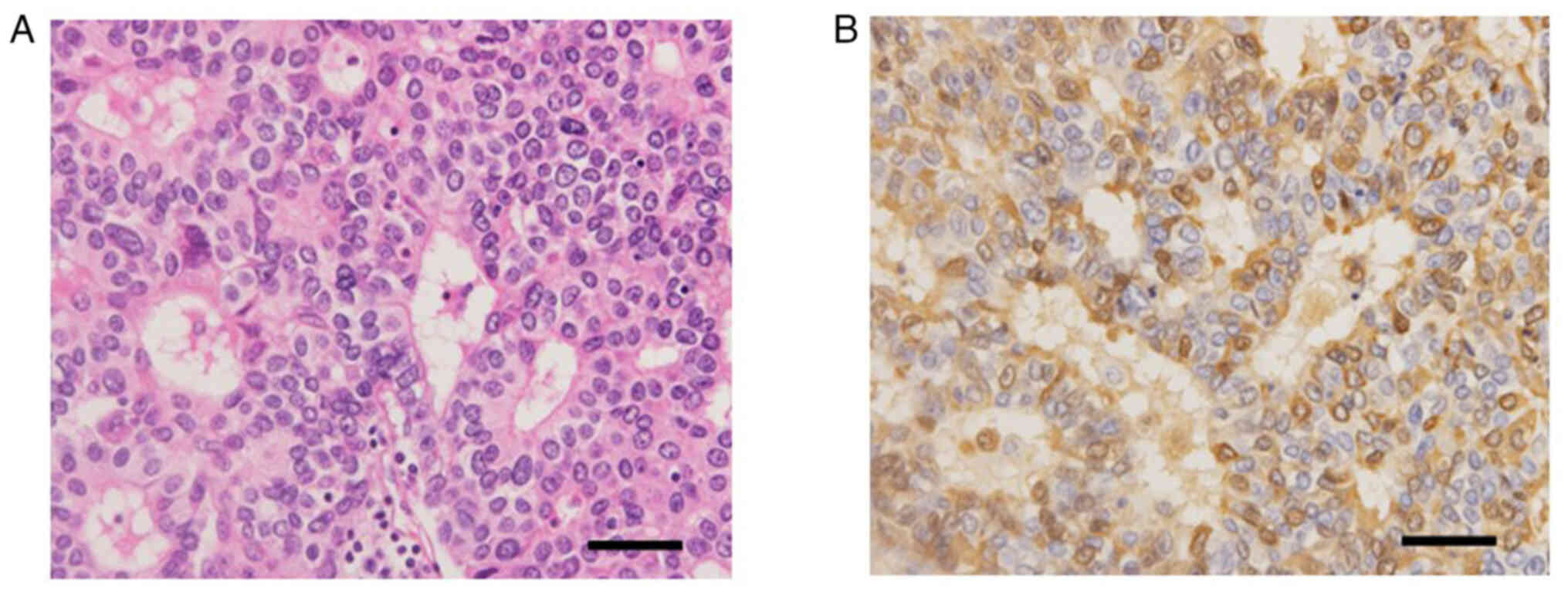
and the hepatoid cellular appearance on microscopy with hematoxylin
and eosin staining (4) (Fig. 2A), AFPGC was suspected and serum AFP
levels were measured. AFP levels were as high as 3,763 ng/ml
(normal range, 0–10 ng/ml) in preoperative serum and rapidly
decreased to 2,567 ng/ml on postoperative day 27.
Immunohistochemistry (IHC) using anti-human AFP monoclonal antibody
(cat. no. 738291; Nichirei Bioscience) showed diffuse and strong
immunoreactivity of tumor cells, confirming the diagnosis of AFPGC
(Fig. 2B). IHC staining was
performed with an automated IHC analyzer (Histostainer 48A;
Nichirei Bioscience) according to the manufacturer's
instructions.
Serum AFP levels rose to 4,484 ng/ml two months
after the surgery, during which the patient was undergoing adjuvant
chemotherapy with S-1 (tegafur/gimeracil/oteracil).
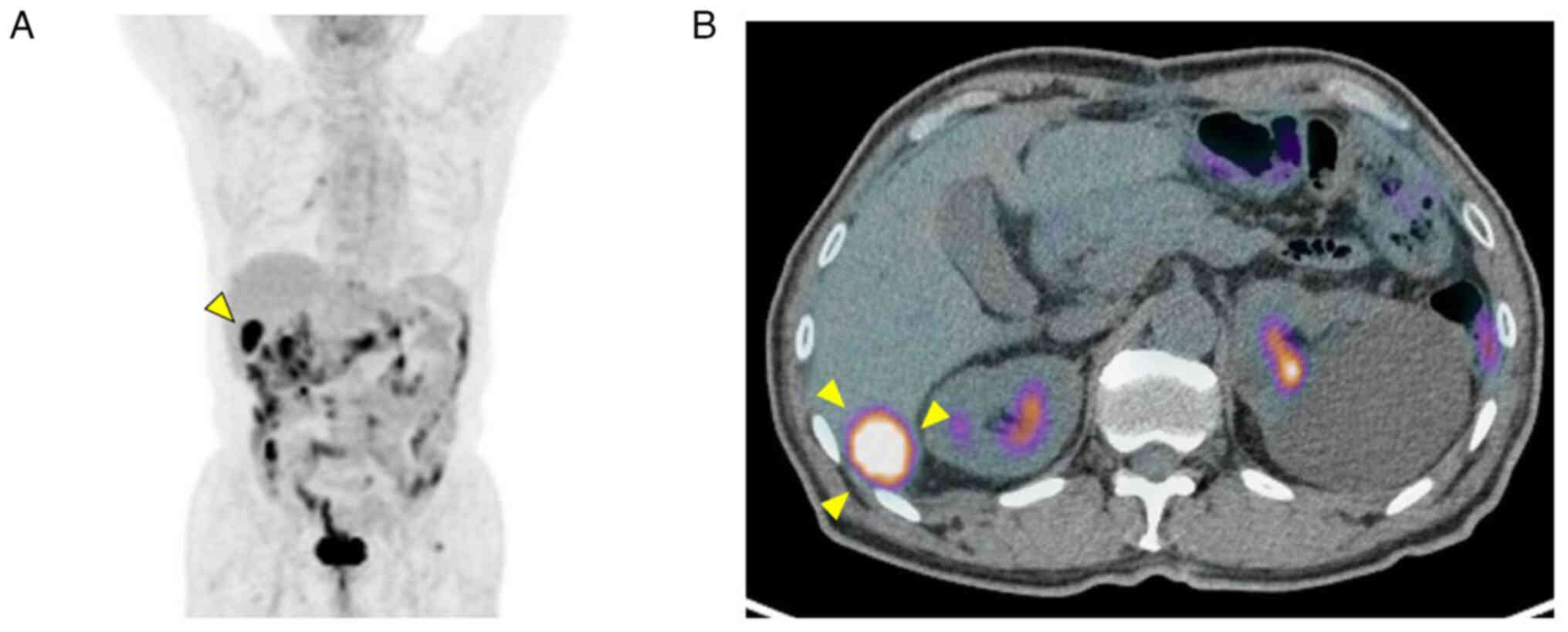
Contrast-enhanced computed tomography (CT) revealed metastasis to
the posterior segment of the liver and
18F-fluorodeoxyglucose positron emission tomography
indicated that the LM was a single recurrent focus (Fig. 3).
Systemic chemotherapy was started on the basis of
the ‘watch-and-wait’ strategy (14). The patient underwent chemotherapy
with one cycle of the SP regimen (S-1/cisplatin) and six cycles of
the SOX regimen (S-1/oxaliplatin). Although serum AFP levels
steadily decreased with the chemotherapeutic cycles and no new
metastasis appeared during the treatment, the extent of shrinkage
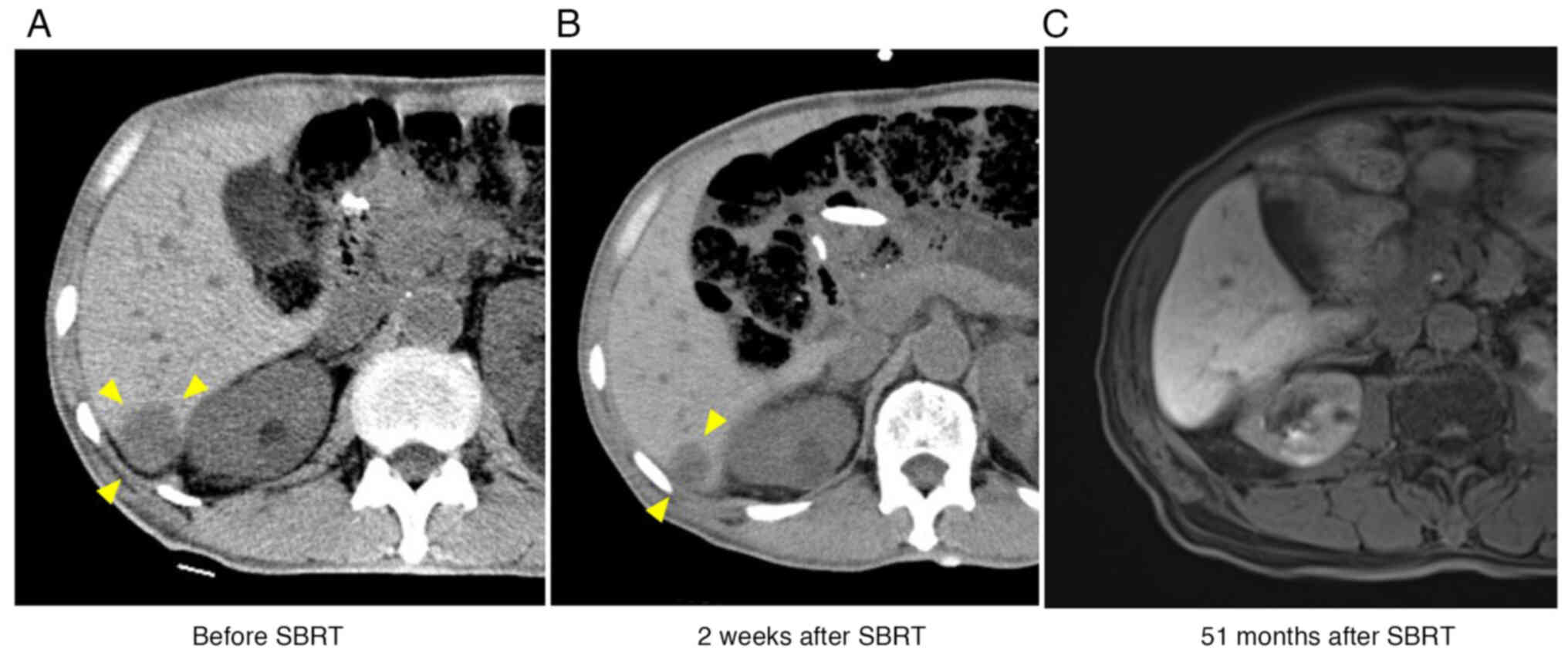
of the metastatic tumor was modest (Fig. 4A).
It was hypothesized that the introduction of local
therapy would enable high-quality disease control and prolong
patient survival. Treatment options for local control, including
surgery, radiofrequency ablation and transcatheter embolization,
were thus proposed. The patient finally selected radiotherapy
because he preferred minimally invasive therapy and underwent SBRT
(dose covering 90% of the planning target volume, 48 Gy; maximal
point dose, 61.3 Gy; in four fractions) for LM on an outpatient
basis.
CT revealed a significant response two weeks after
the completion of SBRT (Fig. 4B).
Magnetic resonance imaging demonstrated that the metastasis
developed to radiological CR two years later and sustained CR
thereafter (Fig. 4C). Serum AFP
levels also continued to decrease and were normalized seven months
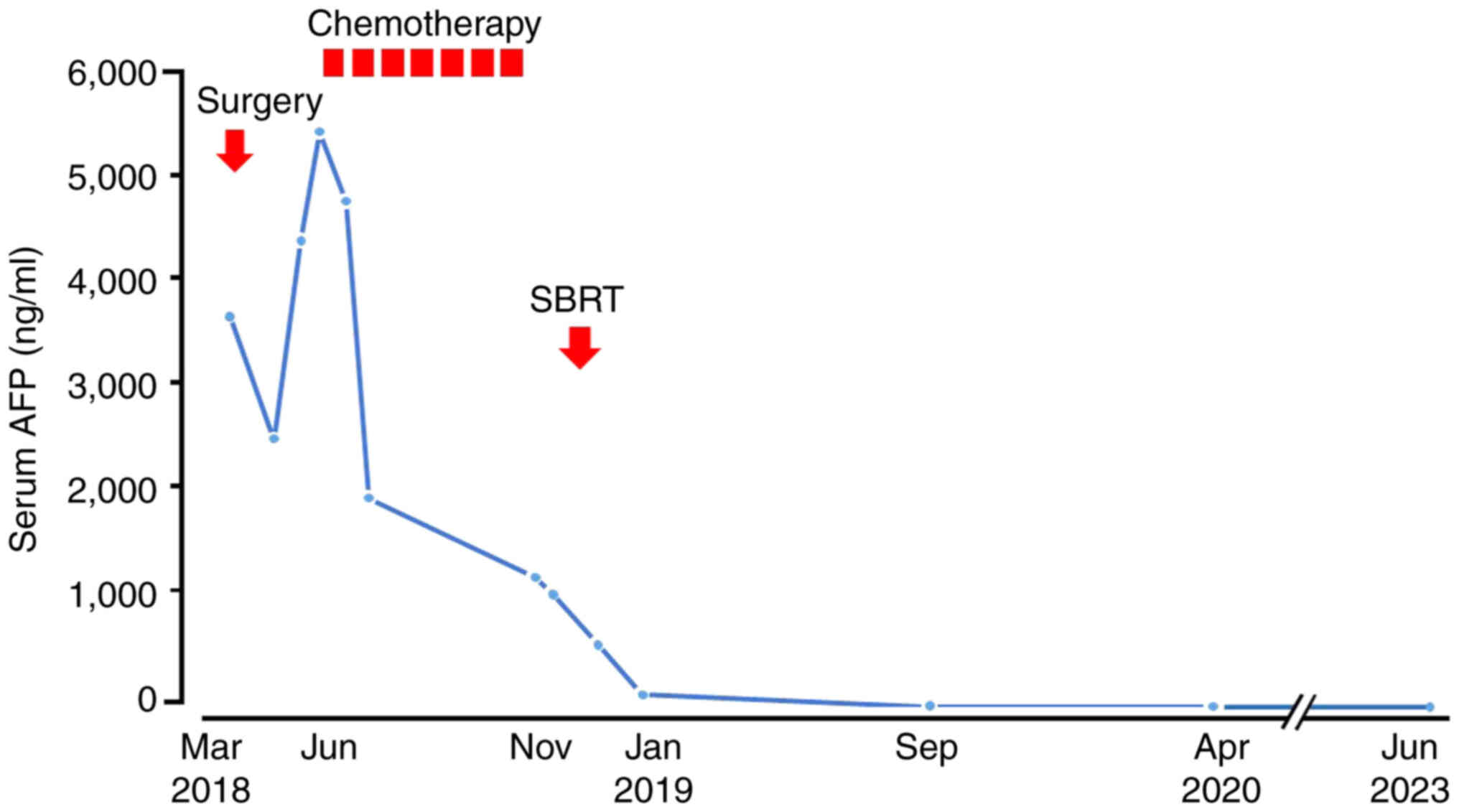
after the start of SBRT. The time course of serum AFP level changes
is shown in Fig. 5. The patient has
been followed up thereafter and is surviving with no evidence of
recurrence 62 months after the diagnosis of LM.
Literature review
To select studies addressing radiotherapy for AFPGC,
a literature search was performed using the PubMed database,
designating ‘alpha-fetoprotein/AFP’, ‘esophagogastric
cancer/stomach cancer’ and ‘radiotherapy/radiation’ as the key
words. The search was not limited to LM because it focused on the
efficacy of radiotherapy for AFPGC. The search provided 33 reports
that were published in English between January 1, 1970 and April
30, 2023; however, careful examination by three researchers (TK, YM
and TT) revealed that only two reports mentioned radiotherapy for
AFPGC and were in accordance with the research purpose of the
present study. One was a case report of a patient with AFPGC in
whom hilar lymph node recurrence showed CR after external beam
radiotherapy (10). The other was a
report of a patient with AFP-producing Siewert type I esophageal
adenocarcinoma (15). The patient
underwent proton beam therapy for the primary tumor and the
metastases (paracardial lymph nodes and LM). In this case, although
new lesions appeared immediately after a significant response, all
tumors including the new lesions finally showed CR with multimodal
therapy. A summary of the clinicopathological characteristics of
the previously reported cases in comparison with the present case
is presented in Table I.
 | Table I.Summary of reported cases of
radiotherapy for α-fetoprotein-producing esophagogastric
adenocarcinoma. |
Table I.
Summary of reported cases of
radiotherapy for α-fetoprotein-producing esophagogastric
adenocarcinoma.
| Author, year | Age at diagnosis,
years | Sex | Primary | Target | Radiotherapy | Total dose, Gy | Concurrent or
post-radiation chemotherapy | Response | Survival after
initial treatment, months | (Refs.) |
|---|
| Asahi, 2016 | 60 | M | Stomach | Hilar lymph node
metastasis | EBRT | 60 | Yes | CRa | 102 | (10) |
| Miyazaki, 2018 | 50 | M | Lower esophagus | Primary tumor,
paracardial lymph node metastasis and liver metastases | PBT | 72.6 | Yes | NE | 50 | (15) |
| Current case | 76 | M | Stomach | Liver metastasis | SBRT | 48 | No | CR | 62 | - |
Discussion
The present study reported on the case of a patient
with AFPGC who has been surviving for five years following SBRT for
LM. Regardless of the number of metastatic nodules, chemotherapy is
the standard treatment for LM from GC (16) because LM is considered one of the
clinical presentations resulting from the systemic dissemination of
cancer cells and numerous patients with GC and LM are presumed to
have subclinical minute metastases and to be at significant risk of
a second recurrence.
Kodera et al (17) published a literature review on
hepatectomy for LM from GC to clarify the efficacy of the surgery.
They found 17 evaluable studies on this topic and comprehensively
analyzed the survival of 515 eligible patients. Although the
reported median survival widely varied from 9 to 38 months among
the studies, 97 patients survived for five years or longer: The
five-year overall survival was 18.8%. On the basis of these
findings, the authors concluded that hepatectomy may be one of the
treatment options for LM from GC in selected patients and to be
considered particularly in solitary metastasis because 61% of
patients included in their study had a single LM.
Shirasu et al (18) published a retrospective study
addressing the timing of surgery for LM from GC. In that study, 24
patients with GC and liver oligometastasis (two or three
metastases) who underwent treatment for LM were enrolled and
divided into the hepatectomy group (n=9) and the chemotherapy group
(n=15) on the basis of the initial treatment for LM. The median
overall survival of the hepatectomy group was 24 months, being
shorter than that of the chemotherapy group (38 months). They also
found that the chemotherapy group included three patients who
underwent conversion hepatectomy (hepatectomy following initial
chemotherapy) and the survival of the conversion hepatectomy
patients was excellent, with all three surviving in the follow-up
period.
The abovementioned studies provide clinically
important points regarding the treatment decision-making for LM
from GC, i.e., chemotherapy is recommended in general as an initial
treatment for LM from GC, and hepatectomy may be considered for
selected patients, particularly those presenting with single or
oligo liver metastasis who show a significant response following
the preceding chemotherapy.
In the present case, SBRT was selected as an
additional local therapy instead of hepatectomy. This choice was
based on the patient's preference for low invasiveness. Clinical
evidence of the efficacy of radiotherapy for LM is much less than
that of surgery. In a literature review performed as part of the
present study, no articles focusing on LM from GC were found.
However, several pivotal studies have given us a hint that SBRT may
be a rational therapy for patients with LM. A multi-institutional
phase I/II study from the US (19)
showed the safety and effectiveness of high-dose SBRT (60 Gy) in 47
patients with liver oligometastasis; a serious adverse event was
reported in only one patient, and the two-year in-field control
rate was as high as 92%. Furthermore, there was only one randomized
phase II study that addressed the impact of SBRT on the survival of
patients with oligometastatic cancer (20). Overall survival, which was set as
the primary endpoint, was compared between the palliative radiation
group and the SBRT with the radical doses (30–60 Gy) group in that
study. The SBRT group showed longer overall survival than the
palliative radiation group (41 vs. 28 months, median survival
time), although the difference was not statistically significant.
The abovementioned findings have demonstrated that SBRT has high
local control ability for LM, suggesting that the high-level local
disease control by SBRT may contribute to prolonging survival even
in patients with LM.
Although SBRT resulted in CR in the present case, it
remains elusive whether the high efficacy of SBRT in this patient
with AFPGC can be generalized in LM from common types of GCs.
Radiosensitivity is less clinically important in SBRT than in
conventional radiotherapy because SBRT is a type of radioablative
therapy that uses high-dose irradiation. However, the
radiosensitivity of various tumors may have to be taken into
consideration when selecting radiotherapy as a cancer treatment
option. The current literature review revealed that data on the
radiosensitivity of AFPGC are scarce: There are only two papers
reporting the outcomes for patients with AFPGC who underwent
radiotherapy. Although publication bias should be considered, it is
noteworthy that the two patients showed a good response to
radiotherapy.
Germ cell tumors, including yolk sac tumors, are
representative malignancies that have high radiosensitivity. AFP is
a fetal protein that is produced in the yolk sac and fetal liver.
The fact that germ cell tumor and AFPGC share a common ontogenetic
protein expression allows for the hypothesis that AFPGC cells may
acquire radiosensitivity in the process of malignant
transformation. Indeed, Ushiku et al (21) have shown that spalt-like
transcription factor 4, an embryonic stem cell marker, is expressed
in AFPGCs, as well as fetal stomach and germ cell tumors, and have
claimed that AFPGC cells are characterized by the phenotype of
retrodifferentiation into fetal gut cells. The molecular mechanisms
underlying AFPGC occurrence remain elusive. More clinical data are
needed to determine whether AFPGC is radiosensitive.
In conclusion, the current study presented a case of
LM from AFPGC. The LM showed CR to SBRT and the patient has been
surviving for five years after the diagnosis of LM. Despite being
an anecdotal case, this case report offers clues that point to the
possibility of the application of SBRT to AFPGC and implies that
this tumor may be radiosensitive. Because AFPGC is rare, clinical
trials to clarify the efficacy of radiotherapy for this disease are
not feasible. Further accumulation of clinical data on a
case-by-case basis is necessary to confirm the efficacy of SBRT for
AFPGC.
Acknowledgements
The authors would like to thank Dr Tatsuya Miyazaki
from the Department of Surgery, Japanese Red Cross Maebashi
Hospital (Maebashi, Japan) for helpful advice on the literature
review. They also thank Ms. Sayoko Fujimoto from the Department of
Nursing, Sanjo General Hospital (Sanjo, Japan) for assistance in
clinical data management.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TK, YM and TT were the attending physicians and
cooperatively conducted the literature review. KN designed this
study and collected the data on this case report. TK and YM drafted
the manuscript. YM, KN and TT prepared the figures included in the
manuscript. MN was responsible for the pathological diagnosis. TK
and KN checked and confirmed the authenticity of the raw data. All
authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of the case report and all accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AFP
|
α-fetoprotein
|
|
AFPGC
|
AFP-producing GC
|
|
CR
|
complete response
|
|
CT
|
computed tomography
|
|
FDG
|
fluorodeoxyglucose
|
|
GC
|
gastric carcinoma
|
|
HCC
|
hepatocellular carcinoma
|
|
IHC
|
immunohistochemistry
|
|
LM
|
liver metastasis
|
|
PET
|
positron emission tomography
|
|
SBRT
|
stereotactic body radiotherapy
|
References
|
1
|
Bergstrand CG and Csar B: Demonstration of
a new protein fraction in serum from the human fetus. Scand J Clin
Lab Invest. 8:1741956. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Smith JB: Alpha-fetoprotein: Occurrence in
certain malignant diseases and review of clinical applications. Med
Clin North Am. 54:797–803. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kodama T, Kameya T, Hirota T, Shimosato Y,
Ohkura H, Mukojima T and Kitaoka H: Production of
alpha-fetoprotein, normal serum proteins, and human chorionic
gonadotropin in stomach cancer: Histologic and immunohistochemical
analyses of 35 cases. Cancer. 48:1647–1655. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ishikura H, Kirimoto K, Shamoto M,
Miyamoto Y, Yamagiwa H, Itoh T and Aizawa M: Hepatoid
adenocarcinomas of the stomach. An analysis of seven cases. Cancer.
58:119–126. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chang YC, Nagasue N, Kohno H, Taniura H,
Uchida M, Yamanoi A, Kimoto T and Nakamura T: Clinicopathologic
features and long-term results of alpha-fetoprotein-producing
gastric cancer. Am J Gastroenterol. 85:1480–1485. 1990.PubMed/NCBI
|
|
6
|
Adachi Y, Tsuchihashi J, Shiraishi N,
Yasuda K, Etoh T and Kitano S: AFP-producing gastric carcinoma:
Multivariate analysis of prognostic factors in 270 patients.
Oncology. 65:95–101. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Inoue M, Sano T, Kuchiba A, Taniguchi H,
Fukagawa T and Katai H: Long-term results of gastrectomy for
alpha-fetoprotein-producing gastric cancer. Br J Surgery.
97:1056–1061. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hirajima S, Komatsu S, Ichikawa D, Kubota
T, Okamoto K, Shiozaki A, Fujiwara H, Konishi H, Ikoma H and Otsuji
E: Liver metastasis is the only independent prognostic factor in
AFP-producing gastric cancer. World J Gastroenterol. 19:6055–6061.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tsurumachi T, Yamamoto H, Watanabe K,
Honda I, Watanabe S, Yamada S, Jingu K, Satomi D and Fujita M:
Resection of liver metastasis from alpha-fetoprotein-producing
early gastric cancer: Report of a case. Surg Today. 27:563–566.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Asahi Y, Kamiyama T, Homma S, Hatanaka KC,
Yokoo H, Nakagawa T, Kamachi H, Nakanishi K, Tahara M, Kakisaka T,
et al: Resection of liver metastasis derived from
alpha-fetoprotein-producing gastric cancer-report of 4 cases. Int
Canc Conf J. 5:98–103. 2016. View Article : Google Scholar
|
|
11
|
Benson AB, D'Angelica MI, Abbott DE, Anaya
DA, Anders R, Are C, Bachini M, Borad M, Brown D, Burgoyne A, et
al: Hepatobiliary cancers, version 2.2021, NCCN clinical practice
guidelines in oncology. J Natl Compr Canc Netw. 19:541–565. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kimura T, Fujiwara T, Kameoka T, Adachi Y
and Kariya S: The current role of stereotactic body radiation
therapy (SBRT) in hepatocellular carcinoma (HCC). Cancers (Basel).
14:43832022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Japanese Gastric Cancer Association, .
Japanese Classification of Gastric Carcinoma-2nd English Edition.
Gastric Cancer. 1:10–24. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fernandez LM, São Julião GP, Figueiredo
NL, Beets GL, van der Valk MJM, Bahadoer RR, Hilling DE,
Meershoek-Klein Kranenbarg E, Roodvoets AGH, Renehan AG, et al:
Conditional recurrence-free survival of clinical complete
responders managed by watch and wait after neoadjuvant
chemoradiotherapy for rectal cancer in the International Watch
& Wait Database: A retrospective, international, multicentre
registry study. Lancet Oncol. 22:43–45. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Miyazaki T, Sohda M, Sakai M, Kumakura Y,
Yoshida T, Kuriyama K, Yokobori T, Miyazaki M, Hirato J, Okumura T,
et al: Multimodality therapy including proton beam therapy for AFP
producing esophageal cancer with multiple liver metastases. Intern
Med. 57:2333–2339. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ajani JA, D'Amico TA, Bentrem DJ, Chao J,
Cooke D, Corvera C, Das P, Enzinger PC, Enzler T, Fanta P, et al:
Gastric cancer, version 2.2022, NCCN clinical practice guidelines
in oncology. J Natl Compr Canc Netw. 20:167–192. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kodera Y, Fujitani K, Fukushima N, Ito S,
Muro K, Ohashi N, Yoshikawa T, Kobayashi D, Tanaka C and Fujiwara
M: Surgical resection of hepatic metastasis from gastric cancer: A
review and new recommendation in the Japanese gastric cancer
treatment guidelines. Gastric Cancer. 17:206–212. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shirasu H, Tsushima T, Kawahira M, Kawai
S, Kawakami T, Kito Y, Yoshida Y, Hamauchi S, Todaka A, Yokota T,
et al: Role of hepatectomy in gastric cancer with multiple
liver-limited metastases. Gastric Cancer. 21:338–344. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rusthoven KE, Kavanagh BD, Cardenes H,
Stieber VW, Burri SH, Feigenberg SJ, Chidel MA, Pugh TJ, Franklin
W, Kane M, et al: Multi-institutional phase I/II trial of
stereotactic body radiation therapy for liver metastases. J Clin
Oncol. 27:1572–1578. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Palma DA, Olson R, Harrow S, Gaede S,
Louie AV, Haasbeek C, Mulroy L, Lock M, Rodrigues GB, Yaremko BP,
et al: Stereotactic ablative radiotherapy versus standard of care
palliative treatment in patients with oligometastatic cancers
(SABR-COMET): a randomised, phase 2, open-label trial. Lancet.
393:2051–2058. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ushiku T, Shinozaki A, Shibahara J,
Iwasaki Y, Tateishi Y, Funata N and Fukayama M: SALL4 represents
fetal gut differentiation of gastric cancer, and is diagnostically
useful in distinguishing hepatoid gastric carcinoma from
hepatocellular carcinoma. Am J Surg Pathol. 34:533–540. 2010.
View Article : Google Scholar : PubMed/NCBI
|