Introduction
Renal cell carcinoma (RCC) is a common cancer in the
kidney that accounts for ~3% of all cancer cases worldwide
(1). Currently, surgery is the main
therapeutic approach for RCC; however, ~30% of patients with RCC
develop distant metastasis following surgery (2). Although cisplatin is an effective
therapeutic agent for certain types of cancer, RCC often exhibits
resistance to cisplatin, and chemotherapy regimens with cisplatin
alone are only effective in 4–6% of patients with RCC (3). Therefore, there is a need to improve
the understanding of the underlying reasons for RCC chemoresistance
and to identify new insights for RCC treatment.
Recent studies have reported that flavonoids have
the power to affect critical biological activities during
tumorigenesis (4–7). The naturally occurring flavonoid,
3,3′,4′,7-tetrahydroxyflavone, usually referred to as fisetin,
occurs in vegetables and fruits, including apples, persimmons,
kiwis, strawberries, grapes, onions and cucumbers. Fisetin displays
pharmacological properties that include anti-inflammatory and
antioxidant effects, and it has been reported to hinder the cell
cycle in HT-29 human colon cancer cells (8). It has also been reported to produce
anti-proliferative effects on prostate cancer (9). Moreover, fisetin suppresses tumor
cells by regulating genes involved in apoptosis (10). Reports have revealed that fisetin
can restrain the proliferation and metastasis of RCC cells by
upregulating MEK/ERK and can increase the expression of
5-hydroxymethylcytosine to impede the viability and migration of
RCC stem cells (11,12). In addition, the combination of
fisetin and cisplatin has been reported to notably increase
apoptosis of the A2780 ovarian cancer resistant cell line (13). Fisetin has also been reported to
reverse cisplatin resistance in lung adenocarcinoma cells (14). However, there is a lack of research
on the effect of fisetin on cisplatin resistance in RCC.
As serine/threonine kinases, the family of
cyclin-dependent kinases (CDKs) are catalytically active upon
binding to their respective regulatory subunits, cyclins, to
regulate several key cellular processes, including cell cycle
progression and transcription. However, when the kinase is
abnormally activated, disordered cell cycle regulation can lead to
uncontrolled cell proliferation, leading to development of cancer.
Thus, CDKs represent a potent target for inhibitory cancer drugs
(15). Among them, CDK6 has been
reported to target and regulate RCC in multiple studies (16–19).
Additionally, fisetin has been reported to regulate the cell cycle
and restrain the expression of CDKs in cancer cells (8), and influence several signaling
pathways, such as the MAPK and PI3K/Akt/mTOR signaling pathways
(20). Furthermore, fisetin has
been reported to promote autophagy by interfering with the mTOR
signaling pathway (21). Therefore,
we hypothesized that fisetin may also display antitumor effects in
RCC and facilitate cisplatin sensitivity through the
CDK6/PI3K/Akt/mTOR signaling pathway.
The present study determined the inhibitory role of
fisetin on the development of RCC by evaluating cell proliferation,
apoptosis and cell cycle arrest. Subsequently, the enhancement of
cisplatin sensitivity was assessed using fisetin and cisplatin
co-treatment in RCC cells.
Materials and methods
Cell culture
Human RCC Caki-1 (BFN60700344, ATCC, Shanghai) and
786-O (cat. no. BFN60700343, ATCC, Shanghai) cell lines, derived
from two Caucasian males with different stages of RCC, were
selected based on our previous research (11,22).
The cell lines were cultured in DMEM (Gibco; Thermo Fisher
Scientific, Inc.), supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin/streptomycin (Nanjing KeyGen
Biotech Co., Ltd.). The cells were maintained at 37°C in an
incubator (SANYO Electric Co., Ltd.) with 5% CO2.
Fisetin, with a purity of >98%, was obtained from Nanjing Best
Biotechnology Co., Ltd (cat. no. D50546; 500 mg). The
cisplatin-resistant cells were established by repeated subculturing
with gradual increase of Cisplatin(Best Biotechnology Co., Ltd.;
D50445-1 ml; 1, 2, 4, 6, 8 and 10 µM) over 6 months, and finally 10
µM cisplatin was used in the cisplatin and fisetin + cisplatin
treatment group.
Cell transfection
CDK6 cDNA was cloned into pcDNA3.0 (Invitrogen™;
Thermo Fisher Scientific, Inc.). A total of 1.5×105
cells/well were seeded into 24-well plates 24 h before plasmid
transfection. Transfection was performed using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) in accordance with the manufacturer's
instructions. Caki-1 cells and 786-O cells were transfected with
4.5 µg pcDNA3.1-CDK6 or pcDNA3.1-entry at 37°C for 48 h. An empty
vector (pcDNA3.1-entry) was used as the negative control. At 48 h
post-transfection, reverse transcription (RT)-quantitative (q)PCR
was used to detect the efficiency.
Cell Counting Kit-8 (CCK-8) assay
The CCK-8 assay kit (Beyotime Institute of
Biotechnology) was used to assess RCC cell proliferation. A total
of 5×103 cells/well (100 µl/well) were seeded in a
96-well plate and were routinely cultured for 24 h. Subsequently,
100 µl fisetin-containing medium was added to each well to adjust
the final concentration of fisetin to 20, 40 and 60 µM. These
concentrations were chosen based on previous studies (23,24).
After incubation for 0, 12, 24 and 48 h at 37°C, 10 µl CCK-8
solution was added to each well and maintained for 2 h at 37°C.
Finally, the optical density values were read at 450 nm.
Colony formation assay
RCC cells were placed at a concentration of 1,000
cells/well in a 6-well plate and cultured at 37°C. After 14 days,
RCC cells were washed and fixed in 4% paraformaldehyde, followed by
staining with leuco crystal violet (Wuhan Servicebio Technology
Co., Ltd.). After washing and drying, the number of colonies
>0.3 mm in diameter was counted by naked eye.
Cell cycle analysis
A total of 5×106 cells were collected and
washed with cold PBS, followed by overnight fixation with 70%
ethanol at 4°C. Cells were centrifuged at 300 × g for 10 min at
4°C, washed with cold PBS and then the supernatant was discarded.
RNase A (100 µl) was then added to resuspend the cells and
maintained at 37°C for 30 min. PI solution (400 µl) was added and
thoroughly mixed with the cells, and the mixture was placed in the
dark at 4°C for 30 min. Flow cytometry (Attune NxT flow cytometer,
Thermo Fisher. Scientific, Inc.; FlowJo V10.10.0, link: http://www.flowjo.com/) was then used to analyze cells
in different cell cycle phases.
Western blot analysis
RIPA buffer (Nanjing Best Biotechnology Co., Ltd.)
supplemented with 1% PMSF (Nanjing Best Biotechnology Co, Ltd.) was
used to isolate total proteins from RCC cells. The concentration of
proteins was detected using the BCA kit (Beyotime Institute of
Biotechnology), and then SDS-PAGE was used to separate the proteins
(50 micrograms per lane. Due to the wide range of molecular weights
of proteins involved in this study, the gel concentrations were
divided into the following: 6% for more than 200 kDa; 8% for
100–200 kDa; 10% for 40–60 kDa; 12% was used for 20 to 40 kDa),
which were transferred to PVDF membranes (MilliporeSigma). Blocking
was performed using 5% skim milk powder at room temperature for 1
h. Overnight at 4°C, the membranes were incubated with primary
antibodies against cyclin B1 (4138, Cell Signaling Technology,
Inc.; dilution: 1:1,000), p21 (2947, Cell Signaling Technology,
Inc.; 1:1,000), p27 (3686, Cell Signaling Technology, Inc.;
dilution: 1:1,000), Bax (41162; Cell Signaling Technology, Inc.;
dilution: 1:1,000), Bcl2 (sc-7382, Santa Cruz Biotechnology, Inc.;
dilution: 1:800), Cleaved caspase −3 (9664, Cell Signaling
Technology, Inc.; 1:1,000), Cleaved caspase −9 (20750, Cell
Signaling Technology, Inc.; dilution: 1:1,000), CDK6 (sc-7961,
Santa Cruz Biotechnology, Inc.; dilution: 1:800), phosphorylated
(p)-Akt (sc-377556, Santa Cruz Biotechnology, Inc.; 1:800), Akt
(sc-5298, Santa Cruz Biotechnology, Inc.; dilution: 1:800), p-PI3K
(ab278545, Abcam; dilution: 1:200), PI3K (sc-365290, Santa Cruz
Biotechnology, Inc.; dilution: 1:800), p-mTOR (sc-293133, Santa
Cruz Biotechnology, Inc.; 1:800), mTOR (sc-517464; Santa Cruz
Biotechnology, Inc.; dilution: 1:800) and β-actin (sc-81178, Santa
Cruz Biotechnology, Inc.; dilution: 1:800). After three washes with
TBST (2% Tween), membranes were hybridized with HRP-conjugated
secondary antibodies (ab131368, ab99697, ab190369; all Abcam;
dilution: 1:1,000) for 90 min at room temperature. Finally, protein
bands were detected using the ECL Assay Kit (Shanghai Yeasen
Biotechnology Co., Ltd.). Semi-quantitative analysis of WB bands
was performed using ImageJ (V 1.8.0, link: http://imagej.net/software/imagej/).
Cell apoptosis measurement
RCC cell apoptosis was assessed by applying the
Annexin V-FITC kit (BioLegend, Inc.). Briefly, 1×105
cells were suspended in 500 µl binding buffer, then 5 µl Annexin
V-FITC was added and incubated for 10 min at 4°C. Subsequently, 5
µl PI solution was added and incubated for another 15 min at 25°C.
Flow cytometry (Attune NxT flow cytometer, Thermo Fisher.
Scientific, Inc.; FlowJo V10.10.0) was then performed to count the
number of apoptotic cells.
RT-qPCR
A total of 1×107 cells were lysed in 1 ml
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.) and
mixed with 0.2 ml chloroform. Subsequently, 0.5 ml isopropanol was
added to the supernatant after centrifugation at 12,000 × g for 8
min at 4°C. The mixture was centrifuged at 12,000 × g for 10 min at
4°C. The precipitate was collected, washed with 75% ethanol and
dissolved in diethyl pyrocarbonate water. cDNA was then synthesized
using the 1st Strand cDNA Synthesis Kit (Shanghai Yeasen
Biotechnology Co., Ltd.) according to the manufacturer's protocol.
The cDNA levels were then detected using the Applied
Biosystems® 7500 (Thermo Fisher Scientific, Inc.) with
the qPCR SYBR Green Master Mix (Shanghai Yeasen Biotechnology Co.,
Ltd.). Pre-denaturation was performed at 95°C for 30 sec.
Denaturation was performed at 95°C for 10 sec and annealing at 60°C
for 30 sec for 40 cycles. GAPDH was used as an internal reference.
The primers were as follows: CDK6, forward (F)
5′-CGACTGACACTCGCAGCC-3′ and reverse (R)
5′-AGTCCAGAATCATTGCACCTGAG-3′ and GAPDH, F
5′-TCATTTCCTGGTATGACAACGA-3′ and R 5′-GGTCTTACTCCTTGGAGGC-3′. Gene
expression was calculated using GADPH and the 2−ΔΔCq
method (25).
Statistical analysis
All of the data in the present study were analyzed
using GraphPad 8(Dotmatics) and are presented as the mean ±
standard deviation. To compare groups, one-way ANOVA or the
Student's unpaired t test were used. Tukey's HSD (Honestly
Significant Difference) test was used for post hoc testing.
P<0.05 was considered to indicate a statistically significant
difference. Each individual experiment was performed in
triplicate.
Results
Fisetin inhibits the proliferation of
RCC cells and promotes apoptosis
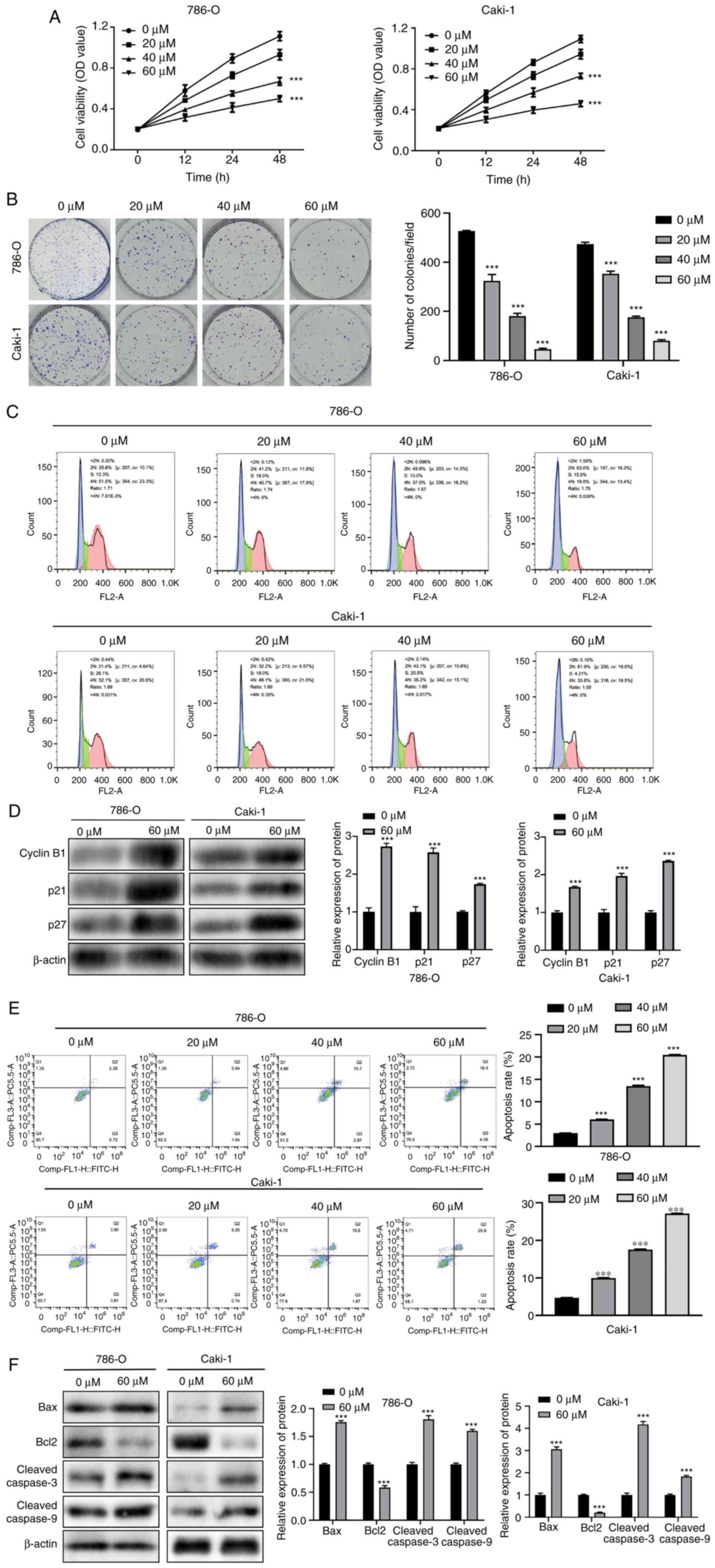
The effects of fisetin on the development of RCC
cells were assessed using different concentrations of fisetin.
First, the CCK-8 assay was used to evaluate the viability of the
786-O and Caki-1 cells. The results revealed that fisetin
significantly inhibited the proliferation of 786-O and Caki-1 cells
in a dose-dependent manner (Fig.
1A). Furthermore, tumor colony formation was significantly
inhibited by fisetin treatment, and the strongest inhibitory
effects were achieved in response to 60 µM fisetin (Fig. 1B). Moreover, with an increase in
fisetin concentration, the cells exhibited a G1 phase
block, indicating that fisetin could deter the proliferation of
786-O and Caki-1 cells and the proliferation inhibition was most
marked in response to 60 µM fisetin (Fig. 1C). The expression levels of cell
cycle-related proteins cyclin B1, p21 and p27 were significantly
increased in the fisetin treatment group compared with those in the
control group (Fig. 1D), with the
cell cycle arrest in the G2/M phase. Apoptosis was also
observed in the 786-O and Caki-1 cells. The findings revealed that
fisetin significantly increased the proportion of apoptotic cells
and promoted RCC cell apoptosis, in comparison with non-treated
cells (Fig. 1E). Furthermore, the
expression levels of the apoptotic activators Bax and
cleaved-caspase 3/9 were significantly increased, whereas the
expression levels of the apoptotic inhibitor Bcl2 were
significantly decreased after fisetin treatment compared with those
in the control group (Fig. 1F).
These findings indicated that fisetin impeded RCC cell
proliferation and enhanced apoptosis. Additionally, the effects of
fisetin on RCC cell development were dose-dependent.
Fisetin enhances cisplatin sensitivity
in RCC cells
The role of fisetin in cisplatin drug resistance was
assessed and it was demonstrated that although fisetin or cisplatin
treatment alone could inhibit 786-O and Caki-1 cell proliferation,
the combination of these two drugs displayed significantly stronger
anti-proliferative effects, in comparison with the control
(Fig. 2A). Therefore, it was
hypothesized that fisetin may enhance cisplatin sensitivity in RCC
cells. To evaluate this hypothesis, the ability of the cells to
form tumorspheres after combination treatment was assessed. The
results demonstrated that the number and size of tumorspheres were
markedly decreased when cells were treated with cisplatin
supplemented with fisetin (Fig.
2B). Furthermore, an augmented inhibitory effect was also
demonstrated in the cell cycle of the two RCC cell lines, as shown
in Fig. 2C, where the proportion of
cells in the G1 phase notably increased in the cisplatin
+ fisetin combination treatment group compared with cisplatin
treatment group. The expression levels of cycle-related proteins
cyclin B1, p21 and p27 were also significantly increased when
treated with this drug combination compared with cisplatin
treatment group (Fig. 2D), inducing
the cell cycle arrest in the G2/M phase. Fisetin also
significantly enhanced the inhibitory impacts of cisplatin on 786-O
and Caki-1 cell apoptosis; the proportion of apoptotic cells was
significantly higher in the group treated with cisplatin combined
with fisetin compared with that treated with cisplatin or fisetin
alone (Fig. 2E), as were the
expression levels of apoptosis activators, Bax and cleaved-caspase
3/9 (Fig. 2F). Conversely, the
expression levels of the apoptotic inhibitor Bcl2 were
significantly reduced when cisplatin was combined with fisetin
compared with cisplatin (Fig. 2F).
Fisetin and cisplatin in combination enhanced the chemosensitivity
of RCC cells and thus demonstrated marked antitumor effects on the
development of RCC cells.
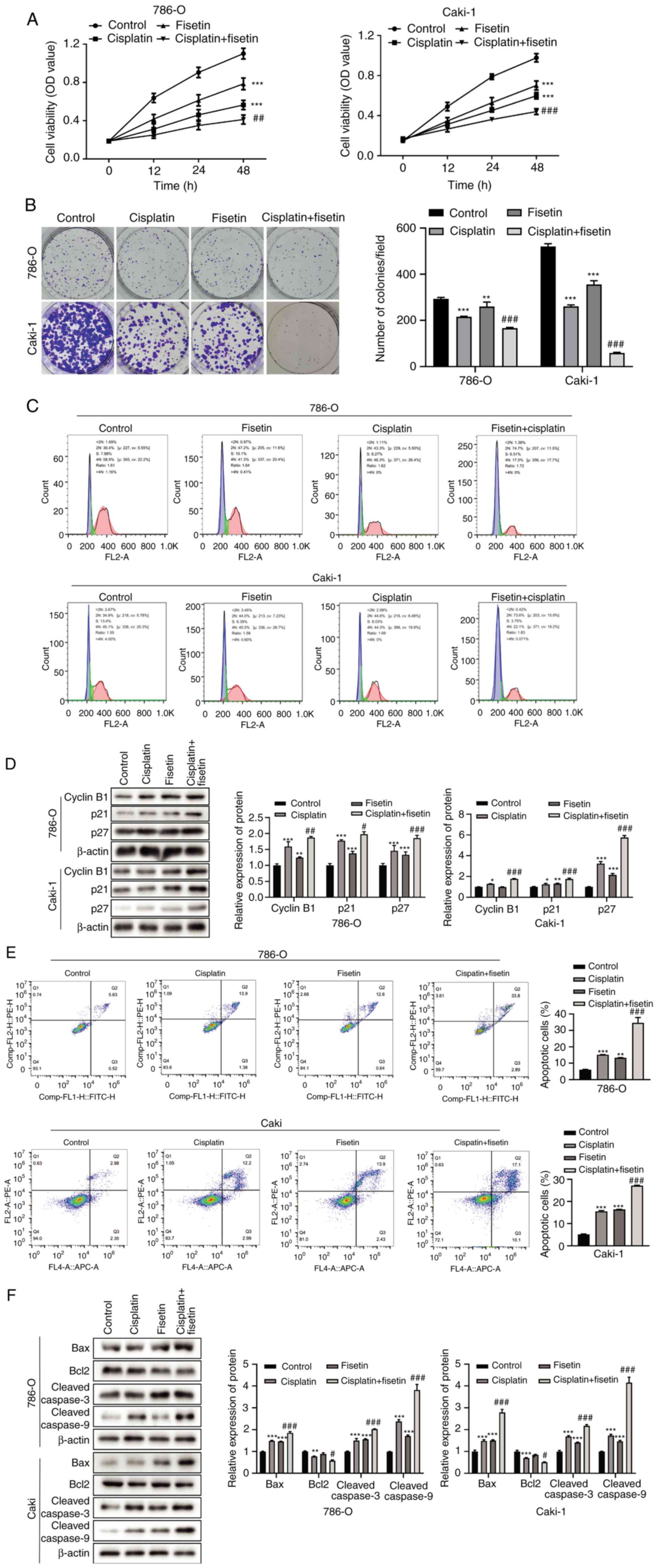 | Figure 2.Fisetin enhances cisplatin
sensitivity in 786-O and Caki-1 RCC cells. (A) Effects of fisetin
and cisplatin combination treatment on RCC cell proliferation.
Fisetin or cisplatin alone significantly inhibited cell
proliferation; however, the combination of these two drugs
demonstrated a stronger inhibitory effect. (B) Colony formation
assays after fisetin and cisplatin combination treatment.
Combination therapy demonstrated marked proliferation suppression
compared with cisplatin or fisetin alone treatment. (C) Effects of
fisetin and cisplatin alone, compared with the combination, on the
cell cycle progression of 786-O and Caki-1 cells. Fisetin and
cisplatin in combination notably increased the proportion of cells
in G1 phase and decreased that in G2/M phase.
(D) Change in the expression levels of cell cycle-related proteins
when fisetin and cisplatin were used in combination. The expression
levels of cyclin B1, p21 and p27 exhibited the greatest increase
following fisetin + cisplatin treatment. (E) Effects of fisetin and
cisplatin alone and combined on apoptosis. Combination treatment
resulted in the greatest increase in the proportion of apoptotic
786-O and Caki-1 cells. (F) Effects of combined fisetin and
cisplatin treatment on apoptosis-associated protein expression.
Fisetin + cisplatin upregulated Bax, cleaved-caspase 3/9, and
downregulated Bcl2 to a larger extent than fisetin or cisplatin
alone. RCC, renal cell carcinoma; OD, optical density. *P<0.05,
**P<0.01, ***P<0.001, vs. Control; #P<0.05,
##P<0.01, ###P<0.001, vs.
cisplatin. |
Fisetin enhances cisplatin sensitivity
through CDK6 in RCC cells
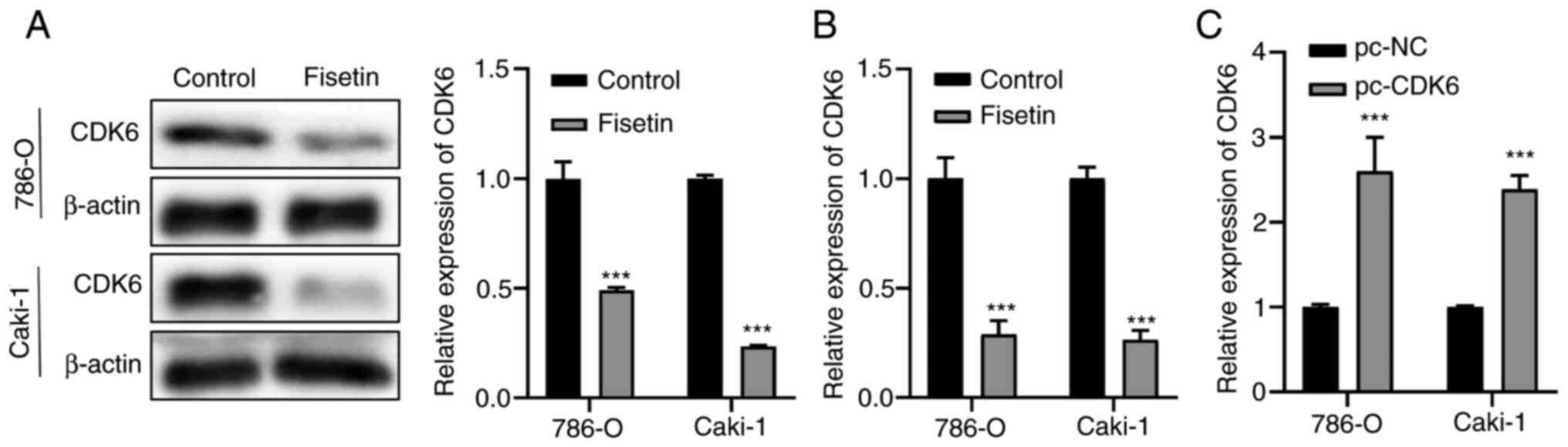
To elucidate the mechanisms underlying the effects
of fisetin, the targets of fisetin were assessed and it was
demonstrated that the protein expression levels of CDK6 were
significantly decreased after treating 786-O and Caki-1 cells with
fisetin compared with those in the control group (Fig. 3A). Subsequently, the mRNA expression
levels of CDK6 were assessed using RT-qPCR and the results
demonstrated that the mRNA expression levels of CDK6 were also
significantly decreased by fisetin treatment compared with those in
the control group (Fig. 3B).
Therefore, we hypothesized that fisetin enhanced the sensitivity of
cisplatin to RCC cells via the inhibition of CDK6 expression.
RT-qPCR results demonstrated that the expression levels of CDK6 in
both cell lines were significantly increased post-transfection with
pcDNA3.1-CDK6 compared with those in the pc-negative control cells
(Fig. 3C).
Fisetin enhances cisplatin sensitivity
in RCC cells via the PI3K/Akt/mTOR signaling pathway
To assess our hypothesis, CDK6 was overexpressed in
786-O and Caki-1 cells. The findings revealed that the expression
levels of p-Akt, p-PI3K and p-mTOR were significantly reduced when
cells were treated with cisplatin and fisetin compared with
cisplatin but were significantly increased after CDK6
overexpression (Fig. 4A). These
results suggested that fisetin may inhibit the PI3K/Akt signaling
pathway by targeting CDK6, thereby enhancing the sensitivity of RCC
cells to cisplatin. Treatment with fisetin combined with cisplatin
significantly inhibited cell proliferation, whereas overexpression
of CDK6 partially reversed the proliferation inhibition caused by
fisetin (Fig. 4B). Moreover,
fisetin and cisplatin treatment significantly reduced tumor colony
formation compared with control and CDK6 overexpression
significantly undermined the inhibitory effect caused by fisetin
compared with cisplatin + fisetin + pc-NC group (Fig. 4C). In addition, combination
treatment notably arrested the cell cycle in the G2 phase for a
longer time and could not normally enter the M phase, whereas
overexpression of CDK6 counteracted the effects of cisplatin and
fisetin combination (Fig. 4D).
Treatment with fisetin and cisplatin also significantly enhanced
the expression levels of cyclin B1, p21 and p27 compared with
control group, and overexpression of CDK6 significantly decreased
the expression levels of these three proteins compared with
cisplatin + fisetin + pc-NC group (Fig.
4E), decreasing the proportion of cells in G2/M
phase. Furthermore, the cisplatin + fisetin treatment group
demonstrated a significant increase in apoptosis compared with
control group, whereas overexpression of CDK6 significantly reduced
the apoptosis-inducing effect caused by this drug combination
compared with cisplatin + fisetin + pc-NC group (Fig. 4F). Cisplatin + fisetin treatment
also significantly increased the expression levels of the
proapoptotic proteins Bax and cleaved-caspase 3/9, and it
significantly reduced the expression levels of the anti-apoptotic
protein Bcl2 compared with control group, whereas overexpression of
CDK6 partially reversed these effects compared with cisplatin +
fisetin + pc-NC group (Fig.
4G).
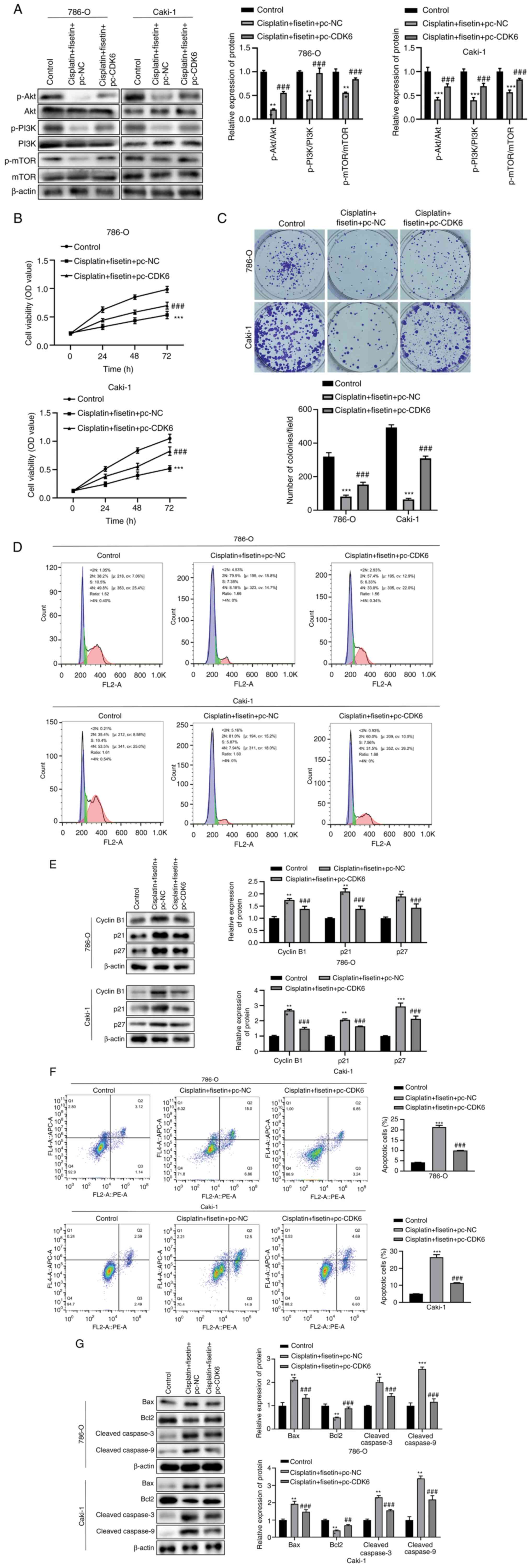 | Figure 4.Fisetin enhances cisplatin
sensitivity in RCC cells via the PI3K/Akt/mTOR signaling pathway to
inhibit cell proliferation, delay the cell cycle and promote
apoptosis. (A) Expression levels of key proteins in the
PI3K/Akt/mTOR signaling pathway assessed following fisetin and
cisplatin treatment. Fisetin and cisplatin significantly decreased
the protein expression levels of p-Akt, p-PI3K and p-mTOR, but CDK6
overexpression increased the levels of p-Akt, p-PI3K and p-mTOR.
(B) RCC cell proliferation after CDK6 overexpression. Fisetin and
cisplatin reduced 786-O and Caki-1 cell proliferation, whereas the
effects were undermined by CDK6 overexpression. (C) Number of
colonies formed significantly increased after CDK6 overexpression.
Fisetin + cisplatin treatment inhibited RCC cell-forming colonies,
whereas CDK6 overexpression demonstrated the opposite effect. (D)
Number of cells remaining in G1 phase notably increased,
whereas they decreased in G2/M phase after the
combination of fisetin and cisplatin treatment. CDK6 overexpression
counteracted effects of fisetin and cisplatin. (E) Overexpression
of CDK6 neutralized the effects of fisetin and cisplatin on the
expression levels of cell cycle-associated proteins. The expression
levels of cyclin B1, p21 and p27 were significantly reduced after
CDK6 overexpression. (F) Proportion of apoptotic RCC cells notably
reduced after CDK6 overexpression, which undermined the
proapoptotic role of fisetin and cisplatin. (G) Overexpression of
CDK6 neutralized the effects of fisetin and cisplatin on the
expression levels of apoptosis-associated proteins. CDK6
significantly downregulated Bax, cleaved-caspase 3 and
cleaved-caspase 9 in both 786-O cells and Caki-1 cells, whereas the
expression levels of Bcl2 were significantly increased after CDK6
overexpression. RCC, renal cell carcinoma; pc, pcDNA; NC, negative
control; OD, optical density; p, phosphorylated. *P<0.05,
**P<0.01, ***P<0.001, vs. Control; ##P<0.01,
###P<0.001, vs. cisplatin + fisetin + pc-NC. |
Discussion
RCC is a common kidney neoplasm that ranks second in
urinary tumor mortality, accounting for >90% of kidney cancer
cases worldwide (26). Chemotherapy
is widely regarded as the most effective treatment method for RCC
because of its simplicity and fast reaction (27,28).
Nevertheless, cancer cells can lack sensitivity to chemotherapy and
the gradual development of drug resistance has presented challenges
for RCC treatment. As a result, understanding the underlying
mechanisms of drug resistance is essential in developing effective
treatment regimens.
Cisplatin is a highly effective chemotherapy drug.
Even though cisplatin has been demonstrated to be effective in the
treatment of numerous malignancies (29,30),
RCC is insensitive to cisplatin therapy (22), as shown by the low response rate to
cisplatin alone. Therefore, more research into the mechanism of
cisplatin resistance is required.
Fisetin is a naturally occurring flavonoid (31) with antioxidant, antidiabetic,
anti-inflammatory (32), anticancer
(21,33) and neuroprotective properties
(34). Fisetin promotes the
apoptosis of several cancer cell types by constraining COX-2
(35), impeding the Wnt/EGFR
signaling pathway (36),
strengthening caspase-3 cascade reactions, boosting the caspase-3/8
dependent pathway, and amplifying the activity of Ca2+
and caspase-3-dependent endonuclease (37). Fisetin has been shown to serve a
role in RCC development. Fisetin has been reported to reduce A-498,
ACHN and 786-O cell proliferation in a concentration-dependent
manner, and to induce the cell cycle to arrest in the
G2/M phase (33).
Moreover, fisetin restrains RCC cell metastasis by suppressing a
disintegrin and a metalloprotease 9, cathepsin S and cathepsin B,
and increasing the expression of activated ERK (11). However, it remains unknown as to
whether fisetin can influence cisplatin resistance in RCC
cells.
The findings from the present study indicated that
fisetin caused a reduction in the proliferation of RCC cell lines
in a concentration-dependent manner. Fisetin also restrained RCC
cell proliferation by inducing cell cycle arrest. Following fisetin
treatment, the proportion of cells in the G1 phase
increased, whereas the G2/M fraction decreased detected
by flow cytometry and WB. The expression levels of cyclin B1, p21
and p27 were also increased when cells were treated with fisetin.
Apoptotic induction was also observed in RCC cells treated with
fisetin, and this process was concentration-dependent. Fisetin
upregulated the expression levels of Bax and cleaved-caspase 3/9,
whereas the expression levels of Bcl2 were decreased. Furthermore,
fisetin and cisplatin in combination enhanced the antitumor
effects. Fisetin combined with cisplatin demonstrated a greater
induction of proliferation inhibition and apoptosis in RCC cells,
coupled with a notable rise in the expression levels of cyclin B1,
p21 and p27, and the activation of caspase-3 and −9. Furthermore,
it was demonstrated that the expression levels of CDK6 were
inhibited when RCC cells were exposed to fisetin. Moreover, fisetin
increased the expression levels of p-PI3K, p-Akt and p-mTOR,
thereby upregulating the activity of the PI3K/Akt/mTOR signaling
pathway. Overexpression of CDK6 was shown to undermine the effects
of fisetin on cisplatin sensitivity in RCC cells and to counteract
the antitumor effects of the fisetin-cisplatin combination. Taken
together, the results of the present study demonstrated that
fisetin enhanced cisplatin sensitivity through the
CDK6/PI3K/Akt/mTOR signaling pathway in RCC. The findings provide
insight into overcoming chemotherapy resistance in RCC and
highlight that combination therapy may be a promising strategy for
RCC treatment in the future.
Nevertheless, certain limitations exist in the
present work. The efficiency of fisetin and cisplatin combination
therapy in the treatment of tumor-bearing mice has not been defined
yet. Whether this combination regimen has the potential to be used
clinically and the response rate among patients with RCC need to be
further assessed. As for the molecular mechanism, whether fisetin
affects CDK6 expression through direct binding or indirect
regulation should be explored in future work. In addition, the
present study demonstrated that the PI3K signaling pathway may be
involved in the antitumor effect of fisetin, and whether other
tumor-related signaling pathways also affect fisetin-mediated
cisplatin sensitivity should be the subject of follow-up studies
with animal experiments performed to verify the effectiveness
demonstrated in vitro.
In conclusion, increasing concentrations of fisetin
inhibited RCC cell proliferation, and promoted cell cycle arrest
and cell apoptosis. Moreover, fisetin reduced cisplatin resistance
and increased its ability to kill RCC cells. Finally, fisetin and
cisplatin in combination demonstrated greater antitumor effects
than fisetin and cisplatin alone via regulation of the
CDK6/PI3K/Akt/mTOR signaling pathway.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
TJ, YL, YJ and YX discussed the concept of the
paper, investigated the background of the paper, performed the data
analysis and confirm the authenticity of all the raw data. TJ, YL
and YJ wrote the manuscript, and YX revised and improved the
manuscript. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jonasch E, Gao J and Rathmell WK: Renal
cell carcinoma. BMJ. 349:g47972014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Van Poppel H, Becker F, Cadeddu JA, Gill
IS, Janetschek G, Jewett MA, Laguna MP, Marberger M, Montorsi F,
Polascik TJ, et al: Treatment of localised renal cell carcinoma.
Eur Urol. 60:662–672. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tanji N and Yokoyama M: Treatment of
metastatic renal cell carcinoma and renal pelvic cancer. Clin Exp
Nephrol. 15:331–338. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sousa C, Duarte D, Silva-Lima B and
Videira M: Repurposing natural dietary flavonoids in the modulation
of cancer tumorigenesis: Decrypting the molecular targets of
naringenin, hesperetin and myricetin. Nutr Cancer. 74:1188–1202.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Manisha DS, Ratheesh AK, Benny S and
Presanna AT: Heterocyclic and non-heterocyclic arena of
monocarboxylate transporter inhibitors to battle tumorigenesis.
Chem Biol Drug Des. 102:1604–1617. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Duan N, Hu X, Zhou R, Li Y, Wu W and Liu
N: A Review on Dietary Flavonoids as Modulators of the Tumor
Microenvironment. Mol Nutr Food Res. 67:e22004352023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Felice MR, Maugeri A, De Sarro G, Navarra
M and Barreca D: Molecular pathways involved in the anti-cancer
activity of flavonols: A focus on myricetin and kaempferol. Int J
Mol Sci. 23:44112022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lu X, Jung Ji, Cho HJ, Lim DY, Lee HS,
Chun HS, Kwon DY and Park JH: Fisetin inhibits the activities of
cyclin-dependent kinases leading to cell cycle arrest in HT-29
human colon cancer cells. J Nutr. 135:2884–2890. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Haddad AQ, Venkateswaran V, Viswanathan L,
Teahan SJ, Fleshner NE and Klotz LH: Novel antiproliferative
flavonoids induce cell cycle arrest in human prostate cancer cell
lines. Prostate Cancer Prostatic Dis. 9:68–76. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kashyap D, Garg VK, Tuli HS, Yerer MB, Sak
K, Sharma AK, Kumar M, Aggarwal V and Sandhu SS: Fisetin and
quercetin: Promising flavonoids with chemopreventive potential.
Biomolecules. 9:1742019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hsieh MH, Tsai JP, Yang SF, Chiou HL, Lin
CL, Hsieh YH and Chang HR: Fisetin suppresses the proliferation and
metastasis of renal cell carcinoma through Upregulation of
MEK/ERK-Targeting CTSS and ADAM9. Cells. 8:9682019. View Article : Google Scholar
|
|
12
|
Si Y, Liu J, Shen H, Zhang C, Wu Y, Huang
Y, Gong Z, Xue J and Liu T: Fisetin decreases TET1 activity and
CCNY/CDK16 promoter 5hmC levels to inhibit the proliferation and
invasion of renal cancer stem cell. J Cell Mol Med. 23:1095–1105.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jafarzadeh S, Baharara J and Tehranipour
M: Apoptosis induction with combined use of cisplatin and fisetin
in cisplatin-resistant ovarian cancer cells (A2780). Avicenna J Med
Biotechnol. 13:176–182. 2021.PubMed/NCBI
|
|
14
|
Zhuo W, Zhang L, Zhu Y, Zhu B and Chen Z:
Fisetin, a dietary bioflavonoid, reverses acquired
Cisplatin-resistance of lung adenocarcinoma cells through
MAPK/Survivin/Caspase pathway. Am J Transl Res. 7:2045–2052.
2015.PubMed/NCBI
|
|
15
|
Nebenfuehr S, Kollmann K and Sexl V: The
role of CDK6 in cancer. Int J Cancer. 147:2988–2995. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiang J, Han P, Qian J, Zhang S, Wang S,
Cao Q and Shao P: Knockdown of ALPK2 blocks development and
progression of renal cell carcinoma. Exp Cell Res. 392:1120292020.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guo Z, Jia H and Ge J: MiR-206 suppresses
proliferation and epithelial-mesenchymal transition of renal cell
carcinoma by inhibiting CDK6 expression. Hum Cell. 33:750–758.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guo L, Wang D and Zhang Z: MiR-384
represses tumorigenesis by regulating CDK6 and predicts prognosis
of clear cell renal cell carcinoma. J BUON. 23:787–794.
2018.PubMed/NCBI
|
|
19
|
Pan H, Hong Y, Yu B, Li L and Zhang X:
miR-4429 Inhibits Tumor Progression and Epithelial-Mesenchymal
Transition Via Targeting CDK6 in Clear Cell Renal Cell Carcinoma.
Cancer Biother Radiopharm. 34:334–341. 2019.PubMed/NCBI
|
|
20
|
Sundarraj K, Raghunath A, Panneerselvam L
and Perumal E: Fisetin inhibits autophagy in HepG2 Cells via
PI3K/Akt/mTOR and AMPK Pathway. Nutr Cancer. 73:2502–2514. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Farooqi AA, Naureen H, Zahid R, Youssef L,
Attar R and Xu B: Cancer chemopreventive role of fisetin:
Regulation of cell signaling pathways in different cancers.
Pharmacol Res. 172:1057842021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Y, Fang L, Zang Y, Ren J and Xu Z:
CIP2A promotes proliferation, invasion and chemoresistance to
cisplatin in renal cell carcinoma. J Cancer. 9:4029–4038. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Seo SH and Jeong GS: Fisetin inhibits
TNF-α-induced inflammatory action and hydrogen peroxide-induced
oxidative damage in human keratinocyte HaCaT cells through
PI3K/AKT/Nrf-2-mediated heme oxygenase-1 expression. Int
Immunopharmacol. 29:246–253. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chang PR, Liou JW, Chen PY, Gao WY, Wu CL,
Wu MJ and Yen JH: The neuroprotective effects of flavonoid fisetin
against corticosterone-induced cell death through modulation of
ERK, p38, and PI3K/Akt/FOXO3a-dependent pathways in PC12 cells.
Pharmaceutics. 15:23762023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lloyd A, Reeves F, Abu-Ghanem Y and
Challacombe B: Metastasectomy in renal cell carcinoma: Where are we
now? Curr Opin Urol. 32:627–633. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cayetano-Salazar L, Nava-Tapia DA,
Astudillo-Justo KD, Arizmendi-Izazaga A, Sotelo-Leyva C,
Herrera-Martinez M, Villegas-Comonfort S and Navarro-Tito N:
Flavonoids as regulators of TIMPs expression in cancer:
Consequences, opportunities, and challenges. Life Sci.
308:1209322022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Alhaj-Suliman SO, Wafa EI and Salem AK:
Engineering nanosystems to overcome barriers to cancer diagnosis
and treatment. Adv Drug Deliv Rev. 189:1144822022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jafarzadeh E, Montazeri V, Aliebrahimi S,
Sezavar AH, Ghahremani MH and Ostad SN: Combined regimens of
cisplatin and metformin in cancer therapy: A systematic review and
meta-analysis. Life Sci. 304:1206802022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Han Y, Wen P, Li J and Kataoka K: Targeted
nanomedicine in cisplatin-based cancer therapeutics. J Control
Release. 345:709–720. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ravula AR, Teegala SB, Kalakotla S,
Pasangulapati JP, Perumal V and Boyina HK: Fisetin, potential
flavonoid with multifarious targets for treating neurological
disorders: An updated review. Eur J Pharmacol. 910:1744922021.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Simunkova M, Alwasel SH, Alhazza IM,
Jomova K, Kollar V, Rusko M and Valko M: Management of oxidative
stress and other pathologies in Alzheimer's disease. Arch Toxicol.
93:2491–2513. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kubina R, Krzykawski K, Kabała-Dzik A,
Wojtyczka RD, Chodurek E and Dziedzic A: Fisetin, a potent
anticancer flavonol exhibiting cytotoxic activity against
neoplastic malignant cells and cancerous conditions: A scoping,
comprehensive review. Nutrients. 14:26042022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Maher P: Preventing and treating
neurological disorders with the flavonol fisetin. Brain Plast.
6:155–166. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang J and Huang S: Fisetin inhibits the
growth and migration in the A549 human lung cancer cell line via
the ERK1/2 pathway. Exp Ther Med. 15:2667–2673. 2018.PubMed/NCBI
|
|
36
|
Ho CS, Grange RW and Joho RH: Pleiotropic
effects of a disrupted K+ channel gene: Reduced body weight,
impaired motor skill and muscle contraction, but no seizures. Proc
Natl Acad Sci USA. 94:1533–1538. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ying TH, Yang SF, Tsai SJ, Hsieh SC, Huang
YC, Bau DT and Hsieh YH: Fisetin induces apoptosis in human
cervical cancer HeLa cells through ERK1/2-mediated activation of
caspase-8-/caspase-3-dependent pathway. Arch Toxicol. 86:263–273.
2012. View Article : Google Scholar : PubMed/NCBI
|