Introduction
Malignancies in the uterine cervix arise from the
epithelial lining, resulting in squamous carcinoma and
adenocarcinoma (1,2). Cervical cancer, predominantly of
squamous epithelial origin, is linked to high-risk human
papillomavirus (HPV) infection. The high incidence of squamous cell
cervical carcinoma is attributed to HPV, offering an opportunity
for global eradication through HPV vaccination (3). HPV tests, including HPV DNA and
thin-prep cytology test (TCT) screening, aid in the early detection
of cervical squamous epithelial lesions. With the implementation of
the HPV vaccine, cervical squamous cancer rates have been
decreasing. The PATRICIA trial, suggested that following the use of
the bivalent anti-type 16 and 18 HPV vaccine in ~9,000 vaccinated
women aged 15–25 years, high-grade cervical intraepithelial
neoplasia (CIN2+/CIN3+) incidence was reduced compared with that in
controls. Also cross-protection against persistent infection was
found with the non-vaccine oncogenic HPV types 31, 33 and 45 at ~3
years after complete vaccination. CIN2+ associated with HPV-16/18
was reduced by 93% and CIN2+ associated with HPV-31/33/45/52/58 was
reduced by 53% (4,5).
According to the 2020 World Health Organization
Classification (4), cervical
adenocarcinomas form a spectrum from well-differentiated adenoma
malignum (mucinous variant of minimal deviation adenocarcinoma) to
poorly differentiated, invasive gastric-type adenocarcinoma
(6,7). The gastric type cervical cancer
encountered in the present study is often overlooked due to its
rarity. Notably, endocervical carcinoma, specifically gastric-type
cervical adenocarcinoma, is unrelated to HPV infection.
Gastric-type adenocarcinomas (GASs) constitute a heterogeneous
group of tumors with varying morphological features, presenting
challenges in diagnosis (8).
Between January 2015 and January 2023, two cases of gastric
endocervical adenocarcinoma were diagnosed and the patients were
admitted to Lishui Central Hospital (Lishui, China). The present
study describes the pathological findings and immunostaining of
relevant markers, providing a comparison with previously published
data. The profile contributes insights for future diagnoses and
aims to improve the understanding the underlying mechanisms of
tumor genesis.
Immunohistochemically, clinical specimens in the
present study were tested for mucinous adenocarcinoma biomarkers,
along with the mutant forms of tumor suppressor genes tumor protein
p53 (p53) and p16. The K-ras proto-oncogene, frequently mutated in
tumors of various histological origins (9,10), was
assessed using an enriched polymerase chain reaction (PCR) method
for point mutation tests and restriction fragment length
polymorphism, amplification refractory mutation system (ARMS). This
method measured K-ras genotypes, providing data to screen
parameters for diagnosis and predict clinical outcomes in the rare
gastric-type endocervical adenocarcinoma (GEA) tumor.
Case report
Case presentation
Between January 2015 and January 2023, two cases of
GEA were diagnosed at Lishui Central Hospital. The general
information of the two patients is summarized in Table I.
 | Table I.General information on the cases
entering the study. |
Table I.
General information on the cases
entering the study.
| Case no. | Sex | Age, years | Clinical
findings | HPV |
|---|
| 1 | Female | 52 | Watery vaginal
discharge | Negative |
| 2 | Female | 47 | Vaginal bleeding | Negative |
Case 1
A 52-year-old woman observed an increase in vaginal
discharge, which was watery and white, in January 2015. There was
no itching in the external genital area, but discomfort was felt in
the lower abdomen. A pelvic computed tomography (CT) scan showed a
markedly enlarged cervix, with a cystic solid mass measuring
~5.4×4.1×7.6 cm. The boundaries of the mass were unclear, and the
contrast-enhanced scan displayed uneven enhancement. The
possibility of cervical malignancy could not be ruled out. TCT
results were negative, while tumor markers indicated elevated
cancer antigen (CA)125 levels at 497.3 U/ml (normal range, 0–35
U/ml) and CA19-9 levels at 2,339.4 U/ml (normal range, 0–37 U/ml).
A high-risk HPV test using the Hybrid Capture 2 assay (HC2; Digene;
Qiagen, Inc.) was also negative. After admission to the hospital,
the patient underwent a radical removal of the uterus and bilateral
adnexa, with pelvic cavity cleaning.
Upon histological examination, at low magnification,
the tumor appeared to consist of dilated cysts or glands with
stromal infiltration. These cysts were lined by a single- or
multi-layered mucinous epithelium. At high magnification, the cells
showed varying degrees of atypia, with clear or pale cytoplasm,
vesicular nuclei and prominent nucleoli. The cells exhibited
distinct borders. Immunohistochemical markers play a crucial role
in determining the molecular characteristics and behavior of
tumors. In this case, positive expression of cytokeratin (CK)7,
mucin (MUC)5AC, and p53 suggested specific features of the tumor;
in cervical HPV-associated adenocarcinoma, p53 is expressed in its
wild-type form, while in cervical gastric-type adenocarcinoma, p53
is expressed as a mutant variant. While negative results for
estrogen receptor (ER), progesterone receptor (PR) and paired box
protein 8 (PAX8) indicated the absence of markers associated with
other types of tumors or conditions.
The final pathological diagnosis indicated nodular
moderate- to poorly differentiated adenocarcinoma of the gastric
type, infiltrating all layers of the cervical wall without breach
of the serosa. Observations noted cancer thrombi within blood
vessels and nerve involvement.
In July 2021, the patient commenced radiotherapy
using a pelvic intensity-modulated radiation therapy field covering
both the primary focus and pelvic lymphatic drainage area. The
treatment involved the following parameters: 6 MV X-rays;
source-axis distance, 100 cm; 95% planning target volume, 50 Gy/25
fractions, 5 fractions/week; cisplatin, 79 mg/week administered
over five cycles with a cycle per week. Subsequently, the patient
underwent chemotherapy at ~1-month intervals in August, September
and October 2021. The chemotherapy regimen included 500 mg
albumin-bound paclitaxel (day 1) and 48 mg cisplatin (days 1–3),
with one cycle being 3 weeks.
The patient was readmitted to the hospital in March
2022, with symptoms of abdominal distension, abdominal pain and
cessation of gas passage for 1 day. An emergency abdominal CT scan
revealed post-cervical cancer surgery changes, such as thickening
and exudate in the presacral soft tissues and perirectal fascia,
along with widespread thickening of the omentum and fascia. These
findings raised concerns about the possibility of metastasis. The
patient who was alive with disease ultimately declined further
radiation and chemotherapy, and follow-up revealed that the patient
is currently self-administering traditional Chinese medicine.
Case 2
A 47-year-old woman presented to Lishui Central
Hospital in March 2021 with the chief complaint of persistent
vaginal bleeding for 2 months. An ultrasound examination revealed a
low echogenic mass measuring 61×41×39 mm in the posterior wall of
the cervix, with unclear borders. Pelvic magnetic resonance imaging
revealed irregular cervical thickening and multiple internal cystic
lesions, some with separations. Tumor marker levels indicated
potential cervical malignancy, with CA125 at 497.30 U/ml and CA19-9
at 2,339.40 U/ml. The patient underwent a radical removal of the
uterus and bilateral adnexa with pelvic cavity cleaning.
Microscopic examination revealed a tumor consisting
of variable-sized cysts with diverse architectures, ranging from
well-differentiated dilated glands lined by mucinous epithelium to
a cribriform pattern. At high magnification, tumor cells displayed
pale cytoplasm and cytologic atypia, varying from mild to severe.
Lymphovascular invasion was observed, indicating the presence of
tumor cells within lymphatic or blood vessels, which is an
important prognostic factor for tumor spread and metastasis.
Immunohistochemical analysis showed negative results for ER, PR,
and p16, indicating their absence in the tumor cells. Positive
staining was observed for MUC5AC, p53, PAX8 and CK7.
Following surgery, the patient underwent whole
pelvic intensity-modulated radiotherapy of 48 Gy/24 fractions
starting in July 2021, with subsequent sessions 11 days later and
then at ~1-month intervals in August, September and October 2021.
After ruling out contraindications, the patient received
intravenous chemotherapy with 400 mg albumin-bound paclitaxel and
500 mg carboplatin for 3 weeks. However, recurrence occurred 1 year
later. During a telephone follow-up, it was learnt that the patient
who was alive with disease is currently considering whether to
enroll in a clinical trial.
Methods
An analysis was conducted on cases of GEA a rare
tumor encountered at Lishui Central Hospital, with only two cases
identified over an 8-year period.
Pathological findings
It is noteworthy that both patients were in the
menopausal age group, distinguishing them from the more common
squamous cell carcinoma (SCC) observed in younger individuals. The
diagnosis of adenocarcinoma, supported by the presence of cysts
lined with mucinous epithelium and glands with stromal
infiltration, was confirmed as GEA through the immunohistochemical
data. In case 1, GEA was diagnosed based on clinical and
histopathological examination of the lesion obtained from
hysterectomy specimens. Aggressive tumor behavior, characterized by
perineural invasion and intravascular thrombus, was observed. In
case 2, in addition to the morphological features of cervical
adenocarcinoma, lymphovascular invasion was identified in the
tumor.
Microscopic observation
Microscopically, the tumor was composed of cysts of
variable sizes with diverse architectures, from well-differentiated
dilated glands linked by mucinous epithelium to a cribriform
pattern. At low magnification, the tumor was comprised of dilated
cysts or glands with stromal infiltration, lined by a single- or
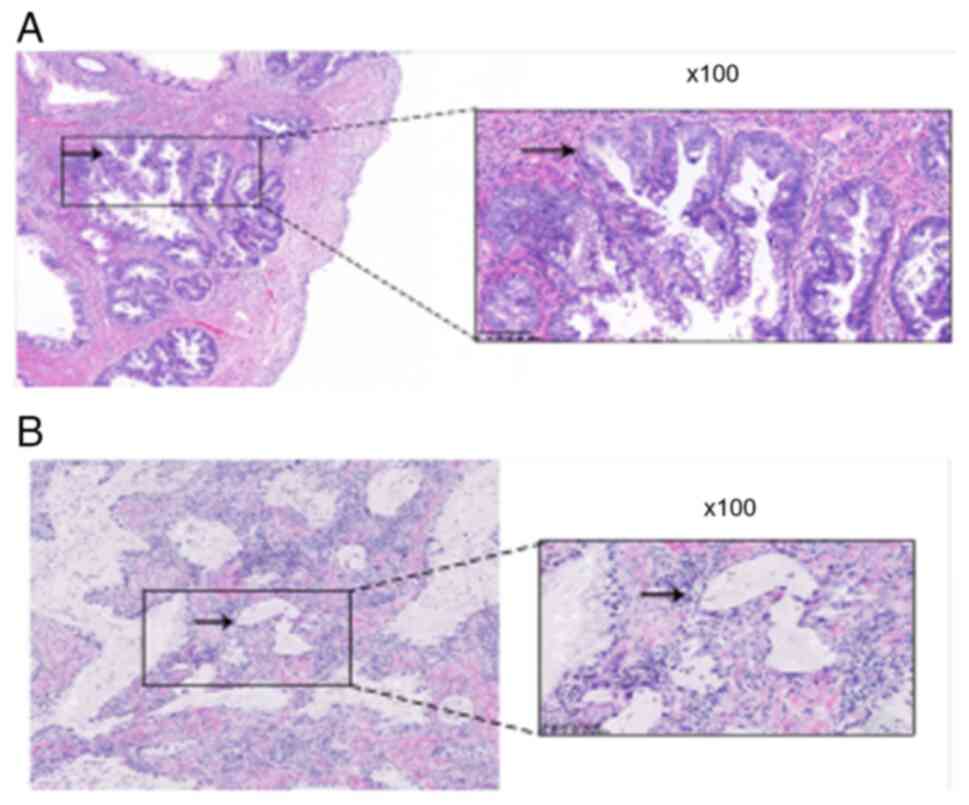
multi-layered mucinous epithelium (Fig.
1A). At high magnification, tumor cells were observed to show
pale cytoplasm and cytologic atypia with mild or severe degrees.
The morphological appearances of the tumor cells differed from
usual HPV-related cervical adenocarcinomas, as apical mitotic
figures and apoptotic bodies were not always present (Fig. 1B). The clinical details are
presented in Table I, and the
representative histopathological findings of the patients are
depicted in Fig. 1. Cervical
surgical specimens for analysis were retrieved from the archival
specimen registry at the Department of Pathology, Lishui Central
Hospital, and microsections of 2 mm were prepared using a Leica
machine (RM2245; Leica Microsystems, Inc.) and mounted on clean
glass slides. Sections were fixed with 4% paraformaldehyde and
stained with hematoxylin and eosin (H&E) at room temperature
for 1 h, before review under a microscope (BX40-72H02; Olympus
Corporation).
Immunohistochemical staining
Table II outlines
the markers to be detected and the sources of the commercial
antibodies employed in this study. Immunohistochemical staining was
conducted according to a previously described method (10,11),
with slight modifications. Microsections were dewaxed, and
antigenic epitopes were decrosslinked by heating at 95° for 5 min.
After drying, the sections were co-incubated with primary
antibodies, diluted at a ratio of 1:100, at room temperature
overnight. Subsequently, these were conjugated with labeled
secondary antibodies and developed using a DAB kit. Tumor
suppressor p53 staining that was entirely negative or strongly and
diffusely positive (with a proportion of tumor cell nuclei >80%)
was considered mutation-type. By contrast, heterogeneous staining
of p53 was considered wild-type (11,12).
Histological markers CK7 and MUC5AC were assessed and showed
cytoplasmic staining. For p16, diffuse cytoplasmic staining was
considered positive, while corresponding focal or patch staining
was deemed negative. Despite HIK1083 being considered as an
immunohistochemical marker of gastric-type differentiation
(13), it was not utilized in this
study due to limitations in departmental funding and resources.
 | Table II.Source of the primary antibodies, and
the immunostaining patterns of the immunohistochemical markers. |
Table II.
Source of the primary antibodies, and
the immunostaining patterns of the immunohistochemical markers.
| Markers detected | Localization
(reactivity) | Source of primary
antibody |
|---|
| CK7 | Cytoplasmic staining
(+) | Fuzhou Maixin
Biotech, Co., Ltd. |
| MUC5AC | Cytoplasmic staining
(+) | Fuzhou Maixin
Biotech, Co., Ltd. |
| p53 | Nuclear staining
(+) | Fuzhou Maixin
Biotech, Co., Ltd. |
| PAX8 | Nuclear staining
(+)a | Origene
Technologies |
| p16 | Nuclear/cytoplasm
staining (−) | Yichen Biotechnology,
Ltd. |
| ER | Nuclear staining
(−) | Roche Diagnostics
(Shanghai), Ltd. |
| PR | Nuclear staining
(−) | Yichen
Biotechnology, Ltd. |
| PD-L1 | Membrane staining
(−) | Amoy Diagnostics,
Co., Ltd. |
Detection of mutant proto-oncogene
K-ras with ARMS-based PCR
Primer design and ARMS PCR protocol
The sequences of the ARMS primers used for the
mutation detection are as previously described (9). Each PCR utilized one ARMS primer and a
common reverse primer for mutation detection.
PCR amplification
The test was conducted as previously reported
(14), with some modifications. For
each reaction, 5 µl of a supernatant containing genomic DNA as a
template was added to a final volume of 50 µl. This volume included
1 µl of a 25 pmol reverse primer, 1 µl of a 12.5 pmol control
forward primer, 1 µl of a 25 pmol control reverse primer, 50 mM
KCl, 10 mM Tris-HCl (pH 8.3), 1.2 mM MgCl2, 200 µM deoxynucleoside
triphosphates and 2 units of Taq DNA polymerase. The reaction was
performed in a DNA Thermolyne (Thermo Fisher Scientific, Inc.) with
the following program: Initial denaturation at 95°C for 5 min,
followed by 25 cycles of denaturation at 95°C for 25 sec, annealing
at 64°C for 20 sec and elongation at 72°C for 20 sec. Subsequently,
30 cycles were performed with denaturation at 95°C for 25 sec,
annealing at 64°C for 30 sec and elongation at 72°C for 20 sec. The
signals of fluorescence dyes 6-carboxyfluorescein and
hexachloro-fluorescein. No signals were registered when the system
cooled to 60°C. The amount of amplified product was plotted against
time to obtain a real-time curve. The amplification products were
verified by loading on a 0.8% agarose gel for electrophoresis.
Results
Only two cases of GEA were identified in the Lishui
Central Hospital over an 8-year period from January 2015 to January
2023, aligning with the low incidence of this tumor type. Notably,
both patients were in the menopausal age group, unlike the more
common SCC observed in younger individuals.
Immunohistochemistry
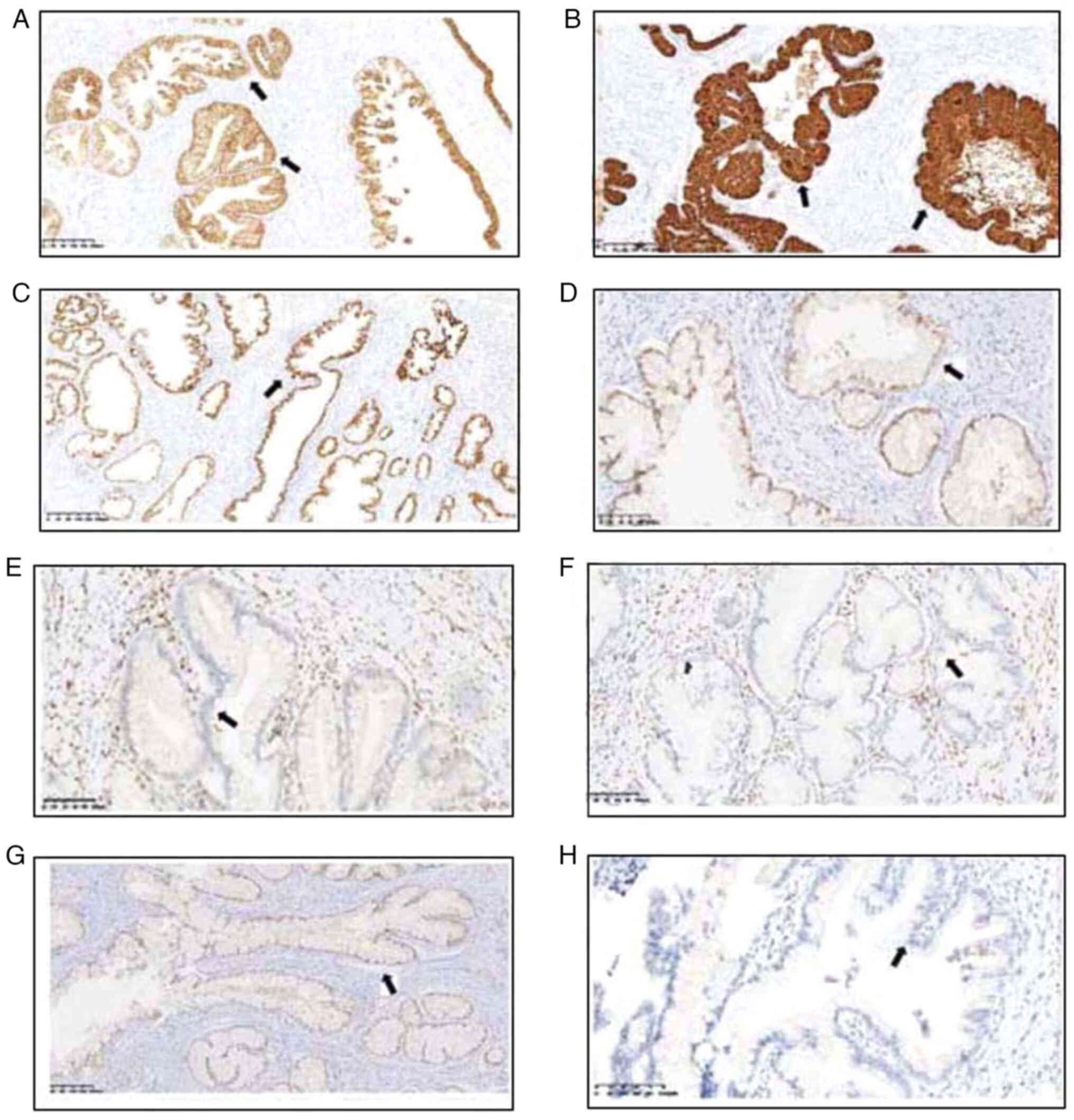
Relevant markers associated with carcinogenesis were
detected immunohistochemically, the data and the immunostaining
pattern are detailed in Table II.
The results were classified as negative, focally positive (<50%
cell staining) or diffusely positive (≥50% cell staining). In both
patients, common findings included several markers being diffusely
or focally positive, such as CK7, MUC5AC and mutant tumor
suppressor p53 (Fig. 2). Negative
markers included ER, PR, and PAX8. Programmed death-ligand 1
(PD-L1) testing was conducted, and the tests yielded negative
results. In case 2, negative results were found for the ER, PR, p16
and PD-L1 immunohistochemical markers, while positive expression
was found for CK7, MUC5AC, PAX8, and mutant p53. The data suggested
an epithelial origin and a mucinous cell phenotype.
Positive staining for mutant p53 encoding protein
using a specific antibody indicated abnormal expression (Fig. 3). Dysregulation or mutations in the
p53 gene can lead to loss of its tumor suppressor function,
contributing to tumor development and progression (15,16).
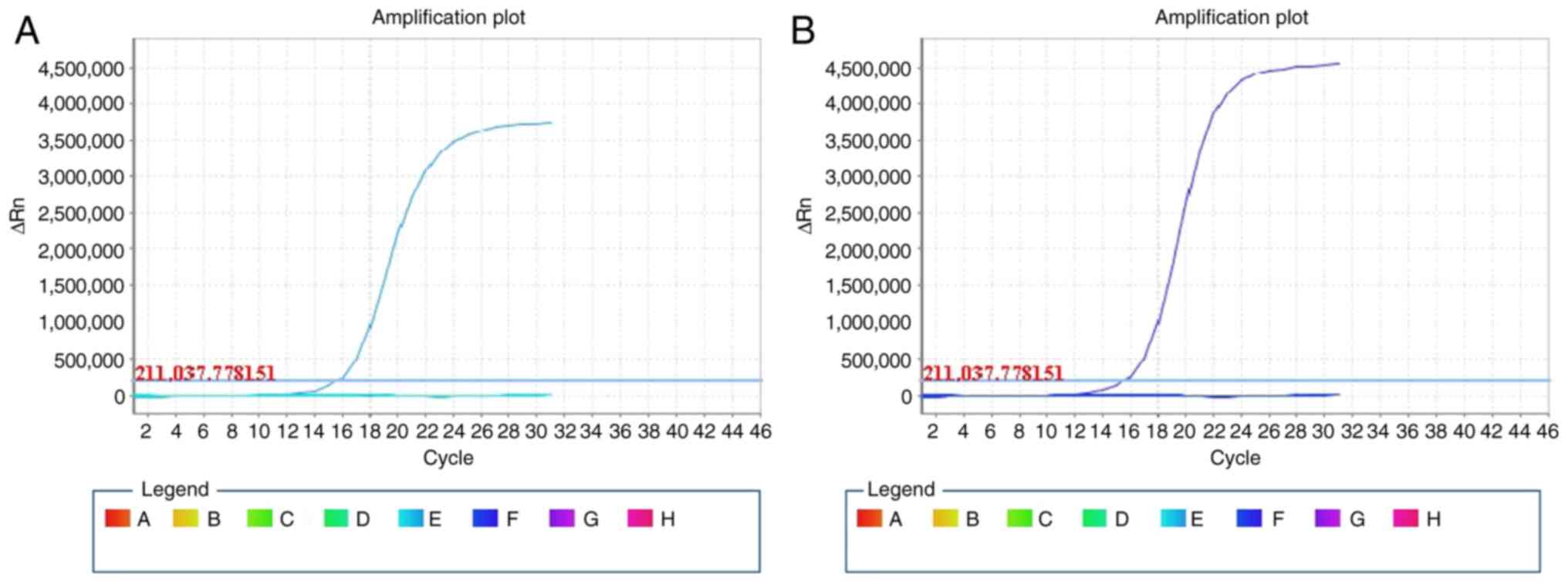 | Figure 3.Amplification refractory mutation
system-based PCR measurement of wild-type and mutant-type K-ras. (A
and B) Real-time amplification dynamic curves of the PCR amplified
DNA samples from the lesions of (A) patient 1 and (B) patient 2.
The wild-type and the following mutations were tested for: The
nucleotide coding for glycine on codons 12 and 13 to mutated to
aspartate, alanine, valine, serine, arginine, or cycteine; the
nucleotide coding for glutamine on codon 61, mutated to lysine,
leucine, arginine or histidine; the nucleotide coding for lysine on
codon 117, mutated to asparagine; and the nucleotide coding for
alanine on codon 146, mutated to threonine, valine or proline. PCR,
polymerase chain reaction. The square symbols with different colors
were defined as the wells in the plate for amplification of the
wild-type and the five mutants of K-ras. ΔRn on y-axis is the
difference of fluorescence intensity during any time point of
amplification against the baseline level. Data are representative
of independent tests repeated at least three times. |
ARMS-based PCR
The analysis of K-ras oncogene mutations in the two
GEA patients, as indicated by the slope shape curve for wild-type
K-ras and the plateau shape for mutant K-ras, suggested that K-ras
was of the wild type. No mutations in codon 12 and 13 of exon 2,
codon 61 of exon 3 and codon 146 of exon 4 were observed (Fig. 3).
Discussion
In the present study, two cases of GEA were
examined. This tumor type is distinguished from common
adenocarcinoma of the uterine cervix in terms of etiology,
biological behavior and clinical outcome. Morphologically, the
tumor is comprised of numerous glands, varying from well to poorly
formed. Eosinophilic or clear cytoplasm, foamy cytoplasm, vesicular
nuclei and prominent nucleoli characterize the tumor cells
(17).
It has been postulated that lobular endocervical
glandular hyperplasia may serve as a precursor to gastric lesions
in GEA. Minimal deviation adenocarcinoma is considered an extremely
well-differentiated form of gastric-type adenocarcinoma. Compared
with common cervical adenocarcinomas, gastric-type adenocarcinoma
is associated with a poorer prognosis (18). By raising awareness of precursor and
well-differentiated forms of GEA, an earlier diagnosis could
potentially be facilitated by pathologists, leading to prompt
treatment and improved patient outcomes.
In the present study, the two patients with GEA both
experienced an unfavorable clinical outcome, as observed after
diagnosis. The clinical manifestations and the pathological
findings were resembling, as per immunohistochemical findings, the
profile was similar except that PAX8 was positive in case 2
(18); this may reflect a variation
in histological differentiation, but was not related to their
prognosis. Both patients had signs of tumor expansion, notably in
case 2, where lymphovascular invasion was observed. The two
patients underwent radical removal of the uterine cervix and
surrounding tissues, and were administered radio- and chemotherapy
afterwards. The patient in case 1 was readmitted after the first
round of therapy. Both patients manifested a poor prognosis as
suggested by signs of metastasis and recurrence.
GEA is not associated with HPV. The etiology may
indicate differences in the genesis mechanism. A notable alteration
in known cancer-related genes, as revealed by the present study, is
the mutation of the tumor suppressor p53. This mutation is rare in
other types of cervical cancer, including both SCC and
adenocarcinoma of the cervix (7,19). The
oncogenic products encoded by HPV were not detected in the present
cases, which is commonly observed in the same type of cancer, i.e.
adenocarinoma of other histological origin. At the molecular level,
the occurrence of malignancies is driven by the action of multiple
cancer-related genes. In adenocarcinoma and GEA, the accumulation
of changes in cancer-related genes could replace the tumorigenic
potential of HPV. This substitution propels the initiation of the
carcinogenesis process from the host cells' phenotype.
Morphologically, gastric-type adenocarcinomas show considerable
overlap but must be distinguished from other adenocarcinomas, such
as those originating in the pancreas and biliary tract (17).
Microscopically, adenocarcinoma cells closely
resemble those of pancreaticobiliary mucinous carcinoma rather than
having a gastric origin (17,20).
The tumors in the present study were composed of cysts lined with
mucinous cells. Immunohistochemically, the cases were negative for
p16 and positive for mutant p53. Other positive immunophenotypic
markers include CK7 and MUC5AC. Negative immunomarkers included ER
and PR. Previous reports indicate that ER and PR expression is
absent in the immunophenotype of adenoma malignum (21,22).
In endocervical adenocarcinoma, MUC5AC showed
positive expression to varying degrees in the majority of samples
analyzed, with some cases being negative similar to previously
reported results (23–25).
Similar to previously reported findings, CK7 was
positive in the tumors from the present patients (26,27).
PD-L1 was negative in the present study, suggesting the status of
the host antitumor immunity. High expression of the immune response
checkpoint factor implies a restoration of host immunity against
tumors, and patients whose tumors overexpress PD-L1 have improved
clinical outcomes with anti-PD-1-directed therapy (28).
PAX8 immunoreactivity has proven useful in
distinguishing non-gynecological adenocarcinomas. While there have
been several studies about GAS (29,30),
little is known about the distinguishing features of GAS.
Therefore, more studies on the diagnosis of GAS should be reported
in the future. PAX8, a paired-box gene crucial in the embryogenesis
of the thyroid gland, kidney and Mullerian system, is positive in
normal thyroid, renal and Mullerian epithelia, as well as in most
carcinomas arising in these organs (29). It has been demonstrated that nuclear
PAX8 staining is present in a large number of non-serous ovarian
epithelial neoplasms and cervical epithelial lesions (29).
In the present study, PAX8 was found to be negative
only in case 1. In the female genital tract, most adenocarcinomas
of the ovary, fallopian tube, endometrium and cervix are positive
for PAX8. However, primary mucinous adenocarcinomas of the ovary
are usually negative, or at most, they exhibit focal weak
immunoreactivity. Cervical adenocarcinomas are less likely to be
positive than endometrial adenocarcinomas and non-mucinous ovarian
adenocarcinomas (30).
Mutant K-ras was not detected in the present study
using specimens from two cases of GEA. Given the small amount of
material used, the role of a ras gene mutation in the genesis of
this specific type of tumor cannot be excluded. Further validation
with materials from more cases is required. It has been reported
that the frequency of codon 12 mutations of K-ras is high in
primary adenocarcinomas of the pancreas, with >80% of the
examined carcinomas harboring a point mutation (9,10).
In summary, several relevant histochemical markers
have been detected in the present study. The findings generally
align with the profile of GEA, but some indicators may vary in
different reports. This prompts further screening for more specific
markers for the diagnosis of GEA, given its status as a rare
clinical entity.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JZ and XZ conceived the idea of investigation,
designed the study and prepared the first draft of the manuscript.
JZ, XZ, WM, YZ, LY, JJ and MZ reviewed the specimens, confirmed the
diagnosis, analyzed the data, and corrected the manuscript. JZ
performed the immunohistochemical staining and collected the
clinical specimens. JZ, XZ and WM confirm the authenticity of all
the raw data. All authors have read and approved the final
manuscript, and approved the submission.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Lishui Central Hospital (Lishui, China; approval no. 2023-526).
Patient consent for publication
The patient provided written informed consent for
publication of the case study described.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Adegoke O, Kulasingam S and Virnig B:
Cervical cancer trends in the United States: A 35-year
population-based analysis. J Womens Health (Larchmt). 21:1031–1037.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guo F, Cofie LE and Berenson AB: Cervical
cancer incidence in young U.S. females after human papillomavirus
vaccine introduction. Am J Prev Med. 55:197–204. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bewley S: HPV vaccination and cervical
cancer screening. Lancet. 399:19392022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Michels KB and zur Hausen H: HPV vaccine
for all. Lancet. 374:268–270. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Paavonen J, Naud P, Salmerón J, Wheeler
CM, Chow SN, Apter D, Kitchener H, Castellsague X, Teixeira JC,
Skinner SR, et al: Efficacy of human papillomavirus (HPV)-16/18
AS04-adjuvanted vaccine against cervical infection and precancer
caused by oncogenic HPV types (PATRICIA): Final analysis of a
double-blind, randomised study in young women. Lancet. 374:301–314.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cree IA, White VA, Indave BI and Lokuhetty
D: Revising the WHO classification: Female genital tract tumours.
Histopathology. 76:151–156. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kojima A, Mikami Y, Sudo T, Yamaguchi S,
Kusanagi Y, Ito M and Nishimura R: Gastric morphology and
immunophenotype predict poor outcome in mucinous adenocarcinoma of
the uterine cervix. Am J Surg Pathol. 31:664–672. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mikami Y and McCluggage WG: Endocervical
glandular lesions exhibiting gastric differentiation: An emerging
spectrum of benign, premalignant and malignant lesions. Adv Anat
Pathol. 20:227–237. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Carpenter KM, Durrant LG, Morgan K,
Bennett D, Hardcastle JD and Kalsheker NA: Greater frequency of
K-ras Val-12 mutation in colorectal cancer as detected with
sensitive methods. Clin Chem. 42 (6 Pt 1):904–909. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hruban RH, van Mansfeld AD, Offerhaus GJ,
van Weering DH, Allison DC, Goodman SN, Kensler TW, Bose KK,
Cameron JL and Bos JL: K-ras oncogene activation in adenocarcinoma
of the human pancreas. A study of 82 carcinomas using a combination
of mutant-enriched polymerase chain reaction analysis and
allele-specific oligonucleotide hybridization. Am J Pathol.
143:545–554. 1993.PubMed/NCBI
|
|
11
|
Staratschek-Jox A, Kotkowski S, Belge G,
Rudiger T, Bullerdiek J, Diehl V and Wolf J: Detection of
Epstein-Barr Virus in Hodgkin-ReedSternberg Cells: No evidence for
the persistence of integrated viral fragments inLatent membrane
protein-1 (LMP-1)-negative classical Hodgkin's disease. Am J
Pathol. 156:209–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Carleton C, Hoang L, Sah S, Kiyokawa T,
Karamurzin YS, Talia KL, Park KJ and McCluggage WG: A detailed
immunohistochemical analysis of a large series of cervical and
vaginal gastric-type adenocarcinomas. Am J Surg Pathol. 40:636–644.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao S, Hayasaka T, Osakabe M, Kato N,
Nakahara K, Kurachi H, Fukase M, Katayama Y, Yaegashi N and
Motoyama T: Mucin expression in nonneoplastic and neoplastic
glandular epithelia of the uterine cervix. Int J Gynecol Pathol.
22:393–397. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fan XY, Hu ZY, Xu FH, Yan ZQ, Guo SQ and
Li ZM: Rapid Detection of rpoB gene mutations in rifampin-resistant
mycobacterium tuberculosis isolates in shanghai by using the
amplification refractory mutation system. J Clin Microbiol.
41:993–997. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Levine AJ, Hu W and Feng Z: The P53
pathway: What questions remain to be explored? Cell Death Differ.
13:1027–1036. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Muller PA and Vousden KH: p53 mutations in
cancer. Nat Cell Biol. 15:2–8. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Talia KL and McCluggage WG: The developing
spectrum of gastric-type cervical glandular lesions. Pathology.
50:122–133. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park KJ: Cervical adenocarcinoma:
Integration of HPV status, pattern of invasion, morphology and
molecular markers into classification. Histopathology. 76:112–127.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ishii K, Hosaka N, Toki T, Momose M,
Hidaka E, Tsuchiya S and Katsuyama T: A new view of the so-called
adenoma malignum of the uterine cervix. Virchows Arch. 432:315–322.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chou YY, Lin MC and Huang LW: Human
papillomavirus-unrelated gastric type of cervical adenocarcinoma
presenting with a metastatic ovarian tumor: Report of a case. J Low
Genit Tract Dis. 17:218–222. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Karamurzin YS, Kiyokawa T, Parkash V,
Jotwani AR, Patel P, Pike MC, Soslow RA and Park KJ: Gastric-type
endocervical adenocarcinoma. An aggressive tumor with unusual
metastatic patterns and poor prognosis. Am J Surg Pathol.
39:1449–1457. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Toki T, Shiozawa T, Hosaka N, Ishii K,
Nikaido T and Fujii S: Minimal deviation adenocarcinoma of the
uterine cervix has abnormal expression of sex steroid receptors,
CA125, and gastric mucin. Int J Gynecol Pathol. 16:111–116. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li H, Jing X, Yu J, Liu J, Zhang T, Chen S
and Zhang X: A combination of cytokeratin 5/6, p63, p40 and MUC5AC
are useful for distinguishing squamous cell carcinoma from
adenocarcinoma of the cervix. Diagn Pathol. 15:1042020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Riethdorf L, O'Connell JT, Riethdorf S,
Cviko A and Crum CP: Differential expression of MUC2 and MUC5AC in
benign and malignant glandular lesions of the cervix uteri.
Virchows Arch. 437:365–371. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hebbar V, Damera G and Sachdev GP:
Differential expression of MUC genes in endometrial and cervical
tissues and tumors. BMC Cancer. 5:1242005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gilks CB, Young RH, Aguirre P, DeLellis RA
and Scully R: Adenoma malignum (minimal deviation adenocarcinoma)
of the uterine cervix. A clinicopathological and
immunohistochemical analysis of 26 cases. Am J Surg Pathol.
13:717–729. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McCluggage WG, Shah R, Connolly LE and
McBride HA: Intestinal-type cervical adenocarcinoma in situ and
adenocarcinoma exhibit a partial enteric immunophenotype with
consistent expression of CDX2. Int J Gynecol Pathol. 27:92–100.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Patel SP and Kurzrock R: PD-L1 expression
as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther.
14:847–856. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Laury AR, Perets R, Piao H, Krane JF,
Barletta JA, French C, Chirieac LR, Lis R, Loda M, Hornick JL, et
al: A comprehensive analysis of PAX8 expression in human epithelial
tumors. Am J Surg Pathol. 35:816–826. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yemelyanova A, Gown AM, Wu LS, Holmes BJ,
Ronnett BM and Vang R: PAX8 expression in uterine adenocarcinomas
and mesonephric proliferations. Int J Gynecol Pathol. 33:492–499.
2014. View Article : Google Scholar : PubMed/NCBI
|