Introduction
Low-grade myofibroblastic sarcoma (LGMS) is a rare
malignant neoplasm in the soft tissues that originates from the
stromal cells and is characterized by atypical myofibroblasts with
fibromatosis-like features (1).
Predominantly affecting middle-aged men, LGMS often presents in the
head and neck regions, although it could be found in other parts of
the body (2). Currently, to the
best of our knowledge, there is no literature reporting knee LGMS.
Due to its painless nature, LGMS is frequently overlooked in
clinical practice and misdiagnosed as a benign lesion or other
diseases with similar symptoms or imaging findings (3). The actual incidence of LGMS may be
under-reported due to unclear diagnostic criteria and a high
potential for misdiagnosis. Reports about clinical details such as
tumor size, method of treatment, and presence or absence of
recurrence (local recurrence, regional recurrence and distant
metastasis) and patient survival are sparse. Furthermore, the
complete clinical picture of LGMS, including mortality rates,
mewthods of treatment and risk factors, remains unclear (4). A population-based study in the USA
reported 49 cases of LGMS with a 5-year overall survival rate of
71.6% (5). Surgery is currently the
primary treatment for LGMS. Due to the rarity of reported cases,
the standardization of its treatment, including surgery,
chemotherapy and radiotherapy, requires further research (6). The present study details a rare
instance of LGMS in the left knee.
Case report
A 75-year-old woman presented to the Department of
Orthopedics at Guangzhou Red Cross Hospital (Guangzhou, China) in
June 2021 with a 15-day history of a painless mass in the left
knee. The patient reported no trauma, fever, joint swelling, weight
loss or systemic symptoms. Physical examination revealed a soft,
non-tender mass in the popliteal fossa, with normal overlying skin
and no knee joint movement limitation. Ultrasound suggested a
potential intramuscular hemangioma in the medial sartorius muscle
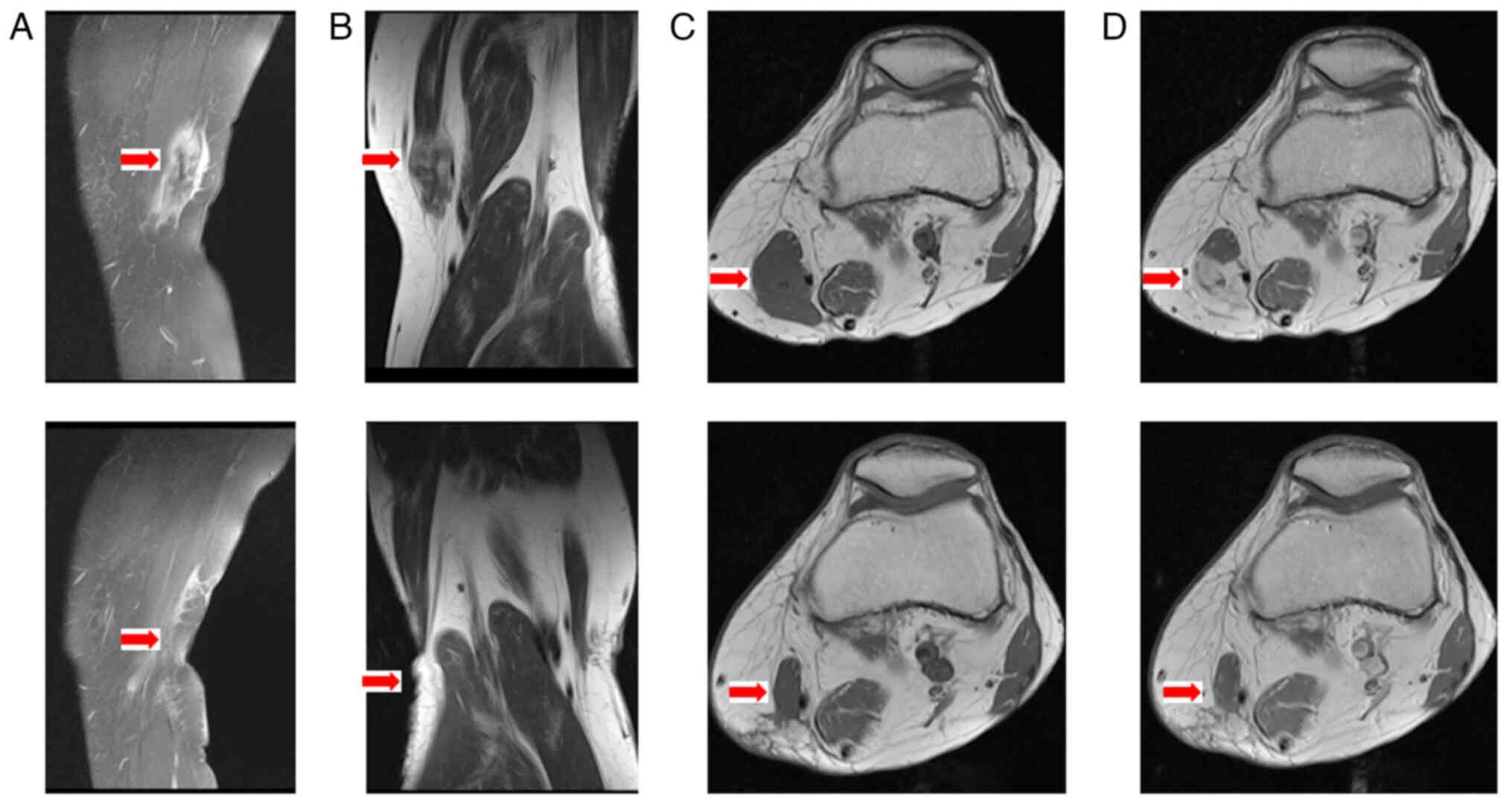
layer. Magnetic resonance imaging (MRI) revealed a well-defined,
heterogeneous 3.9×1.9-cm mass in the deep soft tissues of the
thigh, distinct from the surrounding muscles and bone (Fig. 1). The painless nature, indolent
growth and imaging reports of the mass led to the decision to
perform tumor excision surgery without a pre-operative biopsy.
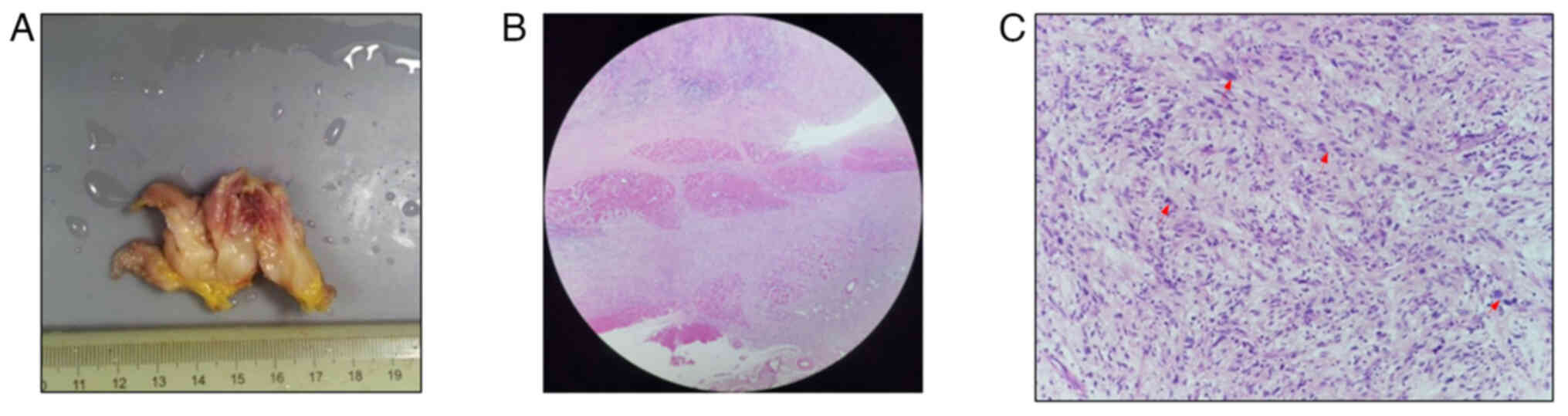
Intraoperatively, the tumor exhibited a unique
morphology, differing from that of conventional lipomas. The
well-encapsulated mass, which was adherent to the surrounding
tissues, was completely excised with clear margins (Fig. 2A). For the microscopic observation,
hematoxylin and eosin (H&E) staining and immunohistochemical
(IHC) staining results were examined using a Nikon Eclipse CI light
microscope (Nikon Corporation). Tumor specimens were fixed in 10%
neutral formalin at room temperature for ~48 h, embedded in
paraffin and then cut into 4-µm thick sections for H&E
staining. The sections were stained with hematoxylin for 3 min and
eosin for 2 min at room temperature. At low magnification
(magnification, ×40; Fig. 2B),
H&E staining showed infiltrative growth into the striated
muscle and adipose tissue of the knee. At higher magnification
(magnification, ×100; Fig. 2C),
Tumor cells, arranged in bundle-like patterns with varying collagen
fibers and patchy collagenization, were fusiform, oval or
irregularly shaped, with lightly eosinophilic cytoplasm. In total,
<5/10 high-power fields contained giant tumor cells and bizarre
giant cells. Chronic inflammatory cell infiltration was observed in
the stroma, with no evident tumor necrosis. IHC was performed
overnight at 4°C using the following primary antibodies (prediluted
by the manufacturer; Guangzhou Aisha Biotechnology Co., Ltd.):
Vimentin (cat. no. IR630), smooth muscle actin (cat. no. IR611),
CD99 (cat. no. IR057), β-catenin (cat. no. IR702), Desmin (cat. no.
IR606), Ki67 (cat. no. IR626), anaplastic lymphoma kinase (ALK;
cat. no. IR641) and S100 (cat. no. IR504). For IHC, the tissues
were fixed in 4% formalin at room temperature for 48 h and
subsequently embedded in paraffin. The tissue was sectioned into
4-µm thick sections. The sections were incubated at 100°C for 20
min in a fully automatic immunohistochemistry instrument for
antigen repair (Roche CC1 immunohistochemistry antigen repair
buffer, High pH; cat. no. 5279801001; Guangzhou Aisha Biotechnology
Co., Ltd.). Endogenous peroxidase activity was quenched with 3%
hydrogen peroxide in methanol before incubation with primary
antibodies. The secondary antibody, obtained from EnVision FLEX/HRP
(prediluted by the manufacturer; cat. no. K4003; Agilent
Technologies, Inc.), was used to incubate sections at room
temperature for 12 min. Subsequently, an EnVision FLEX DAB+
Chromogen detection reagent was applied (cat. no. K5007; Agilent
Technologies, Inc.). The IHC staining results showed positivity for
Vimentin (Fig. 3A), α-smooth muscle
actin (SMA) (Fig. 3B), CD99
(Fig. 3C), β-catenin (few nuclei)
(Fig. 3D), Desmin (focal) (Fig. 3E) and Ki67 (15%) (Fig. 3F), but negative results for
anaplastic lymphoma kinase (Fig.
3G) and S100 (Fig. 3H). These
findings led to a diagnosis of LGMS.
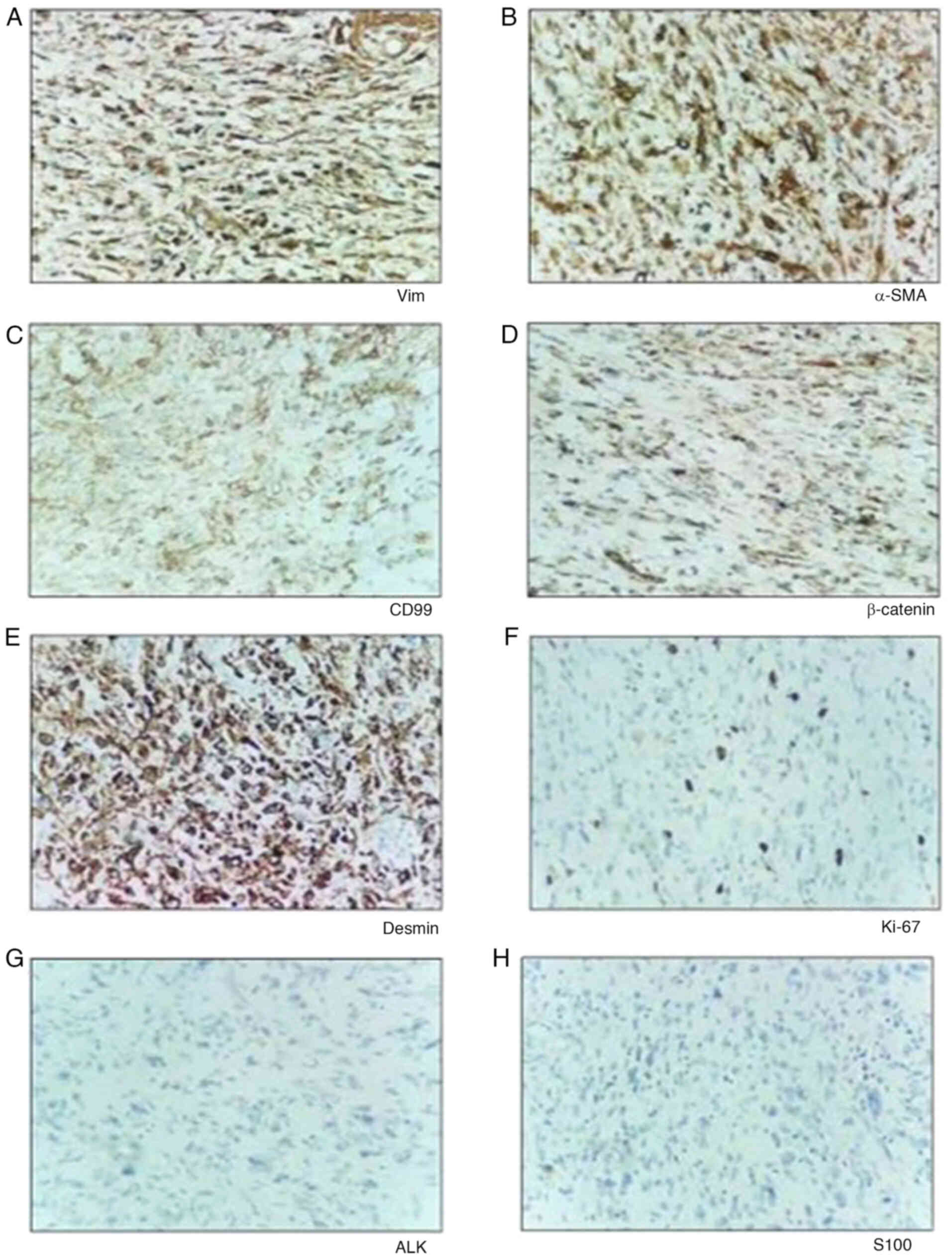 | Figure 3.IHC analysis of tissues. Examination
of the specimen, with IHC staining positive for (A) Vimentin, (B)
α-SMA, (C) CD99, (D) β-catenin, (E) Desmin and (F) Ki-67, and
negative results for (G) ALK and (H) S100, all at ×100
magnification. SMA, smooth muscle actin; ALK, anaplastic lymphoma
kinase; IHC, immunohistochemistry. |
The patient underwent regular post-surgery
evaluations every 3 months. After 1 year, no local recurrence or
distant metastasis was detected. Quality of life (QOL), assessed
using the EQ-5D-5L scale [EQ (visual analog scale) VAS] (7), improved over time, with scores of 80,
90, 94, 95 and 95 at the time of surgery and 3, 6, 9 and 12 months
post-surgery, respectively. The patient expressed satisfaction with
their post-surgery condition during the 1-year follow-up phone
call.
Discussion
Vasudev and Harris (8) initially introduced the concept of LGMS
in 1978, which was later verified by Mentzel et al in 1998
(9). In 2002, the World Health
Organization recognized LGMS as a distinct entity and classified it
under the fibroblast/myofibroblastic tumor category (10). Predominantly, LGMS occurs in the
head, neck and oral cavity regions, with the tongue being the most
commonly affected site (11). This
tumor is known for its tendency to recur locally, although distant
metastasis is rare. The diagnosis of LGMS in clinical practice is
challenging due to its asymptomatic nature, slow growth, absence of
distinctive biological features and limited diagnostic imaging
options, often leading to misidentification as a benign lesion
(12).
Wang et al (13) reported the clinical and radiographic
characteristics of LGMS in bone. Radiologically, LGMS presents as
extensive, infiltrative or worm-like bone destruction on X-rays and
computed tomography (CT) scans, characterized by poorly defined
lesion margins and cortical bone erosion. In the soft tissues, LGMS
manifests as slightly irregular masses of varying densities that
are poorly demarcated from adjacent tissues and lacking of specific
features. MRI findings include a uniform or high signal on
T1-weighted images and a uniform or uneven high signal on
T2-weighted images. Enhanced scans typically show uniform or uneven
signal enhancement. Pathological immunohistochemistry is crucial
for LGMS diagnosis, which is often considered a diagnosis of
exclusion (14). Microscopically,
the tumor cells are elongated or star-shaped, with blurred
cytoplasmic borders and mild acidophilia. The nuclei are elongated
or wavy, containing evenly distributed chromatin. Some nuclei may
appear slightly swollen, vacuolated and contain small nucleoli.
LGMS is characterized by its diffuse infiltrative growth pattern.
By contrast, low-grade malignant fibromyoblastoma features sparsely
arranged cells with spindle-shaped cytoplasm, indistinct borders
and mild acidophilia. The fusiform nuclei in these cells might
display vacuolization, small nucleoli and notches, or they may be
slender and wavy, resembling neural differentiation (15). Areas of collagen degeneration are
also observed in LGMS. The tumor is enriched with thin-walled
capillaries, and the presence of inflammatory cells such as
lymphocytes and plasma cells is not significant. The
immunophenotype of LGMS is varied, generally showing positivity for
at least one myogenic marker, such as Desmin, α-SMA, Vimentin or
Calponin (16). Usually, LGMS is an
atypical tumor consisting of myofibroblasts and often expresses
Vimentin. In the present case, the tumor was positive for Vimentin,
Desmin and α-SMA, while Calponin expression was not evident.
The differential diagnosis of LGMS includes
leiomyosarcoma, fibrosarcoma, fibromatosis and inflammatory
myofibroblastic tumors (IMTs) (17). Leiomyosarcoma is a malignant spindle
cell tumor that exhibits characteristics of smooth muscle; it is
often identified by the presence of fusiform cells arranged in
alternating fascicles. These cells have longitudinally fibrillary
cytoplasm and cigar-shaped vesicular nuclei with paranuclear
vacuolation. IHC tests for leiomyosarcoma usually yield positive
results for α-SMA, Desmin and h-caldesmon. Fibrosarcoma is a type
of neoplasm that consists of malignant spindle cells with
fibroblastic differentiation; it is characterized by a herringbone
fascicular architecture and spindle-shaped cells with elongated,
tapered nuclei and minimal cytoplasm. Unlike myofibroblasts,
fibrosarcoma cells do not show any myoid differentiation, as there
is no immunohistochemical evidence of fibronectin, SMA or calponin.
Fibromatosis is characterized by the presence of a prominent nodule
that has a tendency to invade nearby tissue. The tumor cells in
fibromatosis do not exhibit atypia or mitosis, and they only test
positive for vimentin. Similar to fibrosarcoma, fibromatosis cells
do not display myoid differentiation and do not express actin or
SMA (2,18). IMT has a more distinct border, and
under light microscopy, it exhibits a diverse cellular composition.
In addition to spindle-shaped cells with fibromyoblastic
characteristics, IMT may contain fibroblasts, histiocytes, plasma
cells, lymphocytes and eosinophils, showing mucinous, vascular and
inflammatory changes similar to nodular fasciitis. By contrast,
low-grade malignant fibromyoblastoma typically exhibits
infiltrative growth, predominantly consisting of fibromyoblast
cells, with infrequent infiltration of inflammatory cells (19).
In vivo molecular imaging currently offers
unique advantages in tumor diagnosis; it allows for high spatial
resolution at reduced costs, and the capacity to detect sensitive,
high-resolution light signals in deep tissues, which could aid in
the diagnosis and differentiation of LGMS (20). While LGMS often presents as a
slow-growing, painless mass, it is still classified as a low-grade
malignancy, prone to local recurrence and distant metastasis. To
the best of our knowledge, the current report presents the first
case of LGMS in the knee. The standard treatment for LGMS remains
as surgical resection, with some patients receiving postoperative
radiotherapy and chemotherapy, although the efficacy of these
treatments is debated (21). In the
present case, the mass was completely and widely excised with clear
margins. Patient QOL was assessed using the EQ-VAS on the day of
surgery and at 3, 6, 9 and 12 months post-surgery, with scores of
80, 90, 94, 95 and 95, respectively. At the 1-year follow-up, the
patient reported full recovery of the ROM, with no recurrence or
metastasis observed upon regular reexamination.
Peng et al (22) documented a case where a patient with
LGMS underwent two cycles of adjuvant chemotherapy post-surgery,
resulting in no recurrence over 5 years. Conversely, Maruyama et
al (23) observed a higher
recurrence rate of LGMS following postoperative radiotherapy
compared with surgery alone, suggesting radiotherapy should be
avoided post-surgery. However, Mamikunian et al (24) suggested that radiotherapy is less
likely to induce recurrence, advocating its use as adjuvant
therapy, particularly for malignant tumors with or without adverse
pathological features. Chemoradiotherapy, however, is not
recommended as a routine treatment for patients with
margin-negative LGMS and may be considered for those with positive
margins or recurrent disease (25).
In conclusion, LGMS, although relatively rare, tends
to exhibit infiltrative growth and a high likelihood of local
recurrence, while distant metastasis remains infrequent. The early
presentation of LGMS is often atypical and easily overlooked,
leading to frequent misdiagnoses in clinical settings and
subsequent delays in receiving appropriate treatment. In cases
where LGMS is suspected based on patient symptoms and imaging
results, biopsy and surgical excision are viable approaches. A
definitive diagnosis is typically established through a combination
of postoperative pathology and immunohistochemical analysis. In
instances where immunohistochemistry yields atypical results,
molecular diagnostic methods may be employed. The decision to use
adjuvant chemoradiotherapy post-surgery is influenced by several
factors, including the tumor's location, the patient's overall
health and the presence of metastases. Due to the risk of
recurrence and potential metastases, it is crucial to maintain
long-term follow-up with the patient. This vigilance helps in
timely detection and management of any recurrence, thus playing a
key role in the patient's ongoing care and treatment.
Acknowledgements
The authors would like to thank Dr Yuchao Xiong
(Department of Radiology, Guangzhou Red Cross Hospital) for
providing radiography consultations and Dr Bo Chen (Department of
Pathology, Guangzhou Red Cross Hospital) for pathology consultation
services.
Funding
This research was supported by the Frontier Technology Fund of
Guangzhou Red Cross Hospital (fund no. 01.00064–007).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
WW designed and conceived the study, and revised the
manuscript. TC was responsible for collecting clinical, imaging and
pathological data of the patient, and was responsible for the
conception, design, content and writing of the manuscript. SL
operated on the patients, provided the surgical details described
in the manuscript and was responsible for the conception, design,
content and revision of the manuscript. JZ contributed to the
writing of the manuscript, the conception of the study and the
collection of pathological images. TC, SL and WW confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided specific written informed
consent, which included the acquirement of clinical data and
pictures, in the form of an anonymous document for publication
purposes.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yonezawa H, Yamamoto N, Hayashi K,
Takeuchi A, Miwa S, Igarashi K, Langit MB, Kimura H, Shimozaki S,
Kato T, et al: Low-grade myofibroblastic sarcoma of the levator
scapulae muscle: A case report and literature review. BMC
Musculoskelet Disord. 21:8362020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Myong NH and Min JW: Low-grade
myofibroblastic sarcoma arising in fibroadenoma of the breast-A
case report. Diagn Pathol. 11:332016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schwerzmann MC, Dettmer MS, Baumhoer D,
Iizuka T and Suter VGA: A rare low-grade myofibroblastic sarcoma in
lower jaw with the resemblance to benign lesions. BMC Oral Health.
22:3802022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maruyama T, Nakasone T, Nimura F,
Matayoshi A, Kawano T, Nishihara K and Arasaki A: Indolent growth
of low-grade myofibroblastic sarcoma of the cheek mimics benign
lesions: A case report and literature review. Oncol Lett.
13:4307–4314. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim JH, Choi W, Cho HS, Lee KS, Park JK
and Kim BK: Surgical treatment and long-term outcomes of low-grade
myofibroblastic sarcoma: A single-center case series of 15
patients. World J Surg Oncol. 19:3392021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin Y, Gao X, Liu Z, Liu Z, Li Y, Liang R,
Liao Z and Ye J: Effective treatment of low-grade myofibroblastic
sarcoma with apatinib: A case report and literature review.
Pharmgenomics Pers Med. 15:573–582. 2022.PubMed/NCBI
|
|
7
|
Zhu J, Yan XX, Liu CC, Wang H, Wang L, Cao
SM, Liao XZ, Xi YF, Ji Y, Lei L, et al: Comparing EQ-5D-3L and
EQ-5D-5L performance in common cancers: Suggestions for instrument
choosing. Qual Life Res. 30:841–854. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vasudev KS and Harris M: A sarcoma of
myofibroblasts: An ultrastructural study. Arch Pathol Lab Med.
102:185–188. 1978.PubMed/NCBI
|
|
9
|
Mentzel T, Dry S, Katenkamp D and Fletcher
CD: Low-grade myofibroblastic sarcoma: Analysis of 18 cases in the
spectrum of myofibroblastic tumors. Am J Surg Pathol. 22:1228–1238.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gao G, Liu Y, Ao Y, Wang J and Xu Y:
Low-grade myofibroblastic sarcoma of the proximal femur: A case
report and literature review. Medicine (Baltimore). 101:e317152022.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mikami Y, Fujii S, Kohashi KI, Yamada Y,
Moriyama M, Kawano S, Nakamura S, Oda Y and Kiyoshima T: Low-grade
myofibroblastic sarcoma arising in the tip of the tongue with
intravascular invasion: A case report. Oncol Lett. 16:3889–3894.
2018.PubMed/NCBI
|
|
12
|
Niu R, Wang JF, Zhang DC, Shao XL, Qiu C
and Wang YT: Low-grade myofibroblastic sarcoma of gastric cardia on
18F-FDG positron emission tomography/computed tomography: An
extremely rare case report. Medicine (Baltimore). 97:e97202018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang L, Li LX, Chen DQ, Yang L, Li SK and
Cheng C: Low-grade Myofibroblastic sarcoma: Clinical and imaging
findings. BMC Med Imaging. 19:362019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jayasooriya PR, Athukorala C, Attygalla M,
Mendis BRRN and Lombardi T: Low-Grade myofibroblastic sarcoma of
the oral cavity: A report of three cases illustrating an emerging
disease in children. Dermatopathology (Basel). 8:1–9. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Taweevisit M and Thorner PS: Distinctive
features of low-grade myofibroblastic sarcoma on aspiration
cytology: A case report. Cytopathology. 29:578–581. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ghosh A, Bandopadhyay A and Sarkar R:
Low-grade myofibroblastic sarcoma of maxillary sinus and buccal
mucosa: Two rare cases and review of the literature. Indian J
Pathol Microbiol. 62:119–121. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mulay K, Sen M and Honavar SG: Limbal,
low-grade myofibroblastic sarcoma: Case report and literature
review. Indian J Ophthalmol. 68:2538–2540. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tang L, Xu H, Gao H, Yang H, Chen S and
Zhang P: Primary low-grade myofibroblastic sarcoma: A rare case
report of this tumor in the orbit and literature review. Eur J
Ophthalmol. Nov 16–2020.(Epub ahead of print).
|
|
19
|
Song W and Zhu Y: Clinical characteristics
and outcomes of 17 cases of inflammatory myofibroblastic tumor at a
University Hospital in China. Oncol Lett. 21:512020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang S, Ma Y, Ma T and Wang Z: Low-grade
myofibroblastic sarcoma of the orbit: A case report and literature
review. Medicine. 96:e91722017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kito M, Ae K, Okamoto M, Endo M, Ikuta K,
Takeuchi A, Yasuda N, Yasuda T, Imura Y, Morii T, et al: Clinical
outcome of Low-Grade myofibroblastic sarcoma in Japan: A
multicenter study from the Japanese musculoskeletal oncology group.
Cancers (Basel). 15:23142023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Peng L, Tu Y, Li Y and Xiao W: Low-grade
myofibroblastic sarcoma of the pancreas: A case report and
literature review. J Cancer Res Ther. 14 (Suppl):S796–S799. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Maruyama T, Nakasone T, Nimura F,
Matayoshi A, Kawano T, Nishihara K and Arasaki A: Indolent growth
of low-grade myofibroblastic sarcoma of the cheek mimics benign
lesions: A case report and literature review. Oncol Lett.
13:4307–4314. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mamikunian G, Ziegler A, Block A and
Thorpe E: Risk factors for recurrence and the role of radiotherapy
in Low-grade Myofibroblastic Sarcoma: A Systematic Review. Am J
Clin Oncol. 46:420–425. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu Y, Xu G, Wang X, Mao M, Wu H,
Baklaushev VP, Chekhonin VP, Peltzer K, Wang G and Zhang C: Is
there a role for chemotherapy and radiation in the treatment of
patients with low-grade myofibroblastic sarcoma? Clin Transl Oncol.
23:344–352. 2020. View Article : Google Scholar : PubMed/NCBI
|