Introduction
Colorectal cancer is the third most common
malignancy and the second leading cause of cancer-related deaths
worldwide (1). Surgical treatment
is the only method enabling a potential cure for patients with
Stage 1–3 colorectal cancer. Along with the aging of the
population, the number of elderly patients who receive surgical
treatment for colorectal cancer has gradually increased. The
incidence rate of colorectal cancer escalates rapidly with age,
increasing by approximately 30% with each 5-year age increase aged
55 years and older (2). The median
age at diagnosis of colorectal cancer is 69–72 years, with 70% of
colorectal cancer diagnosed in patients over 65 years (3–5).
Approximately 17.5% of colorectal cancer patients are diagnosed
when they are over 80 years (6).
Unfortunately, most elderly patients have comorbidities, such as
cardiovascular or pulmonary diseases, and reduced functional
reserve, which may increase their risks of postoperative morbidity,
mortality, and prognosis and subsequently influence the treatment
choice (e.g., avoiding curative resection).
Sarcopenia was first described in 1988 as a
condition in which muscle mass decreases with age (7). It was reported that more than half of
people aged 80 years or older was sarcopenia (8). Among colorectal cancer patients, the
number of patients with sarcopenia is increasing due to the aging
population (9). Recently,
preoperative sarcopenia has attracted attention as a predictor of
postoperative complications and prognosis. In a recent
meta-analysis, sarcopenia was prevalent in 37% of colorectal cancer
patients, significantly prolonging hospital stay and increasing
postoperative complications and mortality rates (10,11).
Sarcopenia has also been linked with poor long-term outcomes in
patients receiving curative resection for stage 1–3 colorectal
cancer (12) and in patients with
rectal cancer who underwent preoperative chemoradiotherapy
(13,14). Despite increased awareness, a
preventative treatment strategy remains unclear (15).
Although the incidence of colorectal cancer and the
proportion of sarcopenia, in which muscle mass decreases and muscle
strength and physical function decline, increases with aging, the
impact of sarcopenia on short- and long-term outcomes in patients
with colorectal cancer aged 80 years or older who received curative
surgery remains still unclear. The purpose of this study was to
demonstrate the impact of preoperative elder sarcopenia on the
short- and long-term outcomes of curative surgery for treating
colorectal cancer in patients older than 80 years.
Materials and methods
Patients
Between January 2016 and December 2020, a
retrospective investigation was conducted on 106 consecutive
colorectal cancer patients aged 80 years or older who were intended
to receive curative resection at Saitama cancer center in Saitama,
Japan. Patients with recurrent colorectal cancers, patients who
received palliative surgery from the beginning, and patients with
synchronous metastases (Stage 4) were excluded.
Skeletal Muscle Tissue
Measurement
The total muscle cross-sectional area (skeletal
muscles, including psoas, paravertebral, and abdominal wall
muscles) at the middle level of the third lumbar (L3) vertebra was
assessed using SYNAPSE VINCENT software (Fujifilm Co., Tokyo,
Japan) before surgery. An intensity window was used from −29 to 150
Hounsfield units for skeletal muscle selection. The skeletal muscle
index (SMI) (cm2/m2) was calculated as
follows:
SMI (cm2/m2)=L3 total muscle
cross-sectional area (cm2)/height2
(m2)
Sarcopenia was defined as SMI <52.4
cm2/m2 for the men and <38.5
cm2/m2 for the women (16). Elder sarcopenia was defined as SMI
<38.3 cm2/m2 for the men and <29.9
cm2/m2 for the women, below the range of SMI
in the previous report (16), which
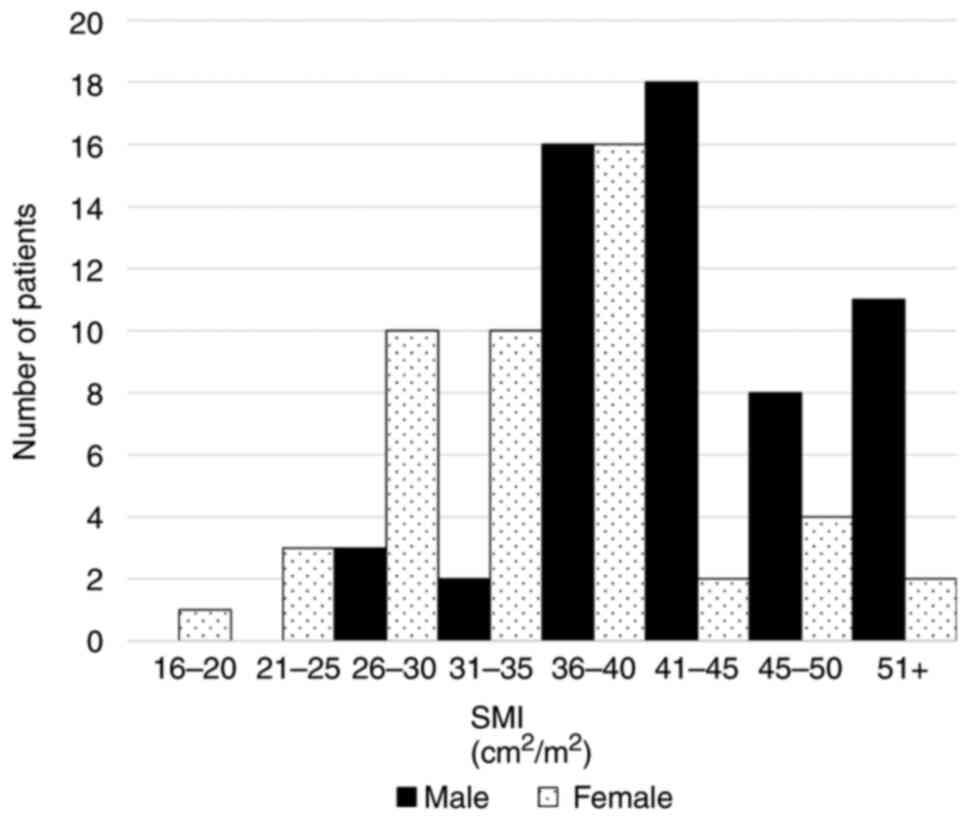
nearly a quartile of this study population. Histogram of SMI
according to sex was demonstrated in Fig. 1
Outcomes
The clinicopathological and operative data, as well
as the short-term outcomes (including morbidity, mortality,
postoperative oral intake, and postoperative hospital stay), and
long-term outcomes (such as relapse-free survival (RFS) and overall
survival (OS)), were investigated. Robotic rectal surgery was
considered a laparoscopic technique and included in laparoscopic
surgery. The results of the pathological examination were
classified according to the TNM classification of The International
Union Against Cancer (UICC) (17).
The resumption of oral intake was decided by the operating surgeon
according to the patient's general condition, including factors
such as good bowel movement, no abdominal distention, no fever, and
laboratory data. Patients were discharged when they had
demonstrated sufficient oral intake, no complications or
well-controlled complications, and did not exhibit excessive
anxiety about leaving the hospital. The Clavien-Dindo (CD)
classification was used to classify postoperative complications. CD
classification grade 3 and 4 are major complications (18). Short-term morbidity and mortality
were defined as 30-day or in-hospital morbidity and mortality. As
the follow-up, blood tests were performed every three months after
the surgery, and a CT of the abdomen combined with imaging of the
pelvis and chest was performed every six months after the surgery.
Recurrence was defined as the presence of locoregional recurrence,
distant metastases, or death from colorectal cancer. The study was
conducted in accordance with the ethical guidelines of the
Declaration of Helsinki and approved by the Ethics Committee of
Saitama Cancer Center (No. 1071). The need for informed consent was
waived because of the retrospective nature of this study.
Statistical analysis
For categorical variables, data were presented as
frequencies and percentages, and Fisher's exact probability test or
chi-square test was applied to evaluate the significance of
differences in proportions. Continuous variables were shown as mean
± standard deviation (SD) and were estimated using the unpaired
Student t-test. To investigate and compare the relapse-free
survival and the overall survival, a Kaplan-Meier analysis was
used, and the log-rank test was used to compare different curves.
Propensity score-matching was performed to reduce imbalances
between patients at baseline. A ratio of 1:1 nearest neighbor
matching method with confounding covariates including gender, age,
ASA-PS (American Society of Anesthesiologists physical status),
comorbidities, procedure, tumor location, pT stage, pN stage,
Stage, and morbidity was used for matching. And matching with a
predefined caliper width equal to 0.2 of the standard deviation of
the logit model yielded the final matched pairs. Statistical
analyses were performed using R software (version 4.3.1: http://www.R-project.org). Associations were
considered significant when P<0.05.
Results
Out of 106 elderly patients with colorectal cancer
intended to receive curative resection, there were 82 patients with
sarcopenia (77.4%) and the remaining 24 patients without
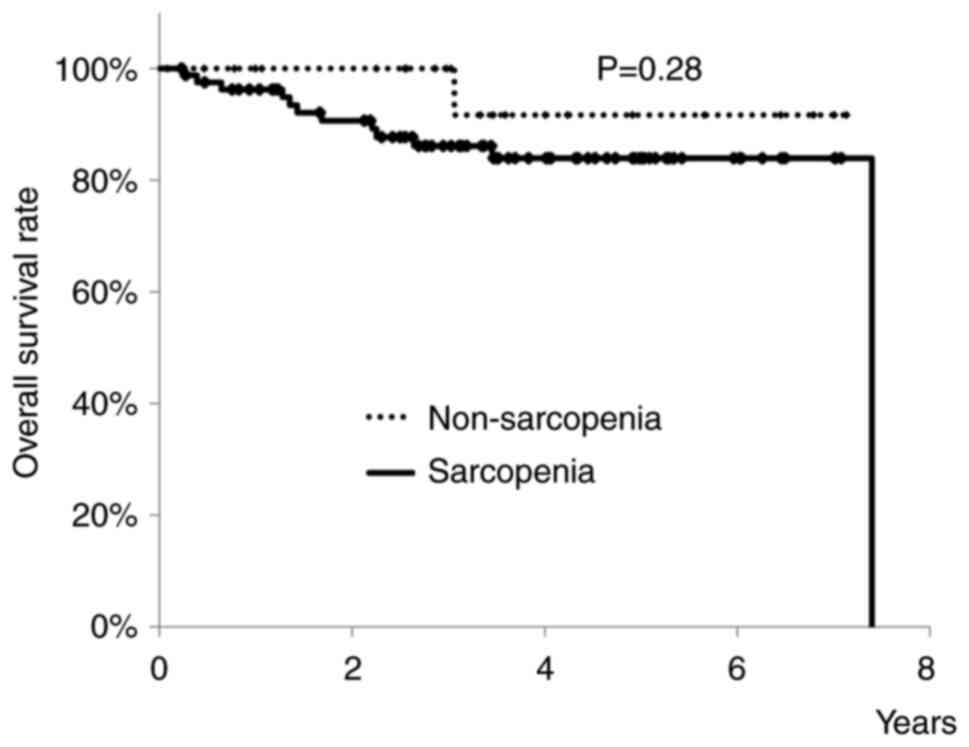
sarcopenia. There were 58 men and 48 women. OS rates of patients
with or without sarcopenia are shown in Fig. 2. Three-year OS rates of elderly
patients with sarcopenia was 86.1%, and that of those without was
100%. There was no significant difference between the two groups
(P=0.28). Next, to investigate whether or not sarcopenia affected
the prognosis of elderly colorectal cancer patients who were
intended to receive curatively resected surgery, we examined
elderly colorectal cancer patients with elder sarcopenia.
There were 27 patients (25.5%) with elder sarcopenia
and the remaining 79 patients without elder sarcopenia. About a
quarter of elderly colorectal cancer patients had elder sarcopenia.
Table I summarizes differences in
characteristics of patients with and without elder sarcopenia. BMI
was significantly smaller in patients with elder sarcopenia
(P<0.01). And there was a tendency for a higher frequency of
comorbidities in patients without elder sarcopenia (P=0.084).
Pulmonary comorbidity was the most common in elderly colorectal
cancer patients. There were no significant differences in gender,
age, preoperative plasma albumin concentration, and ASA-PS. More
than a quarter of elderly patients had a history of other
malignancies.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic | Elder sarcopenia
(n=27) | Non-elder sarcopenia
(n=79) | P-value |
|---|
| Sex |
|
| 1.0 |
|
Male | 15 | 43 |
|
|
Female | 12 | 36 |
|
| Mean age, years
(range) | 84.0 (80–91) | 83.1 (80–90) | 0.15 |
| Mean ± SD Body mass
index, kg/m2 | 19.7±2.5 | 22.5±2.9 | <0.01 |
| Albumin | 3.8±0.44 | 3.8±0.53 | 0.97 |
| ASA-PS |
|
| 1.0 |
| 2 | 25 | 72 |
|
| 3 | 2 | 7 |
|
| Comorbidities |
|
|
|
|
Overall, n (%) | 16 (59.2) | 61 (77.2) | 0.08 |
|
Cardiovascular | 3 | 13 | 0.76 |
|
Pulmonary | 5 | 22 | 0.45 |
|
Renal | 1 | 4 | 1 |
|
Cerebrovascular | 2 | 16 | 0.15 |
|
Diabetes mellitus | 5 | 18 | 0.79 |
| Has a history of
other malignancies, n (%) | 7 (25.9) | 28 (35.4) | 0.64 |
Operative and pathological data are shown in
Table II. There was a tendency for
patients with elder sarcopenia to receive laparoscopic surgery
(P=0.072). Operative time and the amount of blood loss were not
statistically different between the two groups. As for pathological
data, tumor location, pT stage, and number of harvested lymph nodes
were not statistically different between the two groups.
Significantly, more patients with elder sarcopenia had advanced
stages (P=0.029) because of the higher number of lymph node
metastasis cases.
 | Table II.Operative and pathological data. |
Table II.
Operative and pathological data.
| Parameter | Elder sarcopenia
(n=27) | Non-elder
sarcopenia (n=79) | P-value |
|---|
| Procedure |
|
| 0.07 |
|
Open | 3 | 24 |
|
|
Laparoscopic/Robotic | 24 | 55 |
|
| Mean ±
SD operative time, min | 251.1±128.7 | 243.9±91.5 |
|
| Mean ± SD blood
loss, ml | 174.9±465.5 | 135.4±225.1 |
|
| Tumor location |
|
| 0.61 |
|
Colon | 20 | 62 |
|
|
Rectum | 7 | 17 |
|
| Histology |
|
| 1.0 |
|
Well/moderately
differentiated | 26 | 75 |
|
|
Others | 1 | 4 |
|
| pT stage |
|
| 0.64 |
|
Tis | 2 | 3 |
|
| T1 | 1 | 19 |
|
| T2 | 6 | 9 |
|
| T3 | 17 | 41 |
|
| T4 | 1 | 7 |
|
| pN stage |
|
| <0.01 |
| N0 | 13 | 62 |
|
| N1 | 9 | 11 |
|
| N2 | 5 | 6 |
|
| Stage (TNM
classification) |
|
| 0.03 |
| 0 | 2 | 3 |
|
| 1 | 4 | 24 |
|
| 2 | 7 | 34 |
|
| 3 | 14 | 18 |
|
| Mean ± SD no. of
harvested lymph nodes | 19.8±9.7 | 22.5±13.8 | 0.35 |
| Cancer cells at the
proximal and distal margins of the specimen |
|
| 1.0 |
| Cancer
negative | 27 | 79 |
|
| Cancer
positive 0 | 0 |
|
|
| R stage |
|
| 1.0 |
| R0 | 27 | 79 |
|
|
R1/2 | 0 | 0 |
|
Postoperative events are shown in Table III. Day until oral intake (4.6±2.2
days vs. 6.0±4.2 days, respectively) and postoperative hospital
stay (11.3±4.1 days vs. 12.9±10.0 days, respectively) in patients
with and without elder sarcopenia were similar (P=0.12, P=0.42,
respectively). Postoperative complications occurred in about a
quarter of elderly patients, and bowel obstruction was the most
common in both groups. CD classification of more than grade 3 was
observed in one case in the patients with elder sarcopenia and 3
cases in those without elder sarcopenia. There was no mortality in
both groups.
 | Table III.Postoperative events. |
Table III.
Postoperative events.
| Events | Elder sarcopenia
(n=27) | Non-elder
sarcopenia (n=79) | P-value |
|---|
| Mean ± SD time
until oral intake, days | 4.6±2.2 | 6.0±4.2 | 0.12 |
| Mean ± SD
postoperative hospital stay, days | 11.3±4.1 | 12.9±10.0 | 0.42 |
| Morbidity (CD
classification grade 1–5) |
|
|
|
| Overall
(%) | 6 (22.2) | 22 (27.8) | 0.62 |
| Wound
infection | 1 | 5 | 1.0 |
|
Intraabdominal abscess | 1 | 0 | 0.26 |
| Urinary
tract infection | 0 | 1 | 1.0 |
|
Pneumonia | 1 | 3 | 1.0 |
|
Enterocolitis | 0 | 1 | 1.0 |
| Bowel
obstruction | 3 | 11 | 1.0 |
|
Bleeding | 0 | 2 | 1.0 |
| Urinary
retention | 0 | 2 | 1.0 |
| CD
classification grade3≦ | 1 | 3 | 1.0 |
| Mortality (%) | 0 (0) | 0 (0) | 1.0 |
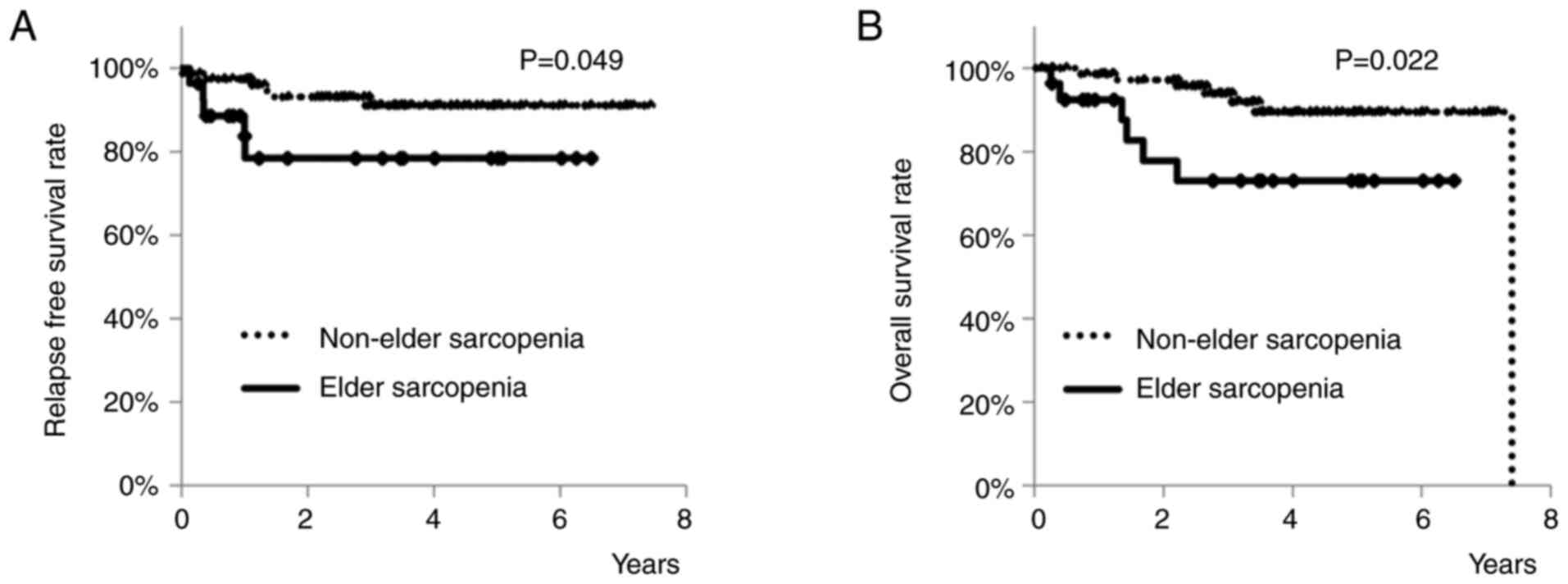
As for the long-term outcomes, RFS rates of patients
with or without elder sarcopenia are shown in Fig. 3A. Three-year RFS rates of elderly
patients with elder sarcopenia was 78.4%, and that of those without
was 91.1%. Patients with elder sarcopenia had a significantly worse
RFS rate than those without (P=0.049). OS rates of patients with or
without elder sarcopenia are shown in Fig. 3B. Three-year OS rates of elderly
patients with elder sarcopenia was 73.0%, and that of those without
was 93.9%. Patients with elder sarcopenia had a significantly worse
OS rate than those without (P=0.022). However, the high rate of
advanced Stage in patients with elder sarcopenia may have caused
the group's worse RFS rate and OS rate.
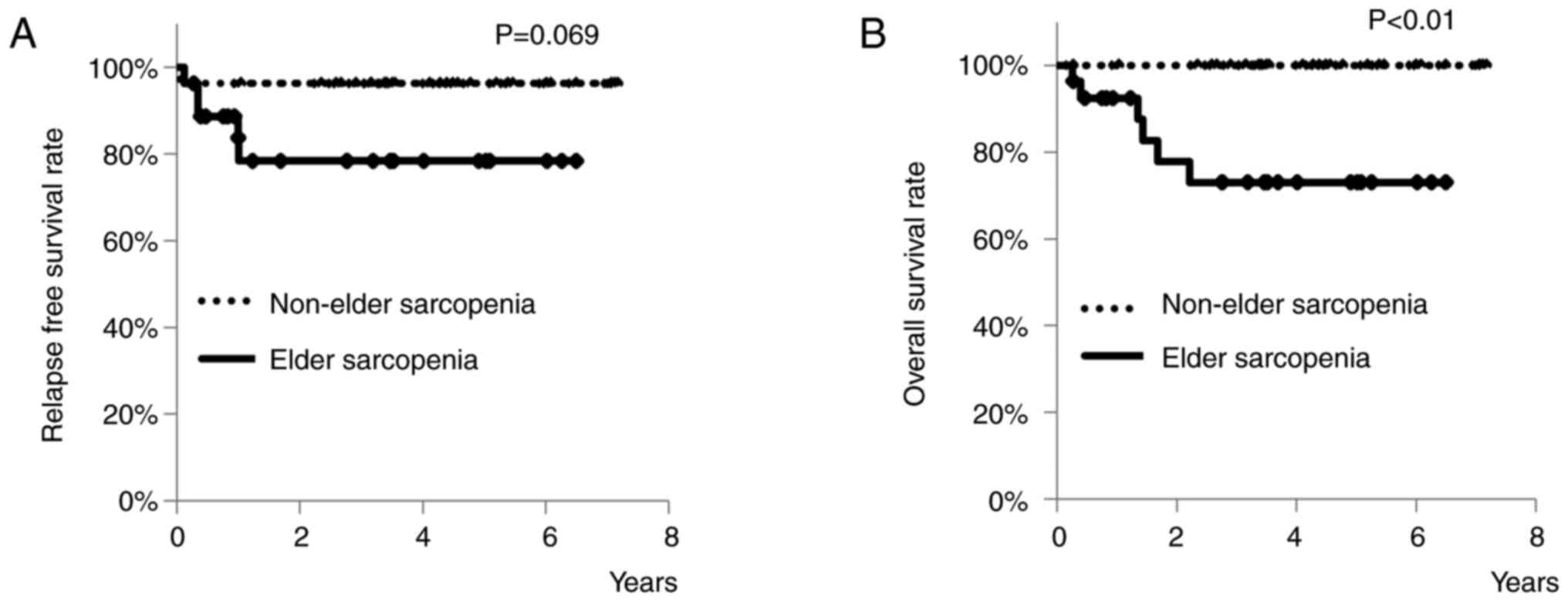
Therefore, a 1-to-1 propensity score matching was
performed to achieve balance between elderly patients with and
without elder sarcopenia. After the matching, 27 patients were
successfully matched in each group. Except for BMI, no
statistically significant differences were found between the two
groups in terms of the measured parameters (Table IV). RFS rates of patients with or
without elder sarcopenia are shown in Fig. 4A. Three-year RFS rates of elderly
patients with elder sarcopenia was 78.4%, and that of those without
was 96.3%. There was a tendency for patients with elder sarcopenia
to have a worse RFS rate than patients without elder sarcopenia
(P=0.069). OS rates of patients with or without elder sarcopenia
are shown in Fig. 4B. Three-year OS
rates of elderly patients with elder sarcopenia was 73.0%, and that
of those without was 100%. Patients with elder sarcopenia had a
significantly worse OS rate than those without (P<0.01).
 | Table IV.Patient data after propensity score
matching. |
Table IV.
Patient data after propensity score
matching.
|
| Propensity-matched
groups |
|
|---|
|
|
|
|
|---|
| Characteristic | Elder sarcopenia
(n=27) | Non-elder
sarcopenia (n=27) | P-value |
|---|
| Sex |
|
| 0.78 |
|
Male | 15 | 17 |
|
|
Female | 12 | 10 |
|
| Mean age, years
(range) | 84.0 (80–91) | 83.7 (80–90) | 0.7 |
| Mean ± SD Body mass
index, kg/m2 | 19.7±2.5 | 22.8±2.5 | <0.01 |
| Mean ± SD albumin,
g/dl | 3.8±0.44 | 3.7±0.46 | 0.45 |
| ASA-PS |
|
|
|
| 2 | 25 | 25 | 1.0 |
| 3 | 2 | 2 |
|
| Comorbidities |
|
|
|
| Overall
(%) | 16 (59.2) | 21 (77.8) | 0.24 |
|
Cardiovascular | 3 | 3 | 1.0 |
|
Pulmonary | 5 | 5 | 1.0 |
|
Renal | 1 | 2 | 1.0 |
|
Cerebrovascular | 2 | 6 | 0.25 |
|
Diabetes mellitus | 5 | 7 | 0.74 |
| History of other
malignancies |
|
|
|
|
Yes | 7 | 8 | 1.0 |
| Procedure |
|
| 0.47 |
|
Open | 3 | 6 |
|
|
Laparoscopic/Robotic | 24 | 21 |
|
| Mean ± SD operative
time, min | 251.1±128.7 | 230.7±95.8 | 0.51 |
| Mean ± SD blood
loss, ml | 174.9±465.5 | 76.3±98.8 | 0.29 |
| Tumor location |
|
| 1.0 |
|
Colon | 20 | 21 |
|
|
Rectum | 7 | 6 |
|
| Histology |
|
| 0.61 |
|
Well/moderately
differentiated | 26 | 24 |
|
|
Others | 1 | 3 |
|
| pT stage |
|
| 0.68 |
|
Tis | 2 | 2 |
|
| T1 | 1 | 1 |
|
| T2 | 6 | 7 |
|
| T3 | 17 | 17 |
|
| T4 | 1 | 0 |
|
| pN stage |
|
| 0.67 |
| N0 | 13 | 15 |
|
| N1 | 9 | 7 |
|
| N2 | 5 | 5 |
|
| Stage (TNM
classification) |
|
| 0.68 |
| 0 | 2 | 2 |
|
| 1 | 4 | 6 |
|
| 2 | 7 | 6 |
|
| 3 | 14 | 13 |
|
| Mean ± SD no. of
harvested lymph nodes | 19.8±9.7 | 26.2±14.3 | 0.06 |
| Proximal margin,
Distal margin |
|
| 1.0 |
|
Negative | 27 | 27 |
|
|
Positive | 0 | 0 |
|
| R stage |
|
| 1.0 |
| R0 | 27 | 27 |
|
|
R1/2 | 0 | 0 |
|
| Mean ± SD time
until oral intake, days | 4.6±2.2 | 4.8±2.8 | 0.83 |
| Mean ± SD
postoperative hospital stay, days | 11.3±4.1 | 12.0±14.7 | 0.78 |
| Morbidity (CD
classification grade 1–5) |
|
|
|
| Overall
(%) | 6 | 4 | 0.73 |
| Wound
infection | 1 | 1 | 1.0 |
|
Intraabdominal abscess | 1 | 0 | 1.0 |
| Urinary
tract infection | 0 | 1 | 1.0 |
|
Pneumonia | 1 | 2 | 1.0 |
|
Enterocolitis | 0 | 0 | 1.0 |
| Bowel
obstruction | 3 | 2 | 1.0 |
|
Bleeding | 0 | 0 | 1.0 |
| Urinary
retention | 0 | 0 | 1.0 |
| CD
classification grade 3≦ | 1 | 2 | 1.0 |
| Mortality (%) | 0 (0) | 0 (0) | 1.0 |
Discussion
We demonstrated in this study that elderly
colorectal cancer patients with elder sarcopenia who received
curative surgery had worse results in terms of decreased
relapse-free survival and overall survival. However, these results
might have been influenced by more advanced colorectal cancer cases
in elderly patients with elder sarcopenia, so we performed a
propensity score matching analysis. Consequently, we found that
elderly colorectal cancer patients with elder sarcopenia who
received curative surgery had worse overall survival, even after
propensity score matching. Regarding postoperative complications,
there were no significant differences observed between elderly
patients with and without elder sarcopenia. Additionally, no
mortality was recorded in this study.
To the best of our knowledge, this is the first
study to report that elder sarcopenia has a negative impact on
long-term survival in elderly colorectal cancer patients aged 80
years or older after curative resection. Previously, Miyamoto et
al revealed that sarcopenia had a negative impact on both RFS
and OS in colorectal cancer patients aged 30–93 years receiving
potentially curative resection (12). Choi et al demonstrated that
sarcopenia was negatively associated with OS in locally advanced
rectal cancer patients who underwent neoadjuvant chemoradiation
therapy followed by surgical resection (19). van Vledder et al demonstrated
that sarcopenia in colorectal cancer patients had a negative impact
on cancer outcomes following resection of colorectal liver
metastasis (20). The precise
mechanisms by which sarcopenia impacts survival rates in patients
with colorectal cancer have not been fully determined (21). Sarcopenia may reflect the increased
metabolic activity of a more aggressive tumor biology, leading to
systemic inflammation and muscle wasting (22). This might explain why skeletal
muscle depletion is a poor prognostic factor. Miyamoto et al
reported that the effect of skeletal muscle depletion on prognosis
appeared to differ with age. This effect is particularly prominent
among young patients, and little difference in skeletal muscle mass
may be seen among older patients (12). So, in our study, although sarcopenia
did not reveal the worse prognosis in elderly colorectal cancer
patients, elder sarcopenia, a quartile of this study population,
has a negative impact on OS in elderly colorectal cancer patients
aged 80 years or older.
This study found no difference in morbidity and
mortality between patients with and without elder sarcopenia.
Postoperative complications are often reported to be strongly
correlated with both disease recurrence and shorter survival in
patients with colorectal cancer (23–25).
Previously, Traeger et al demonstrated sarcopenia had a
significant association with postoperative bowel obstruction and
complications (11). And
Trejo-Avila et al also demonstrated in their meta-analysis
that sarcopenia had an association with total postoperative
complications, postoperative infection, postoperative
cardiopulmonary complications, postoperative mortality, and
prolonged hospital stay after colorectal surgery, and patients with
sarcopenia had significantly shorter overall survival (10). The precise mechanisms by which
sarcopenia impact on surgical morbidity in patients with colorectal
cancer have not been fully determined (21). Trejo-Avila et al described in
the previous report that one potential mechanism proposed was the
hyper-inflammatory response to surgery in patients with sarcopenia,
which might decrease wound healing, increase sepsis, and prolong
the hospital length of stay (10).
In this study, bowel obstruction was the most common prevalent
postoperative complication in both groups, and some infectious
complications also found in both groups. Above mentioned, little
difference in skeletal muscle mass is often seen among older
patients. Therefore, the difference in complication rates did not
exhibit statistical significance, even among elderly colorectal
cancer patients with elder sarcopenia. Additionally, no mortality
was recorded in this study. However, Nishikawa et al
reported cases of postoperative mortality due to pneumonia in
elderly patients aged 80 years or older with colorectal cancer who
underwent curative surgery (26).
Special attention should be given to postoperative complications,
especially infections in elderly patients aged 80 years or older
with colorectal cancer, regardless of the presence of
sarcopenia.
Sarcopenia is the age-related loss of skeletal
muscle mass and strength and impaired muscle function. And
sarcopenia has been increasingly investigated for its predictive
value in cancer patients over the past decade. But the objective
definition of sarcopenia is still controversial (27). Although most reports define
sarcopenia as loss of total skeletal muscle cross-sectional area at
the L3 level, cut-offs differ among reports (16,28,29).
In this study, we chose to use gender-specific cut-off values
rather than quartiles to define the levels of sarcopenia because
the study population was elderly colorectal cancer patients aged 80
years or older. Prado et al demonstrated that a skeletal
muscle index of 52.4 cm2/m2 in men and 38.5
cm2/m2 in women was associated with mortality
(16). Many reports cited this
definition of sarcopenia (14,19,30–32).
Therefore, in this study, we chose to use this index of 52.4
cm2/m2 in men and 38.5
cm2/m2 in women for defining sarcopenia which
could not have significant difference as for OS rates of elderly
patients with colorectal cancer, and defined numerical value below
the lower limit of the range in Prado's report (SMI <38.3
cm2/m2 for the men and <29.9
cm2/m2 for the women) as elder sarcopenia,
which nearly a quartile of this study population and showed
significant difference as for OS rates of elderly patients with
colorectal cancer. This time, the cut-off value from the Western
population was applicable, but a more standardized body composition
index is needed based on not only gender but also ethnicity. There
are some studies that defined sarcopenia in the Japanese population
(13,33). Fujiware et al defined
sarcopenia as 36.2 cm2/m2 in male and 29.6
cm2/m2 in female (33). Further studies with a more
comprehensive definition of sarcopenia are needed to reach more
reliable conclusions.
The present study had some limitations. First, this
study was not a large-scale multicenter randomized trial but a
retrospective study conducted at a single institute with a limited
sample size. We demonstrated the significant differences between
elder sarcopenia patients and non-elder sarcopenia patients as for
overall survival after propensity score matching, although the
number of patients in this study might be small. And regarding
population bias after the PSM method, the remaining patients
without severe sarcopenia are expected to have a better prognosis
due to the low late of advanced stage. Although we employed
propensity score matching to eliminate bias and confounding factors
as much as possible, propensity score matching itself has
limitations, and the influence of some unknown confounding factors
may not be fully excluded. Additionally, selection bias remains a
concern. There is the possibility that some elderly colorectal
cancer patients did not receive curative surgery.
In conclusion, elder sarcopenia is prevalent in a
quarter of elderly patients with colorectal cancer aged 80 years or
older receiving curative surgery. And elder sarcopenia is a poor
prognostic indicator in elderly colorectal cancer patients.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
TNi, TT, NK, RO and TNa contributed to the study
conception and design. Material preparation, data collection and
analysis were performed by TNi and TT. TNi and TT confirm the
authenticity of all the raw data. The first draft of the manuscript
was written by TNi and all authors commented on previous versions
of the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was conducted in accordance with the
ethical guidelines of the Declaration of Helsinki and approved by
the Ethics Committee of Saitama Cancer Center (approval no. 1071).
The need for informed consent was waived because of the
retrospective nature of this study.
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD, Goding Sauer A,
Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA and Jemal
A: Colorectal cancer statistics, 2020. CA Cancer J Clin.
70:145–164. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jacobs L, van der Vlies E, Ten Bokkel
Huinink D, Bloemendal H, Intven M, Smits AB, Weusten BLAM, Siersema
PD, van Lelyveld N and Los M: Tolerability, safety and outcome of
neoadjuvant chemoradiotherapy with capecitabine in Patients Aged
≥70 Years with locally advanced rectal cancer. Clin Colorectal
Cancer. 17:179–186. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Teo NZ and Ngu JCY: Robotic surgery in
elderly patients with colorectal cancer: Review of the current
literature. World J Gastrointest Surg. 15:1040–1047. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
NIH, . SEER Cancer Statistics Review,
1975–2011. Available from. http://seer.cancer.gov/csr/1975_2011
|
|
6
|
Yang L, Ma Q, Yu YY, Wang C, Meng WJ,
Adell G, Albertsson M, Arbman G, Jarlsfelt I, Peng ZH, et al:
Efficacy of surgery and adjuvant therapy in older patients with
colorectal cancer: A STROBE compliant article. Medicine
(Baltimore). 93:e2662014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rosenberg IH: Summary comments:
Epidemiological and methodological problem in determining
nutritional status of older persons. Am J Clin Nutr. 50:1231–1233.
1989. View Article : Google Scholar
|
|
8
|
Baumgartner RN, Koehler KM, Gallagher D,
Romero L, Heymsfield SB, Ross RR, Garry PJ and Lindeman RD:
Epidemiology of sarcopenia among the elderly in New Mexico. Am J
Epidemiol. 147:755–763. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sasaki M, Fukuoka T, Shibutani M, Sugimoto
A, Maeda K and Ohira M: Usefulness of the skeletal muscle index in
postoperative ileus of colorectal cancer patients: A retrospective
cohort study. BMC Surg. 22:4482022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Trejo-Avila M, Bozada-Gutierrez K,
Valenzuela-Salazar C, HerreraEsquivel J and Moreno-Portillo M:
Sarcopenia predicts worse postoperative outcomes and decreased
survival rates in patients with colorectal cancer: A systematic
review and meta-analysis. Int J Colorectal Dis. 36:1077–1096. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Traeger L, Bedrikovetski S, Nguyen TM,
Kwan YX, Lewis M, Moore JW and Sammour T: The impact of
preoperative sarcopenia on postoperative ileus following colorectal
cancer surgery. Tech Coloproctol. 27:1265–1274. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miyamoto Y, Baba Y, Sakamoto Y, Ohuchi M,
Tokunaga R, Kurashige J, Hiyoshi Y, Iwagami S, Yoshida N, Yoshida
M, et al: Sarcopenia is a negative prognostic factor after curative
resection of colorectal cancer. Ann Surg Oncol. 22:2663–2668. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Abe S, Kawai K, Nozawa H, Sasaki K, Murono
K, Emoto S, Kishikawa J, Ishii H, Yokoyama Y, Nagai Y, et al:
Preoperative sarcopenia is a poor prognostic factor in lower rectal
cancer patients undergoing neoadjuvant chemoradiotherapy: A
retrospective study. Int J Clin Oncol. 27:141–153. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chung E, Lee HS, Cho ES, Park EJ, Baik SH,
Lee KY and Kang J: Prognostic significance of sarcopenia and
skeletal muscle mass change during preoperative chemoradiotherapy
in locally advanced rectal cancer. Clin Nutr. 39:820–828. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang X, Wang S, Ji W, Wang H, Zhou K, Jin
Z and Bo L: The effect of prehabilitation on the postoperative
outcomes of patients undergoing colorectal surgery: A systematic
review and meta-analysis. Front Oncol. 12:9582612022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Prado CM, Lieffers JR, McCargar LJ, Reiman
T, Sawyer MB, Martin L and Baracos VE: Prevalence and clinical
implications of sarcopenic obesity in patients with solid tumours
of the respiratory and gastrointestinal tracts: A population-based
study. Lancet Oncol. 9:629–635. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brierley JD, Gospodarowicz MK and
Wittekind C: UICC international union against cancer. TMN
Classification of Malignant Tumours. 8th edition. Wiley-Blackwell;
Chichester: 2017
|
|
18
|
Dindo D, Demartines N and Clavien PA:
Classification of surgical complications: A new proposal with
evaluation in a cohort of 6336 patients and results of a survey.
Ann Surg. 240:205–213. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Choi MH, Oh SN, Lee IK, Oh ST and Won DD:
Sarcopenia is negatively associated with long-term outcomes in
locally advanced rectal cancer. J Cachexia Sarcopenia Muscle.
9:53–59. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
van Vledder MG, Levolger S, Ayez N,
Verhoef C, Tran TC and Ijzermans JN: Body composition and outcome
in patients undergoing resection of colorectal liver metastases. Br
J Surg. 99:550–557. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vergara-Fernandez O, Trejo-Avila M and
Salgado-Nesme N: Sarcopenia in patients with colorectal cancer: A
comprehensive review. World J Clin Cases. 8:1188–1202. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dodson S, Baracos VE, Jatoi A, Evans WJ,
Cella D, Dalton JT and Steiner MS: Muscle wasting in cancer
cachexia: Clinical implications, diagnosis, and emerging treatment
strategies. Annu Rev Med. 62:265–279. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
McSorley ST, Horgan PG and McMillan DC:
The impact of the type and severity of postoperative complications
on longterm outcomes following surgery for colorectal cancer: A
systematic review and meta-analysis. Crit Rev Oncol Hematol.
97:168–177. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ptok H, Marusch F, Meyer F, Schubert D,
Gastinger I and Lippert H; Study Group Colon/Rectum Carcinoma
(Primary Tumour), : Impact of anastomotic leakage on oncological
outcome after rectal cancer resection. Br J Surg. 94:1548–1554.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Khuri SF, Henderson WG, DePalma RG, Mosca
C, Healey NA and Kumbhani DJ; Participants in the VA National
Surgical Quality Improvement Program, : Determinants of long-term
survival after major surgery and the adverse effect of
postoperative complications. Ann Surg. 242:326–343. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nishikawa T, Ishihara S, Hata K, Murono K,
Yasuda K, Otani K, Tanaka T, Kiyomatsu T, Kawai K, Nozawa H, et al:
Short-term outcomes of open vs. laparoscopic surgery in elderly
patients with colorectal cancer. Surg Endsc. 30:5550–5557. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nakanishi R, Oki E, Sasaki S, Hirose K,
Jogo T, Edahiro K, Korehisa S, Taniguchi D, Kudo K, Kurashige J, et
al: Sarcopenia is an independent predictor of complications after
colorectal cancer surgery. Surg Today. 48:151–157. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Takeda Y, Akiyoshi T, Matsueda K, Fukuoka
H, Ogura A, Miki H, Hiyoshi Y, Nagasaki T, Konishi T, Fujimoto Y,
et al: Skeletal muscle loss is an independent negative prognostic
factor in patients with advanced lower rectal cancer treated with
neoadjuvant chemoradiotherapy. PLoS One. 13:e01954062018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Park SE, Hwang IG, Choi CH, Kang H, Kim
BG, Park BK, Cha SJ, Jang JS and Choi JH: Sarcopenia is poor
prognostic factor in older patients with locally advanced rectal
cancer who received preoperative or postoperative
chemoradiotherapy. Medicine (Baltimore). 97:e133632018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hopkins JJ, Reif RL, Bigam DL, Baracos VE,
Eurich DT and Sawyer MB: The impact of muscle and adipose tissue on
long-term survival in patients with stage I to III colorectal
cancer. Dis Colon Rectum. 62:549–560. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Han JS, Ryu H, Park IJ, Kim KW, Shin Y,
Kim SO, Lim SB, Kim CW, Yoon YS, Lee JL, et al: Association of body
composition with long-term survival in non-metastatic rectal cancer
patients. Cancer Res Treat. 52:563–572. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Levolger S, van Vledder MG, Alberda WJ,
Verhoef C, de Bruin RWF, IJzermans JNM and Burger JW: Muscle
wasting and survival following pre-operative chemoradiotherapy for
locally advanced rectal carcinoma. Clin Nutr. 37:1728–1735. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fujiwara N, Nakagawa H, Kudo Y, Tateishi
R, Taguri M, Watadani T, Nakagomi R, Kondo M, Nakatsuka T, Minami
T, et al: Sarcopenia, intramuscular fat deposition, and visceral
adiposity independently predict the outcomes of hepatocellular
carcinoma. J Hepatology. 63:131–140. 2015. View Article : Google Scholar
|