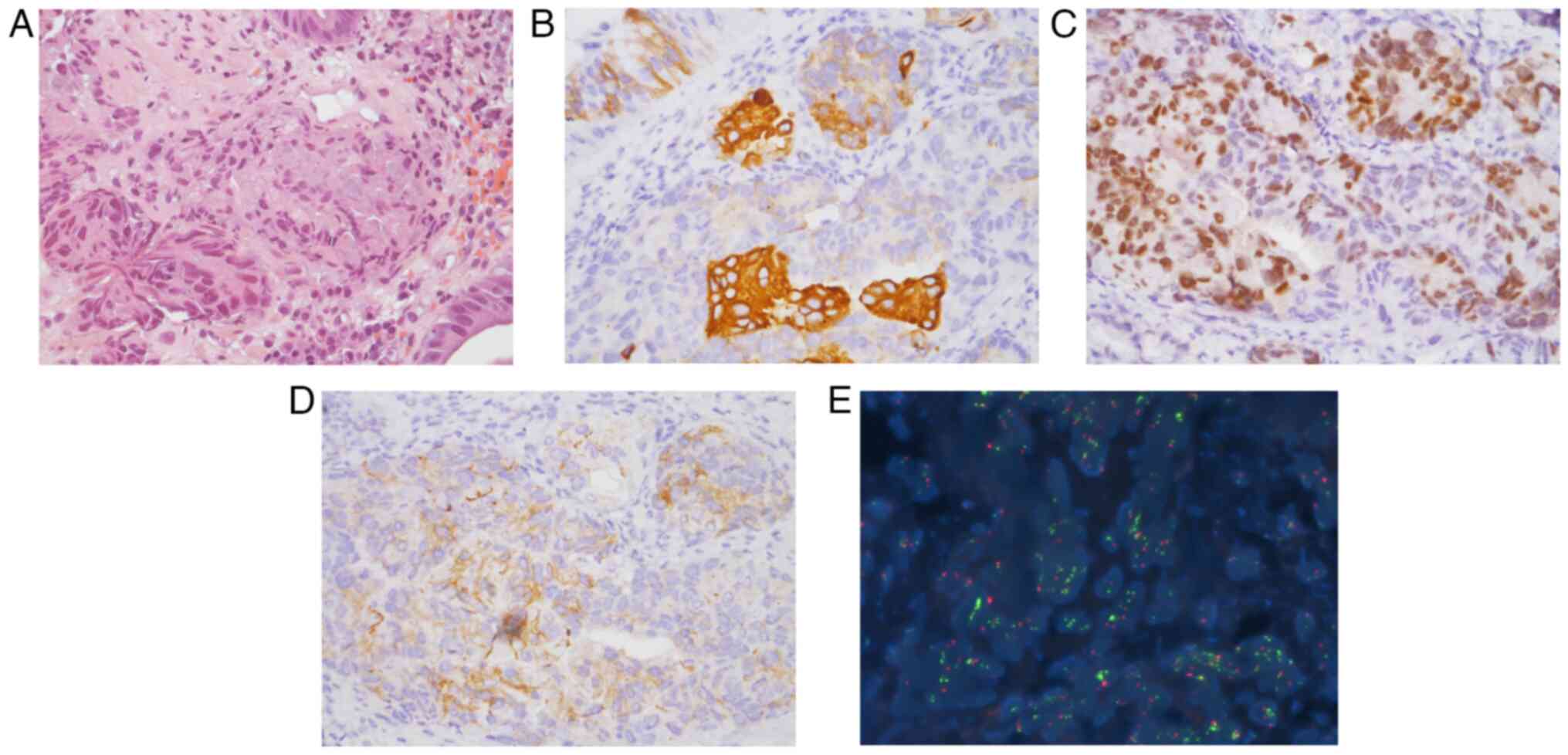
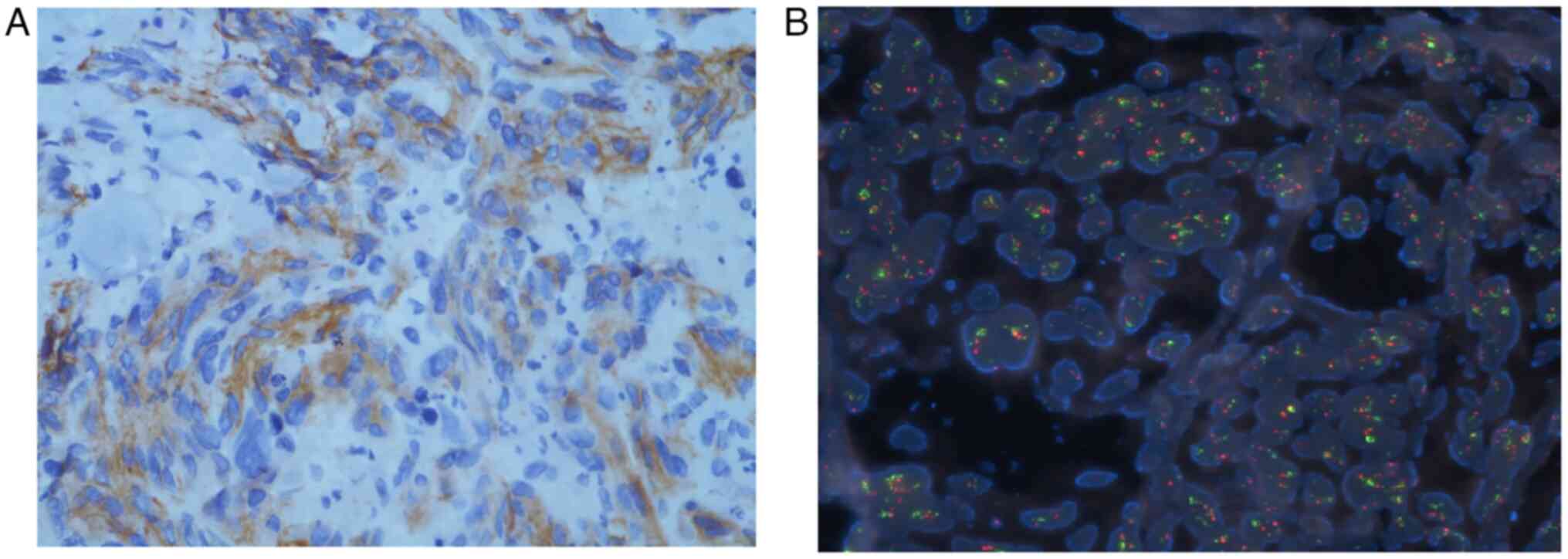
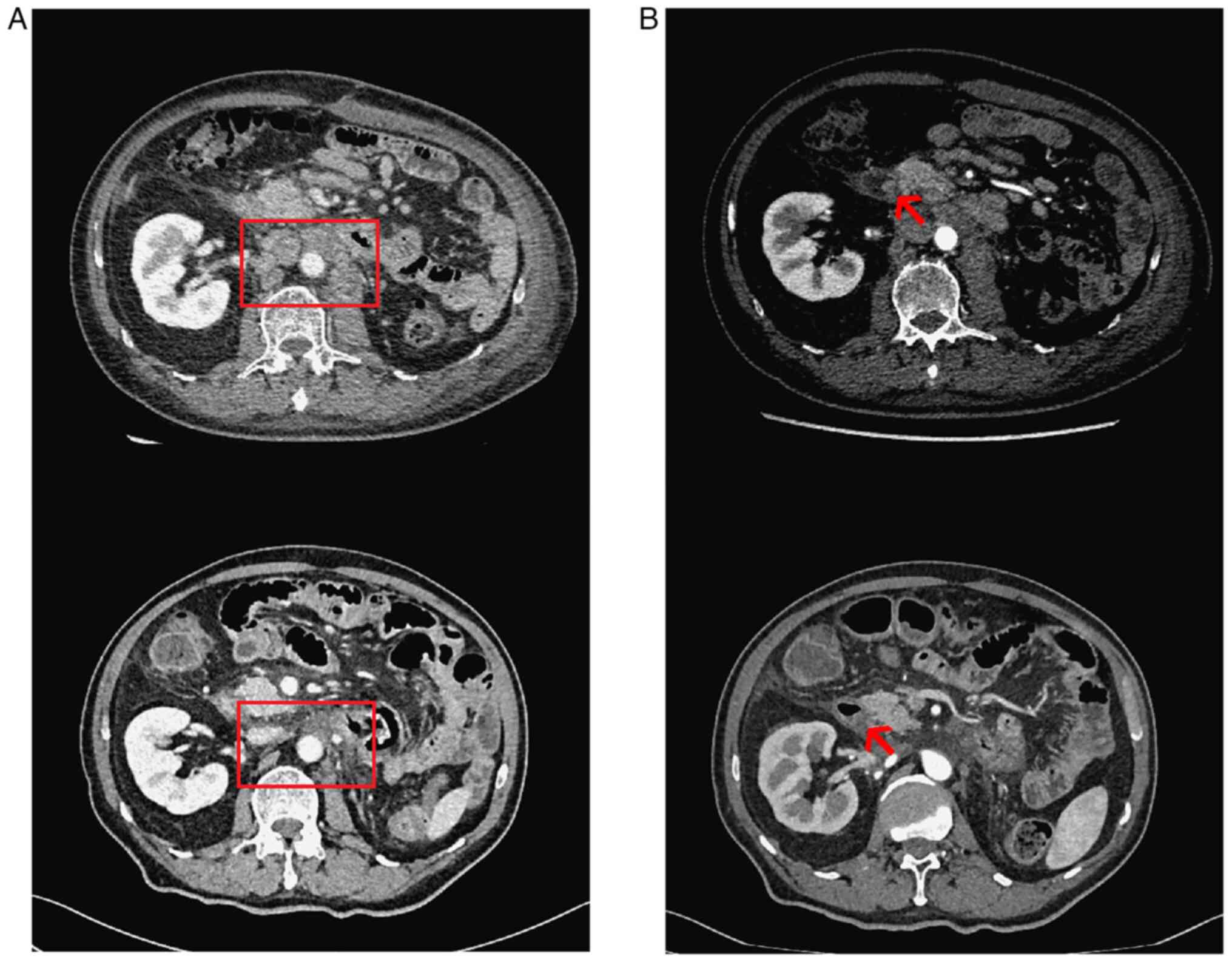
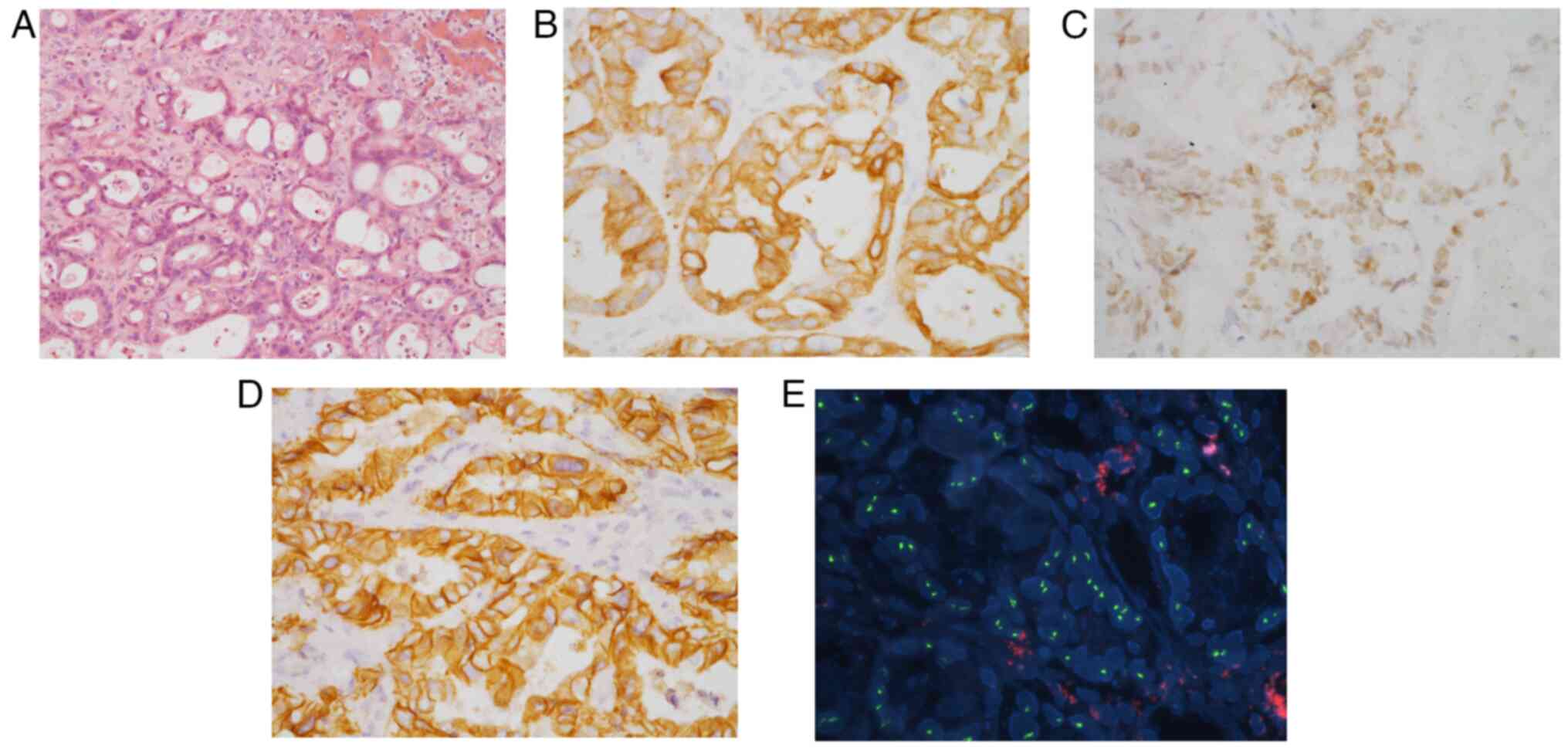
|
1
|
Gay G and Delvaux M: Small-bowel
endoscopy. Endoscopy. 40:140–146. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen WG, Shan GD, Zhang H, Li L, Yue M,
Xiang Z, Cheng Y, Wu CJ, Fang Y and Chen LH: Double-balloon
enteroscopy in small bowel tumors: A Chinese single-center study.
World J Gastroenterol. 19:3665–3671. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou YW, Xia RL, Chen YY, Ma XL and Liu
JY: Clinical features, treatment, and prognosis of different
histological types of primary small bowel adenocarcinoma: A
propensity score matching analysis based on the SEER database. Eur
J Surg Oncol. 47:2108–2118. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Halfdanarson TR, McWilliams RR, Donohue JH
and Quevedo JF: A single-institution experience with 491 cases of
small bowel adenocarcinoma. Am J Surg. 199:797–803. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schrock AB, Devoe CE, McWilliams R, Sun J,
Aparicio T, Stephens PJ, Ross JS, Wilson R, Miller VA, Ali SM and
Overman MJ: Genomic profiling of small-bowel adenocarcinoma. JAMA
Oncol. 3:1546–1553. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oh DY and Bang YJ: HER2-targeted
therapies-a role beyond breast cancer. Nat Rev Clin Oncol.
17:33–48. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): A phase 3,
open-label, randomised controlled trial. Lancet. 376:687–697. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sartore-Bianchi A, Trusolino L, Martino C,
Bencardino K, Lonardi S, Bergamo F, Zagonel V, Leone F, Depetris I,
Martinelli E, et al: Dual-targeted therapy with trastuzumab and
lapatinib in treatment-refractory, KRAS codon 12/13 wild-type,
HER2-positive metastatic colorectal cancer (HERACLES): A
proof-of-concept, multicentre, open-label, phase 2 trial. Lancet
Oncol. 17:738–746. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
National Institutes of Health, National
Cancer Institute, . Common Terminology Criteria for Adverse Events
(CTCAE) v 5.0. 2017.https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5×7.pdfDecember
7–2023
|
|
11
|
Amin MB, Edge SB, Greene FL, Byrd DR,
Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR,
Sullivan DC, et al: AJCC Cancer Staging Manual. 8th ed. Springer;
New York, NY: 2017
|
|
12
|
Harari D and Yarden Y: Molecular
mechanisms underlying ErbB2/HER2 action in breast cancer. Oncogene.
19:6102–6114. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG), . Trastuzumab for early-stage,
HER2-positive breast cancer: A meta-analysis of 13 864 women in
seven randomised trials. Lancet Oncol. 22:1139–1150. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang L, Li W, Lu Z, Lu M, Zhou J, Peng Z,
Zhang X, Wang X, Shen L and Li J: Clinicopathological features of
HER2 positive metastatic colorectal cancer and survival analysis of
anti-HER2 treatment. BMC Cancer. 22:3552022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Richman SD, Southward K, Chambers P, Cross
D, Barrett J, Hemmings G, Taylor M, Wood H, Hutchins G, Foster JM,
et al: HER2 overexpression and amplification as a potential
therapeutic target in colorectal cancer: Analysis of 3256 patients
enrolled in the QUASAR, FOCUS and PICCOLO colorectal cancer trials.
J Pathol. 238:562–570. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang W, Chen Y, Chang W, Ren L, Tang W,
Zheng P, Wu Q, Liu T, Liu Y, Wei Y and Xu J: HER2 positivity as a
biomarker for poor prognosis and unresponsiveness to anti-EGFR
therapy in colorectal cancer. J Cancer Res Clin Oncol.
148:993–1002. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ni S, Wang X, Chang J, Sun H, Weng W, Wang
X, Tan C, Zhang M, Wang L, Huang Z, et al: Human epidermal growth
factor receptor 2 overexpression and amplification in patients with
colorectal cancer: A large-scale retrospective study in Chinese
population. Front Oncol. 12:8427872022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Meric-Bernstam F, Hurwitz H, Raghav KPS,
McWilliams RR, Fakih M, VanderWalde A, Swanton C, Kurzrock R,
Burris H, Sweeney C, et al: Pertuzumab plus trastuzumab for
HER2-amplified metastatic colorectal cancer (MyPathway): An updated
report from a multicentre, open-label, phase 2a, multiple basket
study. Lancet Oncol. 20:518–530. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu T, Wang X, Xin Y, Wang Z, Gong J, Zhang
X, Li Y, Ji C, Sun Y, Zhao F, et al: Trastuzumab combined with
irinotecan in patients with HER2-positive metastatic colorectal
cancer: A phase II single-arm study and exploratory biomarker
analysis. Cancer Res Treat. 55:626–635. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pedersen KS, Foster NR, Overman MJ, Boland
PM, Kim SS, Arrambide KA, Jaszewski BL, Bekaii-Saab T, Graham RP,
Welch J, et al: ZEBRA: A multicenter phase II study of
pembrolizumab in patients with advanced small-bowel adenocarcinoma.
Clin Cancer Res. 27:3641–3648. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pan H, Cheng H, Wang H, Pan H, Cheng H,
Wang H, Ge W, Yuan M, Jiang S, Wan X, et al: Molecular profiling
and identification of prognostic factors in Chinese patients with
small bowel adenocarcinoma. Cancer Sci. 112:4758–4771. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Laforest A, Aparicio T, Zaanan A, Silva
FP, Didelot A, Desbeaux A, Le Corre D, Benhaim L, Pallier K, Aust
D, et al: ERBB2 gene as a potential therapeutic target in small
bowel adenocarcinoma. Eur J Cancer. 50:1740–1746. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hamad A, Singhi AD, Bahary N, McGrath K,
Amarin R, Zeh HJ and Zureikat AH: Neoadjuvant treatment with
trastuzumab and FOLFOX induces a complete pathologic response in a
metastatic ERBB2 (HER2)-amplified duodenal cancer. J Natl Compr
Canc Netw. 15:983–988. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Z, Li W, Wei Y, An L, Su S, Xi C,
Wang K, Hong D and Shi Y: A HER2-mutant patient with late-stage
duodenal adenocarcinoma benefited from anti-HER2 therapy and PD-1
inhibition: A case report. J Gastrointest Oncol. 12:1939–1943.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nishikawa Y, Hoshino N, Horimatsu T,
Funakoshi T, Hida K, Sakai Y, Muto M and Nakayama T: Chemotherapy
for patients with unresectable or metastatic small bowel
adenocarcinoma: A systematic review. Int J Clin Oncol.
25:1441–1449. 2020. View Article : Google Scholar : PubMed/NCBI
|