Introduction
Colorectal cancer (CRC) is the second most prevalent
cause of mortality globally and ranks as the third most frequently
occurring malignancy (1). The
global death rate for CRC is >800,000 individuals annually, with
a persistent upward trend in the incidence and fatalities
associated with CRC (2).
The treatment of early-stage CRC often involves
extensive surgical intervention followed by adjuvant chemotherapy.
This comprehensive approach is associated with favorable prognostic
outcomes (3). However, due to the
absence of readily discernible symptoms during the initial stage of
CRC, at the time of diagnosis, a substantial number of individuals
have advanced or metastatic (m)CRC. This results in a poor
prognosis, characterized by a low 5-year overall survival rate of
5–8% (4).
Chemotherapy is the prevailing therapeutic approach
for managing mCRC; however, it is associated with obstacles,
including limited selectivity, systemic adverse responses and
suboptimal concentration of administered drug at the tumor site
(5,6). Immunotherapy has demonstrated positive
results in treatment of numerous types of malignant cancer, such as
melanoma, renal cell carcinoma and non-small cell lung cancer
(7). Research has demonstrated that
immune checkpoint inhibitors (ICIs) exhibit favorable effectiveness
in individuals with mCRC who possess mismatch repair deficient
(dMMR) or microsatellite instability-high (MSI-H) attributes
(8,9), but only in patients with mismatch
repair deficient (dMMR) or microsatellite instability-high (MSI-H).
However, occurrence of dMMR/MSI-H tumors accounts for only 2–4% of
all cases of mCRC (10).
Most individuals diagnosed with CRC demonstrate MMR
proficient (pMMR) or MS stable (MSS) status. These molecular
characteristics of CRC can lead to a diminished effectiveness of
ICIs in combating the malignancy (11,12).
According to clinical studies, the combination of anti-angiogenic
drugs and ICIs enhances the efficacy of treatment of malignant
tumors (13–15). These drugs possess anti-angiogenic
properties and can hinder the expression of immunosuppressive
molecules; as a result, they contribute to restoration of the
immunosuppressive tumor microenvironment (TME) (13). Hence, using ICIs in conjunction with
tyrosine kinase inhibitors (TKIs) that possess anti-angiogenic
properties may serve as a potential strategy for circumventing
resistance exhibited by MSS or pMMR mCRC towards immunotherapeutic
interventions.
At present, regorafenib and fruquintinib are the
primary TKIs used for the treatment of CRC. In patients with
MSS/pMMR mCRC, the combined therapy of nivolumab and regorafenib
shows a 33% objective remission rate, according to the findings of
a phase 1b clinical trial (16).
Recently, avelumab and regorafenib were the subjects of a phase II
clinical investigation, where the positive response of patients was
limited to achieving a stable disease state (17). Zhang et al (18) reported that the combined use of
fruquintinib and programmed death cell death protein 1 (PD-1)
inhibitors yields notable outcomes in the treatment of advanced MSS
CRC. The aforementioned study revealed an objective effective rate
of 11.8% and a disease control rate of 70%. These findings indicate
favorable therapeutic outcome in the treatment of CRC.
The aforementioned studies indicate that the
combination of ICIs and TKIs holds potential as a viable
therapeutic approach for individuals with advanced or mCRC who
exhibit MSS or pMMR status. However, the number of related studies
is relatively small, and this treatment strategy is currently
mainly applied to small sample size Phase I or II trials, where
patients are usually superselective, and the overall efficacy and
safety of these trials are still unknown (16–18).
There is lack of agreement about the appropriate therapeutic
approach for patients with advanced or metastatic MSS/pMMR CRC.
Therefore, the present systematic review and meta-analysis was
performed to assess relevant literature with data on the combined
use of ICIs and TKIs for the treatment of patients with MSS/pMMR
with advanced-stage or invasive CRC. The aim was to determine if
the treatment is safe and successful, and to offer guidance for
managing CRC.
Materials and methods
Search strategy
The current study aligns with the principles and
recommendations in the Preferred Reporting Items for Systematic
Reviews and Meta-Analyses (PRISMA) guidelines. The systematic
review procedure performed in the present study was registered on
the International Platform of Registered Systematic Review and
Meta-Analysis Protocols (registration no. INPLASY202390019;
doi.org/10.37766/inplasy2023.9.0019).
To ensure comprehensive investigation, an extensive
search was performed across multiple databases, including Embase
(https://www.embase.com/), Web of Science
(https://webofscience.clarivate.cn/),
Cochrane (https://www.cochranelibrary.com/), PubMed (http://www.ncbi.nlm.nih.gov/pubmed) and
ClinicalTrials.gov
(clinicaltrials.gov/). Articles published from January 1, 2003 to
July 28, 2023 were included study. The articles were searched in
all languages and relevant articles were included regardless of
language of publication or primary outcomes. The complete search
terms used for all databases were: ((((Regorafenib) OR
(Fruquintinib)) OR (Tyrosine kinase inhibitors)) AND
((((((((((((immune checkpoint inhibitor) OR (PD-1 Inhibitors)) OR
(programmed cell death protein 1 inhibitor)) OR (PD-L1 Inhibitors))
OR (Programmed Death-Ligand 1 Inhibitors)) OR (CTLA-4 inhibitors))
OR (PD-1-PD-L1 Blockade)) OR (Nivolumab)) OR (Atezolizumab)) OR
(Durvalumab)) OR (Avelumab)) OR (pembrolizumab))) AND
((((((((((((((((Colorectal Neoplasms[Title/Abstract]) OR
(Colorectal Neoplasm[Title/Abstract])) OR (Neoplasm,
Colorectal[Title/Abstract])) OR (Neoplasms, Colorectal[Title/
Abstract])) OR (Colorectal Tumors[Title/Abstract])) OR (Colorectal
Tumor[Title/Abstract])) OR (Tumor, Colorectal[Title/Abstract])) OR
(Tumors, Colorectal[Title/Abstract])) OR (Colorectal
Cancer[Title/Abstract])) OR (Cancer, Colorectal[Title/ Abstract]))
OR (Cancers, Colorectal[Title/Abstract])) OR (Colorectal
Cancers[Title/Abstract])) OR (Colorectal
Carcinoma[Title/Abstract])) OR (Carcinoma,
Colorectal[Title/Abstract])) OR (Carcinomas,
Colorectal[Title/Abstract])) OR (Colorectal
Carcinomas[Title/Abstract])).
Inclusion criteria
To assess the suitability of studies, the population
(P), intervention (I), comparator (C), outcome (O) and study design
(S) framework was used. Utilizing this particular framework
resulted in proficient assessment and determination of the
suitability and qualification of the studies. The criteria were as
follows: P, patients with advanced or metastatic MSS/pMMR CRC; I,
TKIs + ICIs; C, patients with CRC before the study started; O,
adverse reaction rate, disease control rate (DCR) and objective
response rate (ORR); and S, cohort or case-control studies.
Exclusion criteria
Exclusion criteria included: Articles lacking
survival data and studies assessing the combination of ICIs + TKIs
in primary tumors besides CRC. Studies with individuals diagnosed
with CRC or other malignancy that failed to provide separate
findings were also excluded. The analysis also excluded letters to
the editor, reviews, animal studies, case reports and conference
abstracts.
Extraction of data and evaluation of
quality
A total of two researchers, JL and YXZ, performed
the literature screening process. This involved carefully reviewing
the topic, picking relevant articles based on the aforementioned
criteria, and evaluating the abstract and full text of the selected
articles. To assess the quality of cohort and case-control studies,
the Newcastle-Ottawa scale (NOS) was used (19). The NOS is a comprehensive framework
of eight items that are further categorized into three domains:
Population selection, exposure or outcome evaluation, and
comparability. Each item is assigned a numerical score on a scale
ranging from 0–9; scores >5 indicate a high level of quality
(19). The following data were
separately recorded by two researchers (JL and YXZ): Details of the
first author; publication date; country in which the study was
performed; type of research; treatment technique; number of
patients participating in the research; % male patients; and the
median follow-up period of the study. Discussions with a third
researcher (SQL) resolved disagreements between the two
researchers.
Statistical analysis
The current study performed extensive meta-analysis
of pertinent literature to assess a range of clinical outcomes.
Meta-analysis was performed using STATA 16.1 statistical software
(StataCorp LLC, College Station, TX). DCR, ORR, adverse reaction
rate and grade ≥3 adverse reaction rate were assessed. These
outcomes and corresponding 95% confidence intervals (CIs) were
analyzed. To evaluate inter-study heterogeneity, the statistical
measures of the I2 statistic and Cochran's Q test were
used. Values <25% were considered to indicate low levels of
heterogeneity; 25–50% indicated moderate levels of heterogeneity
and values >50 and <75% were considered to indicate high
levels of heterogeneity (20). When
values >75%, sensitivity analysis is conducted on the evaluated
effect size and research heterogeneity to evaluate the stability of
the results, excluding studies with a significant impact on
heterogeneity. The random effect model was used for combined
analysis. Funnel plots were used to assess publication bias. The
identification of potential bias was accomplished by evaluating the
asymmetry of the plots, which was assessed using Egger's and Begg's
tests. P<0.05 was considered to indicate a statistically
significant difference.
Results
Study characteristics
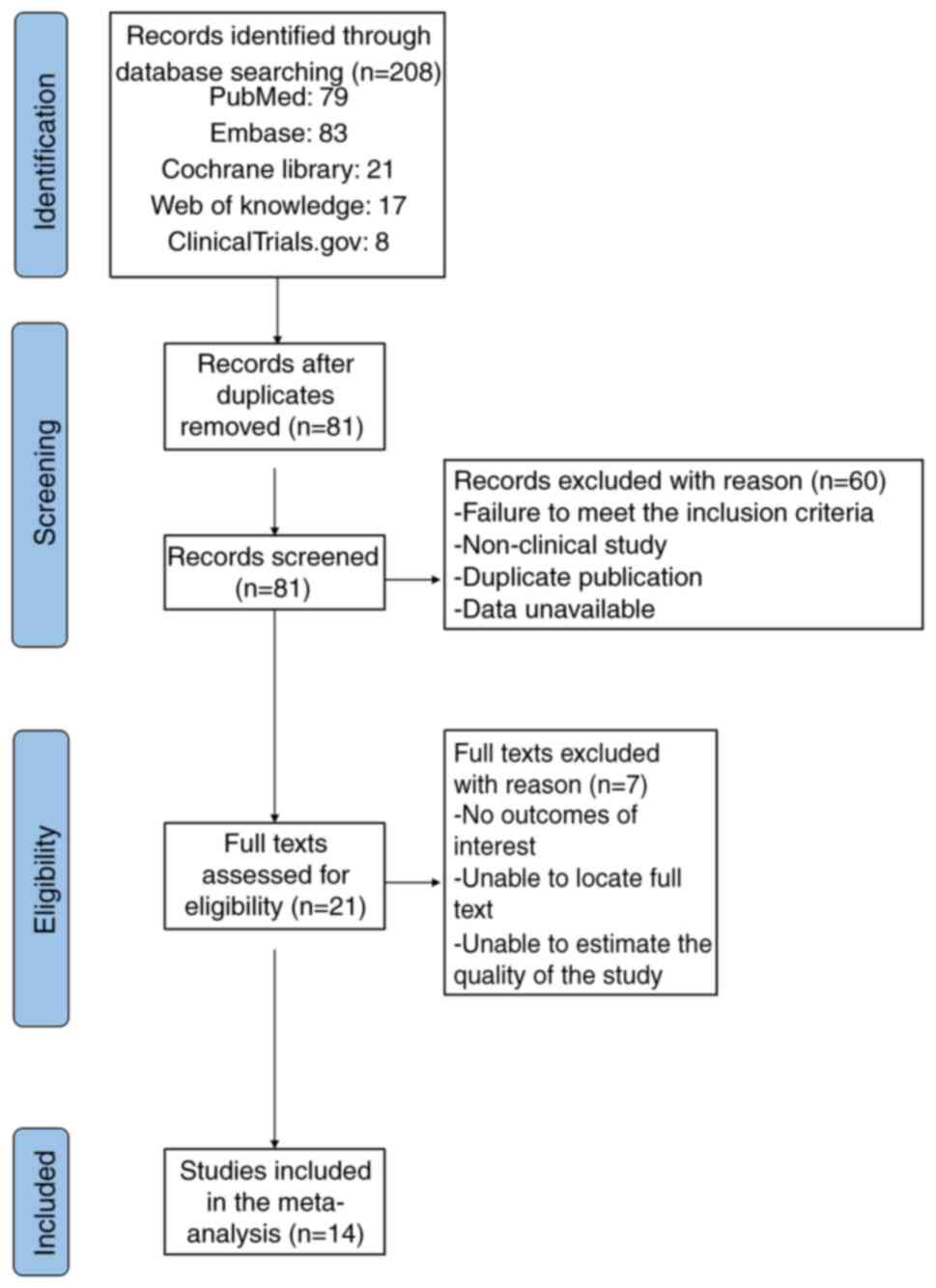
A total of 14 studies, with a sample size of 819
patients, were selected for inclusion after assessing the full-text
articles and extracting relevant data. The selection procedure
adhered to the guidelines outlined in the PRISMA flowchart
(Fig. 1). The present study
analyzed cohort studies (16–18,21–31)
and the pertinent information on the included research is outlined
in Table I.
 | Table I.Characteristics of all studies
included in the meta-analysis. |
Table I.
Characteristics of all studies
included in the meta-analysis.
| First author,
year | Country | Enrollment | PD-1/PD-L1
inhibitor | TKI | Patients | Male, % | Median follow-up,
months | (Refs.) |
|---|
| Fukuoka et
al, 2020 | Japan | Cohort | Nivolumab | Regorafenib | 25 | 72.00 | 6.6 | (16) |
| Cousin et
al, 2021 | France | Cohort | Avelumab | Regorafenib | 43 | 74.00 | 7.2 | (17) |
| Zhang et al,
2022 | China | Cohort | ICI | Fruquintinib | 110 | 57.30 | 9.8 | (18) |
| Chen et al,
2022 | China | Cohort | ICI | Regorafenib | 21 | 37.50 | 16.2 | (21) |
| Wang et al,
2020 | USA | Cohort | Nivolumab and
pembrolizumab | Regorafenib | 18 | 88.90 | 7.0 | (22) |
| He et al,
2023 | China | Cohort | ICI | Regorafenib | 84 | 56.00 | 14.2 | (23) |
| Kim et al,
2022 | USA | Cohort | Nivolumab | Regorafenib | 40 | 53.80 | 7.9 | (24) |
| Li et al,
2020 | China | Cohort | ICI | Regorafenib | 21 | 69.60 | 7.9 | (25) |
| Ma et al,
2023 | China | Cohort | Toripalimab | Fruquintinib | 18 | 63.16 | 9.2 | (26) |
| Ren et al,
2020 | China | Cohort | SHR-1210 | Apatinib | 9 | 30.00 | 8.2 | (27) |
| Sun et al,
2021 | China | Cohort | ICI | Fruquintinib and
regorafenib | 51 | 52.90 | 6.2 | (28) |
| Xu et al,
2022 | China | Cohort | ICI | Regorafenib | 30 | 46.70 | 12.0 | (29) |
| Yang et al,
2022 | China | Cohort | ICI | Regorafenib | 82 | 60.00 | 5.5 | (30) |
| Yu et al,
2021 | China | Cohort | Toripalimab | Regorafenib | 33 | 54.54 | 19.0 | (31) |
Quality evaluation of the included
studies
The present study used the NOS to evaluate the
quality of studies. A score of 5–9 on the NOS suggested a study was
of good quality. All 14 studies reviewed obtained scores ≥7,
indicating a high level of quality (Table II).
 | Table II.Quality assessment using the
Newcastle-Ottawa Scale for cohort studies. |
Table II.
Quality assessment using the
Newcastle-Ottawa Scale for cohort studies.
|
| Selection | Comparability | Outcome |
|
|
|---|
|
|
|
|
|
|
|
|---|
| First author,
year | Representativeness
of the exposed cohort | Selection of the
non-exposed cohort | Ascertainment of
exposure | Demonstration that
outcome of interest was not present at start of study | Comparability of
cohorts on the basis of design or analysis | Assessment of
outcome | Demonstration that
follow-up was long enough for outcomes to occur | Adequacy of
follow-up | Score | (Refs.) |
|---|
| Fukuoka et
al, 2020 | 1 | 1 | 1 | 1 | 2 | 1 | 0 | 1 | 8 | (16) |
| Cousin et
al, 2021 | 1 | 1 | 1 | 1 | 2 | 1 | 0 | 1 | 8 | (17) |
| Zhang et al,
2022 | 1 | 1 | 1 | 1 | 2 | 1 | 0 | 1 | 8 | (18) |
| Chen et al,
2022 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | (21) |
| Wang et al,
2020 | 1 | 1 | 1 | 1 | 2 | 1 | 0 | 1 | 8 | (22) |
| He et al,
2023 | 1 | 1 | 1 | 1 | 2 | 1 | 0 | 1 | 8 | (23) |
| Kim et al,
2022 | 1 | 1 | 1 | 1 | 2 | 1 | 0 | 1 | 8 | (24) |
| Li et al,
2020 | 1 | 1 | 1 | 1 | 2 | 1 | 0 | 1 | 8 | (25) |
| Ma et al,
2023 | 1 | 1 | 1 | 1 | 2 | 1 | 0 | 1 | 8 | (26) |
| Ren et al,
2020 | 1 | 1 | 1 | 1 | 2 | 0 | 0 | 1 | 7 | (27) |
| Sun et al,
2021 | 1 | 1 | 1 | 1 | 2 | 1 | 0 | 1 | 8 | (28) |
| Xu et al,
2022 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | (29) |
| Yang et al,
2022 | 1 | 1 | 1 | 1 | 2 | 1 | 0 | 1 | 8 | (30) |
| Yu et al,
2021 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | (31) |
Meta-analysis
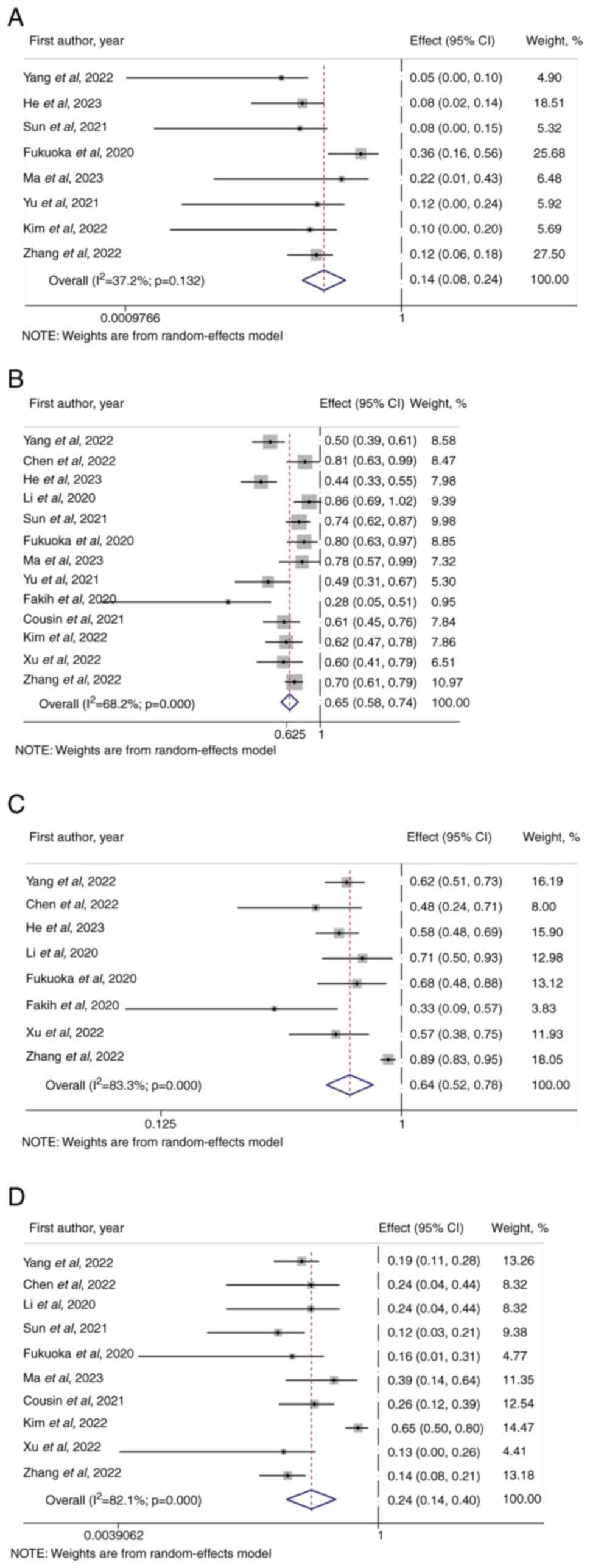
The ORR of the combination therapy of ICIs + TKIs
was 14% (95% CI, 0.08–0.24; Fig.
2A) in patients diagnosed with advanced or metastatic MSS/pMMR
CRC. The DCR was 65% (95% CI, 0.58–0.74; Fig. 2B). The overall incidence of adverse
events of varying severity associated with the combination of ICIs
+ TKIs was 64% (95% CI, 0.52–0.78; Fig.
2C). The incidence of grade ≥3 adverse reactions was 24% (95%
CI, 0.14–0.40; Fig. 2D).
Assessment of publication bias
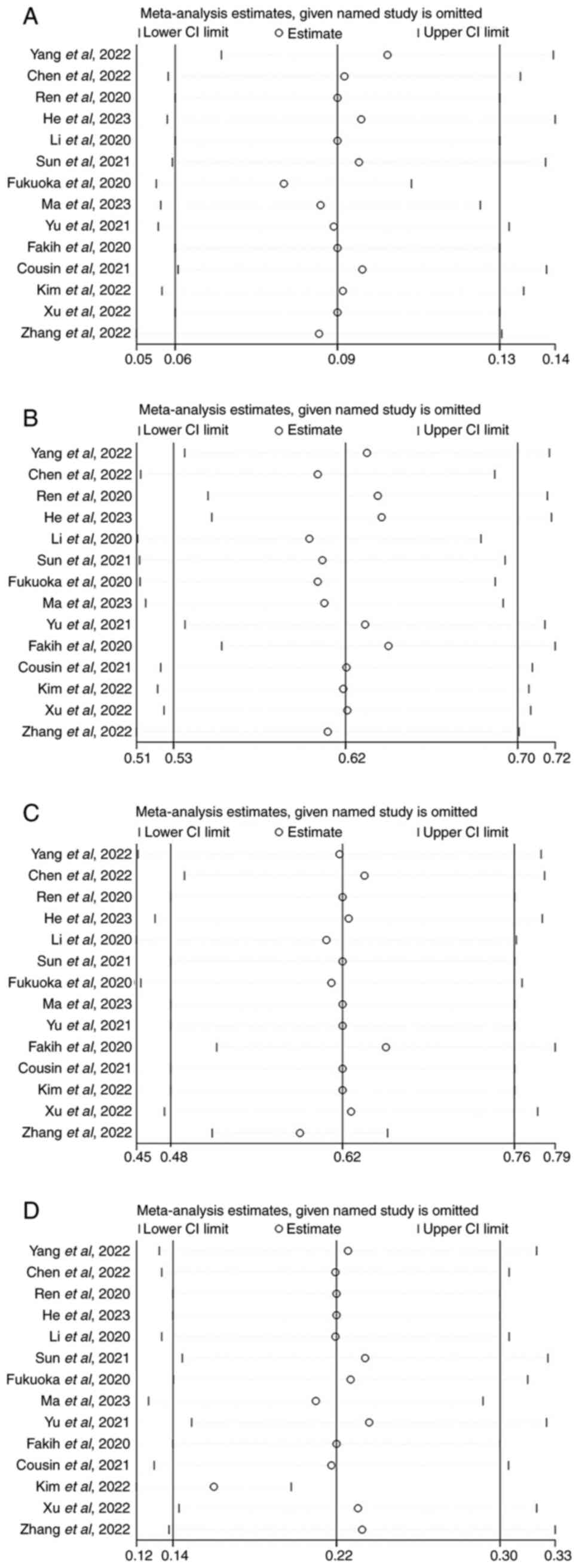
The sensitivity analysis indicated that the
exclusion of individual studies did not yield statistically
significant variations in combined analysis results (Fig. 3A-D). This suggested that the overall
results obtained from the present study may be considered valid and
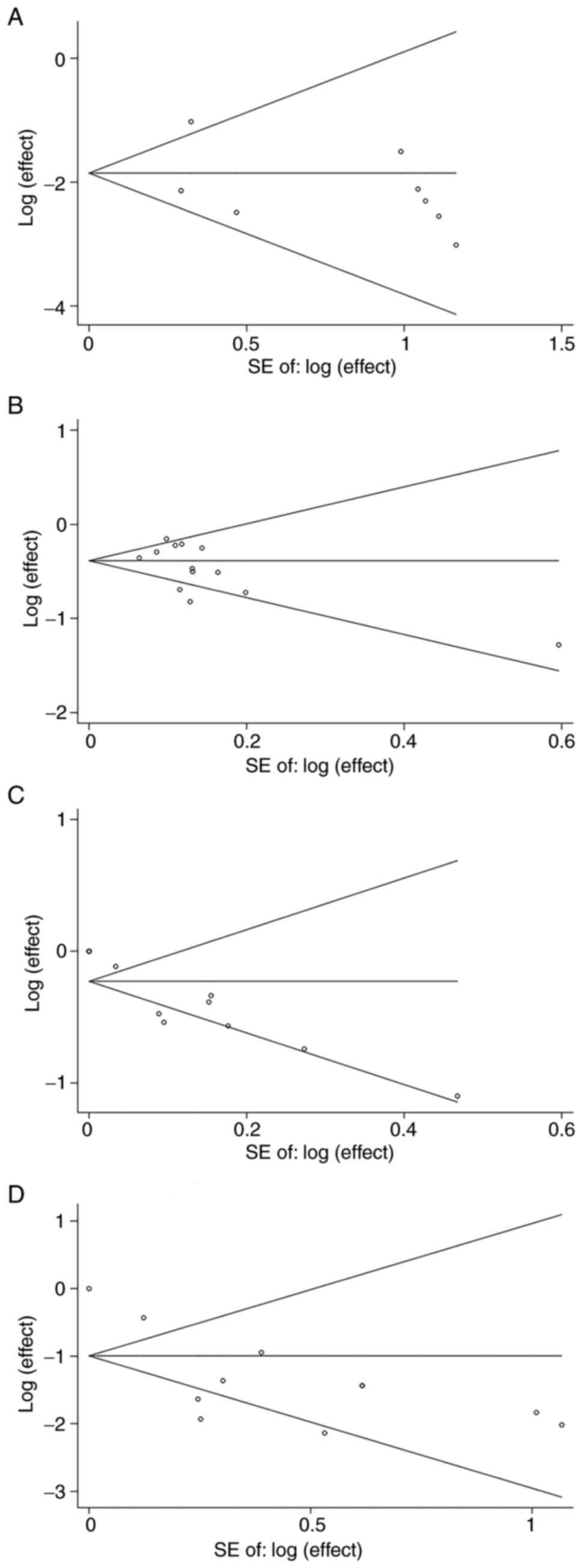
reliable. Furthermore, Begg's and Egger's tests for objective
response and disease control were 0.114 and 0.395, publication bias
was indicated to be absent as there was no apparent asymmetry in
the Begg's funnel plot for objective response (Fig. 4A) and disease control (Fig. 4B). P-values obtained from the Begg's
and Egger's tests for adverse reactions and adverse reaction grade
≥3 were 0.008 and 0.048, respectively. The asymmetry of the Begg's
funnel plot for adverse reactions (Fig.
4C) and adverse reaction grade ≥3 (Fig. 4D) were evident, suggesting presence
of potential publication bias.
Discussion
The relevance of immune inhibitors in the clinical
setting is underscored by their potent anticancer impact, which
extends to several types of solid tumor, such as renal clear cell
carcinoma, liver cancer and melanoma (32). The use of ICIs in managing patients
with MSI-H or dMMR mCRC has received approval from the United
States Food and Drug Administration. This is based on the
compelling evidence from multiple clinical trials (33,34).
MSI-H/dMMR CRC exhibits enhanced tumor antigen production by
elevating tumor mutational burden, which leads to heightened
infiltration of T cells into the TME, rendering these tumors more
responsive to ICIs (35).
Conversely, MSS/pMMR CRC with low tumor mutational burden and
minimal infiltration of T cells exhibits resistance to ICIs
(35).
Researchers are investigating combined strategies to
reverse the MSS immunosuppressive microenvironment of CRC (12,36),
which can lead to benefits from ICI therapy. Clinical study IMblaze
370 reported the combination of atezolizumab (an anti-programmed
cell death ligand 1 antibody) and cobiotinib (a mitogen-activated
extracellular signal-regulated kinase inhibitor), but the results
showed that only 3% of patients were effective with this regimen
(12). In the study CHECKMATE 142,
1/20 patients diagnosed with MSS/pMMR CRC and treated with a
combination of nivolumab and ipilimumab had an objective response
(36). Nevertheless, the challenges
associated with using ICIs for cancers classified as ‘immune
rejection’ or ‘immune desert’ cannot be solved by these connected
study findings.
There is a need to investigate novel treatment
approaches to overcome the immune resistance of MSS/pMMR CRC. Wu
et al (37) assessed the
efficacy of ICIs combined with chemotherapy, anti-VEGF and
anti-EGFR in the treatment of pMMR/non-MSI-H mCRC. ICI-based
combination therapy was revealed to be promising in the treatment
of pMMR/non-MSI-H mCRC with good efficacy and controllable
toxicity. Based on this, the present study focused on the
therapeutic effect of combining anti-VEGF drugs with ICIs on
pMMR/non MSI-H mCRC.
Several studies have demonstrated the potential of
combining anti-VEGF therapy with ICIs as a therapeutic approach to
address drug resistance in MSS/pMMR CRC (13–15).
VEGF promotes angiogenesis, which may result in the proliferation
of immune cells that suppress tumor growth, such as regulatory T
cells and myeloid-derived suppressor cells. Additionally, VEGF can
enhance infiltration of tumor-associated macrophages into the TME
(13). Moreover, VEGF has
immunosuppressive properties via its ability to hinder the
differentiation of progenitor cells derived from CD4+
and CD8+ lymphocytes. Moreover, previous studies have
reported that VEGF possesses the ability to augment T-cell
exhaustion via the upregulation of several molecules on T cells
(14,38). These molecules include cytotoxic T
lymphocyte-associated protein 4, PD-1, lymphocyte activation gene 3
and T-cell immunoglobulin and mucin domain-containing protein 3
(38,39). The present study provided a
theoretical framework for the combined use of angiogenesis
inhibitors and ICIs. In clinical settings, TKIs, such as
regorafenib and fruquintinib, are often used as anti-angiogenic
pharmaceuticals. These medications have been incorporated into the
therapeutic regimen for CRC (28–30).
Yang et al (30) assessed
the combined use of ICIs with regorafenib for treating patients
with advanced or metastatic MSS CRC. In terms of ICIs drug
selection, 39% of patients used Sintilimab, 20% used nivolumab, and
others included: toripalimab (15%), camrelizumab (14%),
pembrolizumab (7%) and tislelizumab (4%). The study reported an ORR
of 4.9% and a DCR of 50%. Furthermore, Ma et al (26) used a combination of toripalimab and
fruquintinib, resulting in notable effectiveness: 4/18 patients in
the final analysis exhibited partial responses, and 10/18 had
stable disease. Nevertheless, further investigation is required to
establish the effectiveness and safety of the combined
administration of TKIs and ICIs.
The present study aimed to perform a comprehensive
review and meta-analysis to assess the efficacy and safety of TKIs
used in conjunction with ICIs for individuals diagnosed with
advanced or metastatic MSS/pMMR CRC. The study involved integrating
analyses with the evaluation of ORR, DCR, incidence of adverse
reactions and incidence of grade ≥3 adverse reactions. The findings
indicated that combining TKI and ICIs may have a favorable
therapeutic outcome and the incidence of grade 3 adverse reactions
was satisfactory. Moreover, this is consistent with previous
clinical studies in terms of therapeutic effect (16–18),
and the therapeutic effect of TKI + ICI combination therapy
provides evidential support for ICIs combined with anti-VEGF
therapy for patients with advanced or metastatic MSS/pMMR CRC. In
terms of the potential mechanism of the combination therapy, VEGFR
and EGFR signaling pathways are related; therefore, the combination
of anti-VEGF and ICIs can block several VEGFR- and EGFR-mediated
signaling pathways in addition to immunosuppression, thereby
inhibiting tumor angiogenesis (25,29).
This would circumvent resistance to immunotherapy interventions
demonstrated by MSS/pMMR mCRC.
Unlike a previous study (37), the present study explored numerous
anti-VEGF drugs in the combination of anti-VEGF and ICI treatment.
TKIs combined with ICIs showed satisfactory results and safety, and
may be a promising strategy for treatment of pMMR/MSS mCRC. In a
study by Fukuoka et al (16), when nivolumab and regorafenib were
combined, the ORR was 36%, and the incidence of adverse reactions
grade >3 was 16%. It was hypothesized that combination of 80 mg
regorafenib and nivolumab could increase the antitumor activity
with controllable security. Furthermore, Cousin et al
(17) combined avelumab and
regorafenib with a DCR value of <60%, but the incidence of grade
3 adverse reactions was 25.6% and the safety was lower than other
combination regimens.
The present study had several limitations. The
analysis included a total of 14 studies, excluding individual case
reports and ongoing studies, all of which were cohort studies. The
selection of subjects, types of drug may have impacted the response
of patients to the investigational drug, necessitating a randomized
controlled clinical study to mitigate the influence of potential
confounding factors. However, the present study used NOS to
evaluate the quality of studies; all 14 studies had scores ≥7,
indicating the high quality of the studies. Nevertheless,
variations in use of TKIs + ICIs across different research studies
contributed to the inherent variability.
Regorafenib and fokuntinib are efficacious oral
angiogenesis inhibitors; however, regorafenib is a multi-targeted
TKI that predominantly exerts its effects on VEGFR2,
platelet-derived growth factor receptor and fibroblast growth
factor receptor tyrosine kinases (40), whereas fokuntinib is a potent and
highly selective VEGFR1/2/3 TKI (41). This suggests that the regulatory
mechanism of active binding sites by the two TKIs is distinct.
Compared with regorafenib, fruquintinib belongs to a new generation
of small molecular TKIs with strong effects. It is highly selective
to VEGFR-1, −2 and −3 but has no obvious inhibitory effect on other
kinase activity, and it has been reported to maintain target
inhibition, minimize toxicity and decrease the incidence of adverse
reactions (42,43). Regorafenib targets multiple pathways
and can inhibit angiogenesis to a greater extent than fruquintinib
and also limit the development of resistance to targeted therapy in
tumor cells (44). Therefore,
regorafenib may have more notable long-term benefits in combination
with ICIs than fruquintinib. In a study by Sun et al
(28), the therapeutic effects of
fruquintinib + ICIs and regorafenib + ICIs were compared. Patients
in the fruquintinib group took 3–5 mg oral fruquintinib, whereas
patients in the regorafenib group took 80–160 mg oral regorafenib
once/day for 21 consecutive days in 28-day cycles. The patients
were injected intravenously with PD-1 inhibitors at the recommended
dose from the first day of taking molecular targeted drugs: 240 mg
toripalimab every 3 weeks; 200 mg nivolumab every 2 weeks and 200
mg sintilimab or camrelizumab every 3 weeks. The study reported
that fruquintinib + ICIs had greater short-term survival benefits
compared with regorafenib + ICIs, and the c group had a lower
incidence of adverse reactions, which is consistent with the
present hypothesis. Furthermore, Chen et al (21) and Li et al (25) reported higher DCRs of 80.9 and 85.7,
respectively. In the study by Chen et al (21) the TKI used was regenifenib and the
ICIs were used according to the recommended doses: 240 mg nivolumab
and 200 mg camrelizumab every 2 weeks and 200 mg sinilimab, 240 mg
toripalimab, 200 mg pembrolizumab and 200 mg tislelizumab every 3
weeks. Li et al (25) used
regeorafenib, patients received an anti PD-1 internally starting on
day 1 of oral regeorafenib according to its recommended dose
response: 240 mg nivolumab every 2 weeks; 200 mg camrelizumab every
2 or 3 weeks and 240 mg toripalimab and 200 mg pembrolizumab and
sinilimab every 3 weeks. Wang et al (22) did not observe any grade ≥3 adverse
reactions with the TKI regorafenib and ICIs nivolumab or
pembrolizumab. The aforementioned results indicated that when the
TKI drugs used are consistent, different ICIs impact on the
efficacy and safety of combination therapy. However, due to
differences in administration time, dosage, subject population, and
ICIs regimen of regorafenib and fruquintinib in the included
studies, the present study did not perform subgroup analyses to
further assess the best treatment plan of TKI combined with
ICIs.
In conclusion, although the present study had
limitations, the systematic review and meta-analysis findings
suggested that the combination of immunosuppressants and TKIs
exhibited favorable effectiveness and notable safety profiles when
used in the management of patients with advanced or metastatic
MSS/pMMR CRC. To validate these findings, it is imperative to
perform rigorous prospective research and randomized controlled
trials in future. These studies should investigate the optimal
regimen and dose of TKI + ICIs, potential biomarkers for patient
selection, identification of predictive biomarkers and the
development of tailored treatments for different subtypes of
MSS/pMMR CRC.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JL and YXZ conceived and designed the study. SQL,
YXZ and JXZ collected data and performed the database search. JL
and YXZ performed statistical analysis. JL, SQL, YXZ and JXZ
drafted the manuscript. JL and SQL confirm the authenticity of all
the raw data. All authors revised the manuscript. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Wagle NS, Cercek A, Smith RA
and Jemal A: Colorectal cancer statistics, 2023. CA Cancer J Clin.
73:233–254. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cervantes A, Adam R, Roselló S, Arnold D,
Normanno N, Taïeb J, Seligmann J, De Baere T, Osterlund P, Yoshino
T, et al: Metastatic colorectal cancer: ESMO clinical practice
guideline for diagnosis, treatment and follow-up. Ann Oncol.
34:10–32. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ciombor KK and Bekaii-Saab T: A
comprehensive review of sequencing and combination strategies of
targeted agents in metastatic colorectal cancer. Oncologist.
23:25–34. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Goel G: Evolution of regorafenib from
bench to bedside in colorectal cancer: Is it an attractive option
or merely a ‘me too’ drug? Cancer Manag Res. 10:425–437. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vogel A, Hofheinz RD, Kubicka S and Arnold
D: Treatment decisions in metastatic colorectal cancer-beyond first
and second line combination therapies. Cancer Treat Rev. 59:54–60.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Morris VK, Kennedy EB, Baxter NN, Benson
AB III, Cercek A, Cho M, Ciombor KK, Cremolini C, Davis A, Deming
DA, et al: Treatment of metastatic colorectal cancer: ASCO
guideline. J Clin Oncol. 41:678–700. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yaghoubi N, Soltani A, Ghazvini K,
Hassanian SM and Hashemy SI: PD-1/PD-L1 blockade as a novel
treatment for colorectal cancer. Biomed Pharmacother. 110:312–318.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
André T, Shiu KK, Kim TW, Jensen BV,
Jensen LH, Punt C, Smith D, Garcia-Carbonero R, Benavides M, Gibbs
P, et al: Pembrolizumab in microsatellite-instability-high advanced
colorectal cancer. N Engl J Med. 383:2207–2218. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sahin IH, Akce M, Alese O, Shaib W,
Lesinski GB, El-Rayes B and Wu C: Immune checkpoint inhibitors for
the treatment of MSI-H/MMR-D colorectal cancer and a perspective on
resistance mechanisms. Br J Cancer. 121:809–818. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Borelli B, Antoniotti C, Carullo M,
Germani MM, Conca V and Masi G: Immune-checkpoint inhibitors (ICIs)
in metastatic colorectal cancer (mCRC) patients beyond
microsatellite instability. Cancers (Basel). 14:49742022.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
André T, Lonardi S, Wong KYM, Lenz HJ,
Gelsomino F, Aglietta M, Morse MA, Van Cutsem E, McDermott R, Hill
A, et al: Nivolumab plus low-dose ipilimumab in previously treated
patients with microsatellite instability-high/mismatch
repair-deficient metastatic colorectal cancer: 4-Year follow-up
from CheckMate 142. Ann Oncol. 33:1052–1060. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eng C, Kim TW, Bendell J, Argilés G,
Tebbutt NC, Di Bartolomeo M, Falcone A, Fakih M, Kozloff M, Segal
NH, et al: Atezolizumab with or without cobimetinib versus
regorafenib in previously treated metastatic colorectal cancer
(IMblaze370): A multicentre, open-label, phase 3, andomized,
controlled trial. Lancet Oncol. 20:849–861. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao S, Ren S, Jiang T, Zhu B, Li X, Zhao
C, Jia Y, Shi J, Zhang L, Liu X, et al: Low-dose apatinib optimizes
tumor microenvironment and potentiates antitumor effect of
PD-1/PD-L1 blockade in lung cancer. Cancer Immunol Res. 7:630–643.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Konecny GE: Inhibition of PD-1 and VEGF in
microsatellite-stable endometrial cancer. Lancet Oncol. 20:612–614.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu RY, Kong PF, Xia LP, Huang Y, Li ZL,
Tang YY, Chen YH, Li X, Senthilkumar R, Zhang HL, et al:
Regorafenib promotes antitumor immunity via inhibiting PD-L1 and
IDO1 expression in melanoma. Clin Cancer Res. 25:4530–4541. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fukuoka S, Hara H, Takahashi N, Kojima T,
Kawazoe A, Asayama M, Yoshii T, Kotani D, Tamura H, Mikamoto Y, et
al: Regorafenib plus nivolumab in patients with advanced gastric or
colorectal cancer: An open-label, dose-escalation, and
dose-expansion phase Ib trial (REGONIVO, EPOC1603). J Clin Oncol.
38:2053–2061. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cousin S, Cantarel C, Guegan JP,
Gomez-Roca C, Metges JP, Adenis A, Pernot S, Bellera C, Kind M,
Auzanneau C, et al: Regorafenib-avelumab combination in patients
with microsatellite stable colorectal cancer (REGOMUNE): A
single-arm, open-label, phase II trial. Clin Cancer Res.
27:2139–2147. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang W, Zhang Z, Lou S, Li D, Ma Z and
Xue L: Efficacy, safety and predictors of combined fruquintinib
with programmed death-1 inhibitors for advanced
microsatellite-stable colorectal cancer: A retrospective study.
Front Oncol. 12:9293422022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stang A: Critical evaluation of the
Newcastle-Ottawa scale for the assessment of the quality of
nonrandomized studies in meta-analyses. Eur J Epidemiol.
25:603–605. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Higgins JP, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen B, Zhao H, Huang J, Lv H, Xu W, Nie
C, Wang J, Zhao J, He Y, Wang S and Chen X: Efficacy of regorafenib
combined with PD-1 inhibitors in elderly patients with advanced
metastatic colorectal cancer. BMC Geriatr. 22:9872022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang C, Chevalier D, Saluja J, Sandhu J,
Lau C and Fakih M: Regorafenib and nivolumab or pembrolizumab
combination and circulating tumor DNA response assessment in
refractory microsatellite stable colorectal cancer. Oncologist.
25:e1188–e1194. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
He WZ, Wang L, Yin CX, Yi JH, Jin YN,
Jiang C, Guo GF and Xia LP: Regorafenib with or without a
programmed cell death protein 1 antibody as third-line treatment
for microsatellite stable metastatic colorectal cancer. Cancer Med.
12:6488–6498. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim RD, Kovari BP, Martinez M, Xie H,
Sahin IH, Mehta R, Strosberg J, Imanirad I, Ghayouri M, Kim YC and
Kim DW: A phase I/Ib study of regorafenib and nivolumab in mismatch
repair proficient advanced refractory colorectal cancer. Eur J
Cancer. 169:93–102. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li J, Cong L, Liu J, Peng L, Wang J, Feng
A, Yue J, Li L and Wang X and Wang X: The efficacy and safety of
regorafenib in combination with anti-PD-1 antibody in refractory
microsatellite stable metastatic colorectal cancer: A retrospective
study. Front Oncol. 10:5941252020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ma S, Chen R, Duan L, Li C, Yang T, Wang J
and Zhao D: Efficacy and safety of toripalimab with fruquintinib in
the third-line treatment of refractory advanced metastatic
colorectal cancer: Results of a single-arm, single-center,
prospective, phase II clinical study. J Gastrointest Oncol.
14:1052–1063. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ren C, Mai ZJ, Jin Y, He MM, Wang ZQ, Luo
HY, Zhang DS, Wu CY, Wang F and Xu RH: Anti-PD-1 antibody SHR-1210
plus apatinib for metastatic colorectal cancer: A prospective,
single-arm, open-label, phase II trial. Am J Cancer Res.
10:2946–2954. 2020.PubMed/NCBI
|
|
28
|
Sun L, Huang S, Li D, Mao Y, Wang Y and Wu
J: Efficacy and safety of fruquintinib plus PD-1 inhibitors versus
regorafenib plus PD-1 inhibitors in refractory microsatellite
stable metastatic colorectal cancer. Front Oncol. 11:7548812021.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu YJ, Zhang P, Hu JL, Liang H, Zhu YY,
Cui Y, Niu P, Xu M and Liu MY: Regorafenib combined with programmed
cell death-1 inhibitor against refractory colorectal cancer and the
platelet-to-lymphocyte ratio's prediction on effectiveness. World J
Gastrointest Oncol. 14:920–934. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang K, Han L, Wu S, Qu X, Li Q, Zhao C,
Zhou J, Jin X, Wang Y, Yan D, et al: Real-world outcomes of
regorafenib combined with immune checkpoint inhibitors in patients
with advanced or metastatic microsatellite stable colorectal
cancer: A multicenter study. Cancer Immunol Immunother.
71:1443–1451. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yu W, Tao Q, Zhang Y, Yi F and Feng L:
Efficacy and safety of regorafenib combined with toripalimab in the
third-line and beyond treatment of advanced colorectal cancer. J
Oncol. 2021:99599462021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Sosman JA,
Atkins MB, Leming PD, et al: Five-year survival and correlates
among patients with advanced melanoma, renal cell carcinoma, or
non-small cell lung cancer treated with nivolumab. JAMA Oncol.
5:1411–1420. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Le DT, Uram JN, Wang H, Bartlett BR,
Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et
al: PD-1 blockade in tumors with mismatch-repair deficiency. N Engl
J Med. 372:2509–2520. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Overman MJ, McDermott R, Leach JL, Lonardi
S, Lenz HJ, Morse MA, Desai J, Hill A, Axelson M, Moss RA, et al:
Nivolumab in patients with metastatic DNA mismatch repair-deficient
or microsatellite instability-high colorectal cancer (CheckMate
142): An open-label, multicentre, phase 2 study. Lancet Oncol.
18:1182–1191. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mlecnik B, Bindea G, Angell HK, Maby P,
Angelova M, Tougeron D, Church SE, Lafontaine L, Fischer M,
Fredriksen T, et al: Integrative analyses of colorectal cancer show
immunoscore is a stronger predictor of patient survival than
microsatellite instability. Immunity. 44:698–711. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lenz HJ, Van Cutsem E, Luisa Limon M, Wong
KYM, Hendlisz A, Aglietta M, García-Alfonso P, Neyns B, Luppi G,
Cardin DB, et al: First-line nivolumab plus low-dose ipilimumab for
microsatellite instability-high/mismatch repair-deficient
metastatic colorectal cancer: The phase II CheckMate 142 study. J
Clin Oncol. 40:161–170. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu Q, Wang Z, Luo Y and Xie X: Efficacy
and safety of immune checkpoint inhibitors in proficient mismatch
repair (pMMR)/non-microsatellite instability-high (non-MSI-H)
metastatic colorectal cancer: A study based on 39 cohorts
incorporating 1723 patients. BMC Immunol. 24:272023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Huang Y, Chen X, Dikov MM, Novitskiy SV,
Mosse CA, Yang L and Carbone DP: Distinct roles of VEGFR-1 and
VEGFR-2 in the aberrant hematopoiesis associated with elevated
levels of VEGF. Blood. 110:624–631. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wada J, Suzuki H, Fuchino R, Yamasaki A,
Nagai S, Yanai K, Koga K, Nakamura M, Tanaka M, Morisaki T and
Katano M: The contribution of vascular endothelial growth factor to
the induction of regulatory T-cells in malignant effusions.
Anticancer Res. 29:881–888. 2009.PubMed/NCBI
|
|
40
|
Loupakis F, Antonuzzo L, Bachet JB, Kuan
FC, Macarulla T, Pietrantonio F, Xu RH, Taniguchi H, Winder T, Yuki
S, et al: Practical considerations in the use of regorafenib in
metastatic colorectal cancer. Ther Adv Med Oncol.
12:17588359209568622020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen Z and Jiang L: The clinical
application of fruquintinib on colorectal cancer. Expert Rev Clin
Pharmacol. 12:713–721. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sun Q, Zhou J, Zhang Z, Guo M, Liang J,
Zhou F, Long J, Zhang W, Yin F, Cai H, et al: Discovery of
fruquintinib, a potent and highly selective small molecule
inhibitor of VEGFR 1, 2, 3 tyrosine kinases for cancer therapy.
Cancer Biol Ther. 15:1635–1645. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gu Y, Wang J, Li K, Zhang L, Ren H, Guo L,
Sai Y, Zhang W and Su W: Preclinical pharmacokinetics and
disposition of a novel selective VEGFR inhibitor fruquintinib
(HMPL-013) and the prediction of its human pharmacokinetics. Cancer
Chemother Pharmacol. 74:95–115. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xu X, Yu Y, Liu M, Liang L and Liu T:
Efficacy and safety of regorafenib and fruquintinib as third-line
treatment for colorectal cancer: A narrative review. Transl Cancer
Res. 11:276–287. 2022. View Article : Google Scholar : PubMed/NCBI
|