Introduction
Malignant mesothelioma is a rare malignant tumor
that occurs in the pleura, peritoneum, pericardium and testicular
membrane, with most cases occurring in the pleura. Asbestos
exposure is considered to be one of the main causes of the disease,
as it has been reported that 78–88% of cases of malignant
mesothelioma in men and 23–65% of cases in women are related to
asbestos exposure (1,2). In addition, in the past few decades,
lung cancer has become the leading cause of cancer death in men
worldwide (3). Asbestos exposure is
a common risk factor for malignant pleural mesothelioma and lung
cancer (4); however, their
coexistence is relatively rare (5–22).
Combination therapy with cisplatin + pemetrexed is an active
regimen for malignant pleural mesothelioma and non-squamous
non-small cell lung cancer (23,24).
However, there are few studies which have reported on patients with
comorbid malignant pleural mesothelioma and lung cancer who were
treated with a common chemotherapeutic regimen, such as cisplatin +
pemetrexed (9,22). Therefore, the present report
describes a patient with lung nodules that grew during the
administration of cisplatin + pemetrexed therapy for malignant
pleural mesothelioma, and they were subsequently diagnosed with
invasive mucinous adenocarcinoma.
Case report
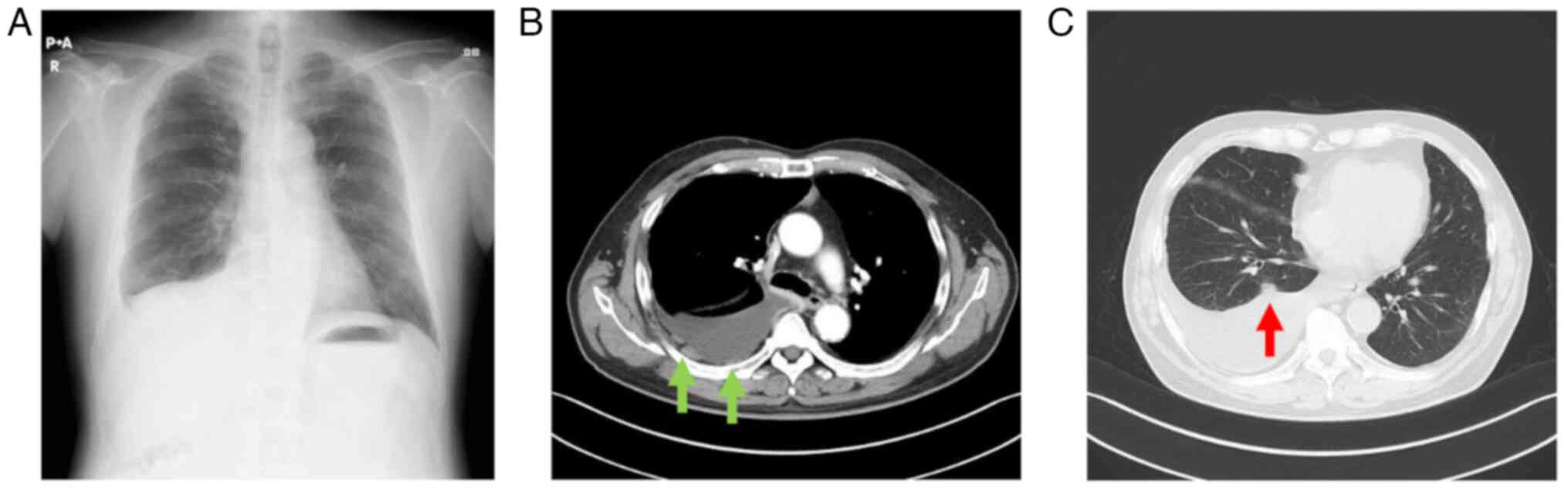
A 74-year-old male patient was referred to Gamagori
City Hospital (Gamagori, Japan) for the evaluation of a blunted
right costophrenic angle observed on a chest X-ray during a routine
annual check-up (Fig. 1A) in
November 2019. The patient had a smoking history of >30 years
and a history of asbestos exposure related to their occupation for
~30 years. Chest computed tomography (CT) performed according to
the standard setting revealed partial thickening of the right
pleura (Fig. 1B), right pleural
effusion and nodules along the right interlobar pleura (Fig. 1C). The initial diagnosis was lung
cancer with carcinomatous pleurisy.
Cytological examination of the pleural effusion
revealed numerous papillary and glandular masses with conspicuous
nucleoli and partial multinucleation (Fig. 2A), suggesting malignant pleural
mesothelioma. All staining was examined using an Olympus BX53 light
microscope (Olympus Corporation). The cytological analysis of the
pleural effusion cell block revealed atypical cells (Fig. 2B and C). Immunohistochemistry was
performed on formalin-fixed and paraffin-embedded 3–4 µm tissue
sections. For fixation, tissues were incubated with 10% formalin at
room temperature for 24 h. Immunohistochemistry was performed
automatically using BOND-III (Leica Microsystems, Inc.) and BOND
Polymer Refine Detection Kit (cat. no. DS9800; Leica Microsystems,
Inc.), according to the manufacturer's instructions. The following
antibodies were used: Cytokeratin (CK)7 (clone OV-TL 12/30; cat.
no. M7018; 1:100; Dako; Agilent Technologies, Inc.), CK5/6 (clone
D5/16 B4; cat. no. M7237; 1:50; Dako; Agilent Technologies, Inc.),
calretinin (clone SP13; cat. no. 413561; ready to use; Nichirei
Corporation), mesothelin (clone 5B2; cat no. NCL-MESO; 1:50; Leica
Microsystems, Inc.), CK20 (clone Ks.20.8; cat. no. M7019; 1:50;
Dako; Agilent Technologies, Inc.), CDX2 (clone DAK-CDX2; cat. no.
M3636; 1:50; Dako; Agilent Technologies, Inc.), napsin A
(polyclonal; cat. no. 418061; ready to use; Nichirei Corporation)
and p40 (clone BA28; cat. no. PA0163; ready to use; Leica
Microsystems, Inc.). The cell block was positive for CK7 (Fig. 2D), CK5/6 (Fig. 2E), calretinin (Fig. 2F) and mesothelin (Fig. 2G), and negative for CK20 (Fig. 2H), CDX2 (Fig. 2I), napsin A (Fig. 2J) and p40 (Fig. 2K) according to immunohistochemistry.
Therefore, the atypical cells were identified as mesothelial cells.
However, determining whether these were reactive mesothelial or
pleural mesothelioma cells was difficult because the mesothelioma
cells in body cavity fluid are generally very diverse.
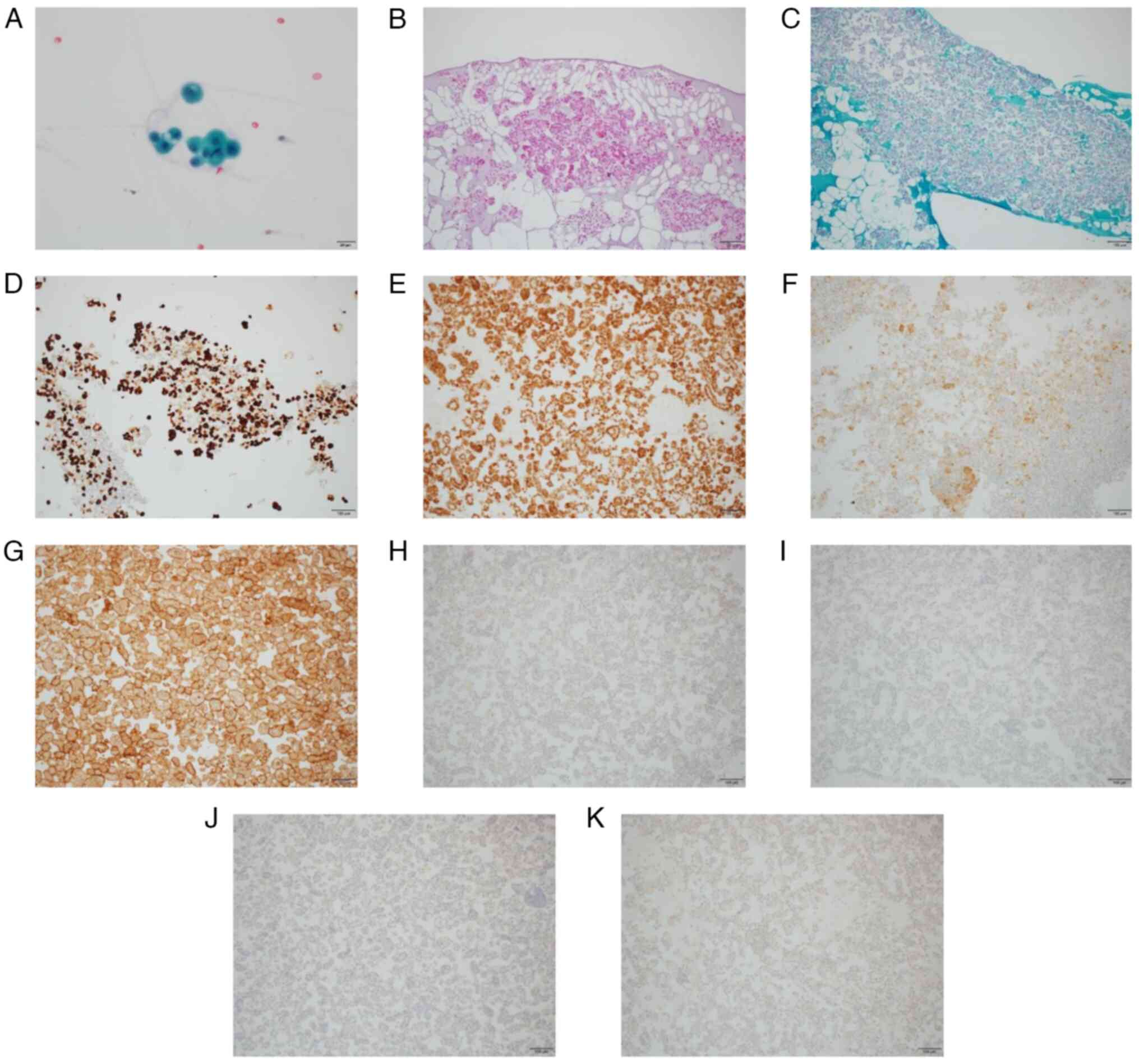 | Figure 2.Pleural fluid cytology. (A) Papillary
and glandular mass with prominent nucleoli and partial
multinucleation on Papanicolaou stain (magnification, ×40). (B)
Hematoxylin and eosin staining of cell block (magnification, ×10).
(C) Periodic acid Schiff staining of cell block (magnification,
×10). Evaluation of the cell block by immunohistochemistry revealed
atypical cells, which were positive for (D) CK7 (magnification,
×10), (E) CK5/6 (magnification, ×10), (F) calretinin
(magnification, ×10) and (G) mesothelin (magnification, ×10), and
negative for (H) CK20 (magnification, ×10), (I) CDX2
(magnification, ×10), (J) napsin A (magnification, ×10) and (K) p40
(magnification, ×10). CK, cytokeratin. |
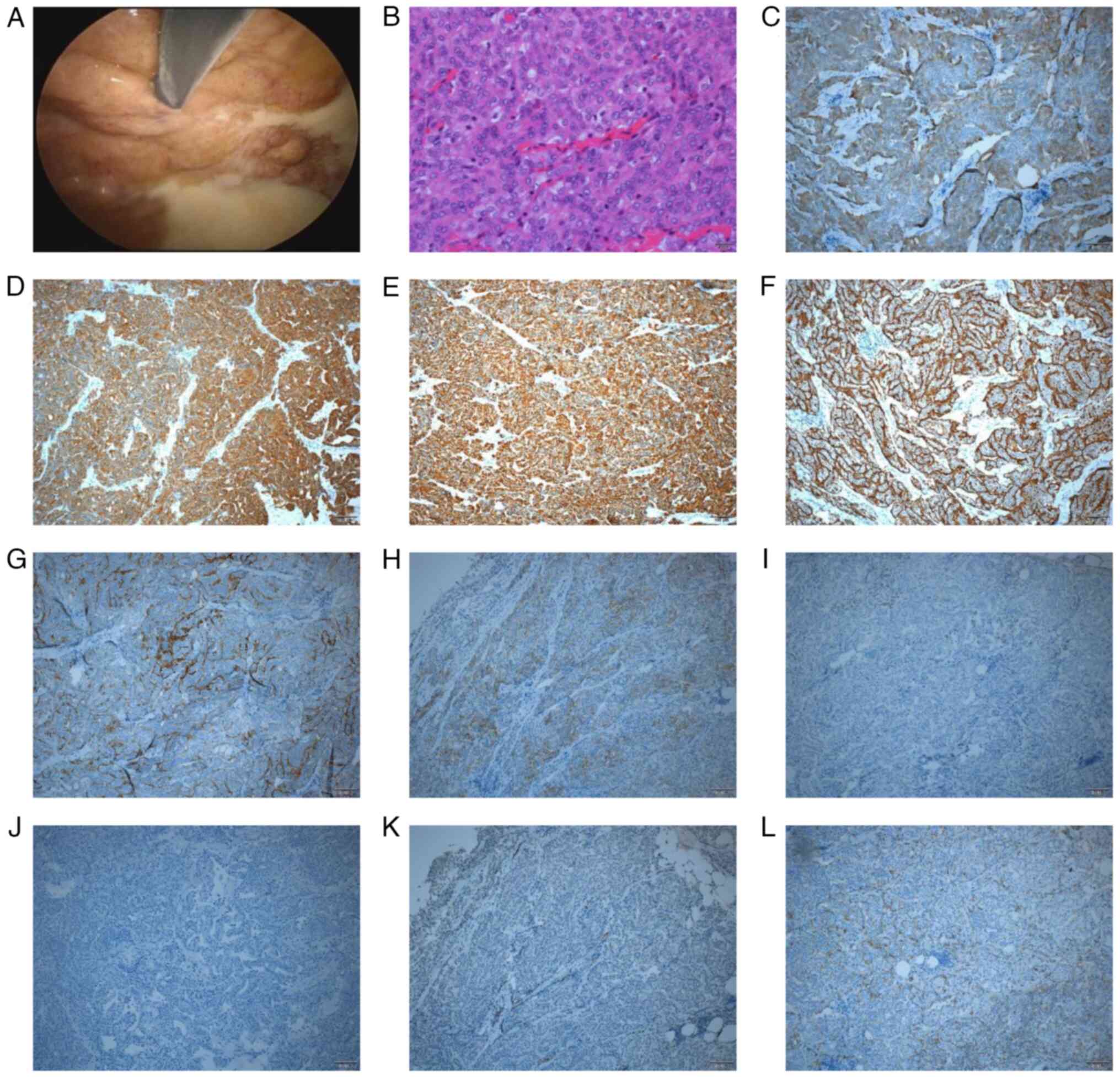
The pleural biopsy specimen obtained by thoracoscopy
(Fig. 3A) at Toyokawa City Hospital
(Toyokawa, Japan) was partially solid and histopathology revealed
medium-sized epithelioid cells with mild/moderate atypia (Fig. 3B). Infiltration into adipose tissue
was also noted. The specimen was positive for calretinin (Fig. 3C), cytokeratin AE1/AE3 (clone AE1/3;
cat. no. IR053; ready to use; Dako; Agilent Technologies, Inc.;
Fig. 3D), CAM 5.2 (clone CAM 5.2;
cat. no. 349205; ready to use; BD Biosciences; Fig. 3E), podoplanin (clone D2-40; cat. no.
413451; ready to use; Nichirei Corporation; Fig. 3F) and epithelial membrane antigen
(clone E29; cat. no. 790-4463; ready to use; Roche Diagnostics;
Fig. 3G); however, the basal
surface was only mildly positive for epithelial cell adhesion
molecule (MOC-31; clone MOC-31; cat. no. 790-4561; ready to use;
Roche Diagnostics; Fig. 3H). The
specimen was negative for thyroid transcription factor-1 (clone
SP141; cat. no. 790-4756; 1:3; Roche Diagnostics; Fig. 3I) and carcinoembryonic antigen
(clone TF3H8-1; cat. no. 760-2507; ready to use; Roche Diagnostics;
Fig. 3J), and mildly positive for
Wilms tumor protein 1 (clone 6F-H2; cat. no. 413861; ready to use;
Nichirei Corporation; Fig. 3K). The
specimen was also mildly positive for sialyated heart development
protein with EGF like domains 1 on the basal surface (clone SKM9-2;
cat. no. 418231; ready to use; Nichirei Corporation; Fig. 3L). Despite the mild positivity for
MOC-31 in most areas, the histologic diagnosis was epithelioid
mesothelioma based on the overall immunostaining results. Bone
scintigraphy and brain-enhanced magnetic resonance imaging (MRI)
performed according to the standard settings showed no overt
findings of distant metastases. Therefore, the patient was
diagnosed with malignant pleural mesothelioma (cT2N0M0, stage IB),
according to the 8th edition of the Union for International Cancer
Control Tumor-Node-Metastasis classification (25).
The patient refused surgery, which is the standard
treatment for stage IB pleural mesothelioma, especially epithelial
mesothelioma (23), due to their
relatively advanced age. Therefore, 2 months after the first visit,
four courses of combination therapy with cisplatin + pemetrexed
(cisplatin supplied by Nichi-Iko Pharmaceutical Co., Ltd.
administered intravenously at 75 mg/m2 on the first day
+ pemetrexed supplied by Eli Lilly Japan K.K. administered
intravenously at 500 mg/m2on the first day, one cycle in
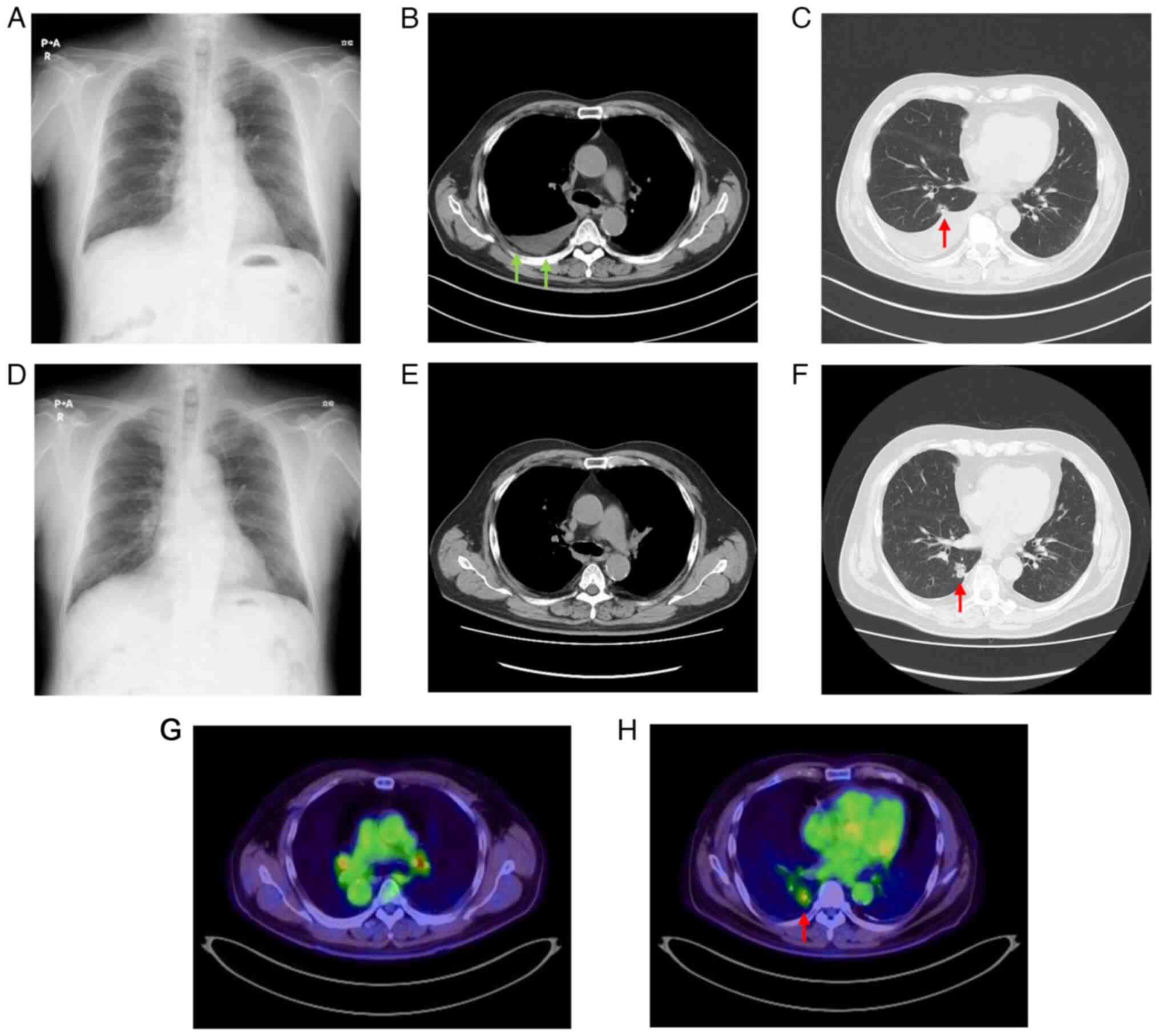
21 days) were administered. Follow-up chest radiography performed
during this time revealed an improvement in the right pleural
effusion (Fig. 4A), and follow-up
CT revealed the reduction of the pleural masses (Fig. 4B). However, the nodule in the S10
segment of the right lower lobe appeared to have slowly grown
during the four courses (Fig. 4C).
Primary lung cancer was suspected based on the follow-up CT
findings; however, the nodule had slightly increased in size. After
four courses, the patient was switched from combination therapy
with cisplatin + pemetrexed to maintenance therapy with pemetrexed
(administered intravenously at 500 mg/m2 on the first
day, one cycle in 21 days), which was discontinued after three
courses due to drug-induced pneumonia.
Follow-up revealed that the pneumonia was resolved
and the pleural mass disappeared (Fig.
4D and E); however, the nodule in the S10 segment of the right
lower lobe had grown ~5 mm in the 18 months since initial diagnosis
(Fig. 4F).
18F-fluorodeoxyglucose positron emission tomography
(Fig. 4G and H) and brain-enhanced
MRI performed according to the standard settings revealed no
evidence of distant metastasis of primary lung tumor or recurrence
of mesothelioma. Therefore, at 20 months after the first visit,
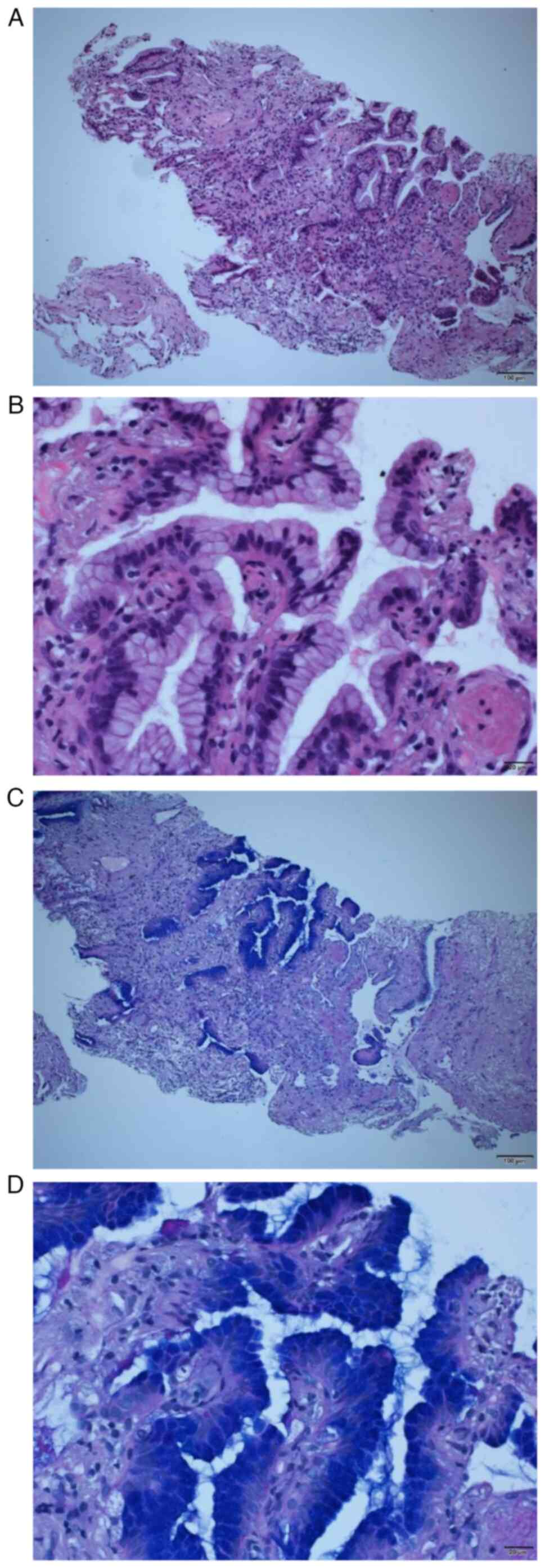
CT-guided biopsy was performed. Pathological examination of the
specimen, performed with hematoxylin and eosin staining with
Carazzi's hematoxylin for 25 min and eosin for 7 min at room
temperature (Fig. 5A and B), and
periodic acid Schiff staining with 1% periodic acid for 10 min,
Schiff's solution for 15 min and Mayer's hematoxylin for 4 min at
room temperature (Fig. 5C and D),
revealed that the alveolar air space was replaced by
papillary-like, highly cylindrical epithelium, suggesting invasive
mucinous adenocarcinoma.
No pleural invasion of adenocarcinoma was observed
in the biopsy specimen. Thus, the patient was diagnosed with lung
adenocarcinoma (cT1aN0M0, stage IA1) in the right lower lobe
(25). The patient was provided
with a full explanation that the standard treatment for stage IA1
non-small cell lung cancer is resection of >1 lung lobe. The
patient refused surgery and underwent stereotactic radiotherapy (3
days, maximum total dose 58.9 Gy), which resulted in the nodule
shrinking and subsequent control of enlargement. At 12 months after
radiotherapy, there was no recurrence of mesothelioma or lung
cancer. Follow-up is presently ongoing to monitor for recurrences
every 3 months and the patient is not undergoing any further
treatments.
Discussion
The present report described the successful
treatment of a patient with simultaneous malignant pleural
mesothelioma and pulmonary adenocarcinoma. In a database analysis
of ~3,800 patients with malignant mesothelioma, lung cancer as a
complication was only reported in 18 (0.5%) patients (26). To the best of our knowledge, 27 such
cases have been previously reported in the literature (5–22), but
none of the reports described the lung cancer as invasive mucinous
adenocarcinoma. Distinguishing between malignant pleural
mesothelioma and advanced-stage lung cancer is challenging
(27), which may be a reason for
the low frequency of reported cases.
The treatment of malignant pleural mesothelioma
requires a multidisciplinary approach and includes surgery,
chemotherapy and radiotherapy. A review by Bou-Samra et al
(28), which analyzed the 2022
National Cancer Database in the US, found no notable changes in the
proportion of patients who underwent surgery, including
extrapleural lung resection, extended pleurectomy, corticectomy,
pleural corticectomy and partial pleurectomy, for malignant pleural
mesothelioma from 2004 to 2020. The authors concluded that the
observed lack of increase in surgical treatment rates may be due to
the lack of a clear evidence-based consensus on surgical approaches
for mesothelioma.
Randomized controlled studies to determine the best
treatment options for malignant pleural mesothelioma are lacking
due to the relative rarity of this diagnosis. The current
management of pleural mesothelioma involves a multidisciplinary
team and expert consensus based on stage and histologic subtype.
Specifically, surgery with neoadjuvant chemotherapy or adjuvant
chemotherapy is recommended for stage I–IIIa and epithelial
histology, whereas systemic therapy and/or supportive care are the
current recommendations for stage III–IV or unresectable
mesothelioma (29). The recently
developed grading system for epithelioid mesothelioma, which is
based on the nuclear atypia, mitotic count and necrosis scores, is
recommended as a prognostic tool by the 2021 World Health
Organization Classification of Thoracic Tumors guidelines (30). However, it remains unclear whether
this grading system can predict prognosis better than clinical
staging and how it can be implemented during treatment
decision-making (31).
Conversely, surgical resection of >1 lung lobe is
the standard treatment for stage I–II non-small cell lung cancer.
In cases where surgery is not feasible for medical reasons, radical
radiotherapy is the first choice (32). Although several recent retrospective
studies were performed using pooled analyses or propensity scores
to evaluate treatment options in cases where resection was possible
(33–36), no randomized controlled trials to
date have reported the comparison of outcomes between surgery and
radical radiotherapy, to the best of our knowledge. In the present
case, radiation therapy was successful in suppressing the
progression of adenocarcinoma. However, this does not mean that
surgery can be avoided or radiotherapy may be preferred in certain
cases.
Combination therapy with cisplatin + pemetrexed, a
commonly used regimen for unresectable malignant pleural
mesothelioma, is also currently used for non-squamous non-small
cell lung cancer. The reported response rate to combination therapy
with cisplatin + pemetrexed is 41.3% for all malignant pleural
mesotheliomas (37) and 44.0% for
all lung adenocarcinomas (38). In
the present case, combination therapy with cisplatin + pemetrexed
was successful for the treatment of mesothelioma; however, the
nodule in the right lower lobe, which was later diagnosed as
adenocarcinoma, continued to grow, indicating a lack of response to
treatment. A literature search found no articles discussing the use
of percutaneous lung needle biopsy in patients with pleural
mesothelioma in remission. Therefore, whether the biopsy method
that allows communication between the thoracic cavity and pleura
poses a risk of worsening the depth of invasion in the event of
recurrence is an issue to be assessed in future research. In such
cases, immune checkpoint inhibitor(s) therapy, such as nivolumab,
which has been approved for non-small cell lung cancer and
malignant pleural mesothelioma, may be considered.
In conclusion, currently available multiple active
regimens for malignant pleural mesothelioma and lung cancer should
aid the treatment of complex cases such as that presented in the
current case report. The long-term impact of these approaches on
recurrence should be evaluated in future studies.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or generated in the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YA conceived and designed the study, and prepared
the draft of the manuscript. YH and TO treated the patient,
analyzed and interpreted the results. YA, TS, MT and TO performed
data collection. YA, TS, YH, MT and TO confirm the authenticity of
all the raw data. All authors reviewed the results, and read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient signed an informed consent form that
included the acquisition of clinical data and images in an
anonymous form for publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yates DH, Corrin B, Stidolph PN and Browne
K: Malignant mesothelioma in south east England:
Clinicopathological experience of 272 cases. Thorax. 52:507–512.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Attanoos RL, Churg A, Galateau-Salle F,
Gibbs AR and Roggli VL: Malignant mesothelioma and its non-asbestos
causes. Arch Pathol Lab Med. 142:753–760. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Global Burden of Disease Cancer
Collaboration, . Fitzmaurice C, Akinyemiju TF, Al Lami FH, Alam T,
Alizadeh-Navaei R, Allen C, Alsharif U, Alvis-Guzman N, Amini E, et
al: Global, regional, and national cancer incidence, mortality,
years of life lost, years lived with disability, and
disability-adjusted life-years for 29 cancer groups, 1990 to 2016:
A systematic analysis for the global burden of disease study. JAMA
Oncol. 4:1553–1568. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Henderson DW, Rödelsperger K, Woitowitz HJ
and Leigh J: After Helsinki: A multidisciplinary review of the
relationship between asbestos exposure and lung cancer, with
emphasis on studies published during 1997–2004. Pathology.
36:517–550. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cagle PT, Wessels R and Greenberg SD:
Concurrent mesothelioma and adenocarcinoma of the lung in a patient
with asbestosis. Mod Pathol. 6:438–441. 1993.PubMed/NCBI
|
|
6
|
Attanoos RL, Thomas DH and Gibbs AR:
Synchronous diffuse malignant mesothelioma and carcinomas in
asbestos-exposed individuals. Histopathology. 43:387–392. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Okumura T, Okada M, Tsuji M, Inoue A and
Ochiai Y: Mesothelioma with lung cancer complicating asbestosis.
Acta Pathol Jpn. 30:579–590. 1980.PubMed/NCBI
|
|
8
|
Allen TC and Moran C: Synchronous
pulmonary carcinoma and pleural diffuse malignant mesothelioma.
Arch Pathol Lab Med. 130:721–724. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Imenpour H, Ivaldi GP, Brianti A,
Pastorino G, Biggi S, Auriati L and Simonassi C: Synchronous
occurrence of pulmonary adenocarcinoma and pleural diffuse
malignant mesothelioma. Pathologica. 105:353–356. 2013.PubMed/NCBI
|
|
10
|
Kishimoto T: A case of triple malignancies
(gastric cancer, lung cancer and malignant pleural mesothelioma)
after asbestos exposure. Nihon Kokyuki Gakkai Zasshi. 41:304–309.
2003.(In Japanese). PubMed/NCBI
|
|
11
|
Maeda R, Isowa N, Onuma H, Miura H,
Tokuyasu H and Kawasaki Y: Minute localized malignant pleural
mesothelioma coexisting with multiple adenocarcinomas. Gen Thorac
Cardiovasc Surg. 58:91–94. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Özbudak İH, Özbudak Ö, Arslan G, Erdoğan A
and Özbılım G: Metachronous malignant mesothelioma and pulmonary
adenocarcinoma. Turk Patoloji Derg. 29:83–86. 2013.PubMed/NCBI
|
|
13
|
Haber SE: Synchronous malignant pleural
mesothelioma and pulmonary carcinoma in a woman without evidence of
asbestos exposure. Resp Med CME. 3:160–161. 2010. View Article : Google Scholar
|
|
14
|
Bianchi C, Bianchi T and Ramani L:
Malignant mesothelioma of the pleura and other malignancies in the
same patient. Tumori. 93:19–22. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee AHS and Soomro IN: Collision tumour of
the pleura composed of small cell carcinoma and malignant
mesothelioma. Histopathology. 45:305–306. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Flood TA, Sekhon HS, Seely JM, Shamji FM
and Gomes MM: Spontaneous pneumothorax and lung carcinoma: Should
one consider synchronous malignant pleural mesothelioma? J Thorac
Oncol. 4:770–772. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tsuzuki T, Ninomiya H, Natori Y and
Ishikawa Y: Coalescent pleural malignant mesothelioma and
adenocarcinoma of the lung, involving only minor asbestos exposure.
Pathol Int. 58:451–455. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Suzuki Y: Correspondence Re: P.T. Cagle,
R. Wessels, and S.D. Greenberg. Concurrent mesothelioma and
adenocarcinoma of the lung in a patient with asbestosis. Mod Pathol
6:438, 1993. Mod Pathol. 7:888–889. 1994.PubMed/NCBI
|
|
19
|
Negi Y, Kuribayashi K, Doi H, Funaguchi N,
Koda Y, Fujimoto E, Mikami K, Minami T, Yokoi T and Kijima T:
Double cancer comprising malignant pleural mesothelioma and
squamous cell carcinoma of the lung treated with radiotherapy: A
case report. Mol Clin Oncol. 9:181–186. 2018.PubMed/NCBI
|
|
20
|
Ito T, Nakanishi K and Goto H: A case of
early-stage malignant pleural mesothelioma accompanied by stage IA
lung adenocarcinoma. Jpn J Chest Surg. 30:503–509. 2016.(In
Japanese). View Article : Google Scholar
|
|
21
|
Niu X, Zhou C, Hu A, Su L, Lin D, Han H
and Lu Y: Malignant mesothelioma without asbestos exposure
diagnosed during EGFR-TKI treatment of lung adenocarcinoma: A case
report. Cancer Treat Res Commun. 27:1003452021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yamazoe M, Tomioka H, Kamada T, Kaneko M
and Katsuyama E: Simultaneous presence of lung adenocarcinoma and
malignant pleural mesothelioma: A case report. Respir Med Case Rep.
26:45–49. 2018.PubMed/NCBI
|
|
23
|
Scherpereel A, Opitz I, Berghmans T,
Psallidas I, Glatzer M, Rigau D, Astoul P, Bölükbas S, Boyd J,
Coolen J, et al: ERS/ESTS/EACTS/ESTRO guidelines for the management
of malignant pleural mesothelioma. Eur Respir J. 55:19009532020.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hendriks LE, Kerr KM, Menis J, Mok TS,
Nestle U, Passaro A, Peters S, Planchard D, Smit EF, Solomon BJ, et
al: Non-oncogene-addicted metastatic non-small-cell lung cancer:
ESMO clinical practice guideline for diagnosis, treatment and
follow-up. Ann Oncol. 34:358–376. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brierley J, Gospodarowicz MK and Wittekind
C: TNM classification of malignant tumours. 8th edition.
Chichester, West Sussex, UK; Hoboken, NJ; John Wiley & Sons,
Inc.: pp. 105–115. 2017
|
|
26
|
Butnor KJ, Brownlee NA, Mahar A, Pavlisko
EN, Sporn TA and Roggli VL: Diffuse malignant mesothelioma and
synchronous lung cancer: A clinicopathological study of 18 cases.
Lung Cancer. 95:1–7. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li Y, Cai B, Wang B, Lv Y, He W, Xie X and
Hou D: Differentiating malignant pleural mesothelioma and
metastatic pleural disease based on a machine learning model with
primary CT signs: A multicentre study. Heliyon. 8:e113832022.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bou-Samra P, Chang A, Azari F, Kennedy G,
Segil A, Guo E, Marmarelis M, Langer C and Singhal S:
Epidemiological, therapeutic, and survival trends in malignant
pleural mesothelioma: A review of the national cancer database.
Cancer Med. 12:12208–12220. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
National Comprehensive Cancer Network, .
NCCN Clinical Practice Guidelines in Oncology Mesothelioma:
Pleural. version 1.2023. https://www.nccn.org/professionals/physician_gls/pdf/meso_pleural.pdfNovember
1–2023
|
|
30
|
Sauter JL, Dacic S, Galateau-Salle F,
Attanoos RL, Butnor KJ, Churg A, Husain AN, Kadota K, Khoor A,
Nicholson AG, et al: The 2021 WHO classification of tumors of the
pleura: Advances since the 2015 classification. J Thorac Oncol.
17:608–622. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schulte JJ and Husain AN: Updates on
grading mesothelioma. Histopathology. 84:153–162. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Postmus PE, Kerr KM, Oudkerk M, Senan S,
Waller DA, Vansteenkiste J, Escriul C and Peters S: ESMO Guidelines
Committee: Early and locally advanced non-small-cell lung cancer
(NSCLC): ESMO clinical practice guidelines for diagnosis, treatment
and follow-up. Ann Oncol. 28 (Suppl 4):iv1–iv21. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chang JY, Senan S, Paul MA, Mehran RJ,
Louie AV, Balter P, Groen HJM, McRae SE, Widder J, Feng L, et al:
Stereotactic ablative radiotherapy versus lobectomy for operable
stage I non-small-cell lung cancer: A pooled analysis of two
randomised trials. Lancet Oncol. 16:630–637. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hamaji M, Chen F, Matsuo Y, Kawaguchi A,
Morita S, Ueki N, Sonobe M, Nagata N, Hiraoka M and Date H:
Video-assisted thoracoscopic lobectomy versus stereotactic
radiotherapy for stage I lung cancer. Ann Thorac Surg.
99:1122–1129. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shirvani SM, Jiang J, Chang JY, Welsh J,
Likhacheva A, Buchholz TA, Swisher SG and Smith BD: Lobectomy,
sublobar resection, and stereotactic ablative radiotherapy for
early-stage non-small cell lung cancers in the elderly. JAMA Surg.
149:1244–1253. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Eba J, Nakamura K, Mizusawa J, Suzuki K,
Nagata Y, Koike T, Hiraoka M, Watanabe S, Ishikura S, Asamura H, et
al: Stereotactic body radiotherapy versus lobectomy for operable
clinical stage IA lung adenocarcinoma: Comparison of survival
outcomes in two clinical trials with propensity score analysis
(JCOG1313-A). Jpn J Clin Oncol. 46:748–753. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vogelzang NJ, Rusthoven JJ, Symanowski J,
Denham C, Kaukel E, Ruffie P, Gatzemeier U, Boyer M, Emri S,
Manegold C, et al: Phase III study of pemetrexed in combination
with cisplatin versus cisplatin alone in patients with malignant
pleural mesothelioma. J Clin Oncol. 21:2636–2644. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kawano Y, Ohyanagi F, Yanagitani N, Kudo
K, Horiike A, Tanimoto A, Nishizawa H, Ichikawa A, Sakatani T,
Nakatomi K, et al: Pemetrexed and cisplatin for advanced
non-squamous non-small cell lung cancer in Japanese patients: Phase
II study. Anticancer Res. 33:3327–3333. 2013.PubMed/NCBI
|