Introduction
A collision tumor consists of two independent tumors of different cell lineages that occur very close to each other in the same body area or organ; however, a clear boundary between the two tumor types is maintained (1,2). The pathogenesis of collision tumors has not been thoroughly investigated and remains uncertain. Three hypotheses have been proposed regarding their development: i) simultaneous proliferation of two distinct cell lines, ii) a common precursor stem cell that differentiates into other cell types that keep their own individual properties and iii) malignant changes in the local microenvironment of the original tumor that promote the growth of a tumor nearby (3). Collision tumors in the liver are also infrequent with an incidence of only 0.1–1% (4), and most of them are asymptomatic or only non-specific symptoms such as abdominal pain and distension (5). The present study reports a case of a liver collision tumor containing HCC and cavernous hemangioma (CH), including an analysis of the clinical symptoms, computed tomography (CT) imaging and histopathological examination findings.
Case report
Case presentation
A routine physical examination of a 71-year-old male patient in March 2023 at Sunshine Union Hospital (Weifang, China) and the ultrasound showed the presence of a liver tumor, and the patient was diagnosed with liver cancer. The patient reported no specific symptoms, such as yellowed skin or sclera, headache or abdominal pain, but had hepatitis B infection on presentation. Laboratory tests demonstrated that the patient's albumin/globulin ratio was low (1.03; reference values: 1.2–2.4) and alkaline phosphatase level was high (183.10 U/l; reference values: 45–125 U/l).
A subsequent CT examination (Fig. 1) demonstrated that in segment IV of the left liver lobe, a slightly low-density circular lesion was observed, with local liver surface bulging. The density of the lesion was uneven, with areas of equal density visible. The boundary between the lesion and the surrounding liver tissue was not clear. In the arterial phase of the contrast-enhanced scan, a ring-enhancing pattern was evident in the lesion, with patchy enhancing areas seen in the periphery of the lesion. No enhancement was observed in the central low-density area of the lesion. The overall size of the lesion was ~4.0×4.2×3.5 cm. In the portal vein phase, the lesion still showed obvious ring enhancement and the enhancing component at the periphery of the lesion was larger compared to the arterial phase. No enhancement was observed in the central low-density area. In the delayed phase, the enhanced area of the lesion further expanded compared with the portal vein phase and no enhancement was observed in the central low-density area. In the lower portion of the lesion, a nodular enhancing lesion was visible in the arterial phase, with a clear demarcation from the main lesion. The size of the enhancing nodule was ~2.4×2.2×1.3 cm. Small non-enhancing areas were observed within the enhancing nodule, with unclear margins. The degree of enhancement in the nodule is significantly reduced in the portal vein phase, exhibiting a ‘fast-in and fast-out’ enhancement pattern. The patient was operated on to remove the tumor 5 weeks days after admission and during the operation the capsule of the tumor was found to be intact, and the boundary with the surrounding tissues was evident. The tumor was completely removed and sent for pathological analysis.
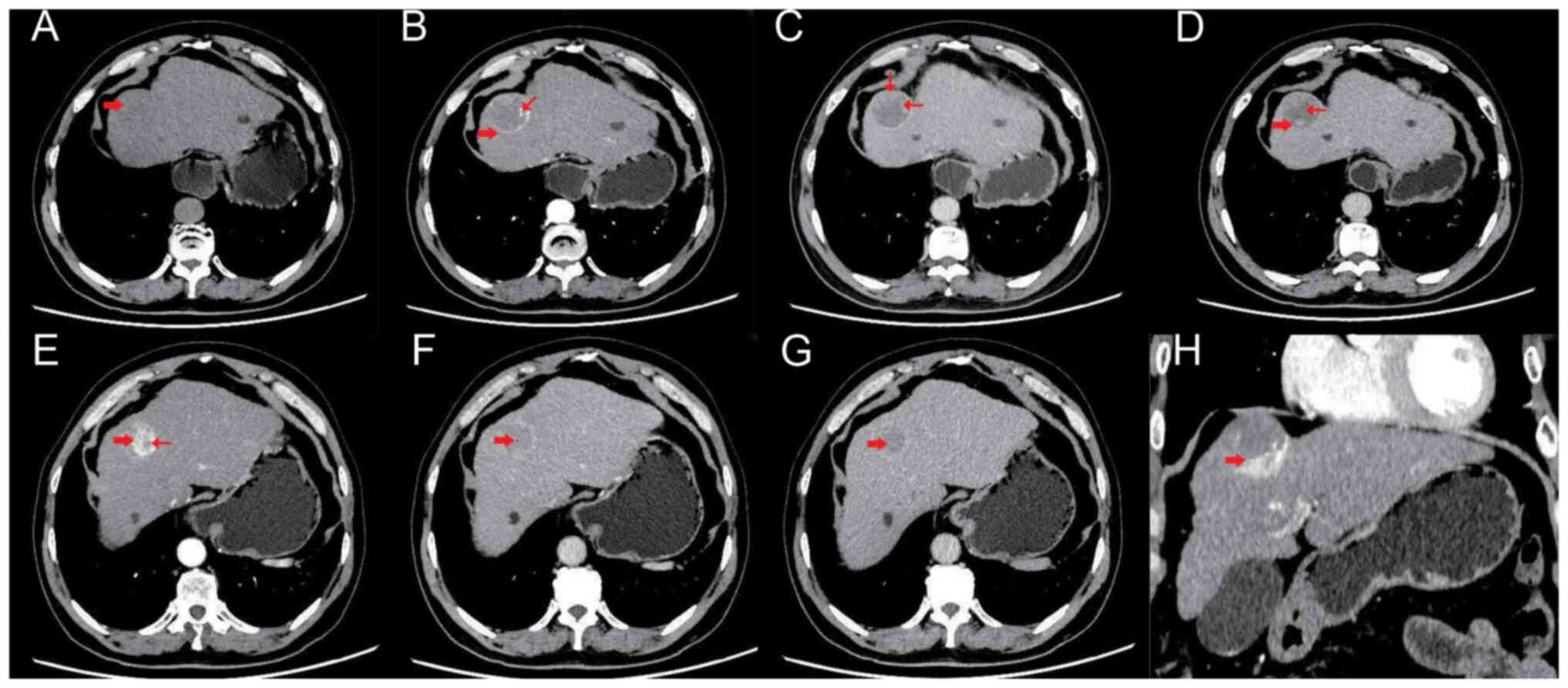 |
Figure 1.
CT scan images of the patient. (A) Segment IV of the left lobe of the liver showed a slightly low-density shadow (thick arrow), uneven internal density and an unclear edge of the lesion. (B) In the arterial phase, circular significant enhancement can be observed in the lesion (thick arrow), while there are focal and patchy areas of obvious enhancement at the periphery of the lesion (thin arrow). No enhancement is observed in the central low-density area of the lesion. (C) In the portal venous phase, evident circular enhancement can still be observed in the lesion, and the solid enhancing component at the periphery of the lesion shows an increased range compared to the arterial phase (thin arrow), but no enhancement was found in the central low-density area. (D) In the delayed phase, the posterior part (thick arrow) of the lesion was further filled than the portal venous phase and the central low-density area was not enhanced (thin arrow). (E) The lower-left part of the lesion showed evident nodular enhancement in the arterial phase (thick arrow) and small non-enhanced areas with unclear edges inside the enhancing nodule (thin arrow), while the boundary between the enhanced nodules and the main lesion was clear. (F) The enhancement degree of the lower-left nodule in the portal venous phase was markedly reduced compared with the arterial phase (thick arrow), displaying a ‘fast- in and fast-out’ pattern. (G) The enhancement degree of the delayed phase of the lower-left nodule of the lesion was close to that in the portal venous phase (thick arrow). (H) The coronal view showed that the abnormally enhanced nodule in the left inferior arterial phase was clearly demarcated from the main lesion (thick arrow).
|
Pathological findings
Macroscopic examination
The tumor varied in color, mainly red or white, with a volume of 4×4×3 cm and a complete surface envelope. The tumor was divided into two parts: One part was red-black and the other was gray-white, while the border between the two parts was evident (Fig. 2).
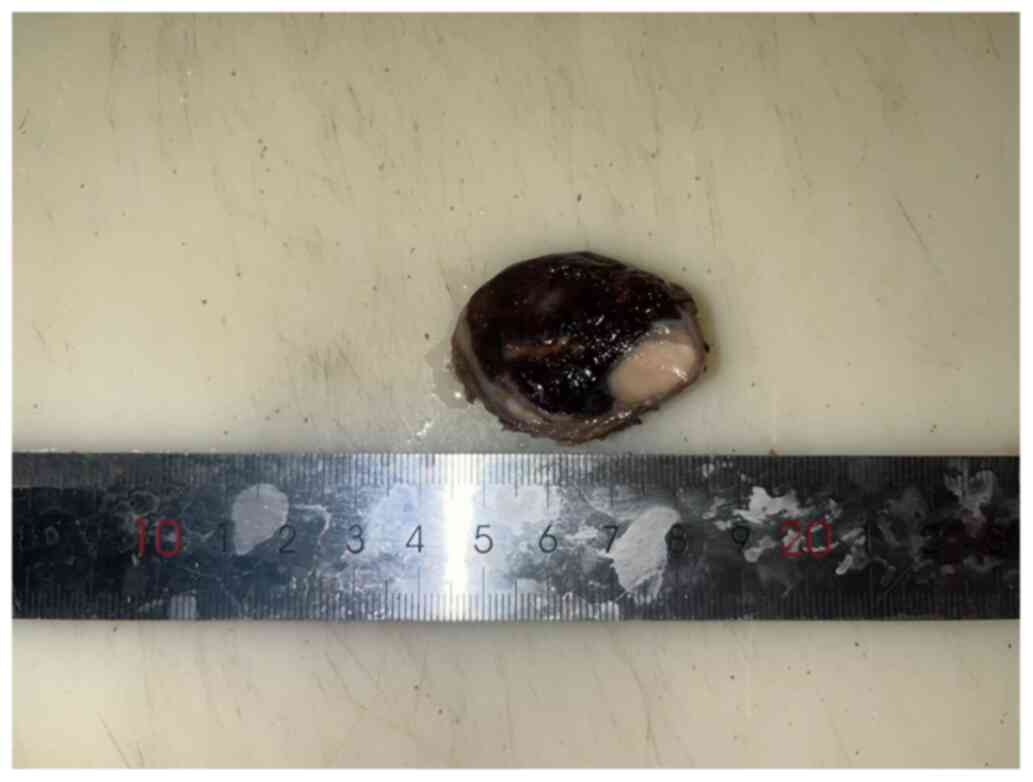 |
Figure 2.
Macroscopic tumor image. Tumor components exhibited clear boundaries and were wrapped together in a distinct capsule.
|
Microscopic observation
Hematoxylin and eosin (H&E) and immunohistochemical staining were examined using an Olympus BX53 light microscope (Olympus Corporation). Tumor specimens were fixed in 10% neutral formalin at room temperature for ~48 h, embedded in paraffin and cut into 4 µm thick sections for H&E staining (hematoxylin 7 min and eosin 4 min at room temperature). At low magnification (magnification, ×40, Fig. 3A), H&E staining demonstrated an intact tumor capsule and a ‘collision’ of HCC and CH, the HCC components demonstrated the common H&E staining pattern of HCC, while the CH components contained a large number of blood clots. The two components did not migrate or mix with each other, while a fibrous interval between the two tumor components was also observed. Cirrhotic nodules of different sizes were observed outside the capsule. At higher magnification (magnification, ×100, ×200, ×400, Fig. 3B-D), HCC cells were eosinophilic and had a similar size and shape with smaller volume, less cytoplasm, more nucleoplasm, an enlarged nucleus, round or oval shape, rough nuclear chromatin, small nucleoli and a mitotic image compared with healthy liver cells. The HCC had lost the cord-like structure of normal liver and was arranged in solid sheets with abundant capillaries and fine fibrous stroma, and large cell dysplasia was mixed with HCC in certain areas. These characteristics are consistent with moderately differentiated HCC. The lumen of the CH was dilated with different sizes and irregular shapes, and was lined with a single layer of flat endothelial cells without cell atypia and smooth muscle components in the wall. Some lumens were cystic and filled with red blood cells.
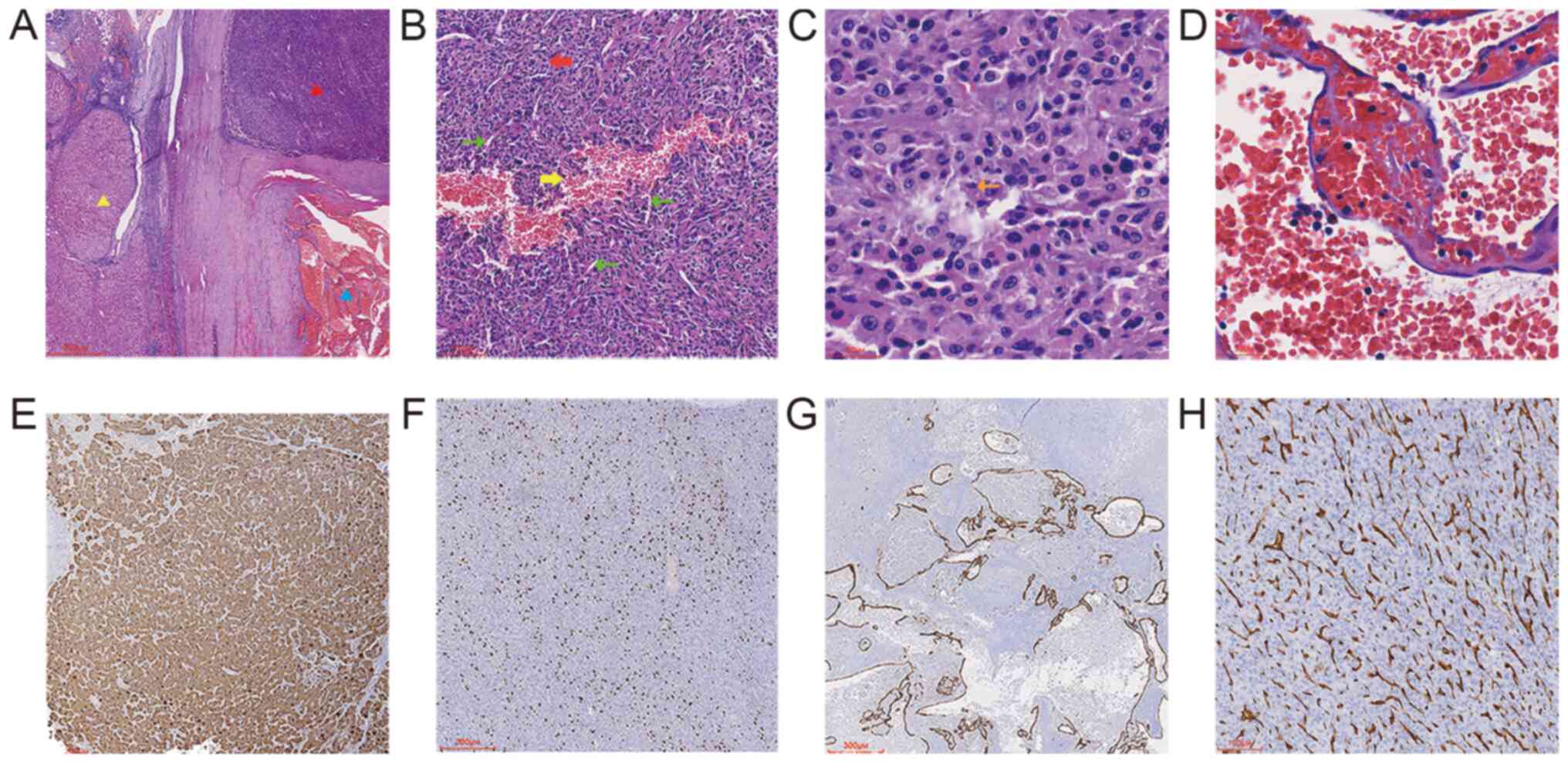 |
Figure 3.
Hematoxylin and eosin and immunohistochemical staining of the tumor. (A) HCC and CH are separated by fibrous tissue (red triangle, HCC; blue triangle, CH; yellow triangle, cirrhotic nodules). Magnification, ×20; scale bar, 600 µm. (B) Partial enlarged view of the tumor highlighting the HCC component. HCC cells lost their normal structure and were arranged in solid sheets (red arrow) with abundant capillaries (green arrow); the local bleeding area can be observed (yellow arrow). Magnification, ×100; scale bar, 100 µm. (C) High magnification image of HCC showing mitotic image (orange arrow). Magnification, ×400; scale bar, 30 µm.(D) High magnification image of CH showing no cell atypia. Magnification, ×400; scale bar, 30 µm. The HCC component exhibited expression of (E) hepatocyte-specific antigen and (F) Ki-67 (30%). (G) The endothelial cells of CH exhibited expression of CD34, and the lumen was dilated with different sizes and irregular shapes. Magnification, ×40; scale bar, 300 µm. (H) CD34 staining in the HCC component, indicating increased vascular density. Magnification, ×100; scale bar, 100 µm. HCC, hepatocellular carcinoma; CH, cavernous hemangioma.
|
Immunohistochemical staining (Fig. 3E-H) was performed overnight at 4°C using the following primary antibodies (prediluted by the manufacturer, Guangzhou LBP Medicine Science & Technology Co., Ltd.): Anti-hepatocyte-specific antigen (HSA; cat. no. IM150), anti-Ki-67 (cat. no. IR098), anti-CD34 (cat. no. IM034). For immunohistochemistry, tissue sections (3 µm) were fixed in 4% formalin at room temperature for 48 h before being embedded in paraffin. These sections were then rehydrated in a descending alcohol series (xylene, 100% ethanol, 95% ethanol, 85% ethanol, ethanol-free water) and underwent antigen retrieval using EDTA antigen retrieval treatment (EnVision FLEX Target Retrieval Solution, High pH; cat. no. K8000; Agilent Technologies, Inc.) in a microwave on high heat for 2 min, followed by incubation at room temperature for 8 min. Endogenous peroxidase activity was quenched with 3% hydrogen peroxide in methanol before incubation with primary antibodies. The secondary antibody was obtained from the EnVision FLEX/HRP (prediluted by the manufacturer; cat. no. K8000; Agilent Technologies, Inc.) and was used to treat sections at room temperature for 25 min. Subsequently, a chromogen detection reagent was applied (EnVision FLEX DAB+ Chromogen; cat. no. K8023 K8000, Agilent Technologies, Inc.). The IHC staining results demonstrated that the HCC tissue expressed HSA, Ki-67 (30%) and CD34 (showing high vascular density). CD34 expression was observed in CH, and partial blood vessel walls were discontinuous.
Pathological diagnosis
The patient was diagnosed with a collision tumor consisting of HCC (moderate differentiation) and CH.
Treatment and follow-up
The patient refused treatment with the targeted drug lenvatinib after the tumor removal operation and received support treatment (antiviral therapy for hepatitis B and anti-liver fibrosis treatment) and nutritional treatment (a high-quality protein diet, avoidance alcohol and overeating, and maintenance of regular bowel movements.). The patient underwent follow-up for 6 months (the last follow-up was conducted in October 2023) after surgery and no recurrence was observed.
Discussion
Liver collision tumors are rare, and the most common form of this tumor consists of HCC and neuroendocrine carcinoma (5–9). Given their rarity, there is currently no definitive conclusion regarding the process of their formation. It has previously been reported that the occurrence of collision tumors is a coincidence (5,10), whereas another study suggested that genetic mutations may contribute to their formation, although this hypothesis is still under debate (4). In previous reports, a number of patients with intrahepatic collision tumors were diagnosed with hepatitis B or C infection (6,11). Therefore, it could be suggested that biological factors, such as hepatitis B or C infection, may also be involved in the formation of intrahepatic collision tumors.
The collision tumor reported in the present study consisted of HCC and CH. HCC is the most common type of primary liver cancer, accounting for 75–85% of cases (12). The prevalence of HCC has been predicted to increase greatly in the next few decades, the number of new cases of liver cancer per year is predicted to increase by 55.0% between 2020 and 2040 with a possible 1.4 million people being diagnosed in 2040 (13). Liver CH is the most common type of benign vascular liver tumor (14). It is usually asymptomatic and is often found accidentally during imaging examinations. CH generally does not require any clinical treatment and patients undergo regular follow-up checks. Although HCC is the most common type of primary malignant tumor of the liver and CH is the most common type of benign liver tumor, HCC-CH collision tumors are observed more rarely than the majority of other, infrequent, types of liver collision tumors. To the best of our knowledge, based on a thorough literature search of PubMed (www.pubmed.gov; search terms: Liver collision or hepatocellular collision or hepatocellular carcinoma concomitant), the only previously published case report of an HCC-CH collision tumor in the liver was reported by Ge et al (10) (Table I).
 |
Table I.
Comparison of clinical information of two patients with a collision tumor consisting of HCC and CH.
|
Table I.
Comparison of clinical information of two patients with a collision tumor consisting of HCC and CH.
| |
Case report |
| |
|
| Patient characteristic |
Ge et al, 2015 (10) |
Present study |
| Sex |
Male |
Male |
| Age, years |
44 |
71 |
| Ethnicity |
Chinese |
Chinese |
| Tumor components |
HCC, CH and concomitant hepatic angiomyolipoma |
HCC and CH |
| Maximum tumor diameter, cm |
9 |
4 |
| Symptoms |
Asymptomatic |
Asymptomatic |
| Hepatitis diagnosis |
Hepatitis B |
Hepatitis B |
| Imaging diagnosis |
HCC |
HCC |
| Follow-up duration and outcome |
21 months and alive |
6 months and alive |
Both the patient from the present case report and the patient in the aforementioned study with an HCC-CH collision tumor had a history of hepatitis B infection. HCC may have numerous causes; however, chronic hepatitis B viral infection is currently reported to be the most common (15). In general, oncogenic viruses do not necessarily lead to the development of cancer; instead it is their interactions with host factors that leads to preneoplastic conditions and subsequently cancer through various mechanisms, such as dysregulation of the immune system (16). Another common point between the present study and the aforementioned study (10) is that both patients were from China, which has a high incidence of hepatitis B and C infections compared with other countries (17,18). Furthermore, neither patient had a record of clinical symptoms associated with the tumor, and both collision tumors were discovered incidentally during health examinations. Several patients with other types of liver collision tumors have not experienced any clinical symptoms (5–8,11,19). Early HCC is typically clinically asymptomatic, and small nodules (<2 cm) are difficult to characterize by radiologic examination (20). Therefore, it could be hypothesized that when hemangioma or other types of tumors collide with HCC, the total tumor volume increases and the tumors are easier to find, resulting in improved survival rates. However, this hypothesis requires verification in future studies.
When an imaging examination shows that there may be two tumor components, the possibility of a collision tumor and the performance of further examinations should be considered, even if the probability of a collision tumor is low. For example, contrast-enhanced CT can distinguish hemangioma from HCC (21). In the patient, the CT revealed another mass other than hepatocellular carcinoma, but the mass did not exhibit typical characteristics of liver hemangioma enhancement (marked enhancement). It only showed localized marked enhancement. However, differentiation can be made through enhanced CT: HCC shows overall significant enhancement in the arterial phase and a noticeable decrease in enhancement in the venous phase, which is a typical ‘fast-in and fast-out’ enhancement pattern. This is distinctly different from the hemangioma in terms of enhancement characteristics (localized persistent enhancement, with an increasing enhancement range) and degree (only showing marked enhancement in the area indicated by the thin arrow in Fig 1B).
The HCC components of the tumor from the present patient demonstrated the common H&E staining pattern of HCC, while the CH components contained a large number of blood clots. Moreover, IHC staining for CD34 in the CH components showed that the vascular wall was discontinuous. Due to the aforementioned finding and the existence of a large number of blood clots around the blood vessel wall, it was believed that the blood vessel was ruptured and the CH volume was enlarged by the squeezed blood (blood that is squeezed out from the site of vascular rupture). Without the outermost capsule of the tumor, the patient may have experienced severe liver bleeding or a liver rupture.
The patient in the present case report had two different tumor components that were wrapped together in the same capsule, but were separated by a distinct border. Such obvious collision tumors in the liver are rare. Due to the existence of the tumor capsule and despite the short follow-up period, it is believed that the patient will have a good prognosis.
The patient in the present case report did not receive postoperative adjuvant therapy. The patient in the study by Ge et al (10) was treated with transarterial chemoembolization after the operation and no recurrence or metastasis was found as of the publication date of the article. The efficacy of this treatment, which is often used for HCC (22), could suggest that the biological characteristics of an HCC-CH collision tumor may be similar to, or even the same as, those of pure liver cancer, but this requires further in-depth study.
In summary, the present case report described a rare case of a collision tumor composed of HCC and CH. A CT scan indicated the presence of a tumor wrapped by a capsule with distinct internal components. However, it can be difficult to comprehensively diagnose this disease by imaging examinations alone; therefore, accurate diagnosis by histopathology is also needed. The tumor was diagnosed using histopathological findings following complete resection. The tumor in the present case report was surrounded by a complete capsule, and complete separation of HCC and CH was observed. The successful course of treatment for HCC-CH collision tumors may be the same as, or similar to, the routine treatment course currently in use for HCC, but further research is needed to validate these treatments.
Acknowledgements
The authors would like to thank Professor Sen Li (Department of General Surgery, Sunshine Union Hospital, Weifang, China) for the meticulous operation on the patient and for providing the tissue.
Funding
The present study was supported by the Research Projects of Weifang Municipal Health Committee (grant no. WFWSJK-2022-239) and the Sunshine Union Hospital Research Project (grant no. 2022YGRH043).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
XY and BW designed and conceived the study, and revised the manuscript. XZ, JB, NS and WG performed the immunohistochemistry/histology examinations and/or CT scans and analyzed the data. SW wrote the manuscript and designed the present study. SW, XY and WG confirm the authenticity of all the raw data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of Sunshine Union Hospital (approval no. 2023-06-0015; Weifang, China). The scope of this approval includes protecting the rights of research participants, safeguarding privacy and confidentiality, as well as ensuring appropriate assessment and management of potential research risks.
Patient consent for publication
The patient provided written informed consent for the present case study to be published.
Competing interests
The authors declare that they have no competing interests.
Authors' information
Author e-mail addresses: SW, 840688955@qq.com; XZ, zhangxinxing-suh@sinosig.com; JB, 1798745462@qq.com; NS, sunnaiying-suh@sinosig.com; WG, guowenjun-suh@sinosig.com; BW, wangbaogui99@163.com; XY, atcom163@163.com.
References
|
1
|
Palomino MZ, Caicedo-Holguín I, Pardo S, Mera AT, Gómez AP and Zorrilla JO: Case report: Tumor collision in the colon, adenocarcinoma-lymphoma. Int J Surg Case Rep. 98:1075732022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Montealegre RA and Barrera JC: Combined mohs micrographic surgery in a collision tumor. Cureus. 14:e267472022.PubMed/NCBI
|
|
3
|
Choe AR, Shim KN, Lim J, Song EM, Tae CH, Jung SA and Jo MS: A collision tumor of the esophagus: Mixed squamous cell carcinoma and neuroendocrine carcinoma. Korean J Gastroenterol. 75:207–211. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yılmaz DB, Bayramoğlu Z, Ünay G, Ayık E, Başsorgun Cİ and Elpek GÖ: Incidental collision tumor of hepatocellular carcinoma and neuroendocrine carcinoma. J Clin Transl Hepatol. 6:339–344. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jeng KS, Huang CC, Chung CS and Chang CF: Liver collision tumor of primary hepatocellular carcinoma and neuroendocrine carcinoma: A rare case report. World J Clin Cases. 10:13129–13137. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Choi GH, Ann SY, Lee SI, Kim SB and Song IH: Collision tumor of hepatocellular carcinoma and neuroendocrine carcinoma involving the liver: Case report and review of the literature. World J Gastroenterol. 22:9229–9234. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nishino H, Hatano E, Seo S, Shibuya S, Anazawa T, Iida T, Masui T, Taura K, Haga H and Uemoto S: Histological features of mixed neuroendocrine carcinoma and hepatocellular carcinoma in the liver: A case report and literature review. Clin J Gastroenterol. 9:272–279. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nakano A, Hirabayashi K, Yamamuro H, Mashiko T, Masuoka Y, Yamamoto S, Ozawa S and Nakagohri T: Combined primary hepatic neuroendocrine carcinoma and hepatocellular carcinoma: Case report and literature review. World J Surg Oncol. 19:782021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Garcia MT, Bejarano PA, Yssa M, Buitrago E and Livingstone A: Tumor of the liver (hepatocellular and high grade neuroendocrine carcinoma): A case report and review of the literature. Virchows Arch. 449:376–381. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ge XW, Zeng HY, Su-Jie A, Du M, Ji Y, Tan YS, Hou YY and Xu JF: Hepatocellular carcinoma with concomitant hepatic angiomyolipoma and cavernous hemangioma in one patient. World J Gastroenterol. 21:3414–3419. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Noh BG, Seo HI, Park YM, Kim S, Hong SB and Lee SJ: Complete resection of large-cell neuroendocrine and hepatocellular carcinoma of the liver: A case report. World J Clin Cases. 10:8277–8283. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gao YX, Ning QQ, Yang PX, Guan YY, Liu PX, Liu ML, Qiao LX, Guo XH, Yang TW and Chen DX: Recent advances in recurrent hepatocellular carcinoma therapy. World J Hepatol. 15:460–476. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lazzaro A and Hartshorn KL: A comprehensive narrative review on the history, current landscape, and future directions of hepatocellular carcinoma (HCC) systemic therapy. Cancers (Basel). 15:25062023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fischer AK, Beckurts KTE, Büttner R and Drebber U: Giant cavernous hemangioma of the liver with satellite nodules: Aspects on tumour/tissue interface: A case report. World J Hepatol. 15:707–714. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kanda T, Goto T, Hirotsu Y, Moriyama M and Omata M: Molecular mechanisms driving progression of liver cirrhosis towards hepatocellular carcinoma in chronic hepatitis b and c infections: A Review. Int J Mol Sci. 20:13582019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Russo FP, Zanetto A, Pinto E, Battistella S, Penzo B, Burra P and Farinati F: Hepatocellular carcinoma in chronic viral hepatitis: Where do we stand? Int J Mol Sci. 23:5002022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang C and Cui F: Expanded screening for chronic hepatitis B virus infection in China. Lancet Glob Health. 10:e171–e172. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang J, Qi JL, Wang XX, Li XH, Jin R, Liu BY, Liu HX and Rao HY: The burden of hepatitis C virus in the world, China, India, and the United States from 1990 to 2019. Front Public Health. 11:10412012023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tamaoka K, Tanemura M, Furukawa K, Mikamori M, Saito T, Ohtsuka M, Suzuki Y, Tei M, Kishi K, Yasuoka H, et al: Primary intrahepatic squamous cell carcinoma with histological collision of adenocarcinoma and squamous cell carcinoma: A case report. Am J Case Rep. 19:1184–1191. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim E and Viatour P: Hepatocellular carcinoma: Old friends and new tricks. Exp Mol Med. 52:1898–1907. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hu S, Lyu X, Li W, Cui X, Liu Q, Xu X, Wang J, Chen L, Zhang X and Yin Y: Radiomics analysis on noncontrast CT for distinguishing hepatic hemangioma (HH) and hepatocellular carcinoma (HCC). Contrast Media Mol Imaging. 2022:76936312022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu HD, Li HL, Huang MS, Yang WZ, Yin GW, Zhong BY, Sun JH, Jin ZC, Chen JJ, Ge NJ, et al: CHANCE001 Investigators: Transarterial chemoembolization with PD-(L)1 inhibitors plus molecular targeted therapies for hepatocellular carcinoma (CHANCE001). Signal Transduct Target Ther. 8:582023. View Article : Google Scholar : PubMed/NCBI
|

















