Background
Endometrial cancer (EC) is one of the three most
common malignant tumors of the female reproductive system and it
ranks sixth in incidence among female malignant tumors globally
(1). The morbidity and mortality
rates of EC in developed countries are higher compared with those
in developing countries (2).
Furthermore, 66,570 new cases of EC and 12,940 EC-related deaths in
the US were estimated for 2021 (3).
In China, the incidence and mortality rates of EC are also
exhibiting gradual increases. Data from the China Cancer Statistics
Report indicated that in 2022, there were 84,520 new cases and
17,543 deaths from cancer in corpus uteri in China (4). In total, ~80% of EC cases are limited
to the uterus at the initial diagnosis and these patients have a
relatively good prognosis with a 5-year survival rate of >95%
(5,6). However, in cases with regional or
distant metastasis, the prognosis is significantly worse (68 and
17%, respectively) (6,7). Paclitaxel plus carboplatin is the
standard first-line treatment for patients with advanced, recurrent
or metastatic EC (8). However, the
effective rate of this treatment is limited and ranges from 7–14%,
with a median overall survival (mOS) time of <1 year (9–12).
Therefore, it is necessary to explore new treatment methods in
order to prolong the survival time of patients with EC.
High-risk factors for EC
At present, the cause of EC remains unknown, but the
related high-risk factors may be divided into several categories,
including reproductive factors, hormonal use, metabolic syndromes
and genetic factors (13).
Reproductive risk factors include nulliparity, early menarche, late
menopause, infertility and anovulatory menstrual cycles (13). There is evidence that, compared with
non-parturient women, the incidence of EC in postpartum women is
reduced by 40% (14). Furthermore,
a large-scale meta-analysis reported that parity (the number of
births after ≥24 weeks of pregnancy) may be associated with a
reduced risk of EC, since the relative risk (RR) of EC decreased
when the parity number increased (15). This outcome may be related to the
protection of progesterone on the endometrium during pregnancy. EC
is a hormone-driven type of cancer and ~80% of EC cases may be
caused by excessive estrogen or lack of progesterone (16). Long-term continuous estrogen
stimulation, including endogenous and exogenous, increases the risk
of hormone-responsive EC. These sources of stimulation include
using only estrogen in women with an intact uterus, selective
estrogen receptor modulators (such as tamoxifen and raloxifene) and
polycystic ovary syndrome (13).
The International Agency for Research on Cancer
suggests that obesity is also a risk factor of EC (17). Furthermore, a Mendelian
randomization study reported that an increase in BMI had a direct
impact on EC risk and the overall impact of SNP alleles associated
with an increase in BMI on EC risk exceeded their predicted impact
on the BMI (18,19). The association of obesity with EC
may be related to elevated estrogen levels, hyperinsulinemia and
chronic inflammation (16,20,21).
There is also evidence that diabetes increases the risk of EC
(14). In a meta-analysis by
Tsilidis et al (22), it was
reported that the overall random impact on the incidence rate of EC
in patients with diabetes was 1.97. EC is also associated with
certain genetic factors. For instance, white women were reported to
have a higher incidence of EC than women of other ethnicities in
the US (23); however, this may
also be due to the socio-economic differences and requires further
study. An Italian study showed that ~5% of patients with EC have a
family history of the disease in a first-degree relative (24). In addition, there are two genetic
syndromes associated with EC, Lynch syndrome and Cowden syndrome
(25–27), with Lynch syndrome (also known as
hereditary non-polyposis colorectal cancer) being the most common
(25,26). It is estimated that up to 70% of
women with Lynch syndrome will develop EC, which is typically
hormone-responsive (25,26).
Certain studies have demonstrated that smoking
reduces the risk of EC (28) and,
compared with non-smokers, current or former smokers have a lower
risk of EC (29,30). This reduced risk may be due to the
mechanistic link between the anti-estrogen effects of smoking and
the risk of EC (14,31). Of note, a study by Aune et al
(32) reported that body height is
significantly associated with the risk of EC [RR, 1.15; 95%
confidence interval (CI), 1.09–1.22]. In summary, obesity is the
main risk factor for EC and therefore, the importance of weight
control to reduce the incidence of EC should be highlighted.
However, additional risk factors such as diabetes, smoking and body
height require further study.
Classification of EC
The classification of cancer is important, since
different classifications may result in different treatment methods
and prognoses. Based on clinical pathology and molecular
characteristics, EC has historically been classified into two
categories of Bokhman histopathology: Type I and type II (33). Type I (endometrioid carcinoma) is
the most common type and accounts for 60–70% of EC cases, is graded
1 or 2 and exhibits high hormone receptor expression (33). These tumors are more likely to be
detected at an early stage due to symptoms such as bleeding and
patients with this type have a good prognosis. Type II accounts for
30–40% of all EC cases, typically includes high-grade endometrioid
carcinoma and other histological types, such as serous or clear
cell carcinoma, and is estrogen-independent (33). Type II is more invasive than type I
and, even with an early diagnosis, the prognosis of type II is poor
(34). However, the traditional
pathological classification has certain limitations. For instance,
certain high-level (grade 3) endometrial carcinoma and serous
carcinoma are not easily distinguishable in terms of morphology.
Furthermore, this classification cannot provide clear targets to
assist in selecting new treatment methods or drugs.
In 2013, The Cancer Genome Atlas (TCGA) research
network introduced a molecular classification system based on new
advances in the understanding of the EC genome landscape (35). TCGA described the four molecular
subgroups of EC as follows (35):
i) Polymerase-ε (POLE) ultra-mutated, which is characterized by
somatic mutations in the exonuclease domain of the DNA replication
enzyme POLE and patients with this subtype have an excellent
prognosis; ii) microsatellite instability hypermutated (MSI-H),
which is characterized by high mutation rates in both sporadic and
hereditary EC that are associated with changes in the mismatch
repair (MMR) system genes, MLH1, MSH2, MSH6 and post-meiotic
segregation 1 homolog 2 (PMS2) and the prognosis of patients with
this subtype is intermediate; iii) copy-number low, which is
characterized by a low mutational load and an intermediate
prognosis, this subtype includes most EC cases and is often
associated with gene mutations in phosphate and tension homology
deleted on chromsome 10, catenin-β1,
phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic α subunit,
AT-rich interactive domain-containing protein 1A and KRAS; and iv)
copy-number high, which includes serous tumors and 25% of
high-grade EC cases, patients with this subtype have a poor
prognosis and the mutation rate of this subtype is the lowest, but
TP53 mutations are frequent. A study of 50 patients with high-grade
endometrial adenocarcinoma demonstrated that the clinical prognosis
of each subgroup was different (36). At 48 months, the
cancer-specific/disease-specific survival rate in the POLE mutation
group was 100%, that in the MSI group was 82%, that in the
copy-number low group was 77.8% and that in the copy-number high
group was 42.9%. Therefore, this classification method reflects
that these subgroups not only have different molecular and
pathological characteristics, but also exhibit significant
differences in clinical outcomes (35,37).
This classification is also an example of tumor precision
treatment. However, molecular subtyping is based on high-throughput
deep sequencing, which is both costly and time-consuming and may
limit the wider clinical application.
Talhouk et al (38) proposed a simple and economical
molecular classification method to replace high-throughput
sequencing (Fig. 1). This method
used immunohistochemistry to detect the expression of the MMR
proteins MSH6 and PMS2 to determine the type of MMR deficiency
(dMMR). Sequencing of the exonuclease domain of the catalytic
subunit of POLE was then conducted to determine the type of POLE
mutant. Finally, cases were divided into p53 mutant-type according
to the p53 immunohistochemistry staining results (staining
2+ or 0) or p53 wild-type (staining 1+). By
this method, EC was then finally divided into dMMR, POLE
ultra-mutated, p53 wild-type or p53 abnormal type, respectively
replacing MSI-H, POLE ultra-mutated, copy-number low or copy-number
high type. Although this new grouping is not completely equivalent
to the TCGA classification, the four survival curves of the
groupings were similar to those of the TCGA classification
(38). The authors of the
aforementioned study suggested that this simple method may be used
on a large scale in the clinic, which is of great significance for
guiding the molecular classification, risk grading and treatment of
EC.
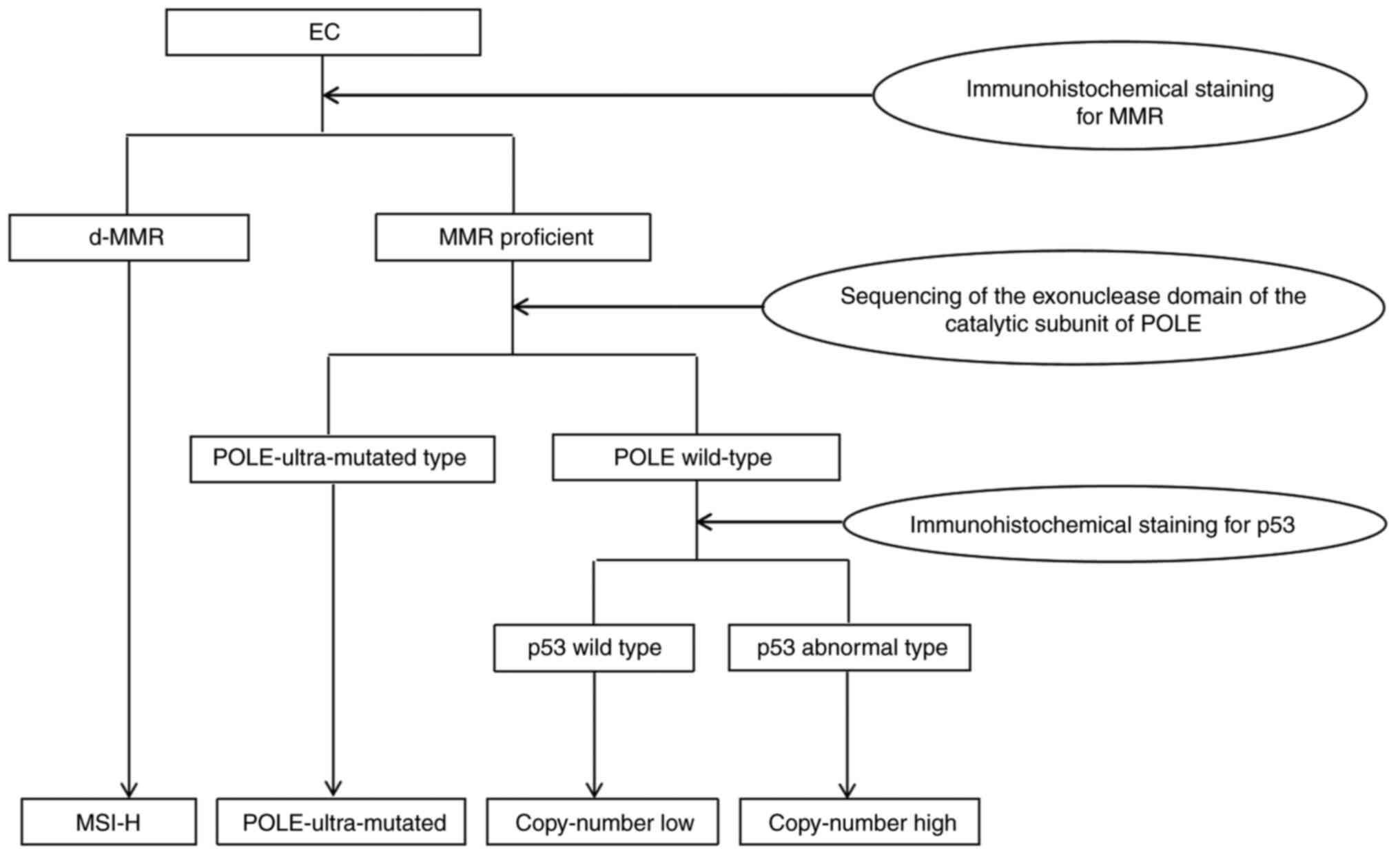 | Figure 1.Simple model for the molecular
classification of EC. Through sequencing of the exonuclease domain
of the catalytic subunit of POLE and immunohistochemical staining
for MMRs and p53, EC can be divided into the dMMR type,
POLE-ultra-mutated type, p53 wild type and p53 abnormal type,
respectively replacing MSI-H, POLE ultramutated, copy-number low
type and copy-number high type in the The Cancer Genome Atlas
Program classification (38). MMR,
mismatch repair proteins; POLE, polymerase epsilon; dMMR, deficient
mismatch repair; MSI-H, microsatellite instability hypermutated;
EC, endometrial cancer. |
Mechanism and application of
immunotherapy
Programmed cell death protein 1 (PD-1) was first
reported by Ishida et al (39) in apoptotic T cells in mice. PD-1
belongs to the CD28 family of proteins and is mainly expressed on
the surface of immune cells, such as activated T cells, B cells and
natural killer cells (40,41). The most notable ligand of PD-1, PD-1
ligand 1 (PD-L1), is frequently expressed in various types of tumor
cell (42). Tumor cells activate
the inhibitory signaling pathway of PD-1/PD-L1, inhibit the
activation of T cells and finally form an immune microenvironment
suitable for tumor cell growth (43,44).
Therefore, immunosuppressive agents against PD-1 or PD-L1 restore
the immune activity of T cells, enhance the immune response and
improve the ability of the immune system to kill tumor cells. This
has been a major breakthrough in the field of tumor treatment in
recent years (45). At present,
PD-1 inhibitors that are effective in cancer treatment include
nivolumab, pembrolizumab and cemiplimab, while PD-L1 inhibitors
include atezolizumab, avelumab and durvalumab (45). Markers related to the efficacy of
immune checkpoint inhibitors include PD-L1, MSI-H or dMMR tumor
mutation burden (TMB). A study by Mo et al (46) demonstrated that 61.3% of patients
with EC expressed PD-L1 in their tumor tissues. Furthermore, the
degree of tissue differentiation was negatively associated with
PD-L1 expression levels (46).
Another previous study has also shown that 25–30% of EC cases have
MSI-H or dMMR (47). A study by
Kautto et al (48)
demonstrated that, compared with proficient MMR (pMMR) tumors, dMMR
tumors had more somatic mutations and produced more neoantigens.
Furthermore, the efficacy of pembrolizumab against dMMR tumors was
significantly higher compared to pMMR tumors (48). The therapeutic effect of PD-1/PD-L1
inhibitors is related to the TMB, as PD-1/PD-L1 inhibitors are more
effective against tumors with a high TMB (35). Among the four molecular subtypes of
EC, the TMB of MSI-H and POLE ultra-mutated subtypes was determined
to be higher compared with that of the other groups (35). In addition, a previous study
reported that the expression rates of PD-1 in POLE-mutant and MSI-H
EC tissues were 73 and 69%, respectively, and that the expression
rates of PD-L1 were 100 and 71%, respectively (49). Another feature of the MSI-H and POLE
ultra-mutated subtypes is that they are rich in tumor-infiltrating
lymphocytes and CD3+ and CD8+ T lymphocytes
(50). Therefore, this suggests
that there are active immune responses in the local
microenvironment of MSI-H and POLE ultra-mutated tumors and that
blocking PD-1/PD-L1 may induce an effective antitumor immune
response (51). As such, the MSI-H
and POLE ultra-mutated subtypes are most likely to benefit from
PD-1/PD-L1 inhibitory therapy.
Application of immunosuppressive agents in
EC
Until now, the first-line treatment for advanced EC
was carboplatin and paclitaxel combined chemotherapy, with an
overall response rate (ORR) of 50–60% and a median progression-free
survival (mPFS) time of 1 year (52,53).
After platinum treatment failed, conventional single drug
chemotherapy was administered, but the outcome was poor. For
instance, doxorubicin and paclitaxel are the most commonly used
second-line treatments for EC and can only provide an mPFS time of
4 months and an mOS time of 1 year (54). Therefore, the exploration of new
therapies to improve the prognosis of patients with advanced EC is
urgently needed. In previous years, there have been a number of
clinical research studies regarding immune checkpoint inhibitors in
EC, which have provided a comprehensive scientific basis for drug
research and development for the treatment of advanced EC (Table I).
 | Table I.Published clinical studies with
results available. |
Table I.
Published clinical studies with
results available.
| First author,
year | Drug | Target | Trial
identifier | Phase | Patient population,
number of patients (n) | Treatment | Findings | Adverse
reactions | (Refs.) |
|---|
| Le et al,
2015 | Pembrolizumab | PD-1 | NCT01876511 | II | Pts with metastatic
cancer with or without dMMR (n=41), including pts with EC
(n=2) | Pembrolizumab 10
mg/kg IV every 14 days | In pts with dMMR,
CRC; ORR, 40.0% (4/10); 20-week PFS, 77.8% (7/9). In pts with pMMR,
CRC; ORR, 0% (0/18); 20-week PFS, 11.1% (2/18). In pts with dMMR,
non-CRC; ORR, 71.4% (5/7); 20-week PFS, 66.7% (4/6) | Rash, itching,
thyroiditis, hypothyroidism, hypophysitis and asymptomatic
pancreatitis | (55) |
| Le et al,
2017 | Pembrolizumab | PD-1 | NCT01876511 | II | Pts with 12 dMMR
tumor types (n=86), including pts with EC (n=15) | Pembrolizumab 10
mg/kg IV every 14 days | In pts with dMMR
ORR, 53.5% (46/86); DCR, 76.7% (66/86) In pts with dMMR of EC ORR,
53.3% (8/15); DCR, 73.3% (11/15) | Hypothyroidism | (57) |
| Ott et al,
2017 | Pembrolizumab | PD-1 | NCT02054806
(KEYNOTE-028) | Ib | Pts with locally
advanced or metastatic PD-L1-positive EC (n=24) | Pembrolizumab 10
mg/kg IV every 2 weeks | ORR, 13.0% (3/23);
PFS, 1.8 months; 6-month PFS, 19%; 12-month PFS, 14.3%; 6-month OS,
67%; 12-month OS, 51% | Fatigue, itching,
fever and anorexia | (58) |
| Marabelle et
al, 2020 | Pembrolizumab | PD-1 | NCT02628067
(KEYNOTE-158) | II | Pts with advanced
MSI-H or dMMR solid tumors (n=233), including pts with EC
(n=49) | Pembrolizumab 200
mg IV once every 3 weeks | ORR, 57.1% (28/49);
PFS, 25.7 months | Fatigue, itching,
diarrhea and weakness | (59) |
| Tamura et
al, 2019 | Nivolumab | PD-1 |
JapicCTI-163212 | II | Pts with
advanced/recurrent uterine cervical cancer (n=20), uterine corpus
cancer (n=23) and soft tissue sarcoma (n=21) | Nivolumab 240 mg IV
every 2 weeks | In pts with EC
(n=22): ORR, 22.7% (5/22); DCR, 68.2% (15/22); mPFS, 3.4 months;
mOS, 8.7 months | Pruritus | (61) |
| Oaknin et
al, 2020 | Dostarlimab | PD-1 | NCT02715284 (GARNET
trial) | I | Pts with defective
mismatch mutation repair EC (n=104) | Dostarlimab 500 mg
IV once every 3 weeks for 4 doses, then 1,000 mg once every 6
weeks | ORR, 42.3% (30/71);
DCR, 57.7% (41/71) | Anemia, colitis and
diarrhea | (63) |
| Liu et al,
2019 | Atezolizumab | PD-L1 | NCT01375842 | I | Pts with
advanced/recurrent epithelial ovarian (n=12) and uterine cancers
(n=15) | In the
dose-expansion phase, atezolizumab 15 mg/kg or 1,200 mg IV every 3
weeks for 16 cycles or 1 year of treatment, whichever occurred
first | ORR, 13.3% (2/15);
mPFS, 1.4 months | Diarrhea and
fatigue | (64) |
| Konstantinopoulos
et al, 2019 | Avelumab | PD-L1 | NCT02912572 | II | Pts with dMMR
(n=15) and pMMR (n=16) recurrent/persistent EC | Avelumab 10 mg/kg
IV every 2 weeks | In pts with dMMR
(n=15): ORR, 26.7% (4/15); PFS6 rate, 40%. In pts with
pMMR/non-POLE (n=16): ORR, 6.25% (1/16); PFS6 rate, 6.25% | Fatigue and
nausea | (65) |
| Antill et
al, 2021 | Durvalumab | PD-L1 | NCT03015129 | II | Pts with advanced
mismatch repair-deficient (n=36) and repair-proficient (n=31)
EC | Durvalumab 1,500 mg
IV every 4 weeks | In pts with dMMR
(n=36): ORR, 47% (17/36); mPFS, 8.3 months. In pts with pMMR
(n=31): ORR, 3% (1/16); mPFS, 1.8 months | Hyperthyroidism and
hypothyroidism | (66) |
| Makker et
al, 2020 | Pembrolizumab +
lenvatinib | PD-L1 | NCT02501096
(KEYNOTE-146) | II | Pts with previously
treated EC (n=108) | Lenvatinib 20 mg
once daily orally plus pembrolizumab 200 mg IV once every 3 weeks,
in 3-week cycles | ORRWk24,
38% (41/108); ORR, 38.9% (42/108); mDOR, 21.2 months; mPFS, 7.4
months; mOS, 16.7 months. In pts with MSI-H/dMMR at 24 weeks
(n=11): ORR, 63.6% (7/11); in pts with MSS/pMMR at 24 weeks (n=94):
ORR, 36.2% (34/94) | Hypertension,
diarrhea, fatigue, decreased appetite, hypothyroidism and
nausea | (71) |
Immune checkpoint inhibitors in
monotherapy
Anti-PD-1. Pembrolizumab
In 2015, Le et al (55) demonstrated the efficacy of the
anti-PD-1 monoclonal antibody pembrolizumab against EC, which
provided the first evidence for the administration of immunotherapy
in advanced EC. The aforementioned study conducted a phase II
clinical trial of 41 patients with metastatic cancer with or
without dMMR, including 2 patients with EC. The 2 patients with EC
achieved partial response (PR) and the ORR and PFS rates were 71
and 67%, respectively. In addition, >5% of patients experienced
adverse events (AEs), including rash or itching (24%), thyroiditis,
hypothyroidism or hypophysitis (10%) and asymptomatic pancreatitis
(15%). To our knowledge, this study was the first to report the
relationship between the tumor microenvironment, genotype and
response to checkpoint inhibitors, which are critical for
identifying predictors of response to immune checkpoint inhibitor
therapy.
In 2016, Mehnert et al (56) reported on a 53-year-old patient with
high-grade metastatic endometrial adenocarcinoma who received 10
mg/kg pembrolizumab treatment every 2 weeks and ultimately achieved
a rapid and sustained (>14 months) clinical response.
In 2017, Le et al (57) published the results of a phase II
clinical trial (NCT01876511) of pembrolizumab as a single-agent
treatment for patients with the dMMR tumor subtype. An ORR of 53%
(46 patients) was observed in the 86 patients enrolled and 21% of
patients reached complete response (CR; 18 patients). The
15-patient EC cohort also exhibited an ORR of 53% (8 patients) and
the disease control rate was 73.3% (11 patients). Throughout the
study, 74% of patients experienced adverse reactions, but the
majority had low-grade reactions. Endocrine disorders, mainly
hypothyroidism, occur in 21% of patients and may be easily treated
by thyroid hormone replacement therapy (57). This study further supported the
hypothesis that dMMR tumors are sensitive to immunosuppressive
agents, regardless of the location of the primary tumor. In the
same year, the results of a phase IB trial, KEYNOTE-028
(NCT02054806), were also published (58). The 24 subjects of this study had
advanced or metastatic PD-L1+ EC. Patients who
progressed after standard treatment received 10 mg/kg intravenous
(IV) pembrolizumab every 2 weeks for up to 24 months or until the
disease progressed or the toxicity was intolerable. Among them, 3
patients achieved PR and 2 patients maintained stable disease (SD).
The ORR was 13% and the 6-month PFS and OS rates were 19.0 and
68.8%, respectively. As for toxicity, minor adverse reactions were
observed in 54.2% of patients, including fatigue, itching, fever
and anorexia. Based on the aforementioned results, the US Food and
Drug Administration (FDA) approved pembrolizumab for the treatment
of solid tumors with MSI-H/dMMR in May 2017. This was the first
antitumor drug approved following a diagnosis by biomarker rather
than tissue type. In 2019, pembrolizumab was added to The National
Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines
for EC, stating that pembrolizumab can be used for the treatment of
EC when accompanied by MSI-H/dMMR recurrence or metastasis that has
not responded to previous treatment (8).
In January 2020, phase II clinical trial
(KEYNOTE-158) results were published involving 27 cases of advanced
MSI-H or dMMR solid tumors, which were consistent with the results
of NCT01876511 (59). Among the 233
patients enrolled, 49 had EC. The ORR of the patients with EC was
57.1%, of which 16% (8 patients) had a CR and 41% (20 patients) had
a PR. The mPFS time was 25.7 months. Of the 233 enrolled patients,
151 (64.8%) had treatment-related AEs, of which 34 (14.6%) had
grade 3–5 AEs. The most common toxicities were fatigue (14.6%),
itching (12.9%), diarrhea (12.0%) and weakness (10.7%). Due to
these AEs, 22 patients (9.4%) had to stop treatment.
Nivolumab
Nivolumab is also an anti-PD-1 immunosuppressant. In
2016, Santin et al (60)
reported on 2 patients with recurrent POLE ultra-mutated and MSH6
hypermutated EC tumors who were unresponsive to conventional
surgery and chemotherapy. During the treatment of these 2 patients,
nivolumab was administered as a single IV drug at a dose of 3 mg/kg
once every 2 weeks. Following computed tomography scanning over
several months, it was confirmed that the patients demonstrated a
sustained clinical reaction to nivolumab and reported no severe
toxicity. In addition, results from a multicenter, open-label
nivolumab phase II clinical trial were released in 2019 (61). The 22 patients in this study with
advanced/recurrent uterine cancer received 240 mg nivolumab every 2
weeks. The primary endpoint was the ORR and the secondary endpoints
included OS, PFS and safety. The resulting ORR was 23%, the mPFS
time was 3.4 months and the 6-month OS rate was 73%. In the uterine
cancer cohort, the most common treatment-related adverse event was
pruritus, which was mostly mild.
Dostarlimab
Dostarlimab (TSR-042) is an effective, selective and
humanized anti-PD-1 immunoglobulin G4 monoclonal antibody, which
has a high affinity for the PD-1 receptor and can effectively block
the binding of PD-1 and PD-L1 (62). To date, the GARNET trial
(NCT02715284) is the only published study to evaluate the curative
effect of dostarlimab in EC and is the largest single study of an
anti-PD-1 monotherapy for advanced or relapsed EC (63). As of the data cut-off point, 104
patients with dMMR EC were enrolled and received dostarlimab
treatment. Among these patients, 71 with measurable lesions at
baseline and a follow-up of ≥6 months were ultimately included in
the analysis. The results indicated an ORR of 42.3% (30 patients),
a CR rate of 12.7% (9 patients) and a PR rate of 29.6% (21
patients). The treatment response to dostarlimab was long-lasting
and the adverse reactions were reported to be tolerable. The most
common treatment-related AEs at level 3 or above were anemia
(2.9%), colitis (1.9%) and diarrhea (1.9%).
Anti-PD-L1
Atezolizumab
The NCT01375842 study by Liu et al (64) was the first to detail the use of
atezolizumab as a single drug treatment for gynecological cancer.
In the aforementioned study, all 15 patients with EC were treated
with atezolizumab in the dose-expansion phase of the study (15
mg/kg atezolizumab, n=1; 1,200 mg atezolizumab, n=14). As of
December 31, 2016, 2 patients showed a PR, 2 patients maintained
SD, 9 patients had progressive disease and 2 patients were not
evaluable. The ORR was 13.3% [95% CI, 1.7–40.5%]. However, all
patients experienced ≥1 AE and 7 patients (46.7%) in the uterine
cancer cohort developed treatment-related AEs. In the uterine
cancer cohort, the most common treatment-related AEs of any grade
were diarrhea (20.0%) and fatigue (13.3%), with no occurrence of
treatment-related grade 4 or 5 AEs. It can therefore be suggested
that atezolizumab is safe for patients with advanced EC and it may
have certain clinical benefits in some patients.
Avelumab
Avelumab, another anti-PD-L1 immunosuppressant, has
also shown promising activity in patients with dMMR EC.
Konstantinopoulos et al (65) published the results of a phase II
clinical trial (NCT02912572) with 33 patients that were divided
into two cohorts, dMMR and pMMR. The co-primary endpoints were the
ORR and PFS rate at 6 months (PFS6). The ORR in the dMMR and
pMMR/non-POLE cohorts was 26.7 and 6.25%, respectively. In the dMMR
cohort, there were 4 patients with objective responses (1 withCR
and 3 with PR). The PFS6 was 40.0% in the dMMR cohort and 6.25% in
the pMMR/non-POLE cohort. Of the 31 patients who started the
regimen, 22 (71%) had treatment-related AEs of any grade, but there
were no grade 4 and 5 treatment-related AEs in any cohort. The most
common adverse reactions were fatigue (35.5%) and nausea
(16.1%).
Durvalumab
Durvalumab is an IgG1 κ monoclonal antibody that
binds to PD-L1 on tumor cells, blocking the interaction with PD-1
on T cells and antigen-presenting cells, thereby alleviating
PD-1/PD-L1-mediated immunosuppression and allowing T cells to
attack tumor cells (66). Results
from the PHAEDRA study demonstrating the activity of durvalumab as
a single agent in a dMMR and pMMR EC cohort were published in 2021
(66). The study included 71
patients with advanced EC, of which 36 were dMMR and 35 were pMMR.
All patients received IV durvalumab at a dose of 1,500 mg every 4
weeks. The ORR of patients with dMMR was 47% (6 cases of CR and 11
cases of PR), while the ORR of patients with pMMR was 3% (1 case of
PR). Furthermore, the mPFS time was 8.3 months in the dMMR cohort,
while it was only 1.8 months in the pMMR cohort. A total of 14
patients reported immune-related AEs, most of which were grade 1 or
2, including hyperthyroidism, hypothyroidism, pneumonia and
hepatitis.
In summary, the reported efficacy of immune
checkpoint inhibitors as monotherapies in treating EC is
considerable. However, the results of the aforementioned PD-1 and
PD-L1 immunosuppressive drug clinical trials are different, which
may be related to various factors, including the size of the
samples, the genotype of the subjects and the choice of observation
indicators. Therefore, if a large-scale clinical study on PD-1 and
PD-L1 immunosuppressive drugs was conducted through a multi-center
collaboration that followed a unified research scheme, jointly
collecting study subjects and conducting an overall analysis, the
clinical trial results would be more robust and reliable.
Immune checkpoint inhibitor-based drug
combinations
As mentioned above, the use of immune checkpoint
inhibitor monotherapy in EC is mainly limited to patients with dMMR
or MSI-H mutations. However, patients with MSI-H/dMMR only account
for 25–30% of cases and 70–75% of patients have microsatellite
stability (MSS)/pMMR (47). The
efficacy of single immune checkpoint inhibitor treatment in
patients with MSS/pMMR is not optimal. Furthermore, with the
increasingly widespread application of immunosuppressants and the
complexity of immune response activation, immunosuppressive drug
resistance is gradually increasing. Therefore, finding an improved
treatment plan for patients with MSS/pMMR is required and
researchers have adopted a joint strategy in the hope of achieving
synergistic benefits and reducing the occurrence of primary or
secondary drug resistance.
Immune checkpoint inhibitors and
angiogenesis inhibitors
Lenvatinib is a kinase inhibitor against VEGFR1-3
and a small molecule targeted drug against angiogenesis (67). In a preclinical model, lenvatinib
reduced the number of tumor-associated macrophages and increased
the proportion of CD8+ T cells, thereby inducing immune
activation (67). In multiple mouse
xenograft models, the combination of anti-PD-1 monoclonal antibody
and levatinib had a more optimal antitumor activity compared with
monotherapy using either drug (68). Therefore, pembrolizumab combined
with lenvatinib was hypothesized to be an effective antitumor
strategy and as such, KEYNOTE-146 (NCT02501096) aimed to study the
safety and initial efficacy of the combined drugs in the treatment
of a variety of advanced solid tumors (69). The phase IB component of the study
established that the maximum tolerated dose and the recommended
phase II dose was 20 mg levatinib orally once a day combined with
200 mg pembrolizumab intravenously every 3 weeks (69). A multi-center, open-label,
single-arm, phase II trial further investigated the efficacy of
lenvatinib plus pembrolizumab in patients with primary advanced or
recurrent EC (70). Between
September 10, 2015 and July 24, 2017, 53 patients were included in
the analysis. Of these patients, 39.6% (21/53) reported an
objective response at week 24 and 30% (16/53) experienced serious
treatment-related AEs. In the final efficacy analysis, the median
follow-up time for 108 patients was 18.7 months at the time of data
cut-off (71). The resulting ORR at
week 24 (ORRWK24) of the 108 patients was 38% (41/108).
Among these patients, 3 achieved CR and 38 achieved PR at week 24.
In the subgroup analysis, the ORRWK24 of patients with
MSS/pMMR (n=94) and MSI-H/dMMR (n=11) was 36.2% (95% CI,
26.5–46.7%) and 63.6% (95% CI, 30.8–89.1%), respectively.
Regardless of the MSI status of the tumor, the median
responseduration was 21.2 months, the mPFS time was 7.4 months and
the mOS time was 16.7 months. Furthermore, 83/124 (66.9%) patients
experienced grade 3 or 4 treatment-related AEs. The most common
adverse reactions were hypertension, diarrhea, fatigue, decreased
appetite, hypothyroidism and nausea. Based on the aforementioned
studies, lenvatinib combined with pambrolizumab was approved by the
FDA for the treatment of advanced EC that was not MSI-H/dMMR and
had progressed following prior therapy.
A 2:1 randomized phase II clinical trial
(NCT03367741) compared the efficacy of a cabozantinib and nivolumab
combination (arm A) vs. nivolumab (arm B) in the treatment of
recurrent EC (72). The primary
endpoint of the study was PFS. The results demonstrated that the
mPFS of arms A and B were 5.3 months (95% CI, 3.5–9.5) and 1.9
months (95% CI, 1.6–3.8), respectively. Furthermore, the ORR was
25% in arm A and 16.7% in arm B and the SD rate was 44.4 and 11.1%,
respectively. Furthermore, the clinical benefit in arm A was
significantly higher compared with that in arm B (P<0.001). The
most common AEs in arm A were diarrhea (47.2%), elevated liver
enzymes (44.4%), fatigue (38.9%), anorexia, hypertension and nausea
(30.6%), which were mainly grade 1 or 2.
Further single arm phase II trials (NCT04042116 and
NCT04157491) of anti-PD-1 drugs combined with other angiogenesis
inhibitors (lucinib and anlotinib) for the treatment of EC are also
under investigation at present (73).
Combination immunotherapy
Since any treatment may eventually result in drug
resistance, there are ongoing efforts to study combined
immunotherapy, which is a combination of immunosuppressive agents
with different mechanisms.
A phase II study by Fumet et al (74) was the first trial to study a
combination of olaparib and dual immunotherapy based on molecular
screening. The study will aim to evaluate the effectiveness and
safety of an olaparib/durvalumab/tremelimumab combination in
patients with several types of solid cancer (n=213) that have at
least one homologous repair gene mutation. Patients initially
receive 300 mg olaparib twice per day. If there is no progress
after receiving olaparib for 6 weeks, the patients receive olaparib
and durvalumab (1,500 mg every 4 weeks) and tremelimumab (75 mg IV
every 4 weeks) immunotherapy within 4 months. Patients are further
administered durvalumab alone until the disease progresses, or
patient death or intolerable toxicity occur or the
patient/researcher decides to stop treatment.
There are currently additional early trials, such as
the combination of nivolumab and ipilimumab (anti-cytotoxic
T-lymphocyte-associated protein 4; NCT03508570 and NCT02982486),
for the treatment of advanced EC (75) and the combination or non-combination
of nivolumab and indoleamine 2,3-dioxygenase inhibitors
(BMS-986205; NCT04106414) (75).
Immune checkpoint inhibitors and
chemotherapy
Preclinical studies have indicated that chemotherapy
may generate immune stimulation, enhance the presentation of tumor
cell-specific antigens and lead to cancer cells triggering immune
responses or increasing their susceptibility to immune system
attack (76,77). These mechanisms lay the biological
foundation for the later clinical research design of using a
combination of chemotherapy and immunotherapy to treat cancer. At
present, there are a number of phase III trials of
immunosuppressive agents combined with carboplatin and paclitaxel
for the treatment of patients with advanced or recurrent EC, such
as dostarlimab (RUBY; NCT03981796), atezolizumab (AtTEnd;
NCT03603184) and pembrolizumab (GY018; NCT02549209) (75). Although these studies do not
consider the MMR status when recruiting patients, differences will
be assessed in a subgroup analysis of patients with MSI-H and MSS
tumors. In addition, a phase III trial of lenvatinib with
pembrolizumab vs. doxorubicin or weekly paclitaxel (NCT03517449) in
the treatment of advanced EC and a first-line lenvatinib with
pembrolizumab vs. carboplatin and paclitaxel chemotherapy
(NCT03884101) trial are currently ongoing (75). To the best of our knowledge, there
are currently no preliminary data reported on the efficacy of
immunotherapy combined with chemotherapy in advanced EC. However,
it is esteemed that their combination provides promising results
for patients with advanced EC.
Other combinations
Radiotherapy is also an important means to treat
malignant tumors. A number of clinical studies have reported that
radiotherapy combined with immunotherapy has an acceptable toxicity
(78) and enhances the immune
response at the irradiated site (79,80).
The PRIMMO study (NCT04214067) is an ongoing randomized phase II
trial evaluating the efficacy of pembrolizumab combined with
low-fraction radiotherapy and immunomodulatory mixtures (vitamin D,
curcumin, lansoprazole, aspirin and low-dose cyclophosphamide) in
patients with pretreated advanced uterine tumors (cervical or
endometrial carcinoma and uterine sarcoma) (81). The main endpoint of the study is the
ORR at week 26.
Netrin-1, a protein upregulated in >80% of
uterine tumors, serves an important role in cancer progression by
regulating cell apoptosis (82).
NP137 is a monoclonal antibody targeting netrin-1 that may reduce
resistance to chemotherapy (83). A
phase IB/II clinical trial (NCT04652076) evaluating the combination
of NP137 with pembrolizumab and/or chemotherapy in the treatment of
locally advanced/metastatic endometrial or cervical cancer has
recently been initiated (73).
Conclusions and perspectives
In previous years, immunotherapy has received
increasing attention in antitumor therapy. When the immune function
of the body functions in a healthy manner, cancerous cells can be
eliminated by the immune response in time and most individuals do
not develop any tumors. When cancerous cells evade surveillance and
elimination by immune cells due to certain changes, tumors may
occur (84). Immunotherapy is aimed
at all aspects of tumor immunity, using the immune response of the
patient to treat tumors, which is safer and more efficient than
other treatment methods and may potentially become a new method for
the treatment of EC (85). However,
following in-depth research on tumor immunotherapy, its drawbacks
have also attracted attention. Indeed, an excessively enhanced
immune response may damage normal tissues. For example, the
gastrointestinal tract, endocrine glands, skin and liver are the
organs most prone to immune-related AEs, while the central nervous
system and cardiovascular, lung, musculoskeletal and blood systems
are less involved (6). In addition,
immune cells recognize tumor cells with a single target, low
specificity and a weak killing effect. In previous studies, it was
reported that immunosuppressive drug monotherapy has certain
effects in the treatment of advanced EC, but the efficacy is not
optimal (55–66). Therefore, the ongoing combined
strategies of targeted therapy, other immunotherapeutic agents,
chemotherapy and radiotherapy may change the therapeutic prospects
of advanced EC. In addition, antiangiogenic agents and
poly(ADP-ribose) polymerase, PI3K/AKT/mTOR, EGFR, MEK,
cyclin-dependent kinase and Wee1 inhibitors have all demonstrated
certain activities, generating promising preliminary data (86) and are therefore research areas
requiring closer attention.
The prognosis of patients with late-stage recurrent
EC is poor. Currently, the NCCN guidelines still consider the
chemotherapy regimen of carboplatin combined with paclitaxel as the
first-line treatment for recurrent disease (87). Pembrolizumab is also listed as a
class 1 treatment option for MSI-H/dMMR endometrial tumors and it
is recommended that MSI-H or dMMR testing are performed for
recurrent endometrial tumors, if not previously tested. As
aforementioned, the effective rate of traditional chemotherapy,
such as paclitaxel and carboplatin in the treatment of patients
with advanced EC, ranges from 7–14% (9–12),
while PD-1/PD-L1 inhibitors have a significant therapeutic effect
on MSI-H/dMMR ECs, with an ORR of ~50% (57,59).
Therefore, after the patients are fully informed of the efficacy,
adverse reactions, medical expenses of immunotherapy and other
related content and agree to the application, the markers related
to the immunotherapy of the patient (including MSI, MMR, TMB and
POLE mutations) can be determined and finally, the most suitable
individualized treatment plan for the patient can be chosen. In
addition, further research is required to elucidate the resistance
mechanism of immunotherapy and for the implementation of
immunotherapy early in the first-line treatment of tumors. In
summary, it is esteemed that immunotherapy can play an increasingly
important role in the treatment of EC and act in combination with
various treatment methods to prolong the survival period and
improve the quality of life of patients.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
LW collected and analyzed data and drafted the
original manuscript. LL analyzed the data and made modifications to
the article. DH collected and analyzed data. YZ edited, reviewed
and revised the manuscript. Data authentication is not applicable.
All authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
EC
|
endometrial cancer
|
|
TCGA
|
The Cancer Genome Atlas
|
|
POLE
|
polymerase ε
|
|
MSI-H
|
microsatellite instability-high
|
|
dMMR
|
mismatch repair deficient
|
|
PD-1
|
programmed cell death protein 1
|
|
PD-L1
|
PD-1 ligand 1
|
|
TMB
|
tumor mutation burden
|
|
pMMR
|
mismatch repair proficient
|
|
ORR
|
overall response rate
|
|
mPFS
|
median progression-free survival
|
|
PR
|
partial response
|
|
CR
|
complete response
|
|
SD
|
stable disease
|
|
NCCN
|
The National Comprehensive Cancer
Network
|
|
AEs
|
adverse events
|
|
PFS6
|
PFS rate at 6 months
|
|
IV
|
intravenous
|
|
MSS
|
microsatellite stability
|
|
ORRWk24
|
ORR at 24 weeks
|
|
mOS
|
median overall survival
|
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gu B, Shang X, Yan M, Li X, Wang W, Wang Q
and Zhang C: Variations in incidence and mortality rates of
endometrial cancer at the global, regional, and national levels,
1990–2019. Gynecol Oncol. 161:573–580. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
American Cancer Society, . Endometrial
cancer survival rates, by stage. https://www.cancer.org/cancer/endometrial-cancer/detection-diagnosis-staging/survival-rates.html
|
|
4
|
Xia C, Dong X, Li H, Cao M, Sun D, He S,
Yang F, Yan X, Zhang S, Li N, et al: Cancer statistics in China and
United States, 2022: Profiles, trends, and determinants. Chin Med J
(Engl). 135:584–590. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Colombo N, Creutzberg C, Amant F, Bosse T,
González-Martín A, Ledermann J, Marth C, Nout R, Querleu D, Mirza
MR, et al: ESMO-ESGO-ESTRO Endometrial Consensus Conference Working
Group. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer:
Diagnosis, treatment and follow-up. Ann Oncol. 27:16–41. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Musacchio L, Boccia SM, Caruso G,
Santangelo G, Fischetti M, Tomao F, Perniola G, Palaia I, Muzii L,
Pignata S, et al: Immune checkpoint inhibitors: A promising choice
for endometrial cancer patients? J Clin Med. 9:17212020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
National Cancer Institute, . Endometrial
cancer treatment Physician Data Query (PDQ). 2021.Available online.
http://www.cancer.gov/cancertopics/pdq/treatment/endometrial/healthprofessional13–August.
2021
|
|
8
|
NCCN Clinical Practice Guidelines in
Oncology, . Available online. https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf13–August.
2020
|
|
9
|
Miller DS, Blessing JA, Lentz SS and
Waggoner SE: A phase II trial of topotecan in patients with
advanced, persistent, or recurrent endometrial carcinoma: A
gynecologic oncology group study. Gynecol Oncol. 87:247–251. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fracasso PM, Blessing JA, Molpus KL, Adler
LM, Sorosky JI and Rose PG: Phase II study of oxaliplatin as
second-line chemotherapy in endometrial carcinoma: A Gynecologic
Oncology Group study. Gynecol Oncol. 103:523–526. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Garcia AA, Blessing JA, Nolte S and Mannel
RS; Gynecologic Oncology Group, : A phase II evaluation of weekly
docetaxel in the treatment of recurrent or persistent endometrial
carcinoma: A study by the Gynecologic Oncology Group. Gynecol
Oncol. 111:22–26. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dizon DS, Blessing JA, McMeekin DS, Sharma
SK, Disilvestro P and Alvarez RD: Phase II trial of ixabepilone as
second-line treatment in advanced endometrial cancer: Gynecologic
oncology group trial 129-P. J Clin Oncol. 27:3104–3108. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Smith RA, von Eschenbach AC, Wender R,
Levin B, Byers T, Rothenberger D, Brooks D, Creasman W, Cohen C,
Runowicz C, et al: American Cancer Society guidelines for the early
detection of cancer: Update of early detection guidelines for
prostate, colorectal, and endometrial cancers. Also: Update
2001-testing for early lung cancer detection. CA Cancer J Clin.
51:38–75; quiz 77–80. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Raglan O, Kalliala I, Markozannes G,
Cividini S, Gunter MJ, Nautiyal J, Gabra H, Paraskevaidis E,
Martin-Hirsch P, Tsilidis KK, et al: Risk factors for endometrial
cancer: An umbrella review of the literature. Int J Cancer.
145:1719–1730. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu QJ, Li YY, Tu C, Zhu J, Qian KQ, Feng
TB, Li C, Wu L and Ma XX: Parity and endometrial cancer risk: A
meta-analysis of epidemiological studies. Sci Rep. 5:142432015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dunneram Y, Greenwood DC and Cade JE:
Diet, menopause and the risk of ovarian, endometrial and breast
cancer. Proc Nutr Soc. 78:438–448. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lauby-Secretan B, Scoccianti C, Loomis D,
Grosse Y, Bianchini F and Straif K; International Agency for
Research on Cancer Handbook Working Group, : Body fatness and
cancer-viewpoint of the IARC working group. N Engl J Med.
375:794–798. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Painter JN, O'Mara TA, Marquart L, Webb
PM, Attia J, Medland SE, Cheng T, Dennis J, Holliday EG, McEvoy M,
et al: Genetic risk score mendelian randomization shows that
obesity measured as body mass index, but not waist: Hip ratio, is
causal for endometrial cancer. Cancer Epidemiol Biomarkers Prev.
25:1503–1510. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nead KT, Sharp SJ, Thompson DJ, Painter
JN, Savage DB, Semple RK, Barker A; Australian National Endometrial
Cancer Study Group (ANECS), ; Perry JR, Attia J, et al: Evidence of
a causal association between insulinemia and endometrial cancer: A
mendelian randomization analysis. J Natl Cancer Inst.
107:djv1782015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Marlson MJ, Thiel KW, Yang S and Leslie
KK: Catch it before it kills: Progesterone, obesity, and the
prevention of endometrial cancer. Discov Med. 14:215–222.
2012.PubMed/NCBI
|
|
21
|
Khandekar MJ, Cohen P and Spiegelman BM:
Molecular mechanisms of cancer development in obesity. Nat Rev
Cancer. 11:886–895. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tsilidis KK, Kasimis JC, Lopez DS, Ntzani
EE and Ioannidis JP: Type 2 diabetes and cancer: umbrella review of
meta-analyses of observational studies. BMJ. 350:g76072015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Buchanan EM, Weinstein LC and Hillson C:
Endometrial cancer. Am Fam Physician. 80:1075–1080. 2009.PubMed/NCBI
|
|
24
|
Parazzini F, La Vecchia C, Moroni S,
Chatenoud L and Ricci E: Family history and the risk of endometrial
cancer. Int J Cancer. 59:460–462. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Aarnio M, Mecklin JP, Aaltonen LA,
Nyström-Lahti M and Järvinen HJ: Life-time risk of different
cancers in hereditary non-polyposis colorectal cancer (HNPCC)
syndrome. Int J Cancer. 64:430–433. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Obermair A, Youlden DR, Young JP, Lindor
NM, Baron JA, Newcomb P, Parry S, Hopper JL, Haile R and Jenkins
MA: Risk of endometrial cancer for women diagnosed with
HNPCC-related colorectal carcinoma. Int J Cancer. 127:2678–2684.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pilarski R, Burt R, Kohlman W, Pho L,
Shannon KM and Swisher E: Cowden syndrome and the PTEN hamartoma
tumor syndrome: Systematic review and revised diagnostic criteria.
J Natl Cancer Inst. 105:1607–1616. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhou B, Yang L, Sun Q, Cong R, Gu H, Tang
N, Zhu H and Wang B: Cigarette smoking and the risk of endometrial
cancer: A meta-analysis. Am J Med. 121:501–508.e3. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Loerbroks A, Schouten LJ, Goldbohm RA and
van den Brandt PA: Alcohol consumption, cigarette smoking, and
endometrial cancer risk: Results from the Netherlands Cohort Study.
Cancer Causes Control. 18:551–560. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lindemann K, Vatten LJ, Ellstrøm-Engh M
and Eskild A: Body mass, diabetes and smoking, and endometrial
cancer risk: A follow-up study. Br J Cancer. 98:1582–1585. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Michnovicz JJ, Hershcopf RJ, Naganuma H,
Bradlow HL and Fishman J: Increased 2-hydroxylation of estradiol as
a possible mechanism for the anti-estrogenic effect of cigarette
smoking. N Engl J Med. 315:1305–1309. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Aune D, Navarro Rosenblatt DA, Chan DS,
Vingeliene S, Abar L, Vieira AR, Greenwood DC, Bandera EV and Norat
T: Anthropometric factors and endometrial cancer risk: A systematic
review and dose-response meta-analysis of prospective studies. Ann
Oncol. 26:1635–1648. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bokhman JV: Two pathogenetic types of
endometrial carcinoma. Gynecol Oncol. 15:10–17. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wilczyński M, Danielska J and Wilczyński
J: An update of the classical Bokhman's dualistic model of
endometrial cancer. Prz Menopauzalny. 15:63–68. 2016.PubMed/NCBI
|
|
35
|
Kandoth C, Schultz N, Cherniack AD, Akbani
R, Liu Y, Shen H, Robertson AG, Pashtan I, Shen R, Benz CC, et al:
Integrated genomic characterization of endometrial carcinoma.
Nature. 497:67–73. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Piulats JM, Guerra E, Gil-Martín M,
Roman-Canal B, Gatius S, Sanz-Pamplona R, Velasco A, Vidal A and
Matias-Guiu X: Molecular approaches for classifying endometrial
carcinoma. Gynecol Oncol. 145:200–207. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Oaknin A, León-Castillo A and Lorusso D:
Progress in the management of endometrial cancer (subtypes,
immunotherapy, alterations in PIK3CA pathway): Data and
perspectives. Curr Opin Oncol. 32:471–480. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Talhouk A, McConechy MK, Leung S, Li-Chang
HH, Kwon JS, Melnyk N, Yang W, Senz J, Boyd N, Karnezis AN, et al:
A clinically applicable molecular-based classification for
endometrial cancers. Br J Cancer. 113:299–310. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ishida Y, Agata Y, Shibahara K and Honjo
T: Induced expression of PD-1, a novel member of the immunoglobulin
gene superfamily, upon programmed cell death. EMBO J. 11:3887–3895.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mamalis A, Garcha M and Jagdeo J:
Targeting the PD-1 pathway: A promising future for the treatment of
melanoma. Arch Dermatol Res. 306:511–519. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Keir ME, Butte MJ, Freeman GJ and Sharpe
AH: PD-1 and its ligands in tolerance and immunity. Annu Rev
Immunol. 26:677–704. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wu Y, Chen W, Xu ZP and Gu W: PD-L1
Distribution and perspective for cancer immunotherapy-blockade,
knockdown, or inhibition. Front Immunol. 10:20222019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Alsaab HO, Sau S, Alzhrani R, Tatiparti K,
Bhise K, Kashaw SK and Iyer AK: PD-1 and PD-L1 checkpoint signaling
inhibition for cancer immunotherapy: Mechanism, combinations, and
clinical outcome. Front Pharmacol. 8:5612017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Pardoll DM: The blockade of immune
checkpoints in cancer immunotherapy. Nat Rev Cancer. 12:252–264.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Post CCB, Westermann AM, Bosse T,
Creutzberg CL and Kroep JR: PARP and PD-1/PD-L1 checkpoint
inhibition in recurrent or metastatic endometrial cancer. Crit Rev
Oncol Hematol. 152:1029732020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mo Z, Liu J, Zhang Q, Chen Z, Mei J, Liu
L, Yang S, Li H, Zhou L and You Z: Expression of PD-1, PD-L1 and
PD-L2 is associated with differentiation status and histological
type of endometrial cancer. Oncol Lett. 12:944–950. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
McMeekin DS, Tritchler DL, Cohn DE, Mutch
DG, Lankes HA, Geller MA, Powell MA, Backes FJ, Landrum LM, Zaino
R, et al: Clinicopathologic significance of mismatch repair defects
in endometrial cancer: An NRG Oncology/Gynecologic oncology group
study. J Clin Oncol. 34:3062–3068. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kautto EA, Bonneville R, Miya J, Yu L,
Krook MA, Reeser JW and Roychowdhury S: Performance evaluation for
rapid detection of pan-cancer microsatellite instability with
MANTIS. Oncotarget. 8:7452–7463. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Eggink FA, Van Gool IC, Leary A, Pollock
PM, Crosbie EJ, Mileshkin L, Jordanova ES, Adam J, Freeman-Mills L,
Church DN, et al: Immunological profiling of molecularly classified
high-risk endometrial cancers identifies POLE-mutant and
microsatellite unstable carcinomas as candidates for checkpoint
inhibition. Oncoimmunology. 6:e12645652016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yamashita H, Nakayama K, Ishikawa M,
Nakamura K, Ishibashi T, Sanuki K, Ono R, Sasamori H, Minamoto T,
Iida K, et al: Microsatellite instability is a biomarker for immune
checkpoint inhibitors in endometrial cancer. Oncotarget.
9:5652–5664. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Piulats JM and Matias-Guiu X:
Immunotherapy in endometrial cancer: In the nick of time. Clin
Cancer Res. 22:5623–5625. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Miller DS, Filiaci VL, Mannel RS, Cohn DE,
Matsumoto T, Tewari KS, DiSilvestro P, Pearl ML, Argenta PA, Powell
MA, et al: Carboplatin and paclitaxel for advanced endometrial
cancer: Final overall survival and adverse event analysis of a
phase III trial (NRG Oncology/GOG0209). J Clin Oncol. 38:3841–3850.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Nomura H, Aoki D, Takahashi F, Katsumata
N, Watanabe Y, Konishi I, Jobo T, Hatae M, Hiura M and Yaegashi N:
Randomized phase II study comparing docetaxel plus cisplatin,
docetaxel plus carboplatin, and paclitaxel plus carboplatin in
patients with advanced or recurrent endometrial carcinoma: A
Japanese Gynecologic Oncology Group study (JGOG2041). Ann Oncol.
22:636–642. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
McMeekin S, Dizon D, Barter J, Scambia G,
Manzyuk L, Lisyanskaya A, Oaknin A, Ringuette S, Mukhopadhyay P,
Rosenberg J, et al: Phase III randomized trial of second-line
ixabepilone versus paclitaxel or doxorubicin in women with advanced
endometrial cancer. Gynecol Oncol. 138:18–23. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Le DT, Uram JN, Wang H, Bartlett BR,
Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et
al: PD-1 Blockade in tumors with mismatch-repair deficiency. N Engl
J Med. 372:2509–2520. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Mehnert JM, Panda A, Zhong H, Hirshfield
K, Damare S, Lane K, Sokol L, Stein MN, Rodriguez-Rodriquez L,
Kaufman HL, et al: Immune activation and response to pembrolizumab
in POLE-mutant endometrial cancer. J Clin Invest. 126:2334–2340.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Le DT, Durham JN, Smith KN, Wang H,
Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, et
al: Mismatch repair deficiency predicts response of solid tumors to
PD-1 blockade. Science. 357:409–413. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ott PA, Bang YJ, Berton-Rigaud D, Elez E,
Pishvaian MJ, Rugo HS, Puzanov I, Mehnert JM, Aung KL, Lopez J, et
al: Safety and antitumor activity of pembrolizumab in advanced
programmed death ligand 1-positive endometrial cancer: Results From
the KEYNOTE-028 study. J Clin Oncol. 35:2535–2541. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Marabelle A, Le DT, Ascierto PA, Di
Giacomo AM, De Jesus-Acosta A, Delord JP, Geva R, Gottfried M,
Penel N, Hansen AR, et al: Efficacy of pembrolizumab in patients
with noncolorectal high microsatellite Instability/Mismatch
repair-deficient cancer: Results from the phase II KEYNOTE-158
study. J Clin Oncol. 38:1–10. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Santin AD, Bellone S, Buza N, Choi J,
Schwartz PE, Schlessinger J and Lifton RP: Regression of
chemotherapy-resistant polymerase ε (POLE) Ultra-mutated and MSH6
Hyper-mutated endometrial tumors with nivolumab. Clin Cancer Res.
22:5682–5687. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Tamura K, Hasegawa K, Katsumata N,
Matsumoto K, Mukai H, Takahashi S, Nomura H and Minami H: Efficacy
and safety of nivolumab in Japanese patients with uterine cervical
cancer, uterine corpus cancer, or soft tissue sarcoma: Multicenter,
open-label phase 2 trial. Cancer Sci. 110:2894–2904. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kasherman L, Ahrari S and Lheureux S:
Dostarlimab in the treatment of recurrent or primary advanced
endometrial cancer. Future Oncol. 17:877–892. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Oaknin A, Tinker AV, Gilbert L, Samouëlian
V, Mathews C, Brown J, Barretina-Ginesta MP, Moreno V, Gravina A,
Abdeddaim C, et al: Clinical activity and safety of the
anti-programmed Death 1 monoclonal antibody dostarlimab for
patients with recurrent or advanced mismatch repair-deficient
endometrial cancer: A nonrandomized phase 1 clinical Trial. JAMA
Oncol. 6:1766–1772. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Liu JF, Gordon M, Veneris J, Braiteh F,
Balmanoukian A, Eder JP, Oaknin A, Hamilton E, Wang Y, Sarkar I, et
al: Safety, clinical activity and biomarker assessments of
atezolizumab from a Phase I study in advanced/recurrent ovarian and
uterine cancers. Gynecol Oncol. 154:314–322. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Konstantinopoulos PA, Luo W, Liu JF,
Gulhan DC, Krasner C, Ishizuka JJ, Gockley AA, Buss M, Growdon WB,
Crowe H, et al: Phase II Study of Avelumab in patients with
mismatch repair deficient and mismatch repair proficient
recurrent/persistent endometrial cancer. J Clin Oncol.
37:2786–2794. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Antill Y, Kok PS, Robledo K, Yip S,
Cummins M, Smith D, Spurdle A, Barnes E, Lee YC, Friedlander M, et
al: Clinical activity of durvalumab for patients with advanced
mismatch repair-deficient and repair-proficient endometrial cancer.
A nonrandomized phase 2 clinical trial. J Immunother Cancer.
9:e0022552021. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Mittica G, Ghisoni E, Giannone G, Aglietta
M, Genta S and Valabrega G: Checkpoint inhibitors in endometrial
cancer: Preclinical rationale and clinical activity. Oncotarget.
8:90532–90544. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kato Y, Tabata K, Kimura T,
Yachie-Kinoshita A, Ozawa Y, Yamada K, Ito J, Tachino S, Hori Y,
Matsuki M, et al: Lenvatinib plus anti-PD-1 antibody combination
treatment activates CD8+ T cells through reduction of
tumor-associated macrophage and activation of the interferon
pathway. PLoS One. 14:e02125132019. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Taylor MH, Lee CH, Makker V, Rasco D,
Dutcus CE, Wu J, Stepan DE, Shumaker RC and Motzer RJ: Phase IB/II
trial of Lenvatinib plus pembrolizumab in patients with advanced
renal cell carcinoma, endometrial cancer, and other selected
advanced solid tumors. J Clin Oncol. 38:1154–1163. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Makker V, Rasco D, Vogelzang NJ, Brose MS,
Cohn AL, Mier J, Di Simone C, Hyman DM, Stepan DE, Dutcus CE, et
al: Lenvatinib plus pembrolizumab in patients with advanced
endometrial cancer: An interim analysis of a multicentre,
open-label, single-arm, phase 2 trial. Lancet Oncol. 20:711–718.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Makker V, Taylor MH, Aghajanian C, Oaknin
A, Mier J, Cohn AL, Romeo M, Bratos R, Brose MS, DiSimone C, et al:
Lenvatinib plus pembrolizumab in patients with advanced endometrial
cancer. J Clin Oncol. 38:2981–2992. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Lheureux S, Matei D, Konstantinopoulos PA,
Block MS, Jewell A, Gaillard S, McHale MS, McCourt CK, Temkin S,
Girda E, et al: A randomized phase II study of cabozantinib and
nivolumab versus nivolumab in recurrent endometrial cancer. J Clin
Oncol. 38:60102020. View Article : Google Scholar
|
|
73
|
Rousset-Rouviere S, Rochigneux P, Chrétien
AS, Fattori S, Gorvel L, Provansal M, Lambaudie E, Olive D and
Sabatier R: Endometrial carcinoma: Immune microenvironment and
emerging treatments in immuno-oncology. Biomedicines. 9:6322021.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Fumet JD, Limagne E, Thibaudin M, Truntzer
C, Bertaut A, Rederstorff E and Ghiringhelli F: Precision medicine
phase II study evaluating the efficacy of a double immunotherapy by
durvalumab and tremelimumab combined with olaparib in patients with
solid cancers and carriers of homologous recombination repair genes
mutation in response or stable after olaparib treatment. BMC
Cancer. 20:7482020. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Green AK, Feinberg J and Makker V: A
review of immune checkpoint blockade therapy in endometrial cancer.
Am Soc Clin Oncol Educ Book. 40:1–7. 2020.PubMed/NCBI
|
|
76
|
Zitvogel L, Kepp O and Kroemer G: Immune
parameters affecting the efficacy of chemotherapeutic regimens. Nat
Rev Clin Oncol. 8:151–160. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Liu WM, Fowler DW, Smith P and Dalgleish
AG: Pre-treatment with chemotherapy can enhance the antigenicity
and immunogenicity of tumours by promoting adaptive immune
responses. Br J Cancer. 102:115–123. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Luke JJ, Lemons JM, Karrison TG, Pitroda
SP, Melotek JM, Zha Y, Al-Hallaq HA, Arina A, Khodarev NN, Janisch
L, et al: Safety and clinical activity of pembrolizumab and
multisite stereotactic body radiotherapy in patients with advanced
solid tumors. J Clin Oncol. 36:1611–1618. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Walle T, Martinez Monge R, Cerwenka A,
Ajona D, Melero I and Lecanda F: Radiation effects on antitumor
immune responses: Current perspectives and challenges. Ther Adv Med
Oncol. 10:17588340177425752018. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Lee L and Matulonis U: Immunotherapy and
radiation combinatorial trials in gynecologic cancer: A potential
synergy? Gynecol Oncol. 154:236–245. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Tuyaerts S, Van Nuffel AMT, Naert E, Van
Dam PA, Vuylsteke P, De Caluwé A, Aspeslagh S, Dirix P, Lippens L,
De Jaeghere E, et al: PRIMMO study protocol: A phase II study
combining PD-1 blockade, radiation and immunomodulation to tackle
cervical and uterine cancer. BMC Cancer. 19:5062019. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Grandin M, Meier M, Delcros JG, Nikodemus
D, Reuten R, Patel TR, Goldschneider D, Orriss G, Krahn N,
Boussouar A, et al: Structural decoding of the Netrin-1/UNC5
interaction and its therapeutical implications in cancers. Cancer
Cell. 29:173–185. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Paradisi A, Creveaux M, Gibert B, Devailly
G, Redoulez E, Neves D, Cleyssac E, Treilleux I, Klein C,
Niederfellner G, et al: Combining chemotherapeutic agents and
netrin-1 interference potentiates cancer cell death. EMBO Mol Med.
5:1821–1834. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Pakish JB and Jazaeri AA: Immunotherapy in
gynecologic cancers: Are We there yet? Curr Treat Options Oncol.
18:592017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Pakish JB, Zhang Q, Chen Z, Liang H,
Chisholm GB, Yuan Y, Mok SC, Broaddus RR, Lu KH and Yates MS:
Immune microenvironment in microsatellite-instable endometrial
cancers: Hereditary or sporadic origin matters. Clin Cancer Res.
23:4473–4481. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Tronconi F, Nero C, Giudice E, Salutari V,
Musacchio L, Ricci C, Carbone MV, Ghizzoni V, Perri MT, Camarda F,
et al: Advanced and recurrent endometrial cancer: State of the art
and future perspectives. Crit Rev Oncol Hematol. 180:1038512022.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Abu-Rustum N, Yashar C, Arend R, Barber E,
Bradley K, Brooks R, Campos SM, Chino J, Chon HS, Chu C, et al:
Uterine Neoplasms, Version 1.2023, NCCN clinical practice
guidelines in oncology. J Natl Compr Canc Netw. 21:181–209. 2023.
View Article : Google Scholar : PubMed/NCBI
|















