Introduction
Pancreatic cancer (PC) is among the most lethal
malignancies and its global mortality and incidence rates have been
rising continuously, with the lowest 5-year survival rate among all
cancers (9%) (1–3). In particular, recent cancer statistics
indicate that China is faced with a rising burden of cases of PC
(4), which merits attention.
Surgery with adjuvant chemotherapy is the only method of curing
this disease, but ~80% of patients are diagnosed with unresectable
or metastatic PC (5). Therefore,
chemotherapy remains the mainstay for advanced PC (6).
According to current guidelines of the National
Comprehensive Cancer Network, first-line regimens for advanced PC
include a combination of fluorouracil + leucovorin + irinotecan +
oxaliplatin (FOLFIRINOX), and nanoparticle albumin-bound paclitaxel
(nab-paclitaxel) plus gemcitabine (AG) (7,8).
Although FOLFIRINOX and AG regimens demonstrated significant
survival advantages in clinical trials, the objective response rate
(ORR) was limited to 23–31.6% (9,10). In
addition, a high level of toxicity was reported among patients
receiving FOLFIRINOX, resulting in it being necessary to use
rigorous patient selection criteria and a low dose intensity of
each agent. In China, AG was considered to be a more practical and
convenient regimen than FOLFIRINOX (11). However, nab-paclitaxel was not
covered for use in PC by health insurance policies in China when
the present study was designed in 2018; therefore, the use of the
AG regimen was limited in Chinese clinical practice at that
time.
Numerous attempts have been made to develop
high-efficacy chemotherapeutic regimens to improve the prognosis of
patients with advanced PC (12–14).
S-1 is a fourth-generation oral fluoropyrimidine, consisting of
tegafur, gimeracil, and oteracil potassium. Due to S-1 being
convenient to administer and highly effective, S-1 has been studied
as a monotherapy in the MPACA-3 and ASPAC-01 trials, and as a
combination therapy in the GEST trial (14–16).
Two phase II trials of S-1 monotherapy reported promising results
with an ORR of 21.1–37.5% and overall survival (OS) of 5.6–9.2
months (14,17). Subsequently, large-scale clinical
trials showed that the ORR of gemcitabine plus S-1 (GS) was 44–48%,
with a median OS of 10–12 months in patients with metastatic PC
(18,19). Notably, a combination of gemcitabine
with a fluoropyrimidine, such as S-1 or capecitabine, was
affordable and more widely used than the AG regimen in China in
2018, particularly for patients with a poor economic status. This
regimen was also recommended as a first-line treatment for advanced
PC in Chinese guidelines since 2018 (20). Although it exhibited a good safety
profile, patient survival was still not adequate (21). Accordingly, alternative agents were
sought to improve patient survival.
Nab-paclitaxel was developed as a solvent-free
paclitaxel formulation that eliminates the risk of hypersensitivity
reactions (22). Owing to its
promising activity, nab-paclitaxel has been approved as a
therapeutic option for various cancers, including breast (23), non-small cell lung cancer (24) and PC (10). Given that nab-paclitaxel and S-1 in
combination with gemcitabine were found to significantly improve
outcomes (25), efforts were made
to develop a novel combination of nab-paclitaxel and S-1 (AS). This
combination was reported to exhibit a synergetic effect with good
tolerability in preclinical models of PC (26,27).
Subsequently, two single-arm phase II trials of AS were performed
in China, which reported noteworthy response rates of 50.0–53.1%
and an OS of 9.4–13.6 months in patients with advanced PC (28,29).
Additionally, the efficacy and safety of this treatment combination
were established in advanced breast cancer and gastric cancer
(30,31). However, the use of nab-paclitaxel
increases the medical expenses of patients with PC. At present,
there is no direct evidence regarding the comparable efficacy of AS
and GS in advanced PC. In view of the potential favorable survival
benefits and controllable safety profiles of the AS regimen, the
present prospective study was designed to compare the efficacy and
safety of AS with that of GS as first-line chemotherapy in patients
with advanced PC. If the more expensive treatment provides no
increase in survival, this treatment may be considered to be
limited in value.
Materials and methods
Study design and participants
The present study (AvGmPC) was an open-label,
multicenter, prospective, randomized clinical study conducted
across three centers (Zhongshan Hospital affiliated to Fudan
University, Huashan Hospital affiliated to Fudan University, and
Ruijin Hospital affiliated to Shanghai Jiaotong University; all
Shanghai, China). Eligible patients were aged between 18 and 75
years with histologically or cytologically confirmed unresectable
locally advanced or metastatic PC; tumor staging was reported using
the eighth edition of the American Joint Committee on Cancer
staging system for PC (32).
Unresectable tumors were initially identified using imaging
techniques and subsequently confirmed by discussion among the
multidisciplinary team responsible for pancreatic care. Additional
eligibility criteria included: No prior history of antitumor
therapy including radiotherapy and chemotherapy; at least one
measurable lesion according to Response Evaluation Criteria In
Solid Tumors version 1.1 (RECIST 1.1) (33); Eastern Cooperative Oncology Group
(ECOG) performance status 0–1; adequate hematologic function, as
indicated by an absolute neutrophil count ≥1.5×109/l, a
platelet count ≥100×109/l and a hemoglobin level ≥100
g/l); adequate hepatic function, as indicated by bilirubin ≤1.5
upper limit of the normal (ULN), alanine aminotransferase and
aspartate aminotransferase ≤2.5 ULN; and adequate renal function
(serum creatinine ≤1 ULN). Patients with recurrent diseases after
surgery were eligible for enrolment. However, patients who had
received other investigational drugs within 4 weeks prior to study
initiation were ineligible for inclusion in the present trial.
Exclusion criteria also comprised: Other malignancies within 5
years, with the exception of cured cervical carcinoma or skin basal
cell carcinoma; uncontrolled brain metastases; congestive heart
failure (New York Heart Association class ≥II); peripheral nerve
injury (Sunderland grade ≥II); allergy or intolerance to study
drugs; severe systemic infection or concomitant diseases; and
pregnant or lactating women.
The study protocol was approved by the Ethics
Committees of Zhongshan Hospital affiliated to Fudan University
(approval no. B2018-260), Huashan Hospital affiliated to Fudan
University [approval no. 2019 (001)] and Ruijin Hospital affiliated
to Shanghai Jiaotong University [approval no. 2019 (143)]. The
study was conducted in accordance with the Declaration of Helsinki.
Written informed consent was obtained from each patient before
enrolment. The study was retrospectively registered with the
Chinese Clinical Trial Registry as ChiCTR1900024588 on July 18,
2019.
Patients were enrolled between December 19, 2018 and
March 8, 2022. The database was closed for final analysis on August
15, 2022.
Randomization and masking
Patients were enrolled by the investigators and
randomly assigned in a 1:1 ratio to receive either AS or GS, using
a central computerized dynamic hierarchical randomization system.
Randomization was stratified according to PC status (locally
advanced vs. metastatic) and baseline CA 19-9 levels (<500 vs.
≥500 U/ml). Patients and investigators were not masked to study
treatment in this open-label trial.
Treatment
Patients randomized to the AS group received
nab-paclitaxel intravenously (125 mg/m2 on days 1 and 8)
and oral S-1 twice daily on days 1-14 at a dose calculated
according to the body surface area (BSA) of the patient (80 mg for
BSA <1.25 m2, 100 mg for BSA 1.25–1.5 m2,
120 mg for BSA ≥1.5 m2) every 3 weeks. Patients assigned
to the GS group received gemcitabine intravenously (1,000
mg/m2 on days 1 and 8) and S-1 at the same doses as
those in the AS group every 3 weeks. Treatment continued until the
occurrence of progressive disease (PD) as evaluated by the
investigators according to RECIST 1.1, any intolerable adverse
events (AEs), or at the discretion of the investigators or
patients. Crossover within the two groups was permitted in the
event of disease progression.
Toxicity was managed with dosing interruption, dose
reduction or supportive care. Dosing interruption was performed
according to protocol guidelines; specifically, the treatment cycle
was delayed until non-hematological (≤ grade 1) and hematological
toxicities were resolved, and an absolute neutrophil count (ANC)
≥1.5×109/l and platelet count (PLT) ≥7×109/l
were achieved. Dose reduction was considered if one of the
following events occurred: i) ANC <0.5×109/l; ii)
three consecutive occurrences of grade 2 ANC reductions
(1.0×109/l < ANC <1.5×109/l); iii)
febrile neutropenia; iv) 0.5×109/l < ANC
<1.0×109/l and 25×109/l< PLT
<50×109/l; v) PLT <25×109/l; vi) ≥
grade 2 peripheral neuropathy or gastrointestinal toxicity. Dose
re-escalation was not allowed after dose reduction.
Assessments
Tumor response was assessed by two independent
oncologists according to RECIST 1.1 guidelines with computed
tomography (CT) or magnetic resonance imaging at baseline, then at
every 6 weeks from the start of the first 3-week cycle, until
disease progression or discontinuation of the treatment protocol.
Patients were followed up for survival until death or study
closure. Safety assessments were performed by investigators at
every clinical visit in accordance with the study protocol. AEs
were classified and graded according to the National Cancer
Institute Common Terminology Criteria for Adverse Events (version
5.0) (34). Physical examinations
and routine laboratory tests, including hematology, liver and
kidney function, CA199 and CA125 tests, were performed prior to
each cycle of chemotherapy.
Outcomes
The primary endpoint was progression-free survival
(PFS), defined as the time between the initiation of treatment and
the observation of disease progression or death due to any cause.
Secondary endpoints included: OS, defined as the time between the
initiation of treatment and the occurrence of death from any cause;
ORR, defined as the proportion of patients achieving a complete
response (CR) or partial response (PR); disease control rate (DCR);
12- and 24-week PFS rates, defined as the percentage of patients
who did not have a PD or had died by week 12 or 24, respectively;
12- and 24-week OS rates, defined as the percentage of patients who
had died by week 12 or 24, respectively; and safety.
Statistical analysis
Based on the results of a previous study (35), it was presumed that the median PFS
of AS and GS groups would be 7.1 and 3.6 months, respectively.
Assuming an enrolment period of 24 months and a follow-up period of
12 months, the PFS was tested by a log-rank test with a
significance level of 5% (two-sided) and 80% power; after
adjustment for a dropout rate of 15%, 106 patients were enrolled in
the study.
Efficacy was assessed in the modified
intent-to-treat (mITT) population, which included all participants
randomly assigned to treatment who received at least one dose of
the assigned trial treatment. Safety was evaluated in the safety
analysis set, which consisted of all patients who had at least one
dose of assigned trial treatment. The Kaplan-Meier method was used
to analyze the PFS and OS, with medians and corresponding 95%
confidence intervals (CIs). Data for patients who were alive and
without disease progression or who were lost to follow-up were
censored for the analysis of PFS at the time of the last imaging
assessment. Patients who received other anticancer therapies, such
as radiotherapy, without disease progression were recorded as
censored. Data for patients who were alive or lost to follow-up
were censored for OS at the time they were last known to be alive.
For post hoc analyses of the interactions between the treatment and
subgroup, the hazard ratio (HR) with two-sided 95% CIs was
estimated using the Cox proportional hazards regression model in
the pre-planned subgroups. ORR, DCR and safety were compared
between the two treatment groups using the Chi-square or Fisher's
exact test. The same stratification factors used in randomization
were used for all stratified analyses. Statistical analyses were
conducted using IBM SPSS software (version 25; IBM Corp.). P≤0.05
was considered to indicate a statistically significant result.
Results
Baseline characteristics
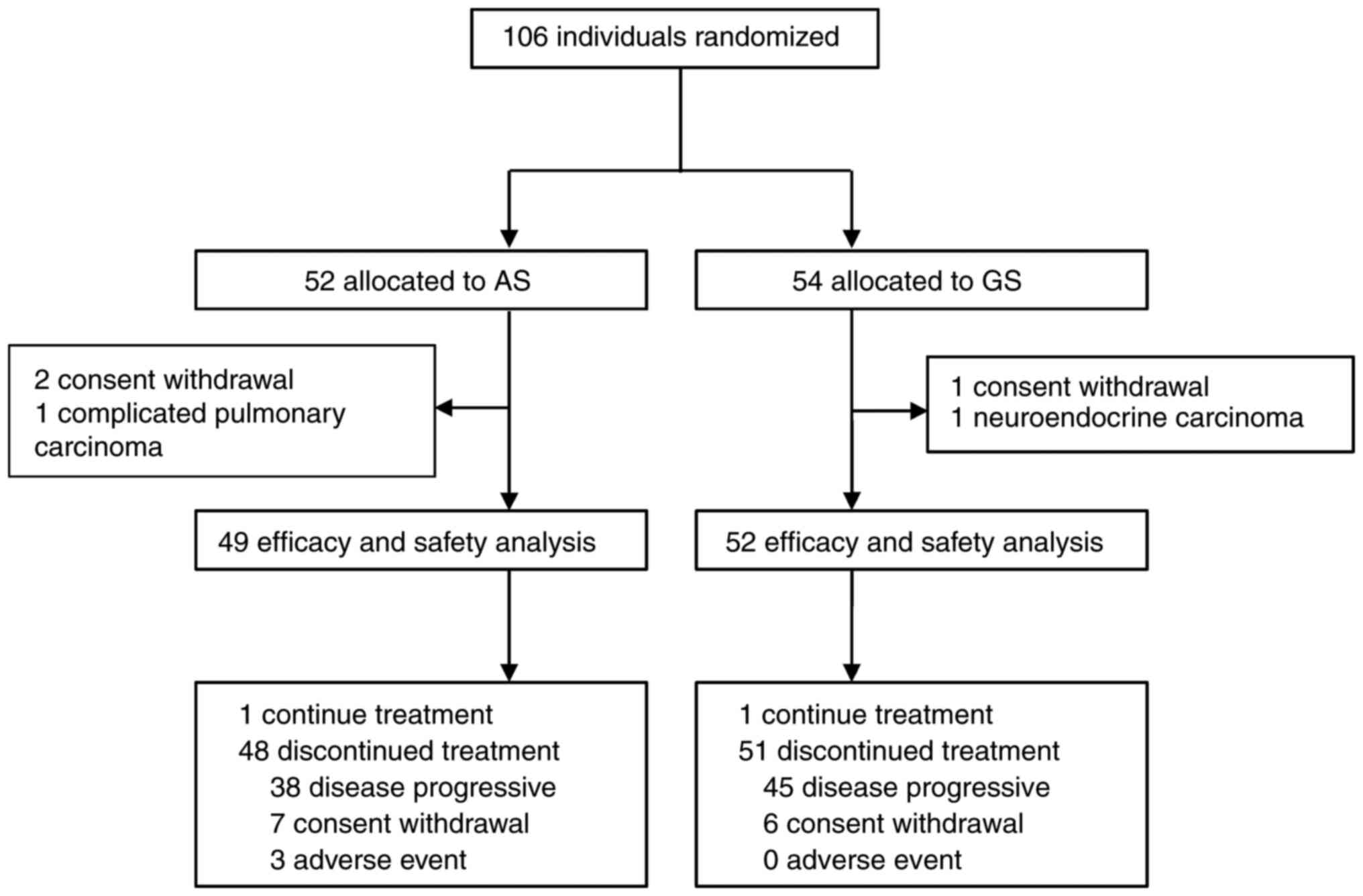
A total of 106 patients from 3 medical centers were
enrolled in the study, of which 52 patients were assigned to the AS
group and 54 patients were assigned to the GS group. However, 5 of
these patients were excluded before treatment initiation due to
neuroendocrine carcinoma (n=1), complicated pulmonary carcinoma
(n=1) and withdrawal before treatment (n=3). Thus, 49 (94.23%) of
52 patients assigned to the AS group and 52 (96.29%) of 54 patients
in the GS group received at least one dose of the study treatment,
and were included in the mITT population. A flow chart of patient
enrolment and the study design is presented in Fig. 1. An ECOG PS score of 1 was reported
in 97.96% of the patients and 65.30% had one or more metastasis
sites in the AS group. Baseline demographics and clinical
characteristics were well-balanced between the groups (Table I). The median age was 63
(interquartile range (IQR) 36–75) years in the AS group and 62 (IQR
39–73) years in the GS group. Most patients were male in the AS
(61.22%) and GS (75.00%) groups.
 | Table I.Patient demographic characteristics
at baseline. |
Table I.
Patient demographic characteristics
at baseline.
| Characteristic | AS group
(n=49) | GS group
(n=52) |
|---|
| Age, years |
|
|
| Median
(IQR) | 63 (36–75) | 62 (39–73) |
| <65,
n (%) | 30 (61.22) | 36 (69.23) |
| ≥65, n
(%) | 19 (38.78) | 16 (30.77) |
| Sex, n (%) |
|
|
|
Male | 30 (61.22) | 39 (75.00) |
|
Female | 19 (38.78) | 13 (25.00) |
| ECOG PS, n (%) |
|
|
| 0 | 1 (2.04) | 1 (1.92) |
| 1 | 48 (97.96) | 51(98.08) |
| CA199a, U/ml, median (range) | 345.00
(2.00–10,000.00) | 276.00
(2.00–10,000.00) |
| CA125b, U/ml, median (range) | 35.50
(7.20–3,505.00) | 37.45
(9.00–852.00) |
| Location, n
(%) |
|
|
|
Pancreatic head | 26 (53.06) | 22 (42.31) |
|
Pancreatic body | 6 (12.24) | 12 (23.08) |
|
Pancreatic tail | 8 (16.33) | 9 (17.31) |
|
Accumulated multiple
sites | 9 (18.37) | 9 (17.31) |
| Stages, n (%) |
|
|
|
III | 17 (34.69) | 20 (38.46) |
| IV | 32 (65.31) | 32 (61.54) |
| Metastasis sites, n
(%) |
|
|
| 0 | 17 (34.69) | 20 (38.46) |
| 1 | 20 (40.81) | 22 (42.31) |
| 2 | 7 (14.29) | 7 (13.46) |
| 3 | 4 (8.16) | 2 (3.85) |
| 4 | 1 (2.04) | 1 (1.92) |
Treatment and subsequent therapy
Of the 106 enrolled patients, 49 in the AS group and
52 in the GS group received at least one dose of the assigned
combination therapy. At the time of the data cutoff in the mITT
population (August 15, 2022), one patient in each group was still
receiving the assigned treatment. The median number of treatment
cycles was 7.00 (IQR 2.50–11.00) for patients in the AS group and
6.50 (IQR 3.25–12.75) for those in the GS group (Table SI). There were 20 patients (14 in
AS group vs. 6 in the GS group) who had not progressed after >6
cycles of combination therapy and were unable to tolerate intensive
regimens who chose to undergo maintenance with S-1, nab-paclitaxel
or gemcitabine monotherapy as decided by the investigators. As the
data cut-off, 99 (98.02%) patients had discontinued the protocol
therapy early (AS, n=48 vs. GS, n=51) due to AEs (AS, n=3 vs. GS,
n=0), disease progression (AS, n=38 vs. GS, n=45) and patient
choice (AS, n=7 vs. GS, n=6; Fig.
1). The relative dose intensities for nab-paclitaxel and S-1 in
the AS regimen and for gemcitabine and S-1 in the GS regimen were
92.30, 93.10, 95.90 and 97.20%, respectively.
Second-line and third-line treatments were
respectively administered to 29 (59.18%) and 8 (16.33%) patients
assigned to the AS group, and 34 (65.38%) and 7 (13.46%) patients
assigned to the GS group (Table
SII). After the discontinuation of their assigned treatment,
the 29 patients in the AS group who received subsequent therapies
with second-line regimens were mostly treated with
gemcitabine-based and radiotherapy-based regimens. By contrast, the
34 patients in the GS group selected mostly nab-paclitaxel-based
regimens and irinotecan/oxaliplatin-based regimens as subsequent
therapies.
Efficacy
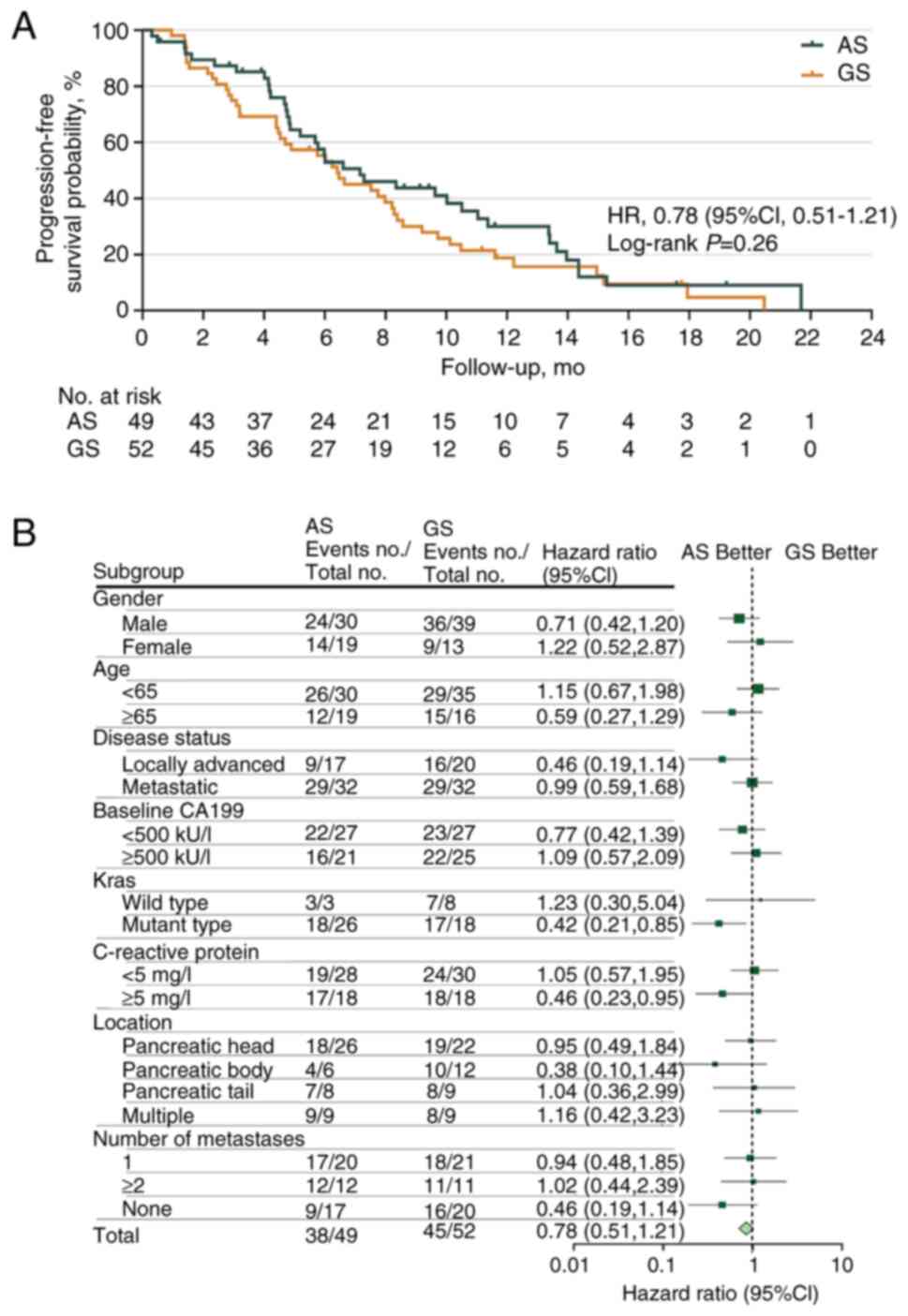
At the data cut-off, the median follow-up period was
11.37 months (95% CI, 9.31–13.24), with 83 PFS events observed [AS,
n=38 (77.55%); GS, n=45 (86.54%)]. At the final analysis, the
median PFS was 7.16 months (95% CI, 5.19–12.32) in the AS group as
compared with 6.41 months (95% CI, 3.72–8.84) in the GS group, with
an HR of 0.78 (95% CI, 0.51–1.21, P=0.264; Fig. 2A). The 12-week PFS rates were 87.29
and 78.85% in the AS and GS groups (P=0.233), respectively. With
regard to 24-week PFS rates, similar results were also observed for
the AS and GS groups (59.65 vs. 57.41%, respectively; P=0.638). A
post hoc subgroup analysis of PFS based on patient characteristics
revealed that patients with KRAS gene mutations (HR 0.42;
95% CI, 0.21–0.85) and baseline C-reactive protein (CRP) ≥5 mg/l
(HR 0.46; 95% CI, 0.23–0.95) were more likely to benefit from the
AS regimen (Fig. 2B).
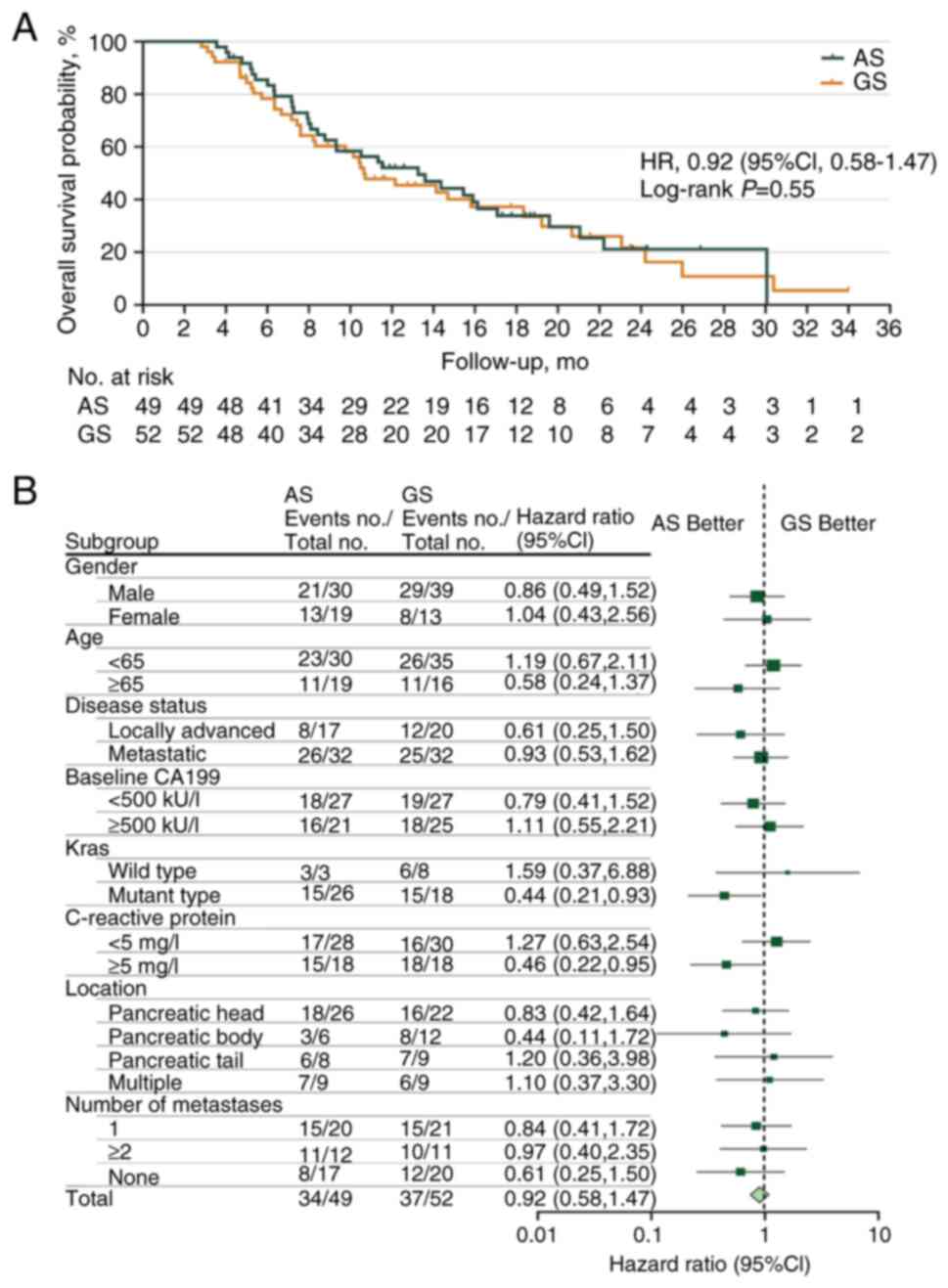
At the time of final analysis, when 71 death events
had been recorded [AS, n=34 (69.39%); GS, n=37 (71.15%)], the
median OS durations were 13.27 months (95% CI, 10.39–18.52) and
10.64 months (95% CI, 5.36–13.59) in the AS and GS groups,
respectively, with an HR of 0.92 (95% CI, 0.58–1.47; P=0.551;
Fig. 3A). The 24-week OS rate was
85.48% in the AS group compared with 78.71% in the GS group. Marked
OS benefits were observed in patients with KRAS gene
mutations [HR 0.44 (95% CI, 0.21–0.93) and high CRP levels (≥5
mg/l) (HR 0.46 (95% CI, 0.22–0.95)] from the AS regimen (Fig. 3B).
No patient in either group achieved a CR as the best
response according to RECIST 1.1 (Table II). However, 22 patients (44.90%)
in the AS group and 8 patients (15.38%) in the GS group had a PR.
Among the evaluated patients, the ORR was significantly higher in
the AS group than in the GS group [44.90% (95% CI, 30.67–59.77%)
vs. 15.38% (95% CI, 6.88–28.08%), respectively; P=0.001]. There
were 20 patients (40.82%) in the AS group and 35 patients (67.31%)
in the GS group who achieved a stable disease; thus, the DCRs in
the AS and GS groups were 85.71% (95% CI, 72.76–94.06%) and 82.69%
(95% CI, 69.67–91.77%), respectively (P=0.678). The maximal
percentage change from baseline in the sum of the longest target
lesion diameters during treatment is presented as a waterfall plot
(Fig. S1).
 | Table II.Tumor response according to RECIST
1.1. |
Table II.
Tumor response according to RECIST
1.1.
| Variable | AS group
(n=49) | GS group
(n=52) | P-value |
|---|
| Objective
response |
|
| 0.001 |
| No. of
patients | 22 | 8 |
|
| % of
patients (95% CI)a | 44.90
(30.67–59.77) | 15.38
(6.88–28.08) |
|
| Best overall
response, n (%) |
|
| 0.005 |
|
Complete response | 0 (0.00) | 0 (0.00) |
|
| Partial
response | 22 (44.90) | 8 (15.38) |
|
| Stable
disease | 20 (40.82) | 35 (67.31) |
|
|
Progressive disease | 5 (10.20) | 8 (15.38) |
|
| Not
evaluated | 2 (4.08) | 1 (1.92) |
|
AEs
The main AEs are summarized in Table III. The number of AEs of any grade
was 46 (93.88%) in the AS group vs. 44 (84.62%) in the GS group.
AEs of grade ≥3 occurred in 34 patients (69.39%) treated with AS
and in 22 patients (42.31%) treated with GS (P=0.009). AEs of any
grade that led to treatment discontinuation were only observed in 3
patients (6.12%) with the AS therapy, for which the events were
myelosuppression (n=3) and peripheral neurotoxicity (n=1). The
frequency of the most common AEs of any grade in the two groups (AS
vs. GS) was generally similar, including neutropenia (55.10 vs.
44.23%), anemia (32.65 vs. 23.08%) and alopecia (42.86 vs. 32.69%).
Most AEs of both groups were grade 1 or 2 and manageable. Dose
reductions occurred in 21 participants (42.85%) with AS and 13
(25.00%) with GS, and all were due to AEs. The most common grade ≥3
hematological toxicity was neutropenia (AS, 44.89% vs. GS, 23.08%;
P=0.020). The non-hematological toxicities with high incidence in
the AS group were peripheral neurotoxicity (46.94%), alopecia
(42.86%) and fatigue (26.53%), and in the GS group were alopecia
(32.69%) and rash (13.46%). The proportion of patients with
peripheral neurotoxicities was higher in the AS group than in the
GS group (46.94 vs. 1.92%, respectively; P<0.001). No
treatment-associated mortalities occurred in either of the
groups.
 | Table III.Major adverse events. |
Table III.
Major adverse events.
|
| All grades | Grades 3–4 |
|---|
|
|
|
|
|---|
| Events | AS group
(n=49) | GS group
(n=52) | P-value | AS group
(n=49) | GS group
(n=52) | P-value |
|---|
| All events, n
(%) | 46 (93.88) | 44 (84.62) | 0.202 | 34 (69.39) | 22 (42.31) | 0.009 |
| Hematology
toxicity, n (%) |
|
|
|
|
|
|
|
Neutropenia | 27 (55.10) | 23 (44.23) | 0.275 | 22 (44.89) | 12 (23.08) | 0.020 |
|
Anemia | 16 (32.65) | 12 (23.08) | 0.283 | 2 (4.08) | 2 (3.85) | 1.000 |
|
Thrombocytopenia | 4 (8.16) | 12 (23.08) | 0.040 | 0 (0) | 5 (9.62) | 0.077 |
| Non-hematological
toxicity, n (%) |
|
|
|
|
|
|
|
Peripheral neurotoxicity | 23 (46.94) | 1 (1.92) | <0.001 | 4 (8.16) | 0 (0) | 0.052 |
|
Alopecia | 21 (42.86) | 17 (32.69) | 0.292 | 0 (0) | 0 (0) | - |
|
Fatigue | 13 (26.53) | 5 (9.62) | 0.026 | 4 (8.16) | 3 (5.77) | 0.710 |
|
Diarrhea | 9 (18.37) | 5 (9.62) | 0.203 | 5 (10.20) | 1 (1.92) | 0.105 |
|
Vomiting | 9 (18.37) | 2 (3.85) | 0.019 | 3 (6.12) | 0 (0) | 0.111 |
| Oral
mucositis | 7 (14.28) | 1 (1.92) | 0.028 | 2 (4.08) | 0 (0) | 0.233 |
|
Rash | 1 (2.04) | 7 (13.46) | 0.061 | 0 (0.00) | 1 (1.92) | 1.000 |
|
Hand-foot syndrome | 7 (14.29) | 1 (1.92) | 0.028 | 0 (0.00) | 0 (0.00) | - |
Discussions
To the best of our knowledge, the present study is
the first multicenter, open-label phase II trial to compare AS with
GS as first-line chemotherapies in patients with advanced PC.
Despite the study failing to meet the primary endpoint of PFS, the
AS regimen showed a numerical improvement of 2.63 months in OS
compared with the GS regimen. More encouragingly, the ORR of
patients treated with AS was significantly higher compared with
that of patients treated with GS. Further analysis revealed that
several subpopulations of patients, including patients who had
KRAS gene mutations and CRP levels ≥5 mg/l, benefited more
from the AS regimen. Additionally, the present study found a
favorable and acceptable safety profile for AS in Chinese patients
with advanced PC. Overall, the data from the present trial provide
an important benchmark for investigation of the AS regimen in a
subset of patients.
Although the results obtained in the present study
did not demonstrate an advantage of AS over GS in terms of PFS
(7.16 vs. 6.41 months), the results were generally comparable with
those of other studies of AS, such as the NPSPAC trial (5.6 months)
(29) and another trial performed
in China (6.3 months) (36). It
should also be noted that the relatively high PFS obtained with the
GS regimen in the present study may be the main reason for the
primary endpoint of PFS not being met. When compared with a
previous retrospective study of GS, with its inherent selection
bias and enrolment of patients only with stage IV disease (35), the improved baseline characteristics
of the patients in the current study, with 38.46% in stage III and
61.54% in stage IV, may have contributed to the longer PFS (6.41
vs. 3.6 months) of the GS regimen. Furthermore, the patients who
received the AS regimen had a numerically higher OS compared with
those who received the GS regimen (13.27 vs. 10.64 months,
respectively), suggesting a possible survival advantage for
patients who were able to adhere to this regimen. Compared with the
OS found in other studies (29,36),
an improved survival benefit was observed in the present study,
which may be due to the use of subsequent therapies in the two
groups. Despite response activity not being a primary endpoint of
the present study, a markedly increased ORR (44.90 vs. 15.38%) was
achieved in patients treated with the AS regimen. Zhang et
al (28) reported that the ORR
was as high as 53.1% among 32 patients with advanced pancreatic
ductal adenocarcinoma receiving the same triweekly regimen, which
was similar to the present results. On the basis of this finding,
the response benefit of AS is suggestive of meaningful clinical
activity in this population. Despite an increased ORR of the AS
regimen compared with the GS regimen being observed in the present
AvGmPC study, the response did not translate into improved survival
benefits, which may be partly explained by more patients in the AS
group discontinuing treatment due to intolerable toxicity. Another
explanation is that crossover within two groups was permitted in
the event of disease progression; thus, the proportion of patients
with second-line use of nab-paclitaxel and gemcitabine was similar,
potentially leading to the OS of the two groups being comparable.
Although these are interesting findings, considering economic
limitations, further study with AS in a larger cohort of advanced
PC requires careful assessment.
Distinct advantages of AS with regard to PFS/OS were
not obtained in the present study population; however, the subgroup
analysis showed that patients with KRAS gene mutations and
elevated CRP levels were more likely to benefit from the AS
regimen. A number of clinical trials have shown significant OS
advantages in KRAS wild-type patients (13.4 vs. 9.1 months;
479 vs. 255 days; and 352 vs. 333 days) (37–39).
The findings of the present study suggest that the AS regimen may
be able to reverse the adverse effects of oncogenic KRAS
mutations. Considering these results, they may be partially
explained by the finding that KRAS mutations increase
nab-paclitaxel uptake by 5–25-fold compared with that in
KRAS wild-type PC cells, which may be associated with
extracellular signal-regulated kinase activation (40). In addition, the subgroup analysis in
the present study showed improvements in PFS and OS among patients
with high CRP levels. This suggests the predictive potential of CRP
as a biomarker for the efficacy of AS. Considering the maximization
of benefits, further study should focus on subpopulations with
KRAS gene mutations and/or elevated CRP levels to explore
the survival benefits of the AS regimen.
In order to identify the less toxic regimen, the
safety of the AS and GS regimens was compared. The safety profiles
of AS in the present study were generally consistent with known
profiles, as previously reported in other studies with similar
populations (29,36). In addition, AS has been shown to be
less safe than nab-paclitaxel or S-1 monotherapy in previous
studies, indicating that this combination might increase the risk
of various AEs including neutropenia, oral mucositis, diarrhea and
vomiting (15,29,41).
Seven patients in the AS group experienced oral mucositis
accompanied by gastrointestinal symptoms, which resulted in
reductions in daily food intake and the recovery of white blood
cell numbers. This may be associated with the status of
dihydropyrimidine dehydrogenase (DPD) and the enzymes that
metabolize paclitaxel; however, the analysis of this data has not
yet been completed. No treatment-associated deaths occurred in
either of the groups in the present study, and no unexpected safety
signals were identified in the study population. Notably, the GS
regimen was associated with a high incidence of thrombocytopenia
and rash. We hypothesize that this result may be partly due to a
generic gemcitabine drug being used. In addition, although a
greater proportion of patients experienced peripheral neuropathy in
the AS group, in most patients, these events could be mitigated
with dose reduction or the suspension of nab-paclitaxel treatment.
In general, the AS regimen was well-tolerated and was delivered
safely in patients with advanced PC.
Overall support for the AS regimen is based on
several considerations: i) A significantly higher ORR and numerical
improvement of 2.6 months in OS was observed in the AS group
compared with the GS group; and subpopulations with KRAS
gene mutations and CRP levels ≥5 mg/l gained more benefit from the
AS regimen. ii) The study being designed on the basis of
retrospective data led to an underestimation of the potential
improvement in PFS for the GS group, and several cases did not
adhere to the protocol due to mucosal reactions, leading to no
significant improvement in PFS being observed between the AS and GS
regimens. iii) Despite there being a greater number of AEs in the
AS group than in the GS group, most AEs were grade 1 or 2 and
manageable. and no treatment-associated deaths occurred. Together,
considering the overall risk-benefit, the AS regimen may be deemed
to be favorable in patients with PC. Importantly, based on these
findings, future studies should focus on exploration of the
efficacy of the AS regimen in selected populations, such as those
with RAS mutations or high CRP levels. Dose adjustments
should also be considered, as it may be necessary to reduce the
initial dose of nab-paclitaxel. Also, the DPD enzyme or the
DPYD gene that encodes it should be investigated to assist
in the dosage selection for S-1, and the impact of taxane
metabolism-associated genetic phenotypes on adverse reactions
require exploration.
Although the present study did not reach the primary
endpoint, it is not possible to make an absolute determination of
whether this is a negative study or a failed study. Several
important limitations of the study should be recognized. Firstly,
the study was conducted in China and included only Asian
participants; it is unclear whether the results can be simply
extrapolated to Western patients because the pharmacokinetics and
pharmacodynamics of S-1 between Western and East Asian patients may
differ (42,43). The prominent factors for
inter-ethnic differences in drug effects between Asian and
Caucasians populations among the three S-1 components are
differences in genetic polymorphisms, cultural differences and
dietary habits. Tegafur (FT) is a prodrug of 5-fluorouracil (5FU),
which is converted to 5FU in vivo mainly in the liver
through hydroxylation by cytochrome P-450 2A6 (CYP2A6), a highly
polymorphic enzyme with a higher frequency of common allelic
variants CYP2A6*4, *7 and *9 in East Asians than Caucasians. As
these variants are associated with reduced enzymatic activity,
reduced activation of FT may explain the lower 5FU exposure in
Asian patients (43). Also, DPD
catabolizes 85% of 5FU to fluoro-β-alanine and has a major
influence on 5FU levels (44);
therefore, the variable inhibition of DPD by gimeracil is likely to
impact 5FU exposure. Secondly, the study had an open-label design
due to the different administration, which may have lead to a
subconscious bias in favor of the experimental group. Nevertheless,
radiological results were required to be assessed by at least two
independent oncologists, which eliminated this limitation and
ensured the quality of the study findings to a large extent.
Thirdly, the high dose in the AS regimen without a dose-escalation
design may have lead to discontinuation due to AEs. However,
patients were allowed to receive supportive care and further
second- or third-line treatment, which made the two groups of
patients more comparable. Finally, due to COVID-19, changes in the
treatment plan and survival data of patients were inevitably
delayed.
Although the study failed to reach the primary
endpoint, the improved response observed in patients with AS may
indicate meaningful clinical benefits in this population. The
promising PFS and OS benefits in certain predefined subsets and
manageable toxicity indicate that the AS regimen is comparable with
GS and a convenient alternative first-line chemotherapy for
advanced PC. However, a larger-scale randomized trial is required
for further evaluation of the AS regimen in the near future.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
The authors would like to thank CSPC Ouyi
Pharmaceutical Co., Ltd. (Shijiazhuang, Hebei, China) for supplying
nab-paclitaxel.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
WL, YZ and TL had full access to all the data and
are responsible for the integrity of the data and the accuracy of
the data analysis. WL, XG, RZ and YZ conceived and designed the
study. XG, YX, TL, LY, JW, DF, JZ, JL, XL, WL, XY, TK, LL, YR, DJ,
WWu and YZ acquired, analyzed and interpretated the data. XG, YX
and XL drafted the manuscript. YX, YR, DJ, WWu, WL and YZ
critically revised the manuscript for important intellectual
content. XX, YJ, LW, DW, WWa, XL and ML performed the statistical
analysis. WL and YZ obtained funding. XG, RZ, LY, JW, DF, JZ, TL,
JL, YR, DJ, WWu, XX, YJ, LW, XY, DW, TK, LL and WWa provided
administrative, technical or material support. XL, WL and YZ
supervised the study. RZ and TL confirm the authenticity of all the
raw data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the ethics
committees of Zhongshan Hospital affiliated to Fudan University
(approval no. B2018-260), Huashan Hospital affiliated to Fudan
University [approval no. 2019 (001)] and Ruijin Hospital affiliated
to Shanghai Jiaotong University (approval no. 2019 (143). The study
was conducted in accordance with the Declaration of Helsinki.
Written informed consent was obtained from all patients before
enrolment. The study is registered with Chinese Clinical Trial
Registry: ChiCTR1900024588.
Patient consent for publication
The subjects gave written informed consent for the
publication of any associated data and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mizrahi JD, Surana R, Valle JW and Shroff
RT: Pancreatic cancer. Lancet. 395:2008–2020. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2022. CA Cancer J Clin. 72:7–33. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Viale PH: The American Cancer Society's
facts & figures: 2020 Edition. J Adv Pract Oncol. 11:135–136.
2020.PubMed/NCBI
|
|
4
|
Cao W, Chen HD, Yu YW, Li N and Chen WQ:
Changing profiles of cancer burden worldwide and in China: A
secondary analysis of the global cancer statistics 2020. Chin Med J
(Engl). 134:783–791. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kamisawa T, Wood LD, Itoi T and Takaori K:
Pancreatic cancer. Lancet. 388:73–85. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Conroy T, Bachet JB, Ayav A, Huguet F,
Lambert A, Caramella C, Maréchal R, Van Laethem JL and Ducreux M:
Current standards and new innovative approaches for treatment of
pancreatic cancer. Eur J Cancer. 57:10–22. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
National Comprehensive Cancer Network
(NCCN), . Clinical Practice Guidelines in Oncology. Pancreatic
Adenocarcinoma. (v. 1 2022). NCCN; Plymouth Meeting, PA: 2022
|
|
8
|
CSCO, . Guidelines of The Chinese Society
of Clinical Oncology (CSCO) pancreatic cancer 2020. People's
Medical Publishing House; Beijing: 2020
|
|
9
|
Conroy T, Desseigne F, Ychou M, Bouche O,
Guimbaud R, Becouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de
la Fouchardiere C, et al: FOLFIRINOX versus gemcitabine for
metastatic pancreatic cancer. N Engl J Med. 364:1817–1825. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Von Hoff DD, Ervin T, Arena FP, Chiorean
EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et
al: Increased survival in pancreatic cancer with nab-paclitaxel
plus gemcitabine. N Engl J Med. 369:1691–1703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cui J, Jiao F, Li Q, Wang Z, Fu D, Liang
J, Liang H, Xia T, Zhang T, Zhang Y, et al: Chinese society of
clinical oncology (CSCO): Clinical guidelines for the diagnosis and
treatment of pancreatic cancer. J National Cancer Center.
2:205–215. 2022. View Article : Google Scholar
|
|
12
|
Oettle H, Riess H, Stieler JM, Heil G,
Schwaner I, Seraphin J, Gorner M, Molle M, Greten TF, Lakner V, et
al: Second-line oxaliplatin, folinic acid, and fluorouracil versus
folinic acid and fluorouracil alone for gemcitabine-refractory
pancreatic cancer: Outcomes from the CONKO-003 trial. J Clin Oncol.
32:2423–2429. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ohba A, Ueno H, Shiba S, Okano N,
Kobayashi T, Nagashima F, Sasahira N, Sasaki M, Imaoka H, Sakamoto
Y, et al: Safety and efficacy of S-IROX (S-1, irinotecan and
oxaliplatin combination therapy) in patients with advanced
pancreatic cancer: A multicenter phase 1b dose-escalation and
dose-expansion clinical trial. Eur J Cancer. 174:40–47. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Go SI, Lee SC, Bae WK, Zang DY, Lee HW,
Jang JS, Ji JH, Kim JH, Park S, Sym SJ, et al: Modified FOLFIRINOX
versus S-1 as second-line chemotherapy in gemcitabine-failed
metastatic pancreatic cancer patients: A randomised controlled
trial (MPACA-3). Eur J Cancer. 157:21–30. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ueno H, Ioka T, Ikeda M, Ohkawa S,
Yanagimoto H, Boku N, Fukutomi A, Sugimori K, Baba H, Yamao K, et
al: Randomized phase III study of gemcitabine plus S-1, S-1 alone,
or gemcitabine alone in patients with locally advanced and
metastatic pancreatic cancer in Japan and Taiwan: GEST study. J
Clin Oncol. 31:1640–1648. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Uesaka K, Boku N, Fukutomi A, Okamura Y,
Konishi M, Matsumoto I, Kaneoka Y, Shimizu Y, Nakamori S, Sakamoto
H, et al: Adjuvant chemotherapy of S-1 versus gemcitabine for
resected pancreatic cancer: A phase 3, open-label, randomised,
non-inferiority trial (JASPAC 01). Lancet. 388:248–257. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Okusaka T, Funakoshi A, Furuse J, Boku N,
Yamao K, Ohkawa S and Saito H: A late phase II study of S-1 for
metastatic pancreatic cancer. Cancer Chemother Pharmacol.
61:615–621. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nakamura K, Yamaguchi T, Ishihara T, Sudo
K, Kato H and Saisho H: Phase II trial of oral S-1 combined with
gemcitabine in metastatic pancreatic cancer. Br J Cancer.
94:1575–1579. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ueno H, Okusaka T, Furuse J, Yamao K,
Funakoshi A, Boku N, Ohkawa S, Yokosuka O, Tanaka K, Moriyasu F, et
al: Multicenter phase II study of gemcitabine and S-1 combination
therapy (GS Therapy) in patients with metastatic pancreatic cancer.
Jpn J Clin Oncol. 41:953–958. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao Y and Yu X: Comprehensive guidelines
for the diagnosis and treatment of pancreatic cancer (2018
edition). J Clin Hepatol. 34:2109–2120. 2018.
|
|
21
|
Song H, Han B, Park CK, Kim JH, Jeon JY,
Kim IG, Kim HJ, Jung JY, Kim JH, Kwon JH, et al: Phase II trial of
gemcitabine and S-1 for patients with advanced pancreatic cancer.
Cancer Chemother Pharmacol. 72:845–852. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Roy V, LaPlant BR, Gross GG, Bane CL and
Palmieri FM; North Central Cancer Treatment Group, : Phase II trial
of weekly nab (nanoparticle albumin-bound)-paclitaxel
(nab-paclitaxel) (Abraxane) in combination with gemcitabine in
patients with metastatic breast cancer (N0531). Ann Oncol.
20:449–453. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schmid P, Adams S, Rugo HS, Schneeweiss A,
Barrios CH, Iwata H, Dieras V, Hegg R, Im SA, Shaw Wright G, et al:
Atezolizumab and Nab-paclitaxel in advanced triple-negative breast
cancer. N Engl J Med. 379:2108–2121. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jotte R, Cappuzzo F, Vynnychenko I,
Stroyakovskiy D, Rodriguez-Abreu D, Hussein M, Soo R, Conter HJ,
Kozuki T, Huang KC, et al: Atezolizumab in combination with
carboplatin and nab-paclitaxel in advanced squamous NSCLC
(IMpower131): Results from a randomized phase III trial. J Thorac
Oncol. 15:1351–1360. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tahara J, Shimizu K, Otsuka N, Akao J,
Takayama Y and Tokushige K: Gemcitabine plus nab-paclitaxel vs.
FOLFIRINOX for patients with advanced pancreatic cancer. Cancer
Chemother Pharmacol. 82:245–250. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Suenaga M, Yamada S, Fujii T, Tanaka C,
Kanda M, Nakayama G, Sugimoto H, Koike M, Fujiwara M and Kodera Y:
S-1 plus nab-paclitaxel is a promising regimen for pancreatic
cancer in a preclinical model. J Surg Oncol. 113:413–419. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li JA, Xu XF, Han X, Fang Y, Shi CY, Jin
DY and Lou WH: Nab-Paclitaxel plus S-1 shows increased antitumor
activity in patient-derived pancreatic cancer xenograft mouse
models. Pancreas. 45:425–433. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang W, Du C, Sun Y, Yang L, Cui C, Jiang
Z, Wang C, Wang J and Zhou A: Nab-paclitaxel plus S-1 as first-line
followed by S-1 maintenance for advanced pancreatic adenocarcinoma:
A single-arm phase II trial. Cancer Chemother Pharmacol.
82:655–660. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shi Y, Zhang S, Han Q, Li J, Yan H, Lv Y,
Shi H, Liu R and Dai G: Nab-paclitaxel plus S-1 in advanced
pancreatic adenocarcinoma (NPSPAC): A single arm, single center,
phase II trial. Oncotarget. 8:92401–92410. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dai YH, Yu XJ, Xu HT, Zhuang L, Zhang MS,
Zou YM, Fu Q, Qiu H and Yuan XL: Nab-paclitaxel plus S-1 versus
oxaliplatin plus S-1 as first-line treatment in advanced gastric
cancer: Results of a multicenter, randomized, phase III trial
(GAPSO study). Ther Adv Med Oncol. 14:175883592211180202022.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Morimoto M, Toba H, Aoyama M, Nakagawa M,
Takechi H, Yoshida T and Tangoku A: Phase 1 Dose-Escalation study
of triweekly Nab-Paclitaxel combined with S-1 for HER2-Negative
metastatic breast cancer. Clin Breast Cancer. 20:448–453. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Amin MB, Greene FL, Edge SB, Compton CC,
Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and
Winchester DP: The Eighth Edition AJCC Cancer Staging Manual:
Continuing to build a bridge from a population-based to a more
‘personalized’ approach to cancer staging. CA Cancer J Clin.
67:93–99. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Freites-Martinez A, Santana N,
Arias-Santiago S and Viera A: Using the common terminology criteria
for adverse events (CTCAE-Version 5.0) to evaluate the severity of
adverse events of anticancer therapies. Actas Dermosifiliogr (Engl
Ed). 112:90–92. 2021.(English, Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xu Y, Guo X, Fan Y, Wang D, Wu W, Wu L,
Liu T, Xu B, Feng Y, Wang Y, et al: Efficacy and safety comparison
of nabpaclitaxel plus S-1 and gemcitabine plus S-1 as first-line
chemotherapy for metastatic pancreatic cancer. Jpn J Clin Oncol.
48:535–541. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zong Y, Yuan J, Peng Z, Lu M, Wang X, Shen
L and Zhou J: Nab-paclitaxel plus S-1 versus nab-paclitaxel plus
gemcitabine as first-line chemotherapy in patients with advanced
pancreatic ductal adenocarcinoma: A randomized study. J Cancer Res
Clin Oncol. 147:1529–1536. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee J, Jang KT, Ki CS, Lim T, Park YS, Lim
HY, Choi DW, Kang WK, Park K and Park JO: Impact of epidermal
growth factor receptor (EGFR) kinase mutations, EGFR gene
amplifications, and KRAS mutations on survival of pancreatic
adenocarcinoma. Cancer. 109:1561–1569. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ogura T, Yamao K, Hara K, Mizuno N,
Hijioka S, Imaoka H, Sawaki A, Niwa Y, Tajika M, Kondo S, et al:
Prognostic value of K-ras mutation status and subtypes in
endoscopic ultrasound-guided fine-needle aspiration specimens from
patients with unresectable pancreatic cancer. J Gastroenterol.
48:640–646. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Philip PA, Azar I, Xiu J, Hall MJ,
Hendifar AE, Lou E, Hwang JJ, Gong J, Feldman R, Ellis M, et al:
Molecular characterization of KRAS Wild-type tumors in patients
with pancreatic adenocarcinoma. Clin Cancer Res. 28:2704–2714.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li R, Ng TSC, Wang SJ, Prytyskach M,
Rodell CB, Mikula H, Kohler RH, Garlin MA, Lauffenburger DA,
Parangi S, et al: Therapeutically reprogrammed nutrient signalling
enhances nanoparticulate albumin bound drug uptake and efficacy in
KRAS-mutant cancer. Nat Nanotechnol. 16:830–839. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ueno H, Okusaka T, Ikeda M, Takezako Y and
Morizane C: An early phase II study of S-1 in patients with
metastatic pancreatic cancer. Oncology. 68:171–178. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Haller DG, Cassidy J, Clarke SJ,
Cunningham D, Van Cutsem E, Hoff PM, Rothenberg ML, Saltz LB,
Schmoll HJ, Allegra C, et al: Potential regional differences for
the tolerability profiles of fluoropyrimidines. J Clin Oncol.
26:2118–2123. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chuah B, Goh BC, Lee SC, Soong R, Lau F,
Mulay M, Dinolfo M, Lim SE, Soo R, Furuie T, et al: Comparison of
the pharmacokinetics and pharmacodynamics of S-1 between Caucasian
and East Asian patients. Cancer Sci. 102:478–483. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Heggie GD, Sommadossi JP, Cross DS, Huster
WJ and Diasio RB: Clinical pharmacokinetics of 5-fluorouracil and
its metabolites in plasma, urine, and bile. Cancer Res.
47:2203–2206. 1987.PubMed/NCBI
|