Introduction
Testicular seminoma is a fairly common malignancy in
adult men that accounts for 55–60% of germ cell tumors, with an age
of onset of 15–40 years old (1,2). The
tumors are typically limited to the testis and have a recurrence
rate after surgery of 4–30% (3).
The treatment methods vary according to the tumor stage, with
radiotherapy and chemotherapy typically administered for metastatic
seminoma, while chemotherapy is preferred for larger metastatic
tumors (4). However, chemotherapy
may not clear the entire tumor, resulting in residual lesions in
some patients, and can also cause long-term toxicity and side
effects including thromboembolic and cardiovascular diseases
(5–7), neurological sequelae (such as hearing
impairment, neuropathy and renal damage) and secondary malignant
tumors (8,9). Therefore, there is currently some
controversy over the choice of treatment options.
The implantation of iodine-125 seeds is a form of
brachytherapy where radioactive seeds are implanted into the tumor
under direct vision or using imaging equipment. The iodine-125
seeds implanted in the tumor continuously produce low-dose
gamma-rays, causing DNA breakage and damage in the tumor through
the production of free radicals. In addition, the gamma-rays can
reduce the increased oxygen ratio in the cells, overcoming
radiation resistance in oxygen-deprived tumor cells, reducing the
number of tumor stem cells and killing the tumor cells, thus
increasing the efficacy of treatment (10). The iodine-125 seeds implanted in the
tumor continuously produce low-dose gamma-rays with a radiation
radius of ~1.7 cm, which is effective for killing cancer cells in
the vicinity while protecting normal tissue (11). In recent years, iodine-125 treatment
has been applied to various types of solid tumor such as prostate,
pancreatic and liver cancer (12),
but its efficacy in the treatment of seminoma has not yet, to the
best of our knowledge, been reported.
In the present study, a patient with retroperitoneal
metastatic seminoma who was treated with iodine-125 seed
implantation combined with carboplatin (AUC7) 3 years previously
was reported.
Case report
In May, 2020, a 45-year-old male visited the
People's Hospital of Chongqing Banan District (Chongqing, China)
after experiencing abdominal pain and distension for 1 month. The
outpatient ultrasound showed a retroperitoneal mass. The patient
had undergone radical resection for testicular cancer due to right
testicular seminoma (stage I) 2 years earlier. After admission, the
serological markers indicated a lactate dehydrogenase (LDH) level
of 326 U/l (reference, 120–250 U/l), β-human chorionic gonadotropin
(HCG) of 426 mIU/ml (reference, 0–10 mIU/ml) and a normal
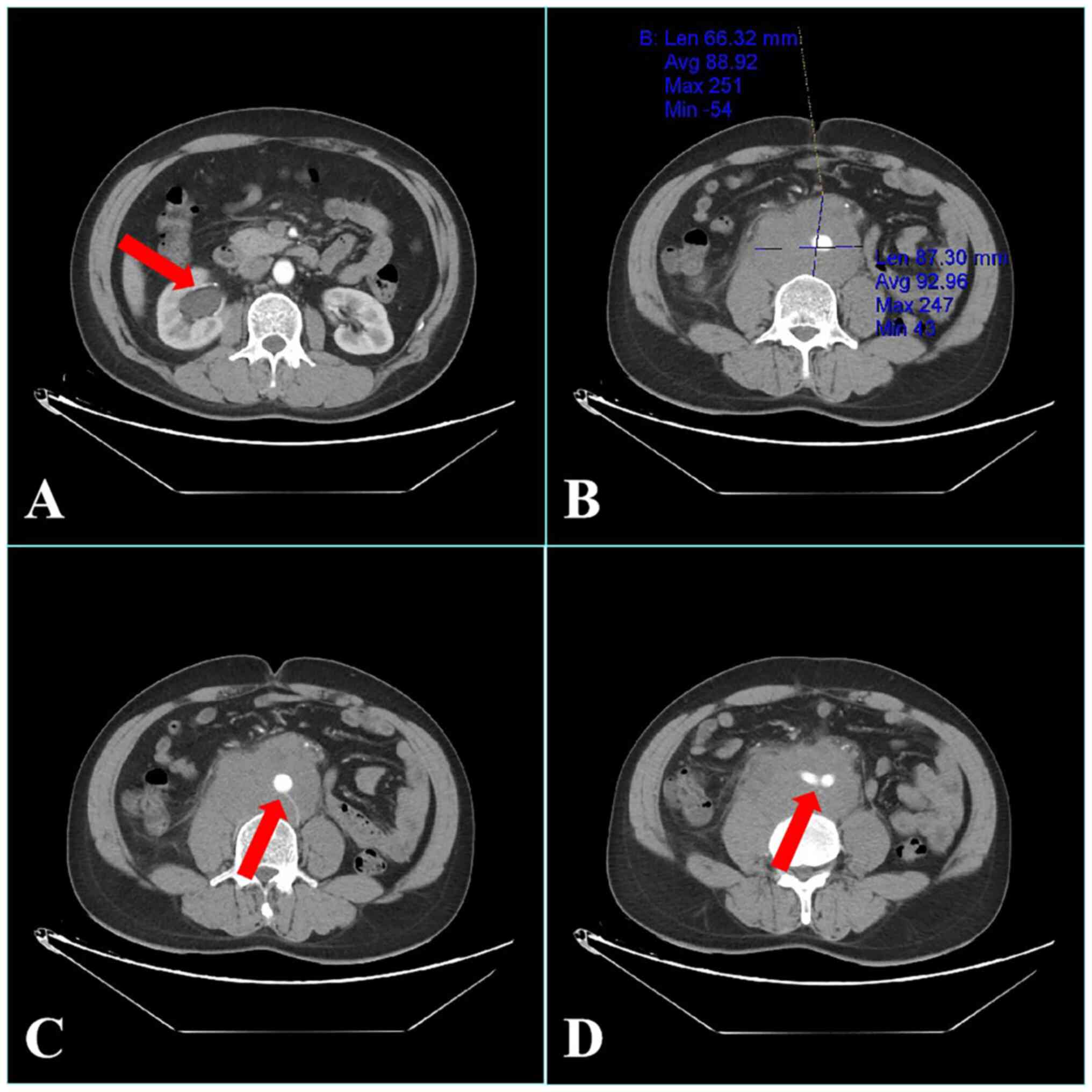
α-fetoprotein level. Enhanced computed tomography (CT) of the
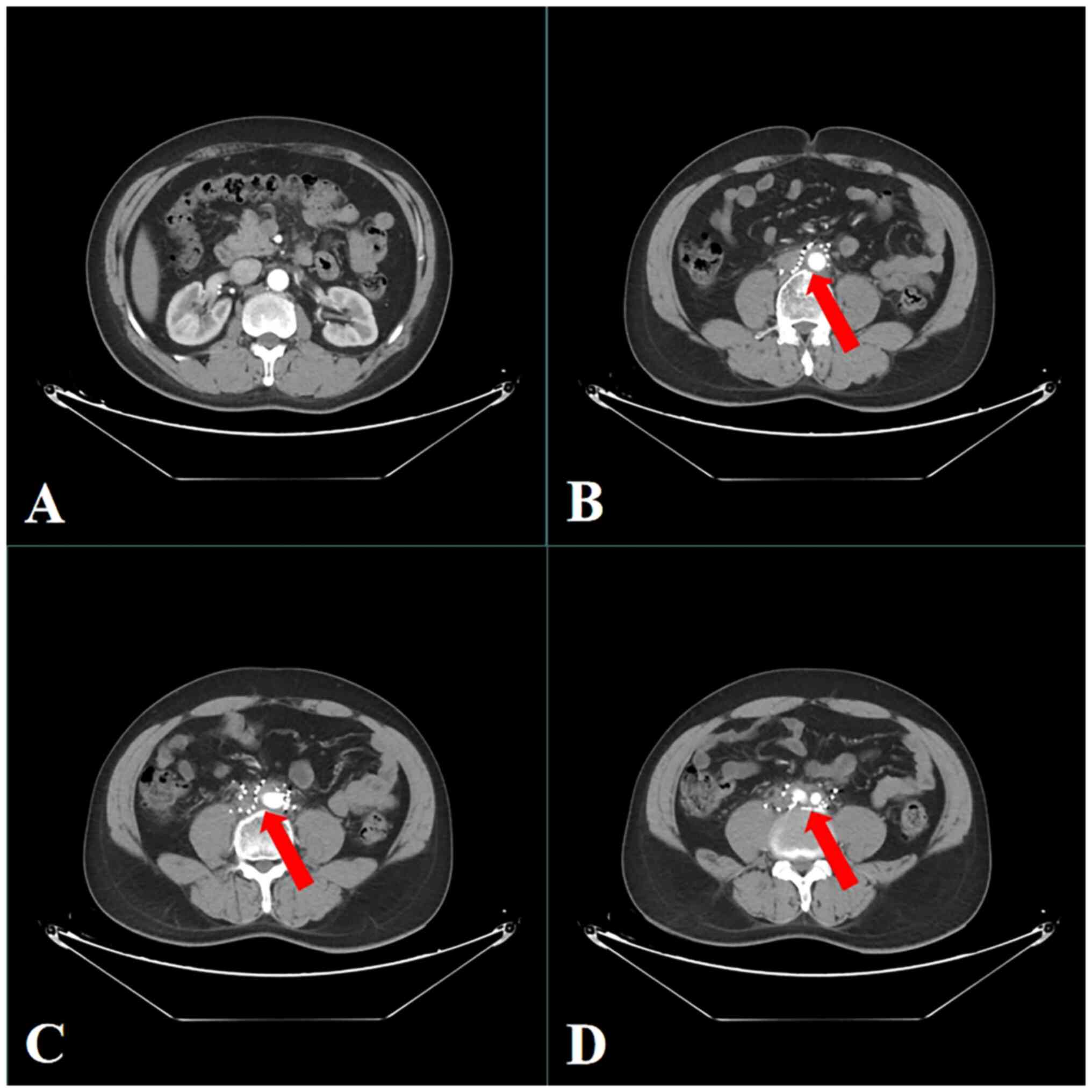
abdomen showed an irregular mass in the retroperitoneum, with a
maximum slice size of 6.6×8.7-cm. The mass surrounded the abdominal
aorta, inferior vena cava and bilateral iliac vessels and had
compressed the right ureter, causing right ureteral obstruction
(Fig. 1). Based on these
examination results, a diagnosis of retroperitoneal metastatic
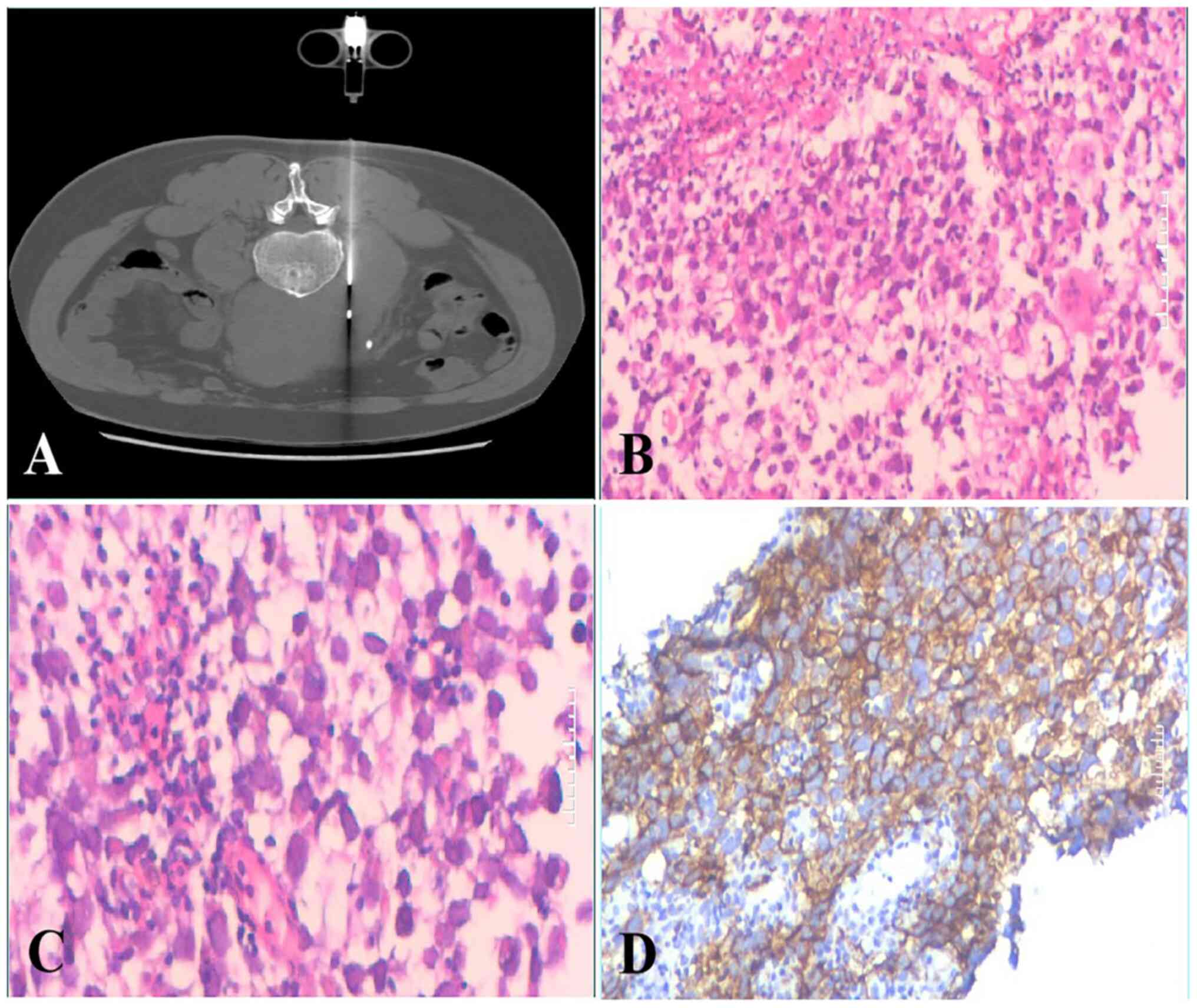
seminoma was considered. A CT-guided puncture biopsy was
subsequently performed in May, 2020 (5 days after admission). The
retroperitoneal puncture tissues were fixed in 10% neutral formalin
at room temperature for 8 h and sent to Chongqing Medical
University (Chongqing, China) for pathological examination. In the
pathological sections, the tumor cells were arranged in a sheet,
nest and cord shape, and the small fiber trabeculae divided the
cell nests, with light cytoplasmic staining and clear envelope
boundaries observed (Fig. 2B and
C). Immunohistochemical staining for CD117 was also positive
(Fig. 2D). The pathological biopsy
therefore indicated recurrence and metastasis of the seminoma. A
multidisciplinary team discussion was then conducted. In terms of
renal obstruction, it was recommended that the patient undergo
ureteral stenting to relieve the compression, but the patient
refused to undergo ureteral implantation as there was no obvious
abnormality in renal function. In terms of the tumor, according to
the American Joint Committee on Cancer (AJCC) Cancer staging
manual, the patient had stage IIC seminoma (13). The treatment guidelines for
treatment for this stage recommend chemotherapy as the first
choice. However, since the tumor was large and surrounded the large
retroperitoneal vessels, surgery was not considered. The tumor was
also not suitable for external radiotherapy after consideration of
the long-term toxicity and side effects of chemotherapy, as well as
the possibility of residual tumor remaining after chemotherapy. A
treatment plan was then formulated, comprising the implantation of
iodine-125 seeds combined with carboplatin (AUC7).
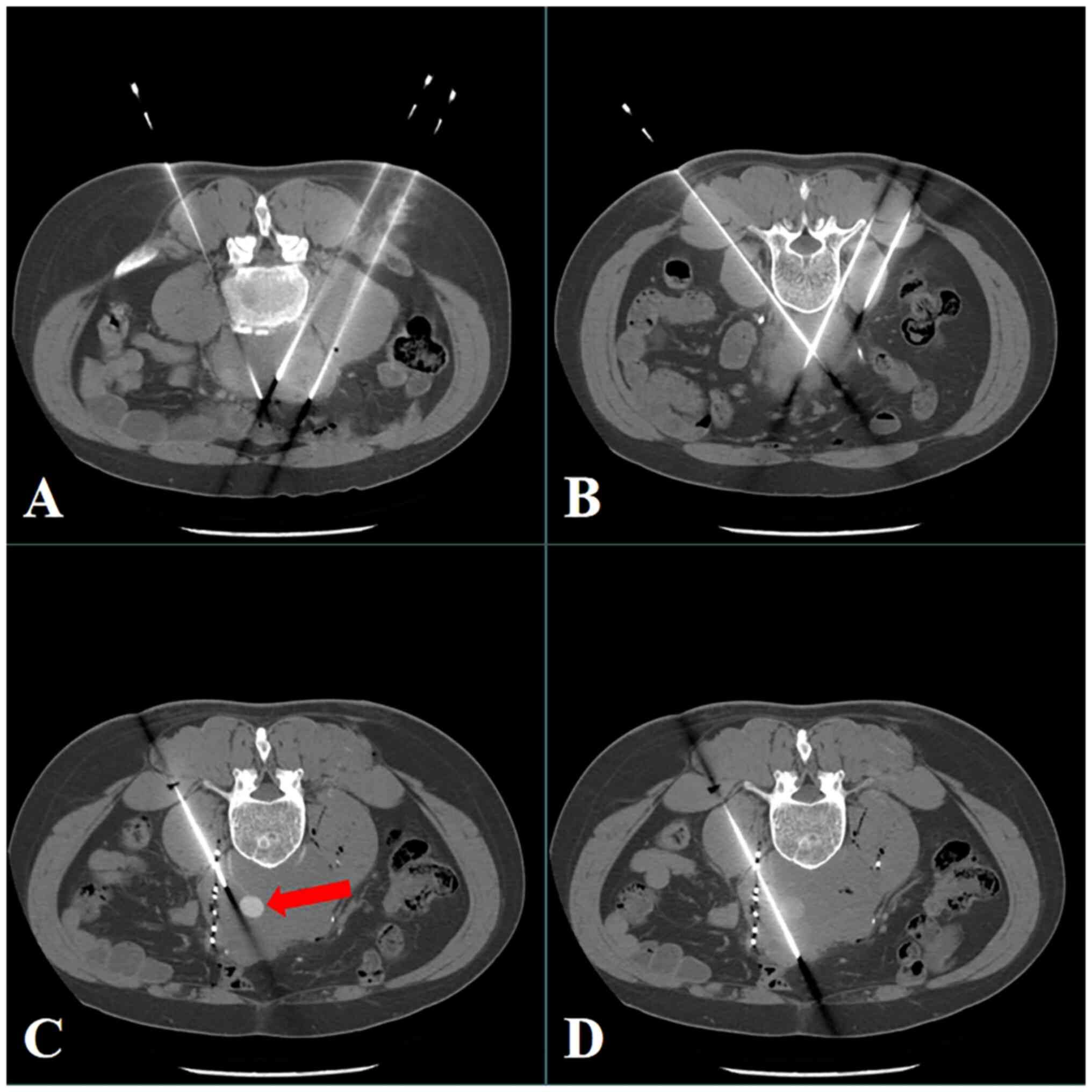
The treatment planning system (TPS) (Beijing Astro
Technology Co. Ltd.,) was used before surgery to determine the
implantation strategy, and percutaneous iodine-125 seed
implantation was conducted under CT guidance in May, 2020 (11 days
after admission). Each implanted iodine-125 seed had an activity of
0.7 mCi, and a dosage of 100 Gy was recommended. During the
procedure, a GE Revolution HD CT (GE Healthcare) was used for
guidance, and a 5-mm slice thickness was selected for spiral
scanning. The procedure was performed under local anesthesia using
the lumbar dorsal approach as the puncture path. Real-time enhanced
scanning was used when the boundary between the tumor and the
abdominal aorta was unclear during the procedure (Fig. 3). Guided puncture was conducted to
avoid the abdominal aorta (intraoperative enhanced image), and
finally, 95 seeds were successfully implanted into the target
lesion using an 18G puncture needle. The postoperative review
indicated an even seed distribution (Fig. 4), and there was no major bleeding or
intestinal injury during the perioperative period. The chemotherapy
consisted of one cycle of carboplatin (AUC7, 871 mg) administered 4
days after surgery, and the patient was discharged successfully
after treatment. Furthermore, the levels of the serological
markers, LDH and β-HCG, decreased progressively following surgery.
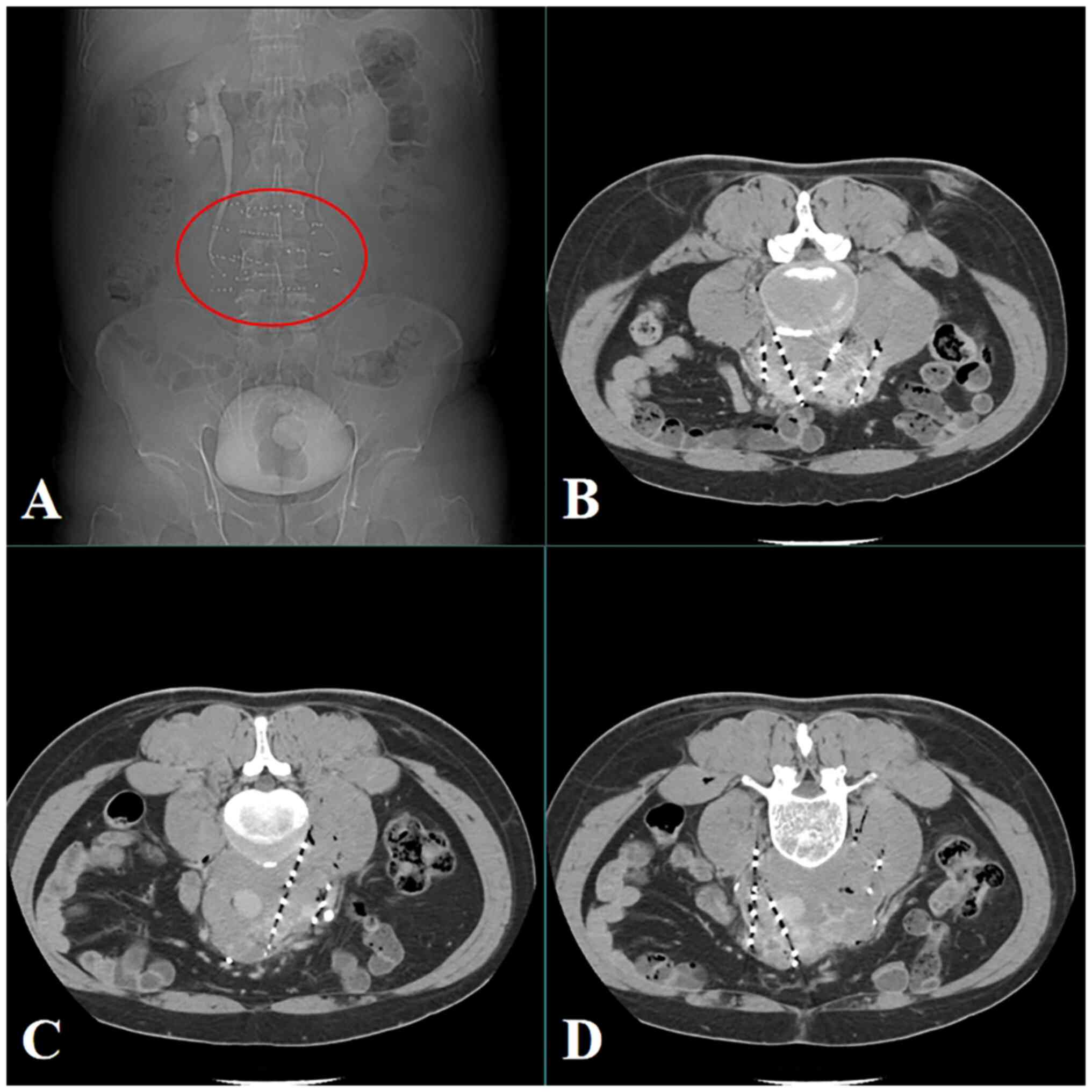
For 6 months after the procedure, CT scans were conducted every 2
months. As per the Response Evaluation Criteria in Solid Tumors
(version 1.1) (14), the lesions
showed complete response 2 months post-surgery (Fig. 5). From 6 months after surgery,
annual CT scans were conducted. Since the last follow-up in August,
2023, the patient's condition has been stable for >3 years.
Discussion
Seminomas have low incidence and high cure rates,
and a study has shown that the long-term cure rate for metastatic
seminoma is >95% (15). The
long-term toxicity and side effects associated with radiotherapy
and chemotherapy may impair the long-term working ability and
quality of life of the patient. Most patients with metastatic
seminoma receive 3–4 cycles of chemotherapy with a high total dose
of cisplatin, typically 300–400 mg/m2. These patients
are at high risk of late toxicity from cisplatin, leading to
cardiovascular disease and increasing the likelihood of secondary
malignancies (16). Therefore,
together with ensuring the efficacy of treatment, reducing the
toxicity and side effects associated with treatment is a current
research focus (17).
In a study of 51 patients with stage II seminoma who
received a single cycle of radiation therapy combined with
carboplatin therapy, 39 patients were found to have the radiation
field reduced from the abdominopelvic field to the para-aortic
region only, and the radiation dose was reduced from 35 to 30 Gy
(18). After a median follow-up of
55 months, none of the patients showed tumor recurrence and no
long-term toxicity or side effects were observed. In another
multicenter phase II trial from the Swiss Clinical Cancer Research
Group and the German Testicular Cancer Study Group, 116 patients
with stage IIA-IIB seminoma (46 patients with stage IIA and 70
patients with stage IIB) were treated with neoadjuvant carboplatin
(AUC7) chemotherapy combined with radiotherapy (30 Gy for stage IIA
and 36 Gy for stage IIB) (19). The
3-year progression-free survival rate was 93.7%, and the incidence
of adverse reactions was very low. Based on the aforementioned
studies, compared with radiotherapy alone, a single cycle of
neoadjuvant carboplatin before radiotherapy can reduce the risk of
recurrence, allowing for a smaller radiation field, and can also
reduce the long-term toxic side effects associated with standard
radiotherapy and chemotherapy regimens.
In addition, a number of studies have shown that
retroperitoneal lymph node dissection can also avoid the toxic side
effects of radiotherapy and chemotherapy, in the treatment of
small-volume metastatic lymph nodes (20–24).
In a retrospective study of 274 patients, 257 underwent unilateral
lymphadenectomy and 17 underwent bilateral lymphadenectomy
(20). In addition, 13 patients
with stage II disease received adjuvant chemotherapy. After a
median follow-up of 55 months, 33 patients relapsed. Among the 113
patients with stage II disease who did not receive chemotherapy, 21
experienced disease recurrence and 81% were cured by surgery alone
and never relapsed. In the COTRIMS trial, 16 patients with IIA/B
seminoma were recruited, 2 of which showed recurrence outside the
surgical area 4 and 6 months after surgery, respectively (21). In an Indiana University experiment,
retroperitoneal lymph node dissection was performed on 67 patients,
yielding an 80.2% 2-year recurrence-free survival rate, with 11
patients reporting recurrence (22), while in the PRIMETEST trial, 10 out
of 33 patients experienced recurrence (23). In the SEMS trial, a total of 55
patients with stage II metastatic seminoma underwent
retroperitoneal lymph node dissection, of which there were 10
instances of recurrence (24). In
another trial, 16 patients received a single cycle of adjuvant
carboplatin (AUC7) combined with retroperitoneal lymph node
dissection, with 1 patient experiencing recurrence (25). The patients in these trials who
relapsed were subsequently successfully treated with salvage
chemotherapy. It can thus be seen that, although retroperitoneal
lymph node dissection can reduce the toxic side effects of
radiotherapy and chemotherapy, the risk of postoperative recurrence
cannot be ignored. Unilateral retroperitoneal lymph node dissection
appears to have a high recurrence rate (up to 30%) (23), which may not be acceptable to
patients; however, bilateral lymph node dissection carries an
increased risk of nerve damage, which may lead to ejaculatory
dysfunction. A more optimal strategy is to use unilateral
retroperitoneal lymph node dissection, preserve the contralateral
ejection nerve meridian and then administer neoadjuvant
chemotherapy. This combined approach appears to be both feasible
and safe.
Other de-escalation treatments include
intensity-modulated proton therapy (IMPT), immunotherapy and
molecular targeted therapy, although their use has only been
reported in a few cases. In a trial of IMPT, the proton therapy
dose was compared with intensity modulated radiation therapy and
volumetric modulated arc therapy photon plans in 10 patients with
stage II seminoma (26). The
results showed that proton therapy reduced the radiation dose to
abdominal and pelvic organs at risk (OAR) and may also reduce the
risk of overall and most secondary OAR cancer. Studies have shown
that immunotherapy based on immune checkpoint inhibitors is
effective against a variety of cancer types (such as lung and liver
cancer), and can be an important alternative to platinum-based
therapy for patients with seminoma (27). Seminoma had a greater prevalence of
somatic mutations in the KIT, KRAS and NRAS genes than in non-tumor
cell tumors (TGCTs), with a mutation frequency of 18% in the KIT
gene, according to an examination of data from The Cancer Genome
Atlas, which included 137 TGCTs (28). There was also a previous case report
of a young male with stage IV chemotherapy-resistant pure seminoma
with upregulated KIT expression who had complete remission
following administration of imatinib mesylate (29).
For patients with larger metastatic lymph nodes
(lymph node diameter >5 cm), platinum-based chemotherapy is a
widely accepted standard treatment. However, some patients may have
residual disease after chemotherapy. The European Society for
Medical Oncology and European Reference Network on Rare Adult Solid
Cancers Clinical Practice Guideline recommend that patients with
complete response after chemotherapy do not require further
treatment (1). Masses <3 cm
should be closely followed up, while masses >3 cm require
positron emission tomography PET-CT examination 6 weeks after
chemotherapy completion; however, the false positive rate of PET is
as high as 75% (30). In cases with
positive test results or continued enlargement of the mass,
pathological biopsy has become the gold standard for the diagnosis
of residual active tumors. If residual active disease occurs,
further surgical treatment, local radiotherapy or changing the
treatment regimen to second-line chemotherapy is required.
Iodine-125 seeds can produce high doses of radiation
(100–120 Gy) in the target tumor, resulting in the continuous
irradiation of the tumor cells and consequent induction of
apoptosis. The surrounding non-tumor tissue receives only a very
low dose and suffers very little damage, thus reducing toxic side
effects to the normal tissue while effectively killing the tumor
cells (31). For seed implantation
around large blood vessels, the operator must have a good operating
technique and good anatomical imaging facilities. If the seed
penetrates or migrates into a large blood vessel, it will be
transferred via blood flow to branching and smaller blood vessels,
which will cause local radiation damage. When no clear boundary
between the tumor and the blood vessel is seen during surgery,
real-time enhanced scanning is used to clarify the boundary between
the large vessels and the tumor. In addition, step-by-step needles
can be used when performing puncture operations, so that important
blood vessels can be avoided during puncture.
The efficacy of the combination of iodine-125 seed
implantation with chemotherapy and immunotherapy for the treatment
of solid tumors has been confirmed by certain clinical studies
(32,33). In a study of liver cancer, the
median OS time of the Transarterial Chemoembolization
(TACE)-iodine-125 group (13.8 months) was significantly higher than
that of the TACE-sorafenib group (8.3 months) (34). In a lung cancer study, iodine-125
seed implantation combined with bronchial arterial
chemoembolization was an effective and safe approach (31). The 6-month objective response and
disease control rates were 71.42 and 92.86%, respectively. Local
control duration ranged from 5 to 12 months, and the median PFS
time was 8 months. Studies have also shown that carboplatin can be
used as an alternative to cisplatin-based combination chemotherapy
to reduce the incidence of pulmonary toxicity, neurological damage
and renal toxicity (35,36).
In the present case report, as the metastatic lymph
nodes were large and surrounded blood vessels, there were no
indications for surgery or direct radiotherapy. Furthermore,
considering the possible long-term toxic side effects of standard
chemotherapy, iodine-125 seed implantation followed by treatment
with carboplatin (AUC7) was the chosen treatment method. During the
3-year postoperative follow-up, the serological markers returned to
normal, the tumor showed complete remission and there was no
evidence of adverse reactions, indicating the efficacy of
iodine-125 seed implantation combined with carboplatin (AUC7)
treatment.
In conclusion, the present case demonstrated the
potent antitumor effect of iodine-125 seed implantation in
seminoma, suggesting its use as an alternative method for local
treatment. Combined treatment with carboplatin can reduce the
toxicity and side effects of standard radiotherapy and
chemotherapy. However, the number of reported cases is currently
small, and the choice of the optimal dose of iodine-125 seeds and
the therapeutic effect of combining them with other treatment
methods requires further exploration.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
LR, YL and LJ contributed equally to the concept,
study design, data analysis and manuscript writing. LR and LJ
confirm the authenticity of all the raw data. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written informed consent for
the publication of this research.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Oldenburg J, Berney DM, Bokemeyer C,
Climent MA, Daugaard G, Gietema JA, De Giorgi U, Haugnes HS,
Huddart RA, Leão R, et al: Testicular seminoma and non-seminoma:
ESMO-EURACAN clinical practice guideline for diagnosis, treatment
and follow-up. Ann Oncol. 33:362–375. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gilligan T, Lin DW, Aggarwal R, Chism D,
Cost N, Derweesh IH, Emamekhoo H, Feldman DR, Geynisman DM, Hancock
SL, et al: Testicular cancer, version 2.2020, NCCN clinical
practice guidelines in oncology. J Natl Compr Canc Netw.
17:1529–1554. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Boormans JL, Sylvester R, Anson-Cartwright
L, Glicksman RM, Hamilton RJ, Hahn E, Daugaard G, Lauritsen J,
Wagner T, Avuzzi B, et al: Prognostic factor risk groups for
clinical stage I seminoma: An individual patient data analysis by
the European association of urology testicular cancer guidelines
panel and guidelines office. Eur Urol Oncol. S2588-9311(23)00232-8.
2023.(Epub ahead of print). View Article : Google Scholar
|
|
4
|
von Amsberg G, Hamilton R and
Papachristofilou A: Clinical stage IIA-IIC seminoma: Radiation
therapy versus systemic chemotherapy versus retroperitoneal lymph
node dissection. Oncol Res Treat. 41:360–363. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Giannatempo P, Greco T, Mariani L, Nicolai
N, Tana S, Farè E, Raggi D, Piva L, Catanzaro M, Biasoni D, et al:
Radiotherapy or chemotherapy for clinical stage IIA and IIB
seminoma: A systematic review and meta-analysis of patient
outcomes. Ann Oncol. 26:657–668. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Naoun N, Bernard-Tessier A and Fizazi K:
Stage II seminoma: Why chemotherapy should remain a standard. Eur
Urol Open Sci. 49:69–70. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wood GE, Chamberlain F, Tran B, Conduit C,
Liow E, Nicol DL, Shamash J, Alifrangis C and Rajan P: Treatment
de-escalation for stage II seminoma. Nat Rev Urol. 20:502–512.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Travis LB, Beard C, Allan JM, Dahl AA,
Feldman DR, Oldenburg J, Daugaard G, Kelly JL, Dolan ME, Hannigan
R, et al: Testicular cancer survivorship: Research strategies and
recommendations. J Natl Cancer Inst. 102:1114–1130. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fung C, Dinh P Jr, Ardeshir-Rouhani-Fard
S, Schaffer K, Fossa SD and Travis LB: Toxicities associated with
cisplatin-based chemotherapy and radiotherapy in long-term
testicular cancer survivors. Adv Urol. 2018:86718322018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kou F, Gao S, Liu S, Wang X, Chen H, Zhu
X, Guo J, Zhang X, Feng A and Liu B: Preliminary clinical efficacy
of iodine-125 seed implantation for the treatment of advanced
malignant lung tumors. J Cancer Res Ther. 15:1567–1573. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen Y, Jiang Y, Ji Z, Jiang P, Xu F,
Zhang Y, Zhang P, Guo F, Li X, Sun H, et al: Dosimetry, efficacy,
and safety of three-dimensional printing noncoplanar
template-assisted and CT-guided 125I seed implantation
for recurrent retroperitoneal lymphatic metastasis after external
beam radiotherapy. Brachytherapy. 19:380–388. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen L, Ying X, Zhang D, Lai L, Wu F, Tu J
and Ji J: Iodine-125 brachytherapy can prolong progression-free
survival of patients with locoregional recurrence and/or residual
hepatocellular carcinoma after radiofrequency ablation. Cancer
Biother Radiopharm. 36:820–826. 2021.PubMed/NCBI
|
|
13
|
Amin MB, Greene FL, Edge SB, Compton CC,
Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and
Winchester DP: The eighth edition AJCC cancer staging manual:
Continuing to build a bridge from a population-based to a more
‘personalized’ approach to cancer staging. CA Cancer J Clin.
267:93–99. 2017. View Article : Google Scholar
|
|
14
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fosså SD, Dahl AA, Thorsen L, Hellesnes R,
Kiserud CE, Tandstad T, Brydøy M, Haugnes HS and Myklebust TÅ:
Mortality and second cancer incidence after treatment for
testicular cancer: Psychosocial health and lifestyle are modifiable
prognostic factors. J Clin Oncol. 40:2588–2599. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Alsyouf M and Daneshmand S: Clinical stage
II seminoma: Management options. World J Urol. 40:343–348. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Honecker F, Aparicio J, Berney D, Beyer J,
Bokemeyer C, Cathomas R, Clarke N, Cohn-Cedermark G, Daugaard G,
Dieckmann KP, et al: ESMO consensus conference on testicular germ
cell cancer: Diagnosis, treatment and follow-up. Ann Oncol.
29:1658–1686. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Horwich A, Dearnaley DP, Sohaib A, Pennert
K and Huddart RA: Neoadjuvant carboplatin before radiotherapy in
stage IIA and IIB seminoma. Ann Oncol. 24:2104–2107. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Papachristofilou A, Bedke J, Hayoz S,
Schratzenstaller U, Pless M, Hentrich M, Krege S, Lorch A,
Aebersold DM, Putora PM, et al: LBA30 single-dose carboplatin
followed by involved-node radiotherapy as curative treatment for
seminoma stage IIA/B: Efficacy results from the international
multicenter phase II trial SAKK 01/10. Ann Oncol. 32 (Suppl
5):S13052021. View Article : Google Scholar
|
|
20
|
Douglawi A, Calaway A, Tachibana I,
Panizzutti Barboza M, Speir R, Masterson T, Adra N, Foster R,
Einhorn L and Cary C: Long-term oncologic outcomes after primary
retroperitoneal lymph node dissection: Minimizing the need for
adjuvant chemotherapy. J Urol. 204:96–103. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Heidenreich A, Paffenholz P, Hartmann F,
Seelemeyer F and Pfister D: Retroperitoneal lymph node dissection
in clinical stage IIA/B metastatic seminoma: Results of the cologne
trial of retroperitoneal lymphadenectomy in metastatic seminoma
(COTRIMS). Eur Urol Oncol. 7:122–127. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tachibana I, Alabd A, Piroozi A, Mahmoud
M, Kern S, Tong Y, Masterson TA, Adra N, Foster R, Hanna NH, et al:
Oncologic outcomes of primary retroperitoneal lymph node dissection
(RPLND) for stage II seminoma: Indiana University experience. J
Clin Oncol. 40 (16 Suppl):e170122022. View Article : Google Scholar
|
|
23
|
Lusch* A, Hiester A, Siemer RG, Paffenholz
P, Heidenreich A and Albers P: PD56-05 the primetest trial-interim
analysis of a phase II trial for primary retroperitoneal lymph node
dissection (RPLND) in stage II A/B seminoma patients without
adjuvant treatment. J Urol. 201 (Suppl 4):e10192019. View Article : Google Scholar
|
|
24
|
Daneshmand S, Cary C, Masterson TA,
Einhorn L, Boorjian SA, Kollmannsberger CK, Schuckman AK, So A,
Black PC, Bagrodia A, et al: SEMS trial: Result of a prospective,
multi-institutional phase II clinical trial of surgery in early
metastatic seminoma. J Clin Oncol. 39 (6 Suppl):S3752021.
View Article : Google Scholar
|
|
25
|
Huddart RA, Reid AH, Mayer E, Sohaib SA
and Nicol D: Clinical outcomes of minimally invasive
retroperitoneal lymph node dissection and single dose carboplatin
for clinical stage IIa seminoma. J Clin Oncol. 37 (7
Suppl):S5302019. View Article : Google Scholar
|
|
26
|
Rønde HS, Kronborg C, Høyer M, Hansen J,
Bak ME, Agergaard SN, Als AB, Agerbæk M, Lauritsen J, Meidahl
Petersen P, et al: Dose comparison of robustly optimized intensity
modulated proton therapy (IMPT) vs IMRT and VMAT photon plans for
testicular seminoma. Acta Oncol. 62:1222–1229. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Savelyeva AV and Medvedev KE: Seminoma
subtypes differ in the organization and functional state of the
immune microenvironment. 3 Biotech. 13:1102023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shen H, Shih J, Hollern DP, Wang L, Bowlby
R, Tickoo SK, Thorsson V, Mungall AJ, Newton Y, Hegde AM, et al:
Integrated molecular characterization of testicular germ cell
tumors. Cell Rep. 23:3392–3406. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pectasides D, Nikolaou M, Pectasides E,
Koumarianou A, Valavanis C and Economopoulos T: Complete response
after imatinib mesylate administration in a patient with
chemoresistant stage IV seminoma. Anticancer Res. 28:2317–2320.
2008.PubMed/NCBI
|
|
30
|
Cathomas R, Klingbiel D, Bernard BD, Lorch
A, Garcia del Muro X, Morelli F, De Giorgi U, Fedyanin M, Oing C,
Haugnes HS, et al: FDG PET scan (PET) positive residual lesions
after chemotherapy (chemo) for metastatic seminoma: Results of an
international global germ cell cancer group (G3) registry. J Clin
Oncol. 35 (15 Suppl):S45212017. View Article : Google Scholar
|
|
31
|
Chen C, Wang W, Yu Z, Tian S, Li Y and
Wang Y: Combination of computed tomography-guided iodine-125
brachytherapy and bronchial arterial chemoembolization for locally
advanced stage III non-small cell lung cancer after failure of
concurrent chemoradiotherapy. Lung Cancer. 146:290–296. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chin J, Rumble RB, Kollmeier M, Heath E,
Efstathiou J, Dorff T, Berman B, Feifer A, Jacques A and Loblaw DA:
Brachytherapy for patients with prostate cancer: American society
of clinical oncology/cancer care ontario joint guideline update. J
Clin Oncol. 35:1737–1743. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sharma R, Sagoo NS, Haider AS, Sharma N,
Haider M, Sharma IK, Igbinigie M, Aya KL, Aoun SG and Vira S:
Iodine-125 radioactive seed brachytherapy as a treatment for spine
and bone metastases: A systematic review and meta-analysis. Surg
Oncol. 38:1016182021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hu HT, Luo JP, Cao GS, Li Z, Jiang M, Guo
CY, Yuan H, Yao QJ, Geng X, Park JH, et al: Hepatocellular
Carcinoma With Portal Vein Tumor Thrombus Treated With
Transarterial Chemoembolization and Sorafenib vs
125iodine implantation. Front Oncol. 11:8069072021.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tookman L, Rashid S, Matakidou A, Phillips
M, Wilson P, Ansell W, Jamal-Hanjani M, Chowdhury S, Harland S,
Sarwar N, et al: Carboplatin AUC 10 for IGCCCG good prognosis
metastatic seminoma. Acta Oncol. 52:987–993. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shamash J, Syed R, Sarker SJ, Sarwar N,
Sharma A, Mutsvangwa K, Coetzee C, Wilson P and Rustin GJ: A phase
II study of carboplatin AUC-10 guided by positron emission
tomography-defined metabolic response in metastatic seminoma. Eur J
Cancer. 115:128–135. 2019. View Article : Google Scholar : PubMed/NCBI
|