Introduction
Distant metastases account for >90% of cancer
deaths (1,2). Despite major advances in drug
development for solid tumors, life-extending systemic therapy is
generally not curative when used as a single modality (3). The oligometastasis hypothesis implies
that patients with limited metastatic burden can benefit from
comprehensive local treatment with the possibility of long-term
disease control, particularly when combined with effective systemic
therapy to prevent new metastases (4). Pioneering prospective studies
conducted in the 2000′s reproducibly confirmed long-term
disease-free survival in 20 to 30% of patients with extracranial
oligometastases long-term follow-up (5). These promising data led to randomized
trials confirming improved progression-free survival and overall
survival when supplementing metastasis-directed therapy to systemic
therapy alone for patients with extracranial oligometastases from
non-small cell lung cancer and mixed primary tumors (6,7).
Based on these data, patients with oligometastases
receiving radiation therapy to all areas of known disease represent
an important subset of contemporary patients referred to radiation
oncology for distant metastases (8). After screening 29 clinical,
pathologic, radiologic and laboratory variables, our group
identified and validated 4 prognostic factors independently
predicting survival in unselected patients with distant metastases
referred to radiation oncology (9,10).
Taken together, the components of the NEAT predictive model
predicting higher survival are higher performance status, normal
serum albumin, less widespread disease and breast, prostate or
kidney primary tumor. A surprising finding was that brain
metastases were not independently associated with poorer outcome
(9).
In a recent analysis, patients with distant
metastases selected for higher dose radiation defined as an
equivalent dose in 2 Gy fractions (EQD2) of ≥40 Gy had a median
survival of 16.0 months compared to 3.8 months for patients treated
with an EQD2 of <40 Gy (8). We
hypothesized that performance status, serum albumin and primary
tumor type would remain robust prognostic factors in a cohort of
patients with oligometastases, including limited brain metastases,
treated with higher dose radiation.
The ESTRO/EORTC consensus oligometastasis
classification proposed 9 distinct oligometastasis categories
(2). There are emerging data
suggesting that patients with induced oligometastases have worse
survival that patients with de novo oligometastases or repeat
oligometastases (11). The purpose
of this work is to report the long-term outcomes of patients with
oligometastases treated with curative intent radiotherapy in the
context of the recently developed ESTRO/EORTC classification
schema.
Materials and methods
Patient population
This retrospective single institution registry study
was approved by the IRB #16-016 with waiver of informed consent.
The study population consists of consecutive adult patients 18
years of age or older with oligometastases from solid tumor
malignancy referred to a single high volume radiation oncologist
(JK) between 1/1/14 to 12/31/21. Oligometastases were defined as 5
or fewer extracranial or intracranial metastatic lesions where all
sites of active disease are amenable to treatment. For synchronous
metastases, the primary tumor ± regional lymph nodes were also
treated with radiotherapy. For metachronous metastases, the primary
tumor was controlled with prior local therapy. Systemic therapy was
integrated with radiotherapy at the discretion of the treating
medical oncologist. Whole body imaging consisted of PET/CT or CT
chest, abdomen and pelvis with bone scan ± brain or spine
imaging.
Treatment and follow-up
Immobilization, simulation, radiation treatment
volumes and schedules were personalized based on location, volume
and organs at risk. Depending on location, volume and organs at
risk, intensity modulated radiation therapy, stereotactic body
radiotherapy and stereotactic radiosurgery were prescribed.
Image-guided radiation therapy was delivered on the Varian TrueBeam
or Varian Edge. Systemic therapy prior to, during or after
radiation was administered at the discretion of the treating
medical oncology and/or urologist.
Patients were followed by radiation oncology and
medical oncology using an integrated electronic medical record
system (EPIC) supplemented by tumor imaging and blood work.
Generally, follow-up was robust with a daily inpatient oncology
huddle attended by radiation oncology and medical oncology
supplementing scheduled outpatient follow-up. The minority of
patients lost to follow-up were supplemented by requests for office
records, phone calls and review of obituary records.
Outcomes
The primary endpoints were overall survival and
progression-free survival measured from date of consultation to
time of event. Our group has previously demonstrated the prognostic
significance of ECOG performance status, primary tumor and
pre-treatment serum albumin on survival in patients with distant
metastases. Additionally, we investigated the prognostic
significance of ESTRO/EORTC oligometastatic disease classification,
age, sex, lesion site, number of metastases treated, whether the
primary tumor was also treated and systemic therapy previously
reported by other authors.
Statistical analysis
Overall survival and progression-free survival were
estimated using the Kaplan-Meier method. Patients lost to follow-up
were censored at last known follow-up. Candidate predictors of
survival were assessed using univariate Cox regression. Variables
with a P-value of <0.10 were entered into multivariate Cox
regression analysis. A P-value of <0.05 was considered
statistically significant. All analyses were performed using Stata
13.1.
Results
Patient and treatment
characteristics
The study population consists of 130 patients with
207 treated distant metastases referred to radiation oncology
between 1/2014 and 12/2021. Key patient characteristics include
median age 71 (range 28 to 96), 54% male, 72% ECOG 0–1 performance
status, median pre-radiation albumin 3.7 g/dl, 35% lung primary,
12% prostate primary, median of 1 distant metastasis treated, 31%
bone metastases, 30% brain metastases (Table I).
 | Table I.Characteristics of 130 patients with
oligometastases. |
Table I.
Characteristics of 130 patients with
oligometastases.
| Variable | % (n) |
|---|
| Age (years) |
|
|
<60 | 19 (25) |
|
60-79 | 55 (72) |
| ≥80 | 26 (34) |
| Sex |
|
| Male | 54 (70) |
|
Female | 46 (60) |
| ECOG performance
status |
|
| 0 | 23 (30) |
| 1 | 48 (63) |
| 2 | 22 (28) |
| 3 or
4 | 7 (9) |
| Category of
oligometastatic disease |
|
| De
novo oligometastases (synchronous oligometastatic, metachronous
oligorecurrence or metachronous oligoprogression) | 77 (100) |
| Repeat
oligometastasis (repeat oligorecurrence, repeat oligopersistence or
repeat oligoprogression) | 11 (14) |
| Induced
oligometastasis (induced oligorecurrence, induced oligopersistence
or induced oligoprogression | 12 (16) |
| Primary
tumora |
|
| Lung | 35 (45) |
|
Prostate | 12 (15) |
|
Breast | 9 (12) |
|
Colorectal | 8 (10) |
|
Endometrial | 8 (10) |
|
Other | 29 (38) |
| Metastasis
location | 207 tumors |
|
Bone | 31 (40) |
|
Brain | 30 (39) |
|
Lung | 22 (28) |
| Distant
Lymph Nodes | 19 (25) |
|
Liver | 10 (13) |
| Adrenal
gland | 3 (4) |
| Albumin (g/dl) |
|
|
≥3.4 | 66 (86) |
|
2.4–3.3 | 24 (31) |
|
<2.4 | 2 (2) |
|
Unknown | 8 (11) |
| Number of
metastases treated |
|
| 0 | 7 (9) |
| 1 | 56 (73) |
|
2-5 | 37 (46) |
| Active primary
tumor requiring treatment |
|
| No | 53 (69) |
|
Yes | 47 (61) |
| Post-RT systemic
therapy |
|
| No | 29 (38) |
|
Yes | 71 (92) |
The most frequent ESTRO oligometastatic groups were
synchronous oligometastases (40%) and metachronous oligorecurrence
(29%) (Table SI). The incidence of
de novo oligometastases was 77% with 11% repeat oligometastasis and
12% induced oligometastases.
A total of 69 patients received stereotactic
radiation with a median dose of 27 Gy (interquartile range 27 to 33
Gy) in a median of 3 fractions (interquartile range 3 to 4). A
total of 84 patients were treated with image-guided radiation
therapy to a median dose of 50 Gy (interquartile range 45 to 59.4
Gy) in a median of 15 fractions (interquartile range 10 to 28
fractions). A small subset of patients underwent surgery (most
commonly craniotomy). Two patients underwent interventional
radiology ablation and 1 patient underwent brachytherapy (20 Gy in
4 fractions). In addition to radiation to distant metastases, 47%
received synchronous treatment to the primary tumor ± regional
lymph nodes.
Prior to radiation, 68% were not actively receiving
systemic therapy while 12% were receiving chemotherapy alone, 7%
hormonal therapy ± adjuncts such as androgen receptor blockade
and/or CDK 4/6 inhibitors, 5% immunotherapy or targeted therapy
alone and 8% chemotherapy in combination with immunotherapy or
targeted therapy. During or following radiation, 74% received
systemic therapy with diverse treatment regimens (Table SII).
Survival outcomes
At a median follow-up among surviving patients of
28.8 months (IQR 16.0 to 56.3 months), a total of 66 patients (49%)
died and 92 patients (71%) experienced disease progression. The
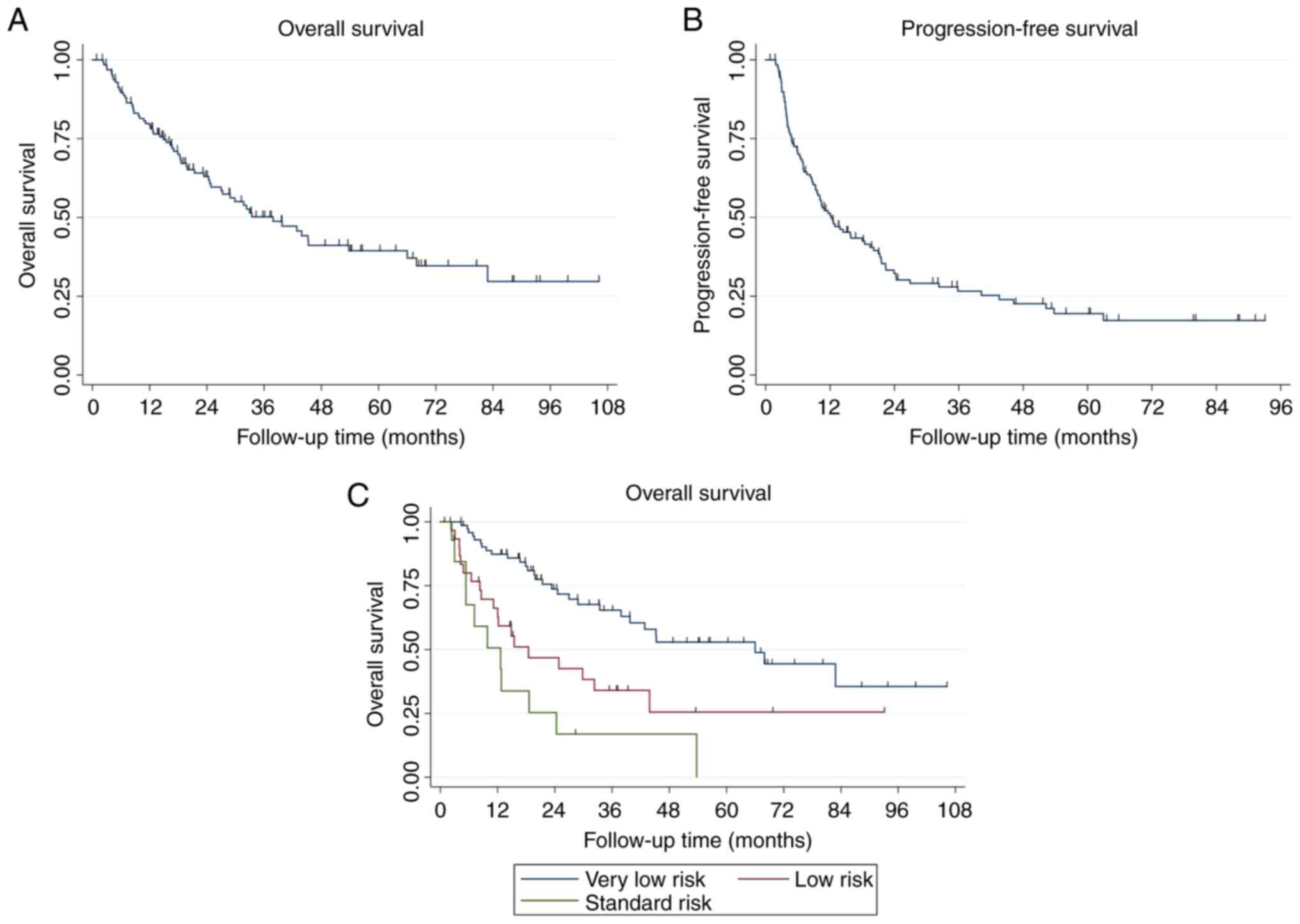
median overall survival is 37.9 months with a 4-year overall
survival of 41.1% (95% CI 30.7–51.3) (Fig. 1A). The median progression-free
survival was 12.3 months with a 4-year progression-free survival of
22.6% (95% CI, 14.9–31.3) (Fig.
1B).
Predictors of survival
On univariate analysis, age, ECOG performance
status, primary prostate, breast or kidney tumor and albumin
predicted overall survival (Table
SIII). Other variables including sex, category of ESTRO/EORTC
oligometastatic disease, metastasis location, number of distant
metastases treated, active primary tumor requiring treatment and
post-radiation systemic therapy were not predictive of overall
survival (Tables SI and SIII).
On multivariate analysis, age [HR 1.05 (1.02–1.08);
CI: 95%; P<0.001], ECOG performance status [HR 1.69 (1.15–2.47);
CI: 95%; P=0.007], primary prostate, breast or kidney tumor [HR
2.79 (1.29–6.03); CI: 95%; P=0.009] and pre-radiation serum albumin
[HR 0.55 (0.35–0.87); CI: 95%; P=0.01] were independently
predictive of overall survival (Table
II). The previously validated NEAT model included ECOG
performance status (0–1 vs. 2 vs. 3–4), primary tumor (favorable
vs. unfavorable) and albumin (≥3.4 vs. 2.4 to 3.3 vs. <2.4).
 | Table II.Multivariate analysis for overall
survival and progression-free survival. |
Table II.
Multivariate analysis for overall
survival and progression-free survival.
| A, Overall
survival |
|---|
|
|---|
| Variable | HR (95% CI) | P-value |
|---|
| Age (years;
continuous) | 1.05
(1.02–1.08) | 0.001 |
| ECOG performance
status (continuous) | 1.69
(1.15–2.47) | 0.007 |
| Primary tumor site
(breast, prostate or kidney vs. other) | 2.79
(1.29–6.03) | 0.009 |
| Albumin (g/dl;
continuous) | 0.55
(0.35–0.87) | 0.011 |
| Induced
oligometastases (no vs. yes) | 1.39
(0.68–2.88) | 0.369 |
|
| B,
Progression-free survival |
|
|
Variable | HR (95%
CI) | P-value |
|
| Albumin (g/dl;
continuous) | 0.59
(0.39–0.88) | 0.009 |
| Primary tumor site
(breast, prostate or kidney vs. other) | 1.90
(1.06–3.38) | 0.031 |
| ECOG performance
status (continuous) | 1.22
(0.90–1.66) | 0.203 |
| No. of metastases
treated (0 to 1 vs. ≥2) | 1.29
(0.81–2.07) | 0.272 |
| Age (years;
continuous) | 1.01
(0.99–1.03) | 0.312 |
| Induced
oligometastases (no vs. yes) | 1.28
(0.67–2.44) | 0.458 |
When applying the NEAT model to this cohort of
patients with oligometastases receiving comprehensive radiation,
the median survivals by risk group for very favorable, favorable
and standard risk groups were 66.0 months, 18.5 months and 12.6
months respectively (P<0.001) (Fig.
1C).
On univariate analysis, albumin, primary tumor site,
induced oligometastases and >1 metastasis treated predicted for
progression-free survival (Table
SIII). The strongest predictors of progression free survival on
multivariate analysis are albumin [HR 0.59 (0.39–0.88); CI: 95%;
P=0.009] and primary prostate, breast or kidney tumor [HR 1.90
(1.06–3.38); CI: 95%; P=0.03] (Table
IIB).
Discussion
This dataset is unique because it represents a large
single physician experience with comprehensive radiation to all
areas of known disease for oligometastases with close long-term
follow-up. In the context of a busy community hospital with
inpatient radiation oncology, the oligometastasis patient
population includes higher proportion of patients with lung
primary, brain metastases, ECOG 2 to 3 performance status and
advanced age. Additionally, radiation administered included not
only stereotactic radiation but also intensity modulated radiation
with an EQD2 ≥40 Gy. Despite including higher risk patients, the
4-year overall survival was 41% and the 4-year progression-free
survival was 23% comparable to published series with more
restrictive eligibility criteria (7,12–18)
(Table III). In previously
published series, the 4-year overall survival was 25 to 58%
(Table III). In the current
study, the median survival was 38 months These results are
reflective of a pragmatic community practice thus broadening access
to oligometastasis treatment to include patients with brain
metastases, those ineligible for stereotactic radiation and
patients with induced oligometastases with disease limited to the
primary site and regional lymph nodes. While patients with brain
metastases were previously considered a poor prognosis cohort,
modern stereotactic techniques can achieve long-term disease
control (19). In this series, site
of metastases including brain metastases did not predict survival.
Finally, the patient population treated in a community hospital
with a busy emergency room skewed older with a median age of 71
compared to prior studies with a median or mean age of 63 to 69
(Table III). On multivariable
analysis, older age was predictive of shorter overall survival.
 | Table III.Selected studies of oligometastases
with long-term follow-up. |
Table III.
Selected studies of oligometastases
with long-term follow-up.
| Study | No. of
patients | Age (years) | Median OS
(months) | Long-term OS | Long-term PFS | Performance
status | Primary tumors | Brain metastasis
(%) | Other patient
selection notes | (Refs.) |
|---|
|
Multi-institutional- | 361 | 63 | 47 | 3-year | 3 year |
| 19% | 0 | - ~40% lung | (15) |
| Duke
University |
| (median) |
| OS 56% | PFS 24% | Not reported | colorectal |
| metastases |
|
|
|
|
|
|
|
|
| 17% lung |
|
|
|
|
|
|
|
|
|
|
| 16% breast |
|
|
|
|
Multi-institutional- | 1,033 | 68 | 44 | 5-year | 5-year | Not reported | 25% lung | 0 | -Induced | (16) |
| University of |
| (mean) |
| OS 35% | PFS 15% |
| 23% colorectal |
|
oligometastases |
|
| Toronto |
|
|
|
|
|
| 13% prostate |
| excluded |
|
|
|
|
|
|
|
|
|
|
| −45% lung |
|
|
|
|
|
|
|
|
|
|
| metastases |
|
| National
Health | 1,422 | 69 | Not | 2-year | 2-year | 95% | 29% prostate | 0 | −1 to 3 | (13) |
| Service
registry |
| (median) | reached | OS 79% | MFS 52% | ECOG 0–1 | 28% colorectal |
| metachronous |
|
|
|
|
|
|
|
|
| 10% renal |
| extracranial |
|
|
|
|
|
|
|
|
|
|
| metastases or |
|
|
|
|
|
|
|
|
|
|
| synchronous |
|
|
|
|
|
|
|
|
|
|
| colorectal
liver |
|
|
|
|
|
|
|
|
|
|
| metastases |
|
|
|
|
|
|
|
|
|
|
| −31% lymph
nodes |
|
|
|
|
|
|
|
|
|
|
| −29% lung |
|
|
|
|
|
|
|
|
|
|
| metastases |
|
| NRG BR-001 | 39 | 63 | Not | 2 year | Not | 92% | 33% breast | 0 | −2 to 4
distant | (18) |
|
|
| (mean) | reached | OS 57% | reported | ECOG 0–1 | 33% lung |
| metastases |
|
|
|
|
|
|
|
|
| 33% prostate |
|
|
|
| SABR-5 | 381 | 68 | Not | 4-year | 4-year | 96% | 32% prostate | 1 | −40% bone | (29) |
|
|
| (mean) | reached | OS 58% | PFS 29% | ECOG 0–1 |
|
| metastases |
|
|
|
|
|
|
|
|
|
|
| −35% lung |
|
|
|
|
|
|
|
|
|
|
| metastases |
|
| SABR-COMET | 66 | 67 to 69 | 50 | 5-year | 5-year | 100% | 21% prostate | 2 (of | −43% lung | (6) |
|
|
| (median) |
| OS 42% | PFS 17% | ECOG 0–1 | 20% breast | metastatic | metastases |
|
|
|
|
|
|
|
|
| 18% lung | lesions) | −35% bone |
|
|
|
|
|
|
|
|
|
|
| metastases |
|
| University of | 147 | 63 | 42 | 5-year | 5-year | 100% | 22% lung | 1 | -Excluded | (17) |
| Pittsburgh |
| (median) |
| OS 43% | MFS 17% | Zubrod 0–1 | 21% colorectal |
| synchronous |
|
|
|
|
|
|
|
|
| 11% head and |
|
oligometastases |
|
|
|
|
|
|
|
|
| neck |
| −52% lung |
|
|
|
|
|
|
|
|
|
|
| metastases |
|
| Vrjie
Universiteit | 309 | 63 | 24 | 4 year | 2-year | KPS | 33% lung | 35 | −29% lymph
node | (14) |
| Brussels |
| (median) |
| OS 25% | PFS 10% | 80 to 100 | 33% colorectal |
| metastases |
|
|
|
|
|
|
|
|
| cancer |
|
|
|
|
|
|
|
|
|
|
| 11% breast |
|
|
|
| Current study | 130 | 71 | 38 | 4-year | 4-year | 72% | 35% lung | 30 | −31% bone |
|
|
|
| (median) |
| OS 38% | PFS 23% | ECOG 0–1 | 12% prostate |
| metastases |
|
|
|
|
|
|
|
|
| 9% breast |
|
|
|
In this present study, the treating radiation
oncologist treated 16 new oligometastases patients per year while
treating an average of 71 new patients with distant metastases per
year (8). These data suggest that
~20% of patients with distant metastases referred to radiation
oncology have oligometastases amenable to metastasis directed
therapy. With respect to patient selection, there were 4-year
survivors across all subgroups examined with the exception of small
cohorts of ECOG 3–4 (n=9), albumin <2.4 (n=2), repeat
oligoprogression (n=5) and induced oligorecurrence (n=2).
Predicting outcome for patients with oligometastases
is particularly important because a basket design is pragmatic for
accrual purposes but is inherently heterogeneous (20). Our group previously developed and
validated the NEAT model for patients with distant metastases
referred to radiation therapy that included oligometastases
(9,10). The prognostic factors contributing
to the NEAT model also independently predict overall survival in
this cohort of patients with oligometastases. Patients with
oligometastases had numerically higher survival by NEAT grouping
compared to the entire cohort of patients with distant metastases
−66.0 vs. 29.5 months for very low risk, 18.5 vs. 11.8 months for
favorable and 12.6 months vs. 4.9 months for intermediate risk.
These findings provide further evidence that treating all areas of
known involvement contributes to improved progression-free survival
and overall survival.
Confirming prior research, patients with induced
oligometastases have a numerically worse overall and
progression-free survival in the present study (11,12).
The notion that synchronous oligometastases may have worse outcomes
than metachronous oligometastases informed design of the
ESTRO/EORTC classification system (2). Due to small numbers, any observed
differences in survival in this study were only marginally
significant on univariate analysis and was not statistically
significant on multivariate analysis. The authors eagerly await
prospective data on the oligometastatic disease classification
through the ongoing OligoCare study for further clarification
regarding the prognostic significance of the ESTRO/EORTC
classification.
The present study highlights the importance of
albumin in predicting survival for patients with distant
metastases, including oligometastases. In a systematic review,
pretreatment serum albumin provides useful prognostic information
across various types of cancer (21). While there is great interest in
emerging biomarkers such as circulating tumor DNA (ctDNA),
comprehensive genomic testing and PD-L1, there is currently only
minimal interest in utilizing serum albumin as a simple, widely
available and low cost biomarker for future radiation oncology
trials investigating metastatic disease (22).
The finding that age impacted survival was not seen
in prior analyses of patients with unselected distant metastases or
oligometastases (9,10). The present study included 26% of
patients age ≥80 and advanced age is a risk factor for all-cause
mortality, particularly during the SARS-CoV-2 pandemic (23). Although 64% of patients age ≥80 in
the present study received systemic therapy, frailty upon
progression certainly limited the availability and effectiveness of
salvage systemic options with earlier initiation of transition to
hospice.
This study should be interpreted in the context of
both strengths and limitations. First, this is a single physician
study performed by an investigator with extensive experience in
safely treating oligometastases for over a decade and interest in
prognostication to inform treatment decisions. Therefore, these
findings may not be generalizable to other settings. Second,
patient numbers were insufficient to make definitive conclusions
about small subsets including individual ESTRO/EOTRC
oligometastatic disease classifications. Although single physician
series are currently out of vogue because of inherently lower
sample sizes, data quality may be higher because each patient is
well known to the treating physician who also performs longitudinal
follow-up. The community hospital setting has unique strengths
because distance travelled is shorter and patients and their
families tend to rely on their local healthcare infrastructure for
acute hospitalizations towards the end of life rather than
traveling to more distant quaternary facilities (24). The recent implementation of
multidisciplinary daily oncology rounds further enhances the
quality of longitudinal follow-up. Finally, it is important to
promote the inclusion of the perspectives of a community oncology
practice serving a racially and economically diverse community with
more limited resources in contrast to major academic centers
(25).
In terms of future directions for treatment of
oligometastases, further drug development would reduce the
incidence of early progression (1,3).
Integrating ctDNA could allow for earlier and more effective use of
salvage therapies, including repeat stereotactic body radiotherapy
(26). Utilizing genomic
information could achieve more precise prognostication to reduce
futile therapy. Optimizing cancer survivorship for long-term
survivors of distant metastases is another important avenue of
research (27,28). Finally, validating accurate
predictors of early widespread progression after metastasis
directed therapy could lead to the design of more effective
treatment strategies and avoiding futile therapy (29).
In conclusion, long-term overall survival is
possible after radical treatment for oligometastases in the real
world setting. The ESTRO classification provides an enriched
nomenclature for oligometastases but is not independently
predictive of overall survival.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JK and AS contributed to the conception and design
of the study. JK, PE, JM, CA and AS contributed to the acquisition,
analysis and interpretation of the data, and contributed to
manuscript drafting and critical revisions on the intellectual
content. All authors have read and approved the final version of
the manuscript and agreed to be accountable for all aspects of the
work. JK and PE confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
This retrospective single institution registry study
was approved by the Good Samaritan University Hospital (approval
no. IRB #16-016) with a waiver for informed consent.
Patient consent for publication
Not applicable.
Competing interests
JK served on Advisory Boards for Astra Zeneca and
previously led an educational webinar for Varian. The other authors
declare that they have no competing interests.
References
|
1
|
Gerstberger S, Jiang Q and Ganesh K:
Metastasis. Cell. 186:1564–1579. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guckenberger M, Lievens Y, Bouma AB,
Collette L, Dekker A, deSouza NM, Dingemans AC, Fournier B,
Hurkmans C, Lecouvet FE, et al: Characterisation and classification
of oligometastatic disease: A European society for radiotherapy and
oncology and european organisation for research and treatment of
cancer consensus recommendation. Lancet Oncol. 21:e18–e28. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Haslam A and Prasad V: Estimation of the
percentage of US patients with cancer who are eligible for and
respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw
Open. 2:e1925352019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hellman S and Weichselbaum RR:
Oligometastases. J Clin Oncol. 13:8–10. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Salama JK and Milano MT: Radical
irradiation of extracranial oligometastases. J Clin Oncol.
32:2902–2912. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gomez DR, Tang C, Zhang J, Blumenschein GR
Jr, Hernandez M, Lee JJ, Ye R, Palma DA, Louie AV, Camidge DR, et
al: Local consolidative therapy vs maintenance therapy or
observation for patients with oligometastatic non-small-cell lung
cancer: Long-term results of a multi-institutional, phase II,
randomized study. J Clin Oncol. 37:1558–1565. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Palma DA, Olson R, Harrow S, Gaede S,
Louie AV, Haasbeek C, Mulroy L, Lock M, Rodrigues GB, Yaremko BP,
et al: Stereotactic ablative radiotherapy for the comprehensive
treatment of oligometastatic cancers: Long-term results of the
SABR-COMET phase II randomized trial. J Clin Oncol. 38:2830–2838.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kao J, Farrugia MK, Frontario S, Zucker A,
Copel E, Loscalzo J, Sangal A, Darakchiev B, Singh A and Missios S:
Association of radiation dose intensity with overall survival in
patients with distant metastases. Cancer Med. 10:7934–7942. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kao J, Gold KD, Zarrili G, Copel E,
Silverman AJ, Ramsaran SS, Yens D and Ryu S: Clinical predictors of
survival for patients with stage IV cancer referred to radiation
oncology. PLoS One. 10:e01243292015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zucker A, Tsai CJ, Loscalzo J, Calves P
and Kao J: The NEAT predictive model for survival in patients with
advanced cancer. Cancer Res Treat. 50:1433–1443. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Willmann J, Vlaskou Badra E, Adilovic S,
Ahmadsei M, Christ SM, van Timmeren JE, Kroeze SGC, Mayinger M,
Guckenberger M and Andratschke N: Evaluation of the prognostic
value of the ESTRO EORTC classification of oligometastatic disease
in patients treated with stereotactic body radiotherapy: A
retrospective single center study. Radiother Oncol. 168:256–264.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Baker S, Mou B, Jiang W, Liu M, Bergman
AM, Schellenberg D, Alexander AS, Carolan H, Atrchian S, Berrang T,
et al: Validation of the prognostic utility of ESTRO/EORTC
oligometastatic disease classification: A secondary analysis from
the population-based phase II SABR-5 trial. Int J Radiat Oncol Biol
Phys. 114:849–855. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chalkidou A, Macmillan T, Grzeda MT,
Peacock J, Summers J, Eddy S, Coker B, Patrick H, Powell H, Berry
L, et al: Stereotactic ablative body radiotherapy in patients with
oligometastatic cancers: A prospective, registry-based, single-arm,
observational, evaluation study. Lancet Oncol. 22:98–106. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
de Vin T, Engels B, Gevaert T, Storme G
and De Ridder M: Stereotactic radiotherapy for oligometastatic
cancer: A prognostic model for survival. Ann Oncol. 25:467–471.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hong JC, Ayala-Peacock DN, Lee J,
Blackstock AW, Okunieff P, Sung MW, Weichselbaum RR, Kao J, Urbanic
JJ, Milano MT, et al: Classification for long-term survival in
oligometastatic patients treated with ablative radiotherapy: A
multi-institutional pooled analysis. PLoS One. 13:e01951492018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Poon I, Erler D, Dagan R, Redmond KJ,
Foote M, Badellino S, Biswas T, Louie AV, Lee Y, Atenafu EG, et al:
Evaluation of definitive stereotactic body radiotherapy and
outcomes in adults with extracranial oligometastasis. JAMA Netw
Open. 3:e20263122020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sutera P, Clump DA, Kalash R, D'Ambrosio
D, Mihai A, Wang H, Petro DP, Burton SA and Heron DE: Initial
results of a multicenter phase 2 trial of stereotactic ablative
radiation therapy for oligometastatic cancer. Int J Radiat Oncol
Biol Phys. 103:116–122. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chmura S, Winter KA, Robinson C, Pisansky
TM, Borges V, Al-Hallaq H, Matuszak M, Park SS, Yi S, Hasan Y, et
al: Evaluation of safety of stereotactic body radiotherapy for the
treatment of patients with multiple metastases: Findings from the
NRG-BR001 phase 1 trial. JAMA Oncol. 7:845–852. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Greto D, Scoccianti S, Compagnucci A,
Arilli C, Casati M, Francolini G, Cecchini S, Loi M, Desideri I,
Bordi L, et al: Gamma knife radiosurgery in the management of
single and multiple brain metastases. Clin Neurol Neurosurg.
141:43–47. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tsai CJ: Moving away from counting the
numbers: Leveraging a sensible clinical trial design for
oligometastatic disease and beyond. Int J Radiat Oncol Biol Phys.
114:846–848. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gupta D and Lis CG: Pretreatment serum
albumin as a predictor of cancer survival: A systematic review of
the epidemiological literature. Nutr J. 9:692010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Iyengar P: Local therapy for
oligometastatic disease-cart before the horse? Int J Radiat Oncol
Biol Phys. 114:836–839. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chavez-MacGregor M, Lei X, Zhao H, Scheet
P and Giordano SH: Evaluation of COVID-19 mortality and adverse
outcomes in US patients with or without cancer. JAMA Oncol.
8:69–78. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lamont EB, Hayreh D, Pickett KE, Dignam
JJ, List MA, Stenson KM, Haraf DJ, Brockstein BE, Sellergren SA and
Vokes EE: Is patient travel distance associated with survival on
phase II clinical trials in oncology? J Natl Cancer Inst.
95:1370–1375. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ledesma Vicioso N, Lin D, Gomez DR, Yang
JT, Lee NY, Rimner A, Yamada Y, Zelefsky MJ, Kalman NS, Rutter CE,
et al: Implementation strategies to increase clinical trial
enrollment in a community-academic partnership and impact on
hispanic representation: An interrupted time series analysis. JCO
Oncol Pract. 18:e780–e785. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sanz-Garcia E, Zhao E, Bratman SV and Siu
LL: Monitoring and adapting cancer treatment using circulating
tumor DNA kinetics: Current research, opportunities, and
challenges. Sci Adv. 8:eabi86182022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gallicchio L, Devasia TP, Tonorezos E,
Mollica MA and Mariotto A: Estimation of the number of individuals
living with metastatic cancer in the United States. J Natl Cancer
Inst. 114:1476–1483. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Langbaum T and Smith TJ: Time to study
metastatic-cancer survivorship. N Engl J Med. 380:1300–1302. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Baker S, Mou B, Jiang W, Liu M, Bergman
AM, Schellenberg D, Alexander AS, Carolan H, Atrchian S, Berrang T,
et al: Predictors of early polymetastatic relapse after SABR for up
to 5 oligometastases: A secondary analysis of the phase II SABR-5
trial. Int J Radiat Oncol Biol Phys. 114:856–861. 2022. View Article : Google Scholar : PubMed/NCBI
|