Introduction
In Europe and in the United States, total mesorectal
excision (TME) or tumor-specific mesorectal excision is the
standard treatment after neoadjuvant chemoradiotherapy (NACRT) for
locally advanced rectal cancer (LARC). However, NACRT has not been
shown to improve overall survival (OS) or prognosis because,
although it reduces the local recurrence (LR) rate (range, 5.6–9%),
it does not reduce the multi-organ metastasis rate (30%) (1–4).
Neoadjuvant chemotherapy (NAC) can be considered to avoid adverse
events associated with radiation therapy and improve survival rates
by controlling distant metastases. However, generally, NAC is
considered to have a higher LR rate than NACRT, and there are still
insufficient data on NAC. Hence, NAC is considered insufficient to
prevent LR in cT4b cases (5). To
overcome these issues, total NAC, which applies systemic
chemotherapy before rather than after surgery, has gained attention
(6).
In recent years, cancer immunotherapy, particularly
immune checkpoint inhibitors (ICIs), have been used to treat
various types of solid tumors such as hepatocellular carcinoma and
lung cancer (7,8). Adaptive immune cell therapies, such as
tumor-infiltrating lymphocyte (TIL) therapy for melanoma and
chimeric antigen receptor-T cell therapy for hematological
malignancies, have also been used (9,10).
Immune cell therapy exerts antitumor properties through a mechanism
of action that is different from that of chemo- and radiotherapy,
where LAK cells mediate potent tumor cytolysis without MHC
restrictions independent of the tumor type (11). Positive outcomes are expected when
immune cell therapy is used in combination with other therapies
such as chemotherapy (12). αβ
T-lymphocyte therapy is a method involving notable proliferation
and activation of all lymphocytes, such as T and NK cells, that
target cancer cells and raise overall immunity. The safety and
efficacy of chemo-adoptive immunotherapy (CAIT) for patients with
stage IV or recurrent colorectal cancer have been previously
reported (12). Results showed the
median progression-free survival was 21.3 months, the response rate
was 80% [complete response (CR), 26.7%; partial response (PR),
53.3%], and with almost no adverse events. However, the
significance and mechanism of CAIT have not been sufficiently
evaluated.
Therefore, the safety and efficacy of neoadjuvant
CAIT for LARC was investigated in the present study. In addition,
to explore the mechanism of CAIT, the changes in immune cells in
the peripheral blood and in the tumor microenvironment were
studied.
Patients and methods
Study design
The present study is a prospective, non-randomized,
open-label, single-arm, translational clinical trial carried out in
Japan, and performed in accordance with ethical guidelines for
clinical studies. The Institutional Review Board (IRB) of Juntendo
University approved the protocol, and the current study was
registered with the Japan Registry of Clinical Trials (jRCT; ID,
jRCTc030190248; January 21, 2019). The present study was reviewed
and approved by the relevant Accreditation Committee for
Regenerative Medicine (Tokyo, Japan). The IRBs of all participating
institutions approved the present clinical study (Juntendo
University, Tokyo, Japan; approval no. 2018061; Nippon Medical
School Tokyo, Japan; approval no. 2018-212), and written informed
consent was obtained from every patient. Patient registration
required approval from the ethics committees of all participating
medical institutions, but no patients were registered at Fukuoka
University Hospital.
The safety of neoadjuvant CAIT for LARC was
investigated as a primary endpoint. In addition, efficacy and
immunological response were evaluated as secondary endpoints. The
target sample size was six patients because this was a feasibility
study, and the sample size was not calculated.
Patient selection
Four patients were enrolled between March 2019 and
January 2020 from Juntendo University, and two patients were
enrolled between February and July 2020 from Nippon Medical School.
Eligible patients were ≥20 and ≤80 years old, had histologically
confirmed rectal adenocarcinoma without prior chemo or radiotherapy
for any other cancers, had ≥cT3 stage or had local metastases to
lymph nodes but no distant metastasis and no direct invasion into
the trigone bladder, urethra or sacrum (13). Rectum was defined as the distance
from the second sacral vertebra to the upper edge of the anal
canal. Those patients who met the following criteria were included
in the present study: i) Eastern Cooperative Oncology Group
performance status (ECOG PS) score range, 0–1; ii) neutrophil
count, ≥1,500/mm3; iii) platelet count,
≥100,000/mm3; iv) total bilirubin, ≤2.0 mg/dl; v)
aspartate transferase and alanine transferase, ≤100 IU/l; and vi)
serum creatinine, ≤1.5 mg/dl. Patients were excluded based on the
following exclusion criteria: i) Multiple primary cancers within
the past 5 years; ii) active infection; iii) positive human
immunodeficiency virus or human T-cell lymphotropic virus type I
test result; iv) positive microsatellite instability test; v)
systemic steroid or immunosuppressant administration; vi)
pregnancy; vii) uncontrolled diabetes; viii) interstitial lung
disease; ix) autoimmune disease; x) clinically significant
cardiovascular disease; and xi) any other conditions that made the
patient unsuitable for inclusion in the present study (14).
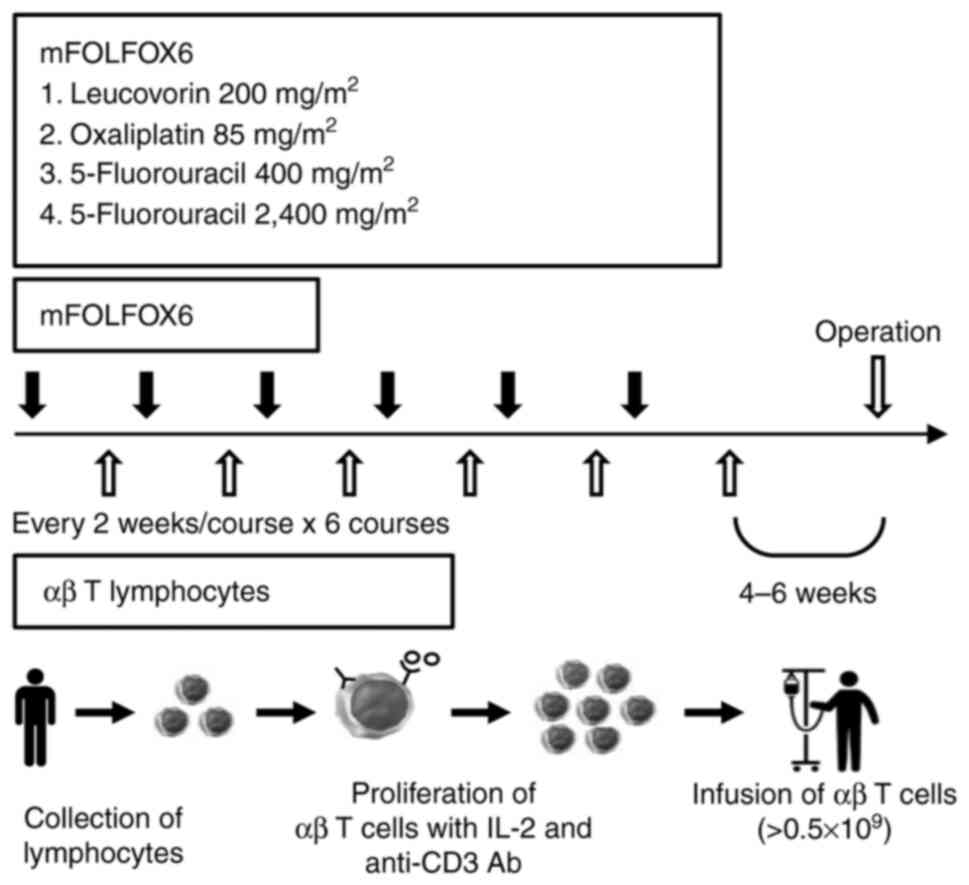
Treatment of neoadjuvant CAIT
Chemotherapy
For advanced rectal cancer, patients received a
FOLFOX6 protocol composed of 85 mg/m2oxaliplatin, 200
mg/m2 leucovorin and 400 mg/m2 fluorouracil
as an intravenous bolus with a 46-h continuous injection of 2,400
mg/m2 fluorouracil on day 1. Patients were administered
six courses of this therapy every 2 weeks.
Immunotherapy
Peripheral blood mononuclear cells (PBMCs) were
harvested using a Vacutainer (Becton, Dickinson and Company) by
centrifugation at 1,500 × g at 4°C for 15 min. A total of
≥0.5×109 αβ T-lymphocytes were cultured ex vivo
with an immobilized antibody to CD3 (muromonab-CD3; Janssen-Cilag
Ltd.; Johnson & Johnson) in a medium containing 1% autologous
serum at 37°C in a 5% CO2 incubator, then cultured in
the presence of 700 IU/ml recombinant interleukin-2
(Proleukin®; Novartis International) for 9 days, and
finally injected intravenously into patients once every 2 weeks,
starting 10 days after mFOLFOX6 therapy initiation. Surface antigen
analysis of the injected cells by flow cytometry (FCM) in FACS
Calibur (Becton, Dickinson and Company) using the FITC-conjugated
antibody to CD8 (clone SK1; BioLegend, Inc.) showed that
CD8+ T cells were the most cultured (range, 48.1–78.7%;
data not shown). Surgery was performed 4–6 weeks after the last αβ
T lymphocyte injection (Fig.
1).
Evaluation of toxicity and efficacy of
neoadjuvant CAIT
Evaluation of toxicity of neoadjuvant CAIT
Safety was evaluated by assessment of all adverse
events, laboratory data, symptoms, objective findings, body weight,
ECOG-PS score, imaging findings and postoperative complications.
Adverse events were evaluated according to the National Institute
Common Terminology Criteria for Adverse Events version 4.0
(15). Laboratory and adverse event
monitoring were performed before all cycles of CAIT. Treatment was
delayed if any of the following were noted on the day of
administration: i) Neutrophil count, <1,000/mm3; ii)
platelet count, <75,000/mm3; iii) active infection
with fever ≥38.0°C; iv) ≥grade 2 peripheral sensory neuropathy
(PSN); and v) ≥grade 3 non-hematological toxicity. The oxaliplatin
dose was reduced to 65 mg/m2 if grade 3–4 neutropenia,
febrile neutropenia or thrombocytopenia, persistent grade 2 or
reversible grade 3 PSN, or any grade 3–4 non-hematological toxicity
occurred. The continuous fluorouracil dose was reduced to 2,000
mg/m2 if grade 4 neutropenia, grade 3–4 febrile
neutropenia or thrombocytopenia, or if any grade 3–4
non-hematological toxicity occurred. The present study was
terminated if grade 3 toxicity persisted after oxaliplatin dose
reduction to 50 mg/m2, continuous injection of 1,600
mg/m2 fluorouracil, or if grade 4 non-hematological
toxicities occurred.
Evaluation of efficacy of neoadjuvant
CAIT
All patients underwent a physical examination;
computed tomography (CT) scans of the chest, abdomen and pelvis,
and magnetic resonance imaging (MRI) of the abdomen and pelvis
before and after neoadjuvant CAIT were performed. Effects were
evaluated by the reduction ratio of tumors, downstaging rate,
histopathological effect, immunopathological effect and prognosis.
After the completion of neoadjuvant CAIT, the maximum tumor
thickness was measured by MRI, and the reduction ratio was
evaluated according to the Response Evaluation Criteria for Solid
Tumors (RECIST) criteria version 1.1 (16). The preoperative reduction ratio was
calculated as the percentage of eligible patients with measurable
lesions who achieved either CR or PR according to RECIST criteria.
Cancer staging was evaluated using the TNM classification version 8
(13). Downstaging was defined as
the reduction in pathological T and N stages from the clinical
stage. Histopathological effects were evaluated according to the
Japanese Classification of Colorectal, Appendiceal and Anal
Carcinoma guidelines (3rd English edition) (17). According to the guidelines, grades
0, 1a, 1b, 2 and 3 indicated no effect, and minimal, mild, moderate
and marked effect, respectively (17).
Assessment of immunological status in
the peripheral blood
Assessment of patient immunological status and
injected immune cells
The number and frequency of immune cells in
peripheral blood samples were examined by FCM from patients before
and after treatment as previously reported (18). The phenotype of PBMCs was analyzed
using monoclonal antibodies (mAbs) against CD3, CD4, CD8, CD45,
CD56, T cell receptor (TCR) PAN αβ, TCR PAN γδ and TCRVγ9 (Beckman
Coulter, Inc.). The isolated PBMCs were used for Foxp3 staining and
cytokine production assays. For Foxp3 staining, Foxp3 was stained
with an anti-Foxp3 mAb (clone 259D; BioLegend, Inc.) after cell
fixation and permeabilization. For the intracellular cytokine
production assay, activated cells were fixed, permeabilized and
then the intracellular cytokines were stained with anti-IFN-γ or
-IL-4 mAbs (Beckman Coulter, Inc.).
Immunohistochemical analysis of tumor
infiltrating lymphocytes (TILs)
TILs in tumor tissue were evaluated by
immunohistochemical analysis, as previously reported (19). TILs were compared between biopsy
tissue collected endoscopically pre- and post-CAIT. TILs were
stained with anti-human CD3, CD4, CD8 and CD56 mAbs (clones PS1,
4B12, 4B11 and ERIC1, respectively; Novocastra Laboratories Ltd.)
and anti-human FOXP3 mAb (clone 236A/E7; eBioscience; Thermo Fisher
Scientific, Inc.). Sections with a thickness of 3 µm were incubated
with primary antibodies diluted 100–200× at room temperature for 15
min for CD3, CD4, and CD8 and overnight for CD56 and Foxp3. After
washing with phosphate buffer containing Tween 20, tumor sections
were incubated with HRP-labeled anti-IgG Ab (Nichirei Biosciences,
Inc.) for 10–30 min, and each positive cell was detected with
3,3′-diaminobenzidine, tetra-hydrochloride. The number of cells
positive for each TIL out of 1,000 lymphocytes was measured to
calculate the positive rate, in %, for every TIL.
Statistical analysis
Statistical analysis was performed using the
unpaired Student's t-test for analysis of changes in immune cell
counts, and P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient characteristics
At Juntendo University, four males (49, 51, 54 and
63 years old, respectively) were enrolled, and at Nippon Medical
School, a 76-year-old male patient and a 63-year-old female patient
were enrolled. The ECOG PS score was 0 for all patients. Four
patients had upper rectal carcinoma and two had lower rectal
carcinoma.
Treatment with neoadjuvant CAIT
Chemotherapy
All six patients completed the six planned cycles.
One patient (16.7%) discontinued oxaliplatin due to an allergic
reaction in the 6th cycle. Two patients (33.3%) required a dose
reduction of oxaliplatin because of peripheral neuropathy or
neutropenia. One patient (16.6%) required a dose reduction of
fluorouracil due to liver dysfunction. The median relative dose
intensity of bolus fluorouracil was 100% (range, 88–100%), of
continuous infusion of fluorouracil was 100% (range, 94.4–100%) and
of oxaliplatin was 98% (range, 94.4–100%).
Immunotherapy
All six patients completed the six planned cycles.
The mean number of cells for each infusion was 5.0×109
cells (range, 1.4–7.8×109 cells).
Safety
The adverse events in the six patients are
summarized in Table I. Only one
patient (16.7%) developed grade 3 hematological toxicity
(neutropenia). No patient experienced ≥grade 3 non-hematological
toxicity. One patient experienced grade 2 hypocalcemia due to
apheresis. No other severe treatment-related adverse events or
deaths were recorded during treatment.
 | Table I.Adverse events during chemo-adaptive
immunotherapy. |
Table I.
Adverse events during chemo-adaptive
immunotherapy.
|
| Event grades |
|---|
|
|
|
|---|
| Hematological and
non-hematological events | Grade 1 | Grade 2 | Grade 3 |
|---|
| Hematological,
n |
|
|
|
| Liver
dysfunction | 3 | 1 | 0 |
|
Neutropenia | 0 | 2 | 1 |
|
Thrombocytopenia | 0 | 1 | 0 |
| Renal
dysfunction | 0 | 1 | 0 |
|
Hypocalcemia (due to
apheresis) | 0 | 1 | 0 |
| Non-hematological,
n |
|
|
|
|
Peripheral neuropathy | 4 | 1 | 0 |
|
Nausea | 1 | 0 | 0 |
|
Allergic reaction | 0 | 1 | 0 |
Efficacy
The median reduction rate was 30.5% (range, 18–40%).
The confirmed response rate was 66.7% [CR, 0%; PR, 66.7% (n=4);
stable disease (SD), 33.3% (n=2); progressive disease (PD), 0%] and
the disease control rate (CR + PR + SD) was 100%. Downstaging was
confirmed in five patients (83%). Regarding histological effects,
two patients were grade 1a, and four were grade 2 (Table II). The clinical stage before
preoperative treatment (cStage), clinical stage after preoperative
treatment (ycStage) and postoperative pathologic stage (ypStage) of
the six patients are shown in Table
II (13).
 | Table II.Effect of neoadjuvant chemo-adaptive
immunotherapy, surgical procedure and postoperative course. |
Table II.
Effect of neoadjuvant chemo-adaptive
immunotherapy, surgical procedure and postoperative course.
| Case |
c-stage/yc-stage | Reduction rate,
% | Degree of
reduction | Surgery | POC
(Clavien-Dindo) | yp-Stage | Histological
effect | Adjuvant
chemotherapy | Recurrence | Outcome
(month) |
|---|
| 1 | IIIc/IIa | 30.0 | PR | R-LAR+DS+BLLND | None | IIa | Grade 2 | None | None | Alive (31) |
| 2 | IIIc/IIIc | 32.0 | PR | R-APR+BLLND | None | IIa | Grade 1a | None | None | Alive (23) |
| 3 | IIIa/IIa | 40.0 | PR | R-LAR+DS+BLLND | Outlet syndrome
(Grade 2) | IIIb | Grade 1a | CAPOX→
Capecitabine | None | Alive (25) |
| 4 | IIIb/IIa | 23.0 | SD | R-LAR+DS+BLLND | Outlet syndrome
(Grade 3b) | IIa | Grade 2 | Capecitabine | None | Alive (24) |
| 5 | IIa/I | 31.4 | PR | Lap-ISR+DS | High output
(Grade2), leakage (Grade1) | I | Grade 2 | None | None | Alive (21) |
| 6 | IIIb/IIa | 18.0 | SD | Lap-LAR+DS | None | IIIb | Grade 2 | mFOLFOX6 | Liver and Lung | Alive (19) |
Surgical procedure and short-term
outcomes
The surgical approaches included robot-assisted
surgery in four cases and laparoscopic surgery in two cases. The
surgical procedure was low anterior resection in four cases,
intersphincteric resection in one case and abdominoperineal
resection in one case. Bilateral lymph node dissection was
performed in four cases. R0 resection was achieved in all the
cases. R0 resection was defined as no evidence of tumor at the
surgical margin, macroscopically or pathologically. Regarding
postoperative complications, grades 2 and 3b outlet obstructions
were observed in two patients (one each), and grade 2 renal
dysfunction due to excessive stoma drainage and grade 1 anastomotic
leakage were observed in one case. Postoperative adjuvant
chemotherapy was administered in three patients. The median
follow-up duration was 24 months. Liver and pulmonary metastases
were observed in one patient 13 months after radical resection
surgery following neoadjuvant chemo--adoptive immunotherapy, and
the remaining five patients had no recurrence (Table II).
Immunological assessment of immune
cells in the peripheral blood and tumors
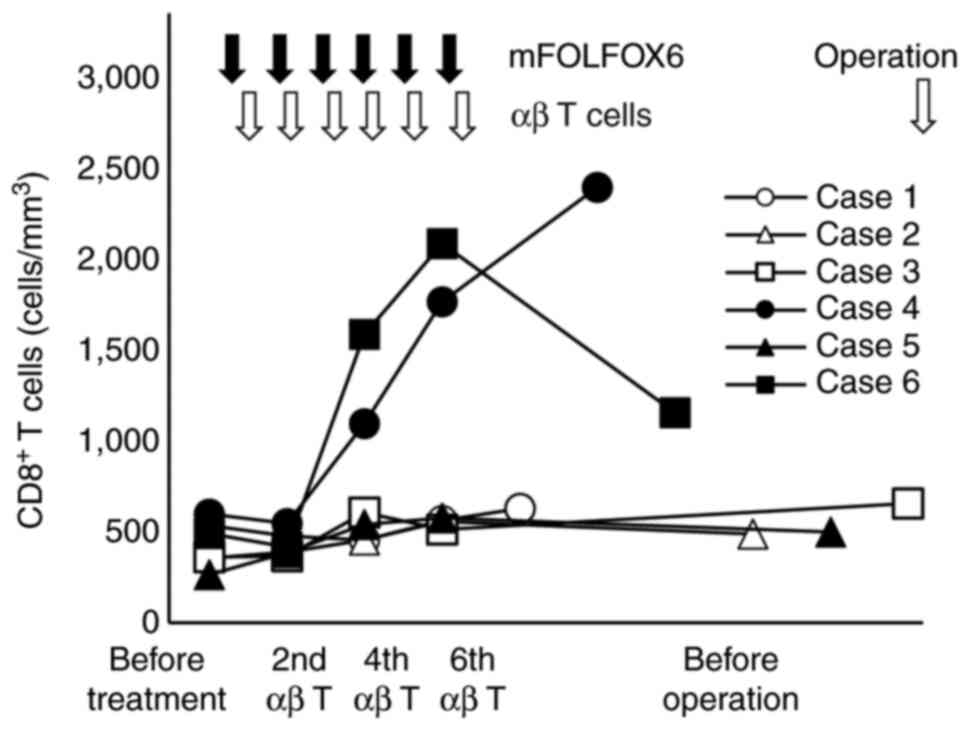
Changes in the number of immune cells, especially T
lymphocytes, in peripheral blood before and after treatment were
investigated using FCM. CD8+ T cells were markedly
increased in cases 4 and 6 at the 6th administration of αβ T cells
and slightly increased in the other four cases (P<0.01). After
CAIT, CD8+ T cells were persistently increased in the
peripheral blood in case 4, but CD8+ T cells promptly
decreased in case 6 (Fig. 2).
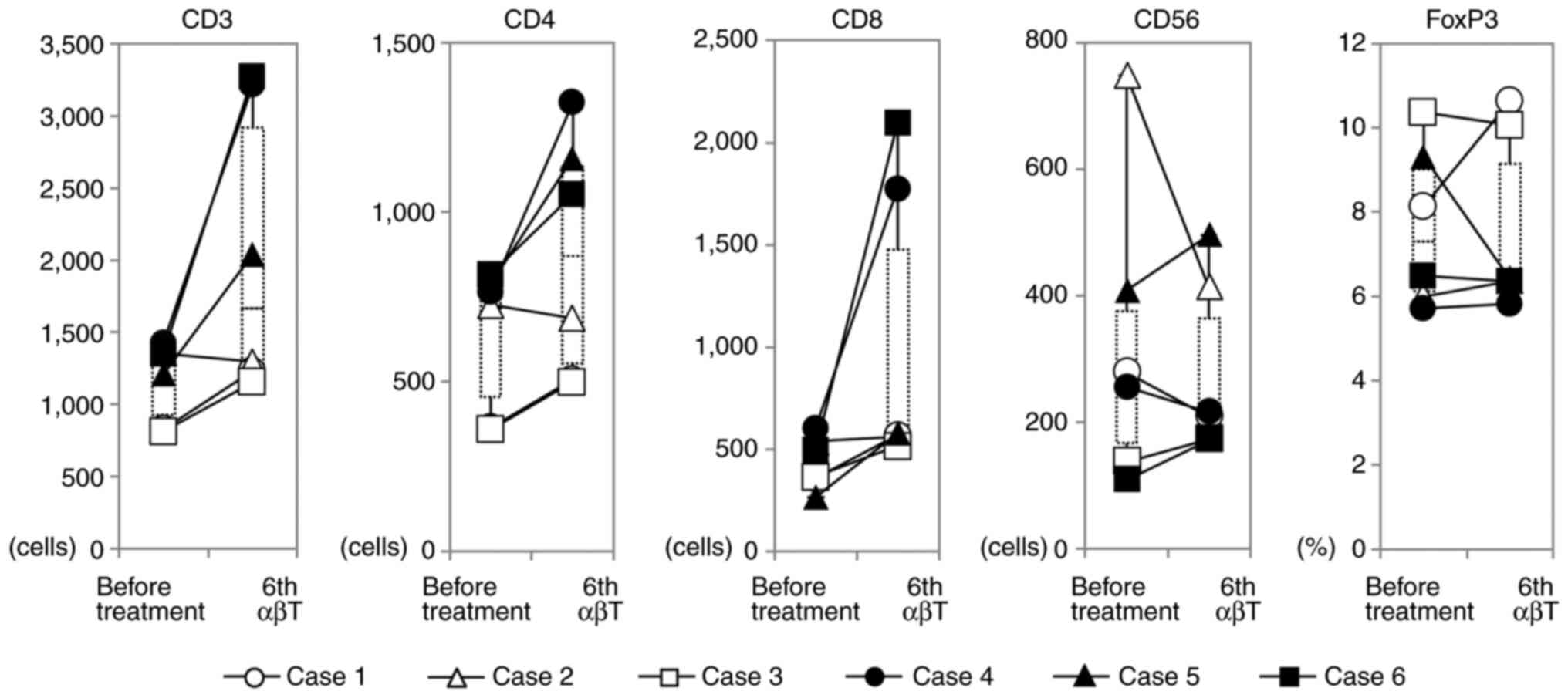
Analysis of CD4+ cells showed a significant increase in
CD4+ T cells as well as CD8+ T cells in case
4, but in the other five cases, CD4+ T cells temporarily
increased and returned to the pretreatment levels after treatment.
In five cases, the number of CD56 positive NK cells did not change
during treatment; however, in case 2, which showed the highest
number of NK cells in peripheral blood before treatment, NK cells
decreased by 50% after treatment (Fig.
3). In the patient with the highest peripheral blood γδ T cell
ratio before treatment, Vγ9γδT cells increased after treatment and
a transient increase in monocytes was observed, although the
present treatment slightly reduced B cells in numerous cases (data
not shown). There was no significant change in the regulatory T
cell ratio in the peripheral blood (Fig. 3).
Immune cell infiltration changes in tumor tissues
due to treatment were evaluated by immunohistochemical analysis
(Fig. 4). In pretreatment biopsy
tissue analysis, the proportion of tumor-infiltrating
CD4+ T cells (range, 21.3–61.2%) was significantly
higher than that of tumor-infilitrating CD8+ cells
(range, 4.7–11.5%) in all cases. In two cases, CAIT enhanced
CD8+ and reduced CD4+ T cell infiltration,
respectively. Enhanced infiltration of both CD4+ and
CD8+ T cells was observed in three cases. In case 6,
infiltration of CD4+ T cells was higher than that of
CD8+ T cells, although the infiltration of
CD8+ T cells was reduced after treatment. The
CD4+:CD8+ T cell ratio decreased after
treatment in five cases (Table
III).
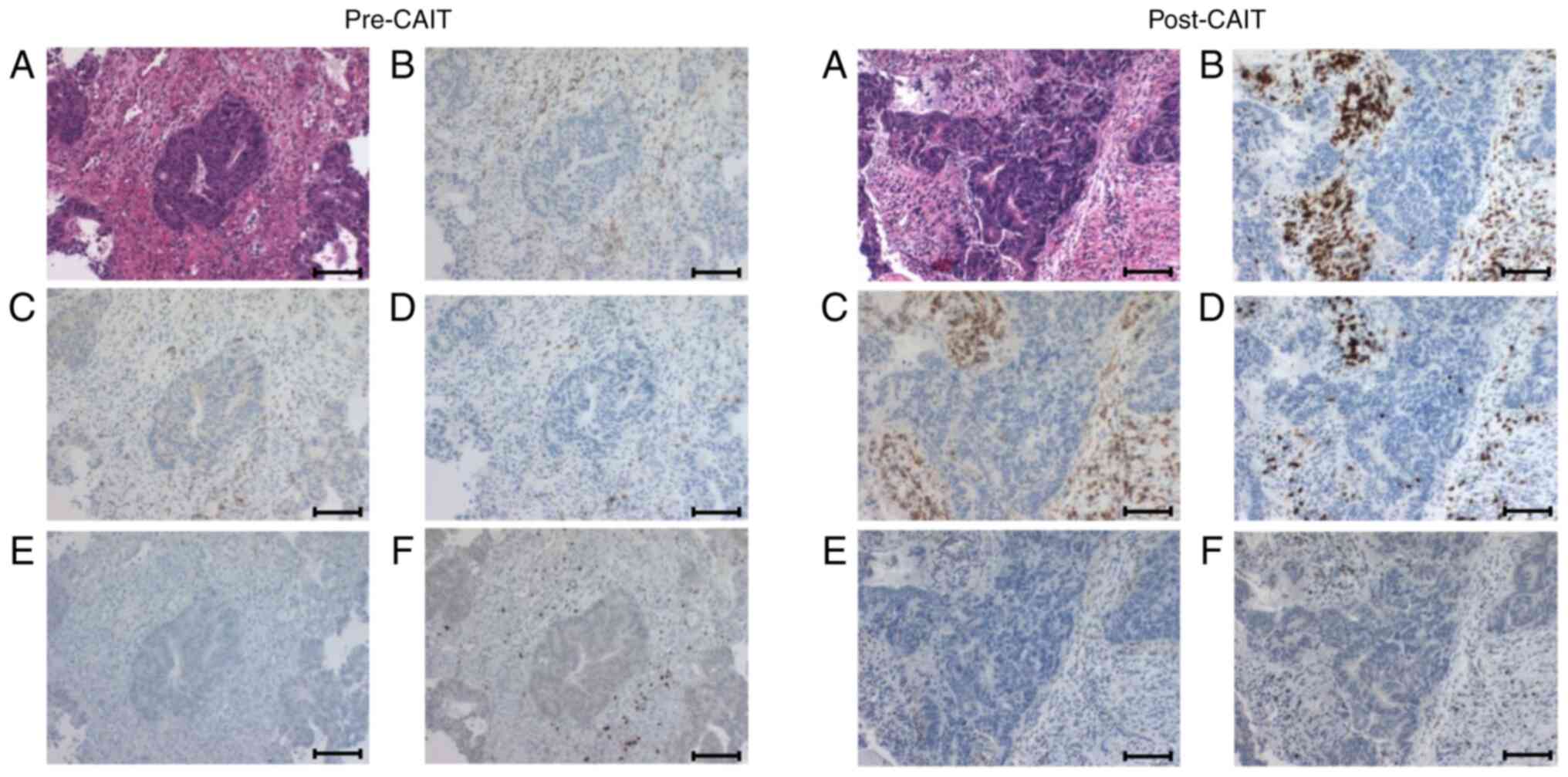 | Figure 4.Immunohistochemical analysis of
various TILs in the tumor microenvironment. Various TIL changes
before and after CAIT in case 3 are shown. (A) Hematoxylin-eosin
staining of the tumor, (B) CD3, (C) CD4, (D) CD8, (E) CD56, (F)
FoxP3. After CAIT, significant infiltration of CD3+,
CD4+ and CD8+ T cells into the tumor stroma
was shown. Cells positive for CD56, an NK cell marker, were rarely
seen in the tumor before CAIT (0.4%) and only slightly after CAIT
(1.4%). Tumor-infiltrating FoxP3+ regulatory T cells
showed no notable difference before and after CAIT (12.4 and 14.9%,
respectively). Scale bar, 100 mm. TILs, tumor infiltrating
lymphocytes; CAIT, chemo-adoptive immunotherapy. |
 | Table III.CD4+ and CD8+ T
cell infiltration changes due to treatment in tumor tissues were
evaluated by immunohistochemical assays. |
Table III.
CD4+ and CD8+ T
cell infiltration changes due to treatment in tumor tissues were
evaluated by immunohistochemical assays.
|
| CD4+ T
cells | CD8+ T
cells |
CD4+/CD8+ T
cells |
|---|
|
|
|
|
|
|---|
| Case | Before, % | After, % | Before, % | After, % | Before, % | After, % |
|---|
| 1 | 21.3a | 40.8 | 4.7 | 33.5 | 4.5 | 1.2 |
| 2 | 26.2 | 33.9 | 5.4 | 9.9 | 4.9 | 3.4 |
| 3 | 24.2 | 42.3 | 11.5 | 29.5 | 2.1 | 1.4 |
| 4 | 61.2 | 34.5 | 8.2 | 20.3 | 7.5 | 1.7 |
| 5 | 56.9 | 19.2 | 7.9 | 20.1 | 7.2 | 1.0 |
| 6 | 29.5 | 51.3 | 11.0 | 6.1 | 2.7 | 8.4 |
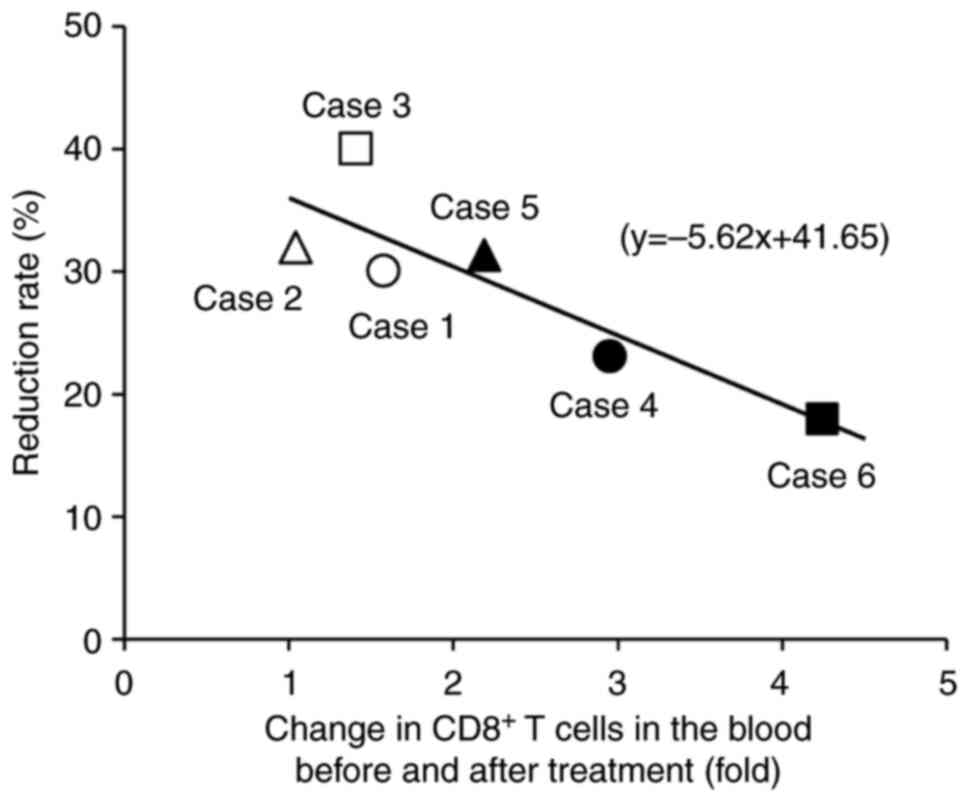
The relationship between antitumor effects, changes
in T cells in the peripheral blood, and the degree of T cell
infiltration were investigated in the tumor tissue. A single
regression analysis showed a significant inverse correlation
between antitumor response rates and the increased rate of
peripheral blood CD8+ T cells after treatment (Fig. 5). Therefore, the smaller the
increase in CD8+ T cells in the peripheral blood, the
greater the reduction in tumor size. However, there was no notable
relationship between changes in peripheral blood T cells and T cell
infiltration into the tumor. Postoperative surveillance using CT
and MRI will be scheduled for ≥5 years.
Discussion
A prospective, non-randomized, open-label,
single-arm, translational clinical trial was conducted to evaluate
the safety of neoadjuvant CAIT for advanced rectal cancer. The
present study suggested that neoadjuvant CAIT can be safely
administered for LARC.
In Europe and in the United States, NACRT + TME +
adjuvant chemotherapy is the standard treatment for LARC (1). In Japan, TME with lateral lymph node
dissection is the standard treatment. Since survival and LR-free
rates in Japan have been reported to be higher than those in Europe
and in the United States, the need for preoperative treatment has
not been well established. However, even in Japan, the number of
facilities that perform NACRT to control LR is increasing. Despite
the positive surgical results in Japan, the option for NAC is
routinely considered to avoid adverse events by radiotherapy and
improve survival rate by controlling distant metastasis. Although
there are few clinical trials on NAC in Japan, a multicenter phase
II trial (FACT trial) verified the efficacy and safety of mFOLFOX6
therapy for stages II and III rectal cancer cases of cT3 and T4
(20–22). The study mentioned serious adverse
events of grade 3–4 were neutropenia (9.6%), leukopenia (1.9%),
thrombocytopenia (1.9%), febrile neutropenia (1.9%), nausea (1.9%),
vomiting (1.9%) and peripheral neuropathy (3.8%) (22). The treatment completion rate was
80.8%, which was higher than that of postoperative adjuvant
chemotherapy containing oxaliplatin in the MOSAIC trial (74.7%)
(23) or the JOIN trial (67.0%)
(24). In the current study, grade
3–4 neutropenia occurred in three patients (50%), thrombocytopenia
in one patient (16.7%) and peripheral neuropathy in one patient
(16.7%). The reason for the high adverse event rate was the small
number of eligible patients. The treatment completion rate in the
current study was 83.3%, which was similar with that of the FACT
trial (84.5%). The median relative dose intensity was 100% for
bolus fluorouracil and continuous infusion of fluorouracil, and 98%
for oxaliplatin, which was higher than those in the FACT trial
(93.2%). In addition, the disease control rate was 100%, and all
patients underwent surgery with an R0 resection. These results were
satisfactory compared with those of other trials (22–24). A
meta-analysis of NAC and NACRT for advanced rectal cancer was
reported in 2021 (25). No notable
differences were found between the two groups regarding the
pathological CR (pCR) rate, N downstaging rate, R0 resection rate,
LR rate, and adverse events related to NAC and NACRT. Based on the
current data, NAC could be considered as a reasonable alternative
to NACRT in patients with T2/3 LARC. However, high-risk cases, such
as T4b, have been reported to have a high LR rate (7.2%) and short
OS (7.5 months) because NAC does not involve radiotherapy (5,25). The
additional use of bevacizumab would contribute to a higher pCR rate
and an improved response rate; however, a high suture failure rate
(27.8%) was reported, which was thought to be caused by the
addition of bevacizumab, the safety of which has not been
established yet (20).
In the present study, changes in immune cells in the
tumor microenvironment and peripheral blood were evaluated. After
treatment, immunohistochemical analysis showed that CD8+
T cells increased in five cases in the tumor microenvironment.
Analysis of the association between pCR rate and TIL in NAC has
shown that the TIL level is a predictor of pCR being independent of
other clinical pathological factors including tumor size, lymph
node status and age, and chemotherapy including treatment with
doxorubicin, cyclophosphamide and/or taxane regimens (26). In the current study, pCR in patients
with rectal cancer receiving neoadjuvant CAIT was not obtained,
although an increased rate of CD8+ T cells was found in
the tumor after treatment. Moreover, high CD8+ TIL and a
high CD8:Foxp3 ratio in residual tumors could predict improved
prognosis in patients with triple-negative breast cancer without
pCR following NAC (27). Therefore,
it is necessary to carefully observe the prognosis of the present
cases with non-pCR because, in most cases, CD8+ T cells
and CD8:Foxp3 ratios were increased after NAC in the tumors. In
colorectal cancer, patients with a low
CD4+:CD8+ T cell ratio in tumors showed a
notably higher 5-year survival rate and better prognosis than
patients with a high CD4+:CD8+ T cell ratio,
regardless of the stage or age (28). In five cases, a
CD4+:CD8+ ratio reduction was observed, but
in case 6, the number of CD8+ T cells decreased post
neoadjuvant CAIT, while the CD4+:CD8+ ratio
increased, and early recurrence was observed. For rectal cancer
treated with neoadjuvant CAIT, these results indicated that the
evaluation of immune cells, especially CD8+ T cells in
the tumor microenvironment, could be useful as a biomarker for
prognosis.
In most cases that received immune cell therapy, an
increase in CD8+ T cell number in peripheral blood after
treatment was previously reported (18). In patients treated with immune cell
therapy combined with mFOLFOX6 as a preoperative therapy for rectal
cancer, the increase in CD8+ T cells in the peripheral
blood was notably lower than that in patients treated with immune
cell therapy alone. In the present study, tumor infiltration of
activated T cells was promoted by mFOLFOX6, and it was reported
that FOLFOX treatment could induce an increase in the frequency and
number of tumor-infiltrating CD8+ T cells in murine
colon cancer models (29).
Furthermore, there was a notable inverse association between the
rate of increase in peripheral blood CD8+ T cells and
the antitumor response rate. These results support the possibility
that intratumoral infiltration of administered immune cells was
promoted by combination with chemotherapy. Although
neoantigen-specific T cells have the potential to cause high tumor
regression (30), it has been
observed that cancer-independent bystander CD8+ T cells
are present in TILs for human colorectal and lung cancers, which
recognize a wide range of epitopes unrelated to cancer (31). These bystander CD8+ T
cells in the peripheral blood were expanded to induce antitumor
immune activity in the present study.
To improve the pCR rate, it may be useful to use
radiation therapy or ICIs in combination with concurrent immune
cell therapy. NACRT, followed by ICI, has been reported to increase
the pCR rate for rectal cancer. In microsatellite stability cases
in which nivolumab, an ICI, was administered after
chemoradiotherapy and before surgery, the pCR rate was 30%
(32). In addition, the usefulness
of immune cell therapy in combination with ICIs was reported for
advanced and recurrent cancers (33).
The current study showed that neoadjuvant CAIT could
be safely used for the treatment of advanced rectal cancer.
However, the current study had several limitations. Due to the
small sample size, statistical analysis was limited and could not
provide definitive conclusions. Additionally, there was an
imbalance between the number of male and female patients, which may
have influenced the results. In the future, a large-cohort study of
the comprehensive immunotherapy for LARC will be carried out to
examine the effect of CAIT. It is crucial to study the
effectiveness of comprehensive immunotherapy combining various
treatments, such as chemoradiotherapy, surgery, ICI, and immune
cell therapy, as the addition of immunotherapy to previously
established treatments may potentially improve the effectiveness of
cancer treatment. In addition, verifying the additional effect of
CAIT compared with standard treatment is key.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the
current study are not publicly available due to privacy reasons but
are available from the corresponding author on reasonable
request.
Authors' contributions
YO, KSu, TI, AM, TaY, TeY, YY, SH and KSa acquired
data. HI, SO, EO, SG, RT and TK carried out immunological analyzing
and interpretation of data. TK and KSa drafted the original
manuscript and guided the study. All authors read and approved the
final manuscript. YO, TK and KSa confirm the authenticity of all
the raw data.
Ethics approval and consent to
participate
The present study was carried out in accordance with
the Declaration of Helsinki. The current study was registered with
the jRCT; ID, jRCTc030190248. The relevant Accreditation Committee
for Regenerative Medicine (Tokyo, Japan) reviewed and approved this
study. The IRBs of all participating institutions approved the
present clinical study (Juntendo University, Tokyo, Japan; approval
no. 2018061; Nippon Medical School, Tokyo, Japan; approval no.
2018-212), and written informed consent was obtained from every
patient.
Patient consent for publication
All the patients enrolled in the present study
agreed for their data to be published.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Benson AB, Venook AP, Al Hawary MM,
Cederquist L, Chen Y, Ciombor KK, Cohen S, Cooper HS, Deming D,
Engstrom PF, et al: Rectal cancer, version 2.2018, NCCN clinical
practice guidelines in oncology. J Natl Compr Canc Netw.
16:874–901. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Peeters KCMJ, Marijnen CAM, Nagtegaal ID,
Kranenbarg EK, Putter H, Wiggers T, Rutten H, Pahlman L, Glimelius
B, Leer JW, et al: The TME trial after a median follow-up of 6
years: Increased local control but no survival benefit in
irradiated patients with resectable rectal carcinoma. Ann Surg.
246:693–701. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Folkesson J, Birgisson H, Pahlman L,
Cedermark B, Glimelius B and Gunnarsson U: Swedish rectal cancer
trial: Long lasting benefits from radiotherapy on survival and
local recurrence rate. J Clin Oncol. 23:5644–5650. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sauer R, Liersch T, Merkel S, Fietkau R,
Hohenberger W, Hess C, Becker H, Raab HR, Villanueva MT, Witzigmann
H, et al: Preoperative versus postoperative chemoradiotherapy for
locally advanced rectal cancer: Results of the German
CAO/ARO/AIO-94 randomized phase III trial after a median follow-up
of 11 years. J Clin Oncol. 30:1926–1933. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tomida A, Uehara K, Hiramatsu K, Maeda A,
Sakamoto E, Okada Y, Kurumiya Y, Nakayama G, Nakamura M, Aiba T, et
al: Neoadjuvant CAPOX and bevacizumab alone for locally advanced
rectal cancer: Long-term results from the N-SOG 03 trial. Int J
Clin Oncol. 24:403–410. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Benson AB, Venook AP, Al-Hawary MM, Arain
MA, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D,
Garrido-Laguna I, et al: NCCN guidelines insights: Rectal cancer,
version 6.2020. J Natl Compr Canc Netw. 18:806–815. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takayama T, Sekine T, Makuuchi M, Yamasaki
S, Kosuge T, Yamamoto J, Shimada K, Sakamoto M, Hirohashi S, Ohashi
Y and Kakizoe T: Adoptive immunotherapy to lower postsurgical
recurrence rates of hepatocellular carcinoma: A randomised trial.
Lancet. 356:802–807. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Iwai K, Soejima K, Kudoh S, Umezato Y,
Kaneko T, Yoshimori K, Tokuda H, Yamaguchi T, Mizoo A, Setoguchi Y,
et al: Extended survival observed in adoptive activated T
lymphocyte immunotherapy for advanced lung cancer: Results of a
multicenter historical cohort study. Cancer Immunol Immunother.
61:1781–1790. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hodi FS, O'Day SJ, McDermott DF, Weber RW,
Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel
JC, et al: Improved survival with ipilimumab in patients with
metastatic melanoma. N Engl J Med. 363:711–723. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Abbasi S, Totmaj MA, Abbasi M, Hajazimian
S, Goleij P, Behroozi J, Shademan B, Isazadeh A and Baradaran B:
Chimeric antigen receptor T (CAR-T) cells: Novel cell therapy for
hematological malignancies. Cancer Med. 12:7844–7858. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mulé JJ, Shu S, Schwarz SL and Rosenberg
SA: Adoptive immunotherapy of established pulmonary metastases with
LAK cells and recombinant interleukin-2. Science. 225:1487–1489.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yoshida Y, Naito M, Yamada T, Aisu N,
Daibo K, Mera T, Tanaka T, Naito K, Yasumoto K, Kamigaki T, et al:
Adoptive chemoimmunotherapy using activated αβ T cells for stage IV
colorectal cancer. Anticancer Res. 36:3741–3746. 2016.PubMed/NCBI
|
|
13
|
Brierley JD, Gospodarowicz MK and
Wittekind C: TNM Classification of Malignant Tumours. 8th edition.
John Wiley & Sons; Hoboken: 2017
|
|
14
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
National Cancer Institute, . Common
Terminology Criteria for Adverse Events v4.0. August
24–2021https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm
|
|
16
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Japanese Society for Cancer of the Colon
Rectum, . Japanese classification of colorectal, appendiceal, and
anal carcinoma: The 3-d, english edition [Secondary Publication]. J
Anus Rectum Colon. 3:175–195. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kamigaki T, Ibe H, Okada S, Matsuda E,
Tanaka M, Oguma E, Kinoshita Y, Ogasawara S, Ono A, Makita K, et
al: Improvement of impaired immunological status of patients with
various types of advanced cancers by autologous immune cell
therapy. Anticancer Res. 35:4535–4543. 2015.PubMed/NCBI
|
|
19
|
Ishii F, Yoshida Y, Yamauchi Y, Aisu N,
Kojima D, Mera T, Kato D, Tanaka T, Naito K, Yasumoto K, et al:
Hepatectomy for liver metastases of colorectal cancer after
adoptive chemoimmunotherapy using activated αβ T-cells. Anticancer
Res. 37:3933–3939. 2017.PubMed/NCBI
|
|
20
|
Uehara K, Hiramatsu K, Maeda A, Sakamoto
E, Inoue M, Kobayashi S, Tojima Y, Yoshioka Y, Nakayama G, Yatsuya
H, et al: Neoadjuvant oxaliplatin and capecitabine and bevacizumab
without radiotherapy for poor-risk rectal cancer: N-SOG 03 phase II
trial. Jpn J Clin Oncol. 43:964–971. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kamiya T, Uehara K, Nakayama G, Ishigure
K, Kobayashi S, Hiramatsu K, Nakayama H, Yamashita K, Sakamoto E,
Tojima Y, et al: Early results of multicenter phase II trial of
perioperative oxaliplatin and capecitabine without radiotherapy for
high-risk rectal cancer: CORONA I study. Eur J Surg Oncol.
42:829–835. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hasegawa H, Okabayashi K, Tsuruta M, Koike
J, Funahashi K, Yokomizo H, Yoshimatsu H, Kan H, Yamada T, Ishida
H, et al: Updated survival results of FACT trial: Multicenter phase
II trial of neoadjuvant chemotherapy with mFOLFOX6 for stage II/III
rectal cancer with a T3/T4 tumor. Ann Oncol. 28:v171–v172. 2017.
View Article : Google Scholar
|
|
23
|
André T, Boni C, Navarro M, Tabernero J,
Hickish T, Topham C, Bonetti A, Clingan P, Bridgewater J, Rivera F
and de Gramont A: Improved overall survival with oxaliplatin,
fluorouracil, and leucovorin as adjuvant treatment in stage II or
III colon cancer in the MOSAIC trial. J Clin Oncol. 27:3109–3116.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kotaka M, Yoshino T, Oba K, Shinozaki K,
Touyama T, Manaka D, Matsui T, Ishigure K, Hasegawa J, Inoue K, et
al: Initial safety report on the tolerability of modified FOLFOX6
as adjuvant therapy in patients with curatively resected stage II
or III colon cancer (JFMC41-1001-C2: JOIN trial). Cancer Chemother
Pharmacol. 76:75–84. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin H, Wang L, Zhong X, Zhang X, Shao L
and Wu J: Meta-analysis of neoadjuvant chemotherapy versus
neoadjuvant chemoradiotherapy for locally advanced rectal cancer.
World J Surg Oncol. 19:1412021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Adams S, Gray RJ, Demaria S, Goldstein L,
Perez EA, Shulman LN, Martino S, Wang M, Jones VE, Saphner TJ, et
al: Prognostic value of tumor-infiltrating lymphocytes in
triple-negative breast cancers from two phase III randomized
adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin
Oncol. 32:2959–2966. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Miyashita M, Sasano H, Tamaki K, Hirakawa
H, Takahashi Y, Nakagawa S, Watanabe G, Tada H, Suzuki A, Ohuchi N
and Ishida T: Prognostic significance of tumor-infiltrating CD8+
and FOXP3+ lymphocytes in residual tumors and alterations in these
parameters after neoadjuvant chemotherapy in triple-negative breast
cancer: A retrospective multicenter study. Breast Cancer Res.
17:1242015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Diederichsen AC, Hjelmborg Jv, Christensen
PB, Zeuthen J and Fenger C: Prognostic value of the CD4+/CD8+ ratio
of tumour infiltrating lymphocytes in colorectal cancer and HLA-DR
expression on tumour cells. Cancer Immunol Immunother. 52:423–428.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mosely SI, Prime JE, Sainson RC, Koopmann
JO, Wang DY, Greenawalt DM, Ahdesmaki MJ, Leyland R, Mullins S,
Pacelli L, et al: Rational selection of syngeneic preclinical tumor
models for immunotherapeutic drug discovery. Cancer Immunol Res.
5:29–41. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li Q and Ding ZY: The ways of isolating
neoantigen-specific T cells. Front Oncol. 10:13472020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Simoni Y, Becht E, Fehlings M, Loh CY, Koo
SL, Teng KWW, Yeong JPS, Nahar R, Zhang T, Kared H, et al:
Bystander CD8+ T cells are abundant and phenotypically distinct in
human tumour infiltrates. Nature. 557:575–579. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bando H, Tsukada Y, Inamori K, Togashi Y,
Koyama S, Kotani D, Fukuoka S, Yuki S, Komatsu Y, Homma S, et al:
Preoperative chemoradiotherapy plus nivolumab before surgery in
patients with microsatellite stable and microsatellite
instability-high locally advanced rectal cancer. Clin Cancer Res.
28:1136–1146. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Takimoto R, Kamigaki T, Gotoda T,
Takahashi T, Okada S, Ibe H, Oguma E and Goto S: Esophageal cancer
responsive to the combination of immune cell therapy and low-dose
nivolumab: Two case reports. J Med Case Rep. 15:1912021. View Article : Google Scholar : PubMed/NCBI
|