Introduction
Squamous cell carcinoma of the head and neck (SCCHN)
comprises tumours of different origin, including mucosa of the
upper airways, the oral cavity and sinuses. Patients have a high
mortality rate (1) and critical
prognostic factors at diagnosis are tumour size and metastatic
lymph nodes in the neck. A major problem for these patients is the
high rate of tumour relapse, where locoregional recurrences develop
in 30–40% of patients and distant metastases in 20–30% (2).
The best current predictive tool for SCCHN survival
is the tumour-node-metastasis (TNM) staging system based on tumour
morphology, without considering patient-specific factors such as
age (3), sex and comorbidity
(4). Immunohistochemistry and
molecular genetics can also provide important information for
prognosis and targeted therapy (5).
However, molecular analyses are uncommon outside of research
institutes and are not performed routinely in the clinical setting,
highlighting the importance of analysing the prognostic impact of
widely available clinicopathological factors that are easily
accessible at the time of diagnosis. Diabetes, characterised by
sustained hyperglycemia, represents one such factor, and is known
to associate with an increased risk for many cancers including
SCCHN, where higher levels of blood glucose and various lipids have
been seen up to 30 years before diagnosis (6). Data from The Cancer Genome Atlas
(TCGA) or other sources, including genomic, transcriptomic and
clinical information have often been used to identify prognostic
indicators in patients with SCCHN, often using computational
algorithms and artificial intelligence (AI) approaches to produce
lists of potential biomarkers, comprising differential expression
of specific mRNAs, microRNAs, lncRNAs etc (7–11).
However, genomic and transcriptomic data are not commonly available
in routine clinical practice, and patient management is therefore
based on clinical, histological and radiological data. In addition,
SCCHN is a heterogenous set of tumours that have different
biologies and prognoses according to their site of origin,
indicating that site-specific factors are involved in patient
outcome (12).
Therefore, we focused on squamous cell carcinoma of
the oral tongue (SCCOT), the most common subgroup of oral SCCHN,
employing machine learning to investigate medical and
clinicopathological data available at diagnosis and identify
parameters that are important to predict recurrence in the routine
clinical situation.
Materials and methods
Material
The study was approved by the Regional Ethical
Committee in Umea, Umeå University, Sweden (dnr 2014-193-32M),
giving consent to achieve clinical information from the files from
patients retrospectively and prospectively. Written informed
consent was achieved from the patients after oral information given
by ENT-staff. Medical records were retrieved for 139 patients with
a verified diagnosis of SCCOT treated during 1997–2019 at the
University Hospital, Umea. Patients lacking clinical data, such as
complete anamnestic information, or not having radiological data
available, and/or not having diagnostic slides available for
re-examination were excluded, leaving 66 patients that were
included in the study. No tumour had positive M-stage and this
factor was thus excluded from further analyses. In addition to the
standard parameters used in TNM and histopathological
classification, and known high-risk factors such as smoking status,
we also retrieved information on common comorbidities such as
allergies, cardiovascular problems and symptoms of metabolic
syndrome. Patient data are summarised in Table I.
 | Table I.Clinicopathological features of
patients with squamous cell carcinoma of the oral tongue
(n=66). |
Table I.
Clinicopathological features of
patients with squamous cell carcinoma of the oral tongue
(n=66).
| Clinicopathological
features | No. (%) |
|---|
| Sex |
|
|
Female | 31 (47.0) |
| Male | 35 (53.0) |
| Age, years |
|
| ≤50 | 23 (34.8) |
|
>50 | 43 (65.2) |
| Localisation |
|
| Oral
tongue, unspecified | 17 (25.8) |
| Lateral
border | 39 (59.1) |
| Tongue
with overgrowth outside the oral tongue | 10 (15.1) |
| T stage |
|
| T1,
T2 | 56 (85.0) |
| T3,
T4 | 9 (13.6) |
|
Missing | 1 (1.5) |
| N stage |
|
| N0 | 60 (90.9) |
| N1,
N2 | 5 (7.6) |
|
Missing | 1 (1.5) |
| Radiology |
|
| Neck
metastasis | 13 (19.7) |
| No neck
metastasis | 48 (72.7) |
| Reactive
nodes | 5 (7.6) |
| Degree of
differentiation |
|
| Poor | 4 (6.1) |
|
Poor-moderate | 14 (21.2) |
|
Moderate | 19 (28.8) |
|
Moderate-high | 27 (40.9) |
|
High | 2 (3.0) |
| Worst pattern of
invasiona |
|
| Broad
pushing fingers; separate tumour islands | 1 (1.5) |
|
Invasive islands (>15
cells⁄island) | 10 (15.1) |
|
Invasive islands (<15
cells⁄island), including single cell invasion | 51 (77.3) |
| Tumour
satellites with ≥1 mm distance from tumour | 4 (6.1) |
| Lymphocytic
responsea |
|
|
Continuous dense rim of
lymphoid tissue | 27 (40.9) |
| Patches
of discontinuous dense | 25 (37.9) |
|
lymphoid infiltrate |
|
| Limited
or no response | 14 (21.2) |
| Smoking |
|
|
Yes | 37 (56.0) |
| No | 25 (37.9) |
|
Unknown | 4 (6.1) |
| Diabetes |
|
|
Yes | 12 (18.2) |
| No | 54 (81.8) |
| Asthma |
|
|
Yes | 8 (12.1) |
| No | 58 (87.9) |
| Cardiovascular
disease |
|
|
Yes | 21 (31.8) |
| No | 45 (68.2) |
| Combination
treatmentb |
|
|
Yes | 50 (75.8) |
| No | 16 (24.2) |
| Recurrence within 2
years |
|
|
Yes | 24 (36.4) |
| No | 42 (63.6) |
| Status |
|
|
Dead | 26 (39.4) |
|
Alive | 40 (60.6) |
Worst pattern of invasion (WPOI) and
Lymphocytic response (LR)
Tumours were also analysed for Worst Pattern of
Invasion (WPOI) and Lymphocytic Response (LR) using
haematoxylin/eosin stained slides. According to the evaluation
scale of Brandwein-Gensler et al (13), WPOI scores range from 1–5 (1=broad
pushing tumour front, 2=pushing tumour fingers and separate tumour
islands, 3=tumour islands comprising >15 cells, 4=tumour islands
comprising <15 cells and 5=tumour satellites found at a distance
of ≥1 mm from the tumour). LR has a three grade-scale, where
1=continuous dense rim of lymphoid tissue, 2=patches of lymphoid
tissue and 3=limited or no lymphocytic response (13).
Principal component analysis
(PCA)
Soft independent modelling of class analogies
(SIMCA) is a multivariate data analysis tool that identifies
patterns and relationships between many variables simultaneously.
We used SIMCA 16 (MKS data analytics solutions, Umeå, Sweden) with
unsupervised principal component analysis (PCA) to identify
clusters or trends in the clinical and histopathological parameters
investigated.
Statistical analysis
Two-tailed Fisher exact tests were used to determine
associations between different clinicopathological features, and
Kaplan-Meier with log rank test was used for survival analysis.
SPSS version 25 was used for calculations and P-values <0.05
were considered statistically significant.
Random forest
We used the python library ‘Lazy Predict’
(https://lazypredict.readthedocs.io/en/latest) for
initial performance assessment of different machine learning models
for our data. Random Forrest (RF) was identified as the best
machine learning model for our data. The RF approach has the
additional advantage of explainability by identifying the specific
features that are principally responsible for prediction. RF is a
machine learning algorithm based on randomly constructed decision
trees using bootstrap aggregating to improve stability and accuracy
(14). Thus, as well as identifying
suitable features for classification, RF also ranks the relative
importance of each variable in the classification scheme. The
importance scores for features are calculated based on their
contribution to the reduction of impurity in the decision trees
that make up the forest. It allows RF to identify and rank features
according to their predictive power. Features with higher
importance scores are considered more significant in the model's
decision-making process. The RF classification model and its
features of importance were calculated using leave-one-out cross
validation (LOOCV) (15).
Results
Principal component analysis
(PCA)
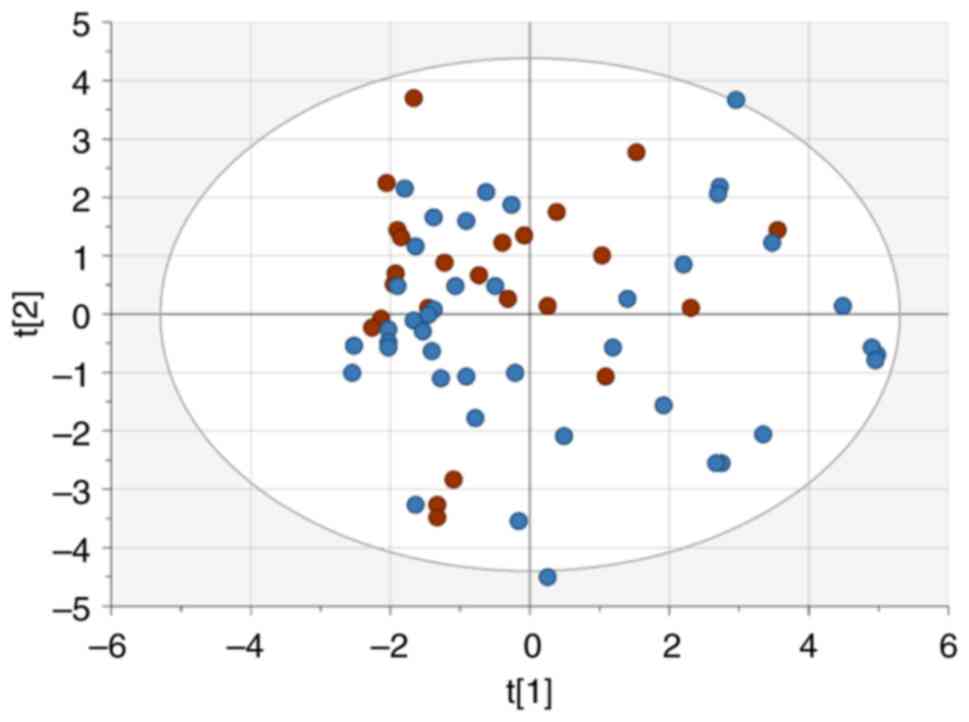
Unsupervised PCA was performed to get an overview of
subject distributions based on clinical, radiological and
histopathological factors available at diagnosis. However, no
distinct clustering of patients with recurrence and those without
was found (Fig. 1).
Statistical analysis
Significant associations were seen between
recurrence and T stage (P=0.001), radiological signs of neck
metastasis (P=0.010) and diabetes (P=0.003) (Table I).
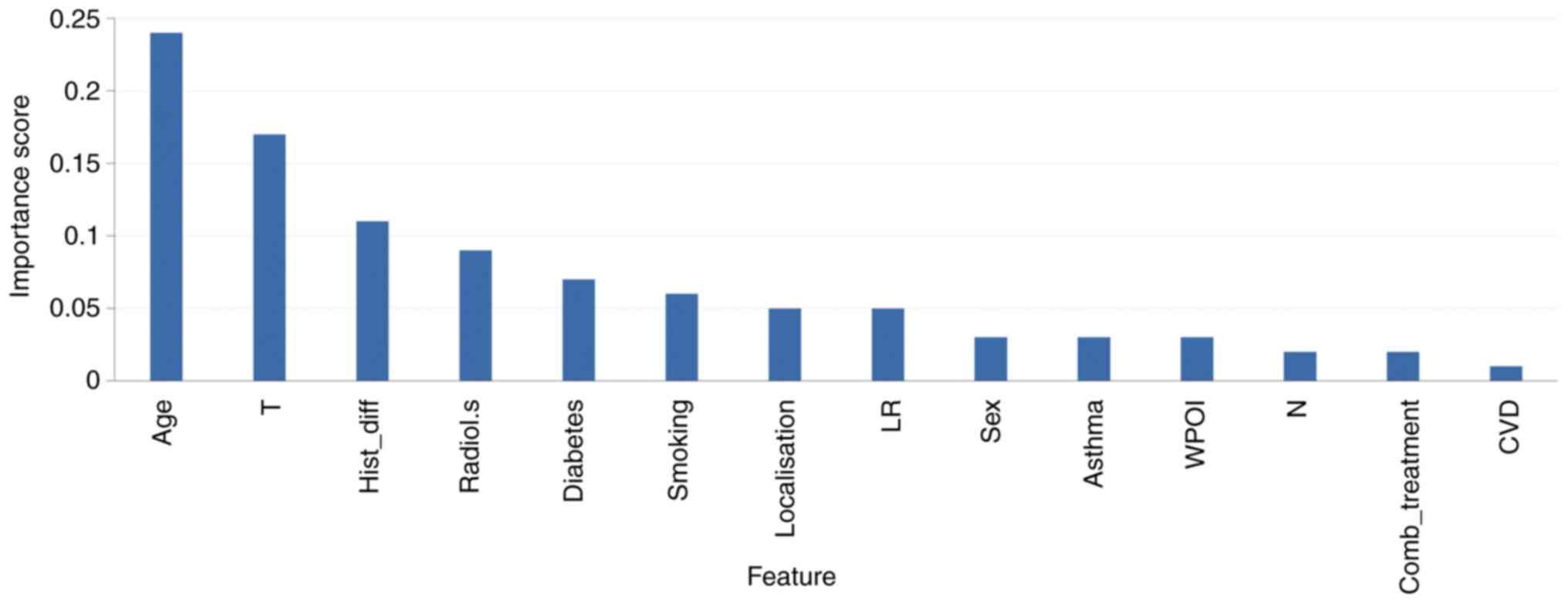
Random forest
The RF classifier was built from 14 clinical and
histopathological features available at diagnosis for the 66
patients (listed in Table II).
Performance was assessed by LOOCV and the optimized classification
RF model (n_estimators=70, max_depth=10) achieved an accuracy of
71.2% (ROC_AUC=0.729, sensitivity=0.583, specificity=0.786,
balanced accuracy=0.685, F-score=0.595) for risk of recurrence. The
three factors that were significantly associated with risk of
recurrence in the statistical analysis-T-stage (high risk),
radiological signs of neck metastasis (high risk), and diabetes
(low risk)-were also among the five most important features in
model building (Fig. 2).
 | Table II.Association between
clinicopathological features and recurrence. |
Table II.
Association between
clinicopathological features and recurrence.
|
| Recurrence |
|
|---|
|
|
|
|
|---|
| Clinicopathological
features | Yes, n | No, n | P-value |
|---|
| Sex |
|
|
|
|
Female | 13 | 18 | 0.446 |
|
Male | 11 | 24 |
|
| Age, years |
|
|
|
|
≤50 | 8 | 15 | >0.999 |
|
>50 | 16 | 27 |
|
| Localisation |
|
|
|
|
Localized tumour on lateral
border or unspecified | 19 | 37 | 0.477 |
|
Overgrowth outside the oral
tongue | 5 | 5 |
|
| T
stagea |
|
|
|
| T1,
T2 | 16 | 40 | 0.001 |
| T3,
T4 | 8 | 1 |
|
| N
stagea |
|
|
|
| N0 | 20 | 40 | 0.058 |
| N1,
N2 | 4 | 1 |
|
| Radiology |
|
|
|
| Neck
metastasis | 9 | 4 | 0.010 |
| No neck
metastasis or reactive nodes | 15 | 38 |
|
| Degree of
differentiation |
|
|
|
| Poorly,
poorly-moderately or moderately differentiated | 15 | 22 | 0.453 |
|
Moderately-well or well
differentiated | 9 | 20 |
|
| WPOI |
|
|
|
| Broad
pushing fingers; separate tumour islands or invasive islands
(>15 cells⁄island) | 2 | 9 | 0.303 |
|
Invasive islands (<15
cells⁄island) or tumour satellites with ≥1 mm distance from
tumour | 22 | 33 |
|
| LR |
|
|
|
|
Continuous dense rim of
lymphoid tissue or patches of discontinuous dense lymphoid
infiltrate | 22 | 30 | 0.066 |
| Limited
or no response | 2 | 12 |
|
|
Smokingb |
|
|
|
|
Yes | 15 | 22 | 0.419 |
| No | 7 | 18 |
|
| Diabetes |
|
|
|
|
Yes | 0 | 12 | 0.003 |
| No | 24 | 30 |
|
| Asthma |
|
|
|
|
Yes | 3 | 5 | >0.999 |
| No | 21 | 37 |
|
| Cardiovascular
disease |
|
|
|
|
Yes | 6 | 15 | 0.422 |
| No | 18 | 27 |
|
| Combination
treatment |
|
|
|
|
Yes | 20 | 30 | 0.375 |
| No | 4 | 12 |
|
Features
That diabetic SCCOT patients showed a lower risk of
recurrence than patients without diabetes was an unexpected result.
More detailed investigation revealed that all diabetic patients
were >50 years old, compared to 57% (31/54) of non-diabetics
(P=0.005), and the majority of diabetics (9/12, 75%) also had
cardiovascular disease, compared to 22% (12/54) of non-diabetics
(P=0.001). Half of the patients with diabetes (6/12) showed a
lymphocytic response (either as a continuous dense rim or as
patches of lymphocytes), compared to 85% (46/54) of patients
without diabetes (P=0.014; Table
III). Of the 12 patients with diabetes, five were treated with
metformin, four with insulin and three with diet only to control
their hyperglycaemia. Information on T- and N-stage was not
available for one diabetic patient, and the remaining 11 patients
all had T1/T2 tumours, compared to 45/54 non-diabetics (P=0.204).
None of the 11 diabetic patients with T1/T2 tumours had a
recurrence, whereas 36% (16/45) of non-diabetics with T1/T2 tumours
had a recurrence (P=0.023). To reduce heterogeneity in
clinicopathological features, we analysed the subgroup of
non-diabetic patients over 50 years of age with a T1/T2 tumour,
comparable to diabetic patients regarding age and T-stage.
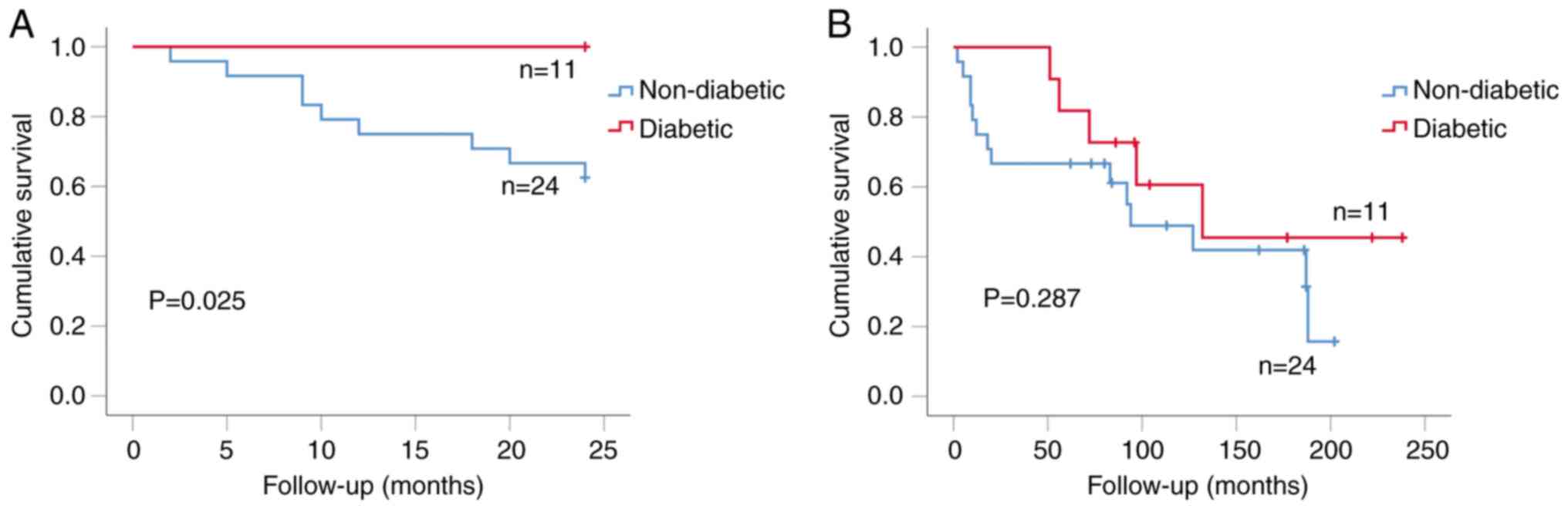
Kaplan-Meier analysis showed that diabetic patients had better
2-year overall survival than non-diabetics (P=0.025; Fig. 3A). Of these patients, 42% (10/24)
non-diabetics developed recurrence, compared to none of the eleven
in the diabetic group (P=0.015, Fisher's exact test). Longer-term
follow up of these diabetic (11)
and non-diabetic (24) patients
using Kaplan-Meier analysis showed that diabetic patients had
better recurrence-free survival than non-diabetics although this
was not statistically significant (P=0.287; Fig. 3B).
 | Table III.Association between
clinicopathological features and diabetes. |
Table III.
Association between
clinicopathological features and diabetes.
|
| Diabetes |
|
|---|
|
|
|
|
|---|
| Clinicopathological
features | Yes, n | No, n | P-value |
|---|
| Sex |
|
|
|
|
Female | 3 | 28 | 0.117 |
|
Male | 9 | 26 |
|
| Age, years |
|
|
|
|
≤50 | 0 | 23 | 0.005 |
|
>50 | 12 | 31 |
|
| Localisation |
|
|
|
|
Localised tumour on lateral
border or unspecified | 12 | 44 | 0.187 |
|
Overgrowth outside the oral
tongue | 0 | 10 |
|
| T
stagea |
|
|
|
| T1,
T2 | 11 | 45 | 0.337 |
| T3,
T4 | 0 | 9 |
|
| N
stagea |
|
|
|
| N0 | 11 | 49 | 0.579 |
| N1,
N2 | 0 | 5 |
|
| Radiology |
|
|
|
| Neck
metastasis | 1 | 12 | 0.434 |
| No neck
metastasis or reactive nodes | 11 | 42 |
|
| Degree of
differentiation |
|
|
|
| Poorly,
poor-moderate or moderately differentiated | 5 | 32 | 0.341 |
|
Moderately-well or well
differentiated | 7 | 22 |
|
| WPOI |
|
|
|
| Broad
pushing fingers; separate tumour islands or invasive islands
(>15 cells⁄island) | 4 | 7 | 0.104 |
|
Invasive islands (<15
cells⁄island) or tumour satellites with ≥1 mm distance from
tumour | 8 | 47 |
|
| LR |
|
|
|
|
Continuous dense rim of
lymphoid tissue or patches of discontinuous dense lymphoid
infiltrate | 6 | 46 | 0.014 |
| Limited
or no response | 6 | 8 |
|
|
Smokingb |
|
|
|
|
Yes | 8 | 29 | 0.501 |
| No | 3 | 22 |
|
| Asthma |
|
|
|
|
Yes | 1 | 7 | >0.999 |
| No | 11 | 47 |
|
| Cardiovascular
disease |
|
|
|
|
Yes | 9 | 12 | 0.001 |
| No | 3 | 42 |
|
| Combination
treatment |
|
|
|
|
Yes | 8 | 42 | 0.465 |
| No | 4 | 12 |
|
Discussion
Tumour recurrence is a major problem for SCCHN
patients, and, with a local recurrence rate of 30–40% (2), there is an urgent need for tools to
predict the risk of relapse to ensure appropriate treatment and
patient monitoring. Many studies have used various approaches to
improve the prognostic prediction for SCCHN patients, including the
ability to estimate outcomes from clinical and genomic data, and AI
and machine learning methods have been applied to large ‘omic’
datasets and other data for this purpose (16–19).
Here, we investigated whether similar machine learning approaches
using only clinically available data at diagnosis are useful for
improving models of risk recurrence in SCCOT, the most common
subgroup of SCCHN tumours. Using consecutive samples of SCCOT and
data available from routine clinical investigations at diagnosis
and medical histories, we identified that the machine learning
model RF is a valuable approach, able to generate a model to
predict SCCOT recurrence with an accuracy of 71.2% using only these
simple data.
Despite the relatively low performance of the
overall model, unlike some AI approaches our modelling procedure
provided information into the specific features responsible for
prediction. Surprisingly, diabetes as a co-morbidity showed a
strong positive influence on outcome; patients with diabetes had
lower rates of recurrence than non-diabetic patients and a
correspondingly better survival. According to the Swedish diabetes
register, approximately half a million people have diabetes, and
85–90% of these have type II diabetes (https://www.diabetes.se/diabetes). In our study, 18%
(12/66) of patients were diabetics (P<0.001 compared to the
general population) and eight had type II diabetes judged by their
medication. Thus, our data are compatible with the general findings
that diabetes is associated with an increased risk of cancer
development, including SCCHN and the oral SCC subtype (20). Paradoxically, our data show that
patients with diabetes have a decreased risk of recurrence.
Hyperglycaemia in diabetes is caused by improper
function or reduced secretion of insulin from pancreas, and the
correlation between diabetes and increased carcinogenesis risk is
therefore thought to be due to hyperinsulinemia, hyperglycaemia and
insulin resistance (21). Despite
the association of hyperglycaemia with increased risk of developing
cancer, none of the diabetic patients we studied showed tumour
recurrence within the first 50 months of follow-up. The link
between diabetes and lack of recurrence was retained when comparing
the diabetic group, all of whom were over 50 years of age and with
a T1/T2 tumour, to the group of non-diabetics who were also over 50
years of age and with a T1/T2 tumour, where 42% of the non-diabetic
patients showed a recurrence compared to none of the diabetic
patients. This presents an apparent paradox, where hyperglycaemia
associated with clinical diabetes is expected to cause an increased
risk of cancer development, yet co-existing diabetes reduces the
risk of cancer recurrence. The most plausible explanation for this
discrepancy between expectation and reality is that patients
diagnosed with diabetes receive one or more treatments and/or
lifestyle changes to control their disease, and are regularly
monitored in wealthy countries to prevent hyperglycaemia. Thus,
advanced health care procedures ensure that diabetics show high
hyperglycaemic levels only before their diagnosis, which is rapidly
and effectively reduced by anti-diabetic therapy.
The majority of newly diagnosed diabetics are
initially treated with Metformin as an anti-glycaemic treatment.
Metformin directly inhibits tumour cell proliferation, including
SCCHN cells (21) and has
cytostatic effects when used therapeutically in non-diabetic women
with operable breast cancer (22).
Metformin users may also have a reduced risk of acquiring cancers
such as colorectal, liver, lung, prostate and breast (23), although recent data suggest that
this issue is controversial and remains to be proven (24). There is also evidence that the
increased risk of recurrence of OSCC seen in type II diabetic
patients is reduced in those who use Metformin (25). In this large retrospective study of
more than 800 patients with oral SCC (OSCC), patients with type II
diabetes showed worse survival. However, when taking Metformin
treatment into account, survival improved significantly. Metformin
has also shown a positive effect on histological grade of dysplasia
in non-diabetics with oral premalignant lesions through its actions
on mTOR signalling (26). Although
these studies imply a specific effect of Metformin on inhibiting
cancer formation and progression (25,26),
only 42% of diabetics in our cohort were treated with Metformin and
the remaining diabetic patients were controlled with insulin (33%)
or dietary modification (25%). As all diabetics were free of
recurrence >50 months of follow-up, the data indicate that not
only Metformin but also other antiglycaemic treatments may have a
positive impact on survival, in keeping with the lack of specific
effects of Metformin versus other methods of control seen in other
studies of Type II diabetic patients with SCCHN (27). That the improved overall survival of
diabetic SCCOT patients was reduced after longer follow-up may be
explained by the majority of diabetics (75%) also suffering from
cardiovascular disease as an additional comorbidity. The effect of
controlling (reducing) circulating glucose levels on tumour growth
is presumably linked to the extreme reliance of cancer cells on
high glucose levels, where tumour cells require high levels of
energy but use the inefficient process of aerobic glycolysis (the
Warburg effect), which has been suggested as a therapeutic avenue
for many cancers, irrespective of diabetes (28). The use of aerobic glycolysis by
tumour cells is also important for DNA repair and helps to promote
resistance to standard genotoxic therapeutic agents (29), which may also help to explain our
observations of improved response in controlled diabetic patients.
In addition to direct effects on tumour cell growth, diabetes also
influences tumour associated altered immune responses. An
inflammatory response has previously been shown to have a positive
prognostic impact in SCCOT (24),
and the majority of non-diabetics in our study (85%) showed a
higher LR than diabetics. That diabetics showed a lower frequency
of CD3+ T-cells due to metabolic inhibition, lactic acidosis
inhibiting T-cell viability and function (30) is in concordance with our results,
where 50% of diabetics showed limited or no LR.
In conclusion, we used clinical, radiological and
histological data available in the routine care at diagnosis of
patients with SCCOT to establish a prognostic model. Using machine
learning to provide a risk of recurrence classification procedure
produced a model with 71.2% accuracy. An unexpected but important
finding from feature importance was that none of the patients with
diabetes showed tumour recurrence after > 50 months follow-up.
Although patients with diabetes are overrepresented among patients
with SCCOT, diabetics showed less recurrence and improved survival
compared to non-diabetics, even after accounting for the
independent prognostic variables of tumour size and patient age at
diagnosis. Even if the number of patients with diabetes and
cardiovascular disease may seem a bit high in our study, 18.2 and
31.8% respectively, there was no selection performed other than the
availability of clinical, histological and radiological data.
Notwithstanding the limitations of our study in
terms of cohort size, the major strength of the present study is
the use of a single subtype of SCCHN, where data imply that
reduction of glucose levels may be beneficial for all patients with
SCCOT, not just patients undergoing treatment for their diabetes.
In addition, irrespective of the genetic and clinical differences
between SCCOT and other SCCHN tumours, our data also indicate that
reduction of glucose levels may be useful for similar tumours in
the head and neck region (larynx, jaw, thyroid, etc), which is an
area for future investigations.
Acknowledgements
Not applicable.
Funding
The present study was supported by Lion's Cancer Research
Foundation, Umeå University, The Swedish Cancer Society (contract
number 23 2775 Pj 01H), Umeå University, Region Västerbotten,
Ministry of Health Czech Republic (MMCI, 00209805) and Czech
Science Foundation (GACR 21-13188S).
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
AMS, LW, PJC and KN designed the study. AMS and LW
retrieved and analysed data. LNS, NS and XG participated in data
analysis. AMS, LW, PJC and KN wrote the manuscript. AMS, LW, LNS,
XG, NS, PJC and KN edited the manuscript. AMS and KN confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
All procedures involving human participants were
performed in accordance with the ethical standards of the
institutional and/or national research committee and with the 1964
Helsinki Declaration and its later amendments. The present study
was approved by the local ethics committee (Regionala
Etikprövningsnämnden, Umeå University, Umeå, Sweden; approval no.
2014-193-32M). Written informed consent was obtained from the
patients after oral information given by Ear, Nose and
Throat-staff.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shah JP and Gil Z: Current concepts in
management of oral cancer-surgery. Oral Oncol. 45:394–401. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Marur S and Forastiere AA: Head and neck
cancer: Changing epidemiology, diagnosis, and treatment. Mayo Clin
Proc. 83:489–501. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lacy PD, Piccirillo JF, Merritt MG and
Zequeira MR: Head and neck squamous cell carcinoma: Better to be
young. Otolaryngol Head Neck Surg. 122:253–258. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bøje CR: Impact of comorbidity on
treatment outcome in head and neck squamous cell carcinoma-a
systematic review. Radiother Oncol. 110:81–90. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bradford CR, Ferlito A, Devaney KO,
Mäkitie AA and Rinaldo A: Prognostic factors in laryngeal squamous
cell carcinoma. Laryngoscope Investig Otolaryngol. 5:74–81. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang Y, Xiao X, Sadeghi F, Feychting M,
Hammar N, Fang F, Zhang Z and Liu Q: Blood metabolic biomarkers and
the risk of head and neck cancer: An epidemiological study in the
Swedish AMORIS Cohort. Cancer Lett. 557:2160912023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gu X, Wang L, Coates PJ, Boldrup L,
Fahraeus R, Wilms T, Norberg-Spaak L, Sgaramella N and Nylander K:
Transfer-RNA-derived fragments are potential prognostic factors in
patients with squamous cell carcinoma of the head and neck. Genes
(Basel). 11:13442020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gu X, Wang L, Boldrup L, Coates P,
Fahraeus R, Sgaramella N, Wilms T and Nylander K: AP001056.1, A
prognosis-related enhancer RNA in squamous cell carcinoma of the
head and neck. Cancers (Basel). 11:3472019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Warner GC, Reis PP, Jurisica I, Sultan M,
Arora S, Macmillan C, Makitie AA, Grénman R, Reid N, Sukhai M, et
al: Molecular classification of oral cancer by cDNA microarrays
identifies overexpressed genes correlated with nodal metastasis.
Int J Cancer. 110:857–868. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang F, Liu Y, Yang Y and Yang K:
Development and validation of a fourteen-innate immunity-related
gene pairs signature for predicting prognosis head and neck
squamous cell carcinoma. BMC Cancer. 20:10152020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou RS, Zhang EX, Sun QF, Ye ZJ, Liu JW,
Zhou DH and Tang Y: Integrated analysis of lncRNA-miRNA-mRNA ceRNA
network in squamous cell carcinoma of tongue. BMC Cancer.
19:7792019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sgaramella N, Gu X, Boldrup L, Coates PJ,
Fåhraeus R, Califano L, Tartaro G, Colella G, Spaak LN, Strom A, et
al: Searching for new targets and treatments in the battle against
squamous cell carcinoma of the head and neck, with specific focus
on tumours of the tongue. Curr Top Med Chem. 18:214–218. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brandwein-Gensler M, Teixeira MS, Lewis
CM, Lee B, Rolnitzky L, Hille JJ, Genden E, Urken ML and Wang BY:
Oral squamous cell carcinoma histologic risk assessment, but not
margin status, is strongly predictive of local disease-free and
overall survival. Am J Surg Pathol. 29:167–178. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Svetnik V, Liaw A, Tong C, Culberson JC,
Sheridan RP and Feuston BP: Random forest: A classification and
regression tool for compound classification and QSAR modeling. J
Chem Inf Comput Sci. 43:1947–1958. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pedregosa F, Varoquaux G, Gramfort A,
Michel V, Thirion B, Grisel O, Blondel M, Prettenhofer P, Weiss R,
Dubourg V, et al: Scikit-learn: Machine learning in python. J Mach
Learn Res. 12:2825–2830. 2011.
|
|
16
|
Alabi RO, Elmusrati M, Sawazaki-Calone I,
Kowalski LP, Haglund C, Coletta RD, Mäkitie AA, Salo T, Almangush A
and Leivo L: Comparison of supervised machine learning
classification techniques in prediction of locoregional recurrences
in early oral tongue cancer. Int J Med Inform. 136:1040682020.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Alabi RO, Elmusrati M, Leivo I, Almangush
A and Mäkitie AA: Machine learning explainability in nasopharyngeal
cancer survival using LIME and SHAP. Sci Rep. 13:89842023.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Alabi RO, Almangush A, Elmusrati M, Leivo
I and Mäkitie A: Measuring the usability and quality of
explanations of a machine learning web-based tool for oral tongue
cancer prognostication. Int J Environ Res Public Health.
19:83662022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Alabi RO, Almangush A, Elmusrati M and
Mäkitie AA: Deep machine learning for oral cancer: From precise
diagnosis to precision medicine. Front Oral Health. 2:7942482022.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Giovannucci E, Harlan DM, Archer MC,
Bergenstal RM, Gapstur SM, Habel LA, Pollak M, Regensteiner JG and
Yee D: Diabetes and cancer, a consensus report. Diabetes Care.
33:1674–1685. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wojciechowska J, Krajewski W, Bolanowski
M, Kręcicki T and Zatoński T: Diabetes and cancer: A review of
current knowledge. Exp Clin Endocrinol Diabetes. 124:263–275. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hadad SM, Coates P, Jordan LB, Dowling RJ,
Chang MC, Done SJ, Purdie CA, Goodwin PJ, Stambolic V,
Moulder-Thompson S, et al: Evidence for biological effects of
metformin in operable breast cancer: Biomarker analysis in a
pre-operative window of opportunity randomized trial. Breast Cancer
Res Treat. 150:149–155. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Noto H, Goto A, Tsujimoto T and Noda M:
Cancer risk in diabetic patients treated with metformin: A
systematic review and meta-analysis. PLoS One. 7:e334112012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dickerman BA, García-Albéniz X, Logan RW,
Denaxas S and Hernán MA: Evaluating metformin strategies for cancer
prevention: A target trial emulation using electronic health
records. Epidemiology. 34:690–699. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hu X, Xiong H, Chen W, Huang L, Mao T,
Yang L, Wang C, Huang D, Wang Z, Yu J, et al: Metformin reduces the
increased risk of oral squamous cell carcinoma recurrence in
patients with type 2 diabetes mellitus: A cohort study with
propensity score analyses. Surg Oncol. 35:453–459. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gutkind JS, Molinolo AA, Wu X, Wang Z,
Nachmanson D, Harismendy O, Alexandrov LB, Wuertz BR, Ondrey FG,
Laronde D, et al: Inhibition of mTOR signaling and clinical
activity of metformin in oral premalignant lesions. JCI Insight.
6:e1470962021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee DJ, McMullen CP, Foreman A, Huang SH,
Lu L, Xu W, de Almeida JR, Liu G, Bratman SV and Goldstein DP:
Impact of metformin on disease control and survival in patients
with head and neck cancer: A retrospective cohort study. J
Otolaryngol Head Neck Surg. 48:342019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Barrea L, Caprio M, Tuccinardi D, Moriconi
E, Di Renzo L, Muscogiuri G, Colao A and Savastano S: Could
ketogenic diet ‘starve’ cancer? Emerging evidence. Crit Rev Food
Sci Nutr. 62:1800–1821. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Marcucci F and Rumio C: Glycolysis-induced
drug resistance in tumors-A response to danger signals? Neoplasia.
23:234–245. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Spanier G, Ugele I, Nieberle F, Symeou L,
Schmidhofer S, Brand A, Meier J, Spoerl S, Krupar R, Rümmele P, et
al: The predictive power of CD3+ T cell infiltration of
oral squamous cell tumors is limited to non-diabetic patients.
Cancer Lett. 499:209–219. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lundqvist L, Stenlund SH, Laurell G and
Nylander K: The importance of stromal inflammation in squamous cell
carcinoma of the tongue. J Oral Pathol Med. 41:379–383. 2012.
View Article : Google Scholar : PubMed/NCBI
|