Introduction
Lung cancer is the leading cause of cancer-related
death worldwide. Non-small cell lung cancer (NSCLC), including
adenocarcinoma, squamous cell carcinoma and large cell carcinoma,
comprises ~85% of all lung cancer cases (1,2).
Advances in recent decades have led to the identification of
numerous oncogenic factors in NSCLC, such as gene mutations in
EGFR, BRAF, ROS proto-oncogene 1, p16INK4a
and human epidermal growth factor receptor 2, and rearrangements in
anaplastic lymphoma kinase and RET (3–6)
Although target therapies and immunotherapies have enhanced the
overall survival time of patients, the occurrence of metastasis and
resistance (primary or acquired) may cause difficulties for
effective second-line treatment options. This has resulted in an
urgent unmet need for NSCLC treatment (7).
Vasculogenic mimicry (VM), a new blood supply
network with abundant extracellular matrix (ECM), is the formation
of microvascular channels by aggressive and metastatic tumor cells
(8). VM is suggested to be
responsible for tumor metastasis and poor prognosis in patients
with cancer, such as lung cancer, colorectal cancer, liver cancer,
sarcoma and melanoma (8–10). VM offers functional perfusion
pathways for rapidly growing tumors by transferring fluid from
leaky vessels and connecting with vasculature, representing a
non-angiogenic pathway. Aggressive tumors possess VM structures,
which are inaccessible to anti-angiogenic therapies, resulting in
cancer resistance (11,12). Analysis of NSCLC tissue samples has
indicated that the levels of VM, slug and vimentin [two key
regulators in epithelial-mesenchymal transition (EMT)] are higher
in NSCLC tissues compared with normal lung tissues (13,14).
Furthermore, CDK5 kinase induces focal adhesion kinase (FAK)/AKT
signaling and subsequent VM formation. Blockade of CDK5 by
inhibitors or siRNA inhibits VM formation and tumor growth in an
NSCLC A549 cell line and animal models (15).
Drug repurposing has emerged as an attractive
approach in combating malignant tumors (16–19).
Doxazosin, an α1-adrenergic blocker for the treatment of
hypertension and the symptoms of benign prostatic hyperplasia, has
been reported to display anticancer activity through the inhibition
of cell proliferation, migration and metastasis, and the induction
of autophagy and apoptosis of cancer cells (20–23).
Furthermore, doxazosin is reported to inhibit vascular endothelial
growth factor (VEGF), suppressing the migration and invasion of
endothelial cells (24). However,
the impact of doxazosin on VM formation has not yet, to the best of
our knowledge, been reported. In the present study, a VM model
containing hollow lumens was established using an NSCLC cell model
and the inhibitory activity of doxazosin was studied. To the best
of our knowledge, the present study is the first report to
elucidate the anti-VM effect of doxazosin.
Materials and methods
Materials
The human NSCLC cell line, A549, was purchased from
the American Type Culture Collection. RPMI 1640 medium, fetal
bovine serum (FBS), penicillin and streptomycin were purchased from
Gibco (Thermo Fisher Scientific, Inc.). The GAPDH (cat. no. 32233)
and fibronectin (cat. no. 18825) antibodies were purchased from
Santa Cruz Biotechnology, Inc. Vascular endothelial (VE)-cadherin
(cat. no. 2500), Ephrin type-A receptor 2 (EpHA2; cat. no. 6997),
phosphorylated (p-)EpHA2Ser897 (cat. no. 6347),
3-phosphoinositide-dependent kinase 1 (PDK1; cat. no. 3062),
p-PDK1Ser241 (cat. no. 3061), AKT (cat. no. 9272),
p-AKTSer473 (cat. no. 4060), mTOR (cat. no. 2972),
mTORSer2448 (cat. no. 2971), P70s6k (cat. no. 9202),
p-P70s6kThr389 (cat. no. 9234), ERK (cat. no. 9102),
p-ERKThr202/Tyr204 (cat. no. 9101) and vimentin (cat.
no. 5741) antibodies were purchased from Cell Signaling
Technologies, Inc. VEGF-A (cat. no. ab1316) antibodies were
purchased from Abcam. Matrix metalloproteinase (MMP)-2 (cat. no.
AB19167), MMP-9 (cat. no. AB19016) and Laminin 5γ2 (cat. no.
MAB19562) antibodies were purchased from Millipore. Matrigel was
purchased from BD Biosciences. Anti-mouse (cat. no. 115-035-062)
and anti-rabbit (cat. no. 111-035-045) IgGs were purchased from
Jackson ImmunoResearch Laboratories, Inc. Doxazosin (cat. no.
D9815), thiazolyl blue tetrazolium blue (MTT; cat. no. M2128) and
sulforhodamine B (SRB; cat. no. S9012) were purchased from
Sigma-Aldrich.
Cell culture
A549 cells were cultured in RPMI 1640 medium
supplemented with 10% (v/v) FBS and 1% (v/v)
penicillin-streptomycin-amphotericin B solution. Cell cultures were
maintained in a 37°C incubator with 5% CO2. Adherent
cell cultures were passaged using 0.05% trypsin-EDTA after reaching
~80% confluence.
MTT assay
After a 24-h treatment with the indicated
concentrations of doxazosin, the cells were incubated with MTT
(final concentration 0.5 mg/ml) for 2 h. Then, the medium was
removed and replaced with 100 µl DMSO to dissolve the formed purple
formazan. An ELISA reader (570 nm) was used to assess the
absorbance values (25).
SRB assay
Firstly, cells were seeded into 96-well plates.
After overnight incubation, cells in partial wells were fixed with
10% trichloroacetic acid (TCA) for 10 min at room temperature and
washed with ddH2O. Cells at this stage represented the
cell population at the time of drug addition (T0). The other cells
were treated with (Tx) or without [control (C) in 0.1% DMSO] the
indicated concentrations of doxazosin for an additional 48 h. Then,
the cells (T0 and Tx) were fixed with 10% TCA for 10 min at room
temperature and washed with ddH2O. All cells were
stained with 0.4% (w/v) SRB in 1% acetic acid for 10 min at room
temperature, and then washed with 1% acetic acid to remove unbound
dye. SRB bound cells were solubilized with 10 mM trizma base. Using
the absorbance (515 nm) measurements for T0, C and Tx, the
percentage of doxazosin effect was calculated as follows:
[1-(Tx-T0)/(C-T0)] ×100% (26,27).
Flow cytometry with propidium iodide
(PI) staining
Cells were harvested by trypsinization, fixed with
70% (v/v) ethanol for 30 min at 4°C and washed with PBS. The cells
were then centrifuged at 500 × g for 10 min at room temperature and
re-suspended with 0.3 ml PI solution containing Triton X-100 (0.1%,
v/v), RNase (100 µg/ml) and PI (80 µg/ml). The cellular DNA content
was analyzed using an FACScan flow cytometer and CellQuest software
(V6.0.4; Becton, Dickinson and Company) (28).
VM formation assays
VM formation assays were performed in 48-well
culture plates coated with 100 µl Matrigel (9.5 mg/ml). Following
Matrigel polymerization at 37°C for 30 min, the cells were seeded
at 3.2×105 cells/ml in serum-free RPMI medium onto the
Matrigel. After cell adhesion to the Matrigel for 4 h (basal
condition, representing non-VM forming condition), 0 or 25 µM
doxazosin was added to the serum-free medium and a medium change
was performed every 2 days at 37°C for the indicated time,
before western blotting and microscopic examination.
Periodic acid Schiff (PAS) staining
and confocal microscopy
Cells were cultured on 18×18 mm glass coverslips
coated with Matrigel at 37°C for 30 min. After 72 h treatment with
different concentration of doxazosin, 3D culture cells were fixed
with 4% paraformaldehyde in PBS for 15 min at room temperature,
then quickly washed with PBS. Vascular channels were stained using
a PAS kit (cat. no. SI-395B; Sigma) for 10 min at room temperature,
washed with PBS for 10 min and treated with Schiff reagent (cat.
no. SI-395B; Sigma-Aldrich) for 20 min at room temperature. The
stained cells were washed with PBS for 15 min and vasculogenic
morphogenesis was visualized using fluorescence microscopy (Zeiss
Axio Imager, M1). Vascular channels were quantified using MetaMorph
software (V7.8.0; Molecular Devices, LLC) (29). For 3D reconstruction, the 3D culture
cells were stained with PAS and observed using a ZEISS Cell
Observer SD Confocal Microscope (Zeiss GmbH) and ZEN software
(V2.3; Zeiss GmbH) (30). The
results were determined as follows: Total tube length=total length
of tube (excluding nodes); mean tube length=(total tube
length)/(number of segments); total tube area=total tube area
(excluding nodes); mean tube area=(total tube area)/(number of
segments); segments=total number of tube segments connecting branch
points and/or ends. IC50 is the half maximal inhibitory
concentration.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR were performed to assess mRNA expression
levels. Corning Cell Recovery Solution (Corning, Inc.) was used to
recover cells from 3D Matrigel cultures according to the
manufacturer's instructions. Total RNA was extracted from the cells
using an RNAspin Mini Kit (Cytivia), then cDNA was reverse
transcribed from the total RNA (0.7 µg) using an iScript cDNA
synthesis kit (Bio-Rad Laboratories, Inc.). The reverse
transcription was performed at 37°C for 60 min, and then at 85°C
for 5 min, according to the manufacturer's protocol. qPCR was
performed using iTag Universal SYBR Green Supermix (Bio-Rad
Laboratories, Inc.) and primer sequences as follows: Human
VEGF-A forward (F), 5′-CTACCTCCACCATGCCAAGT-3′ and reverse
(R), 5′-GCAGTAGCTGCGCTGATAGA-3′; human EPHA2 F,
5′-CCTCTAGTGCCTTCTTTAG-3′ and R, 5′-GAATGTTTGACACCCTCT-3′; human
VE-cadherin F, 5′-CGTGTTCGCCATTGAGAG-3′ and R,
5′-TTCGCCAGTGTCCTTGTC-3′; human N-cadherin F,
5′-AGTACAGAAGCACTGGGATT-3′ and R, 5′-AAGCGTGTTGAAGCATATCAT-3′;
human vimentin F, 5′-AGTCCACTGAGTACCGGAGAC-3′ and R,
5′-CATTTCACGCATCTGGCGTTC-3′; human FAK F,
5′-GTAGCGTGGCGTAAGTTA-3′ and R, 5′-TTCCTTGACAAGTGAATTATGC-3′; and
human GAPDH F, 5′-CAGGGCTGCTTTTAACTCTGGT-3′ and R,
5′-GATTTTGGAGGGATCTCGCT-3′. DNA was amplified with an initial
denaturation at 95°C for 5 min, followed by 40 cycles of 95°C for 5
sec, and 60°C for 30 sec. GAPDH was chosen as the internal
reference. The data were expressed as relative mRNA levels by Cq
values and then subsequently converted to fold change (31).
Western blotting
Corning Cell Recovery Solution (Corning, Inc.) was
used to recover cells from 3D Matrigel cultures according to the
manufacturer's instructions. The cells were harvested, centrifuged
at 500 × g for 10 min at 4°C and lysed in 80 µl ice-cold lysis
buffer (150 mM NaCl, 1% Triton X-100, 20 mM Tris-HCl pH 7.4, 1 mM
EDTA, 1 mM EGTA, 1 mM PMSF, 10 µg/ml leupeptin, 1 mM
Na3VO4, 1 mM NaF and 1 mM dithiothreitol) for
30 min. The concentration of the total protein was quantified using
the Bradford method. Total protein was mixed with sample buffer and
heated at 95°C for 10 min. An equal amount of protein (30 µg) per
lane was separated by 8 or 12% SDS-PAGE, transferred to PVDF
membranes. The membrane was then blocked with 5% skimmed milk for 1
h at room temperature. Protein expression levels were detected with
specific antibodies (1:1,000 dilution for the primary antibodies at
4°C overnight and 1:7,000 dilution for the secondary antibodies at
room temperature for 2 h). The membranes were washed three times
with PBS-T (0.1% Tween 20) for 10 min after incubations with the
primary and secondary antibodies. The immunoreactive proteins were
detected with an enhanced chemiluminescence detection kit
(LumiFlash™ Prime Chemiluminescent Substrate; cat. no. LF01-500;
Visual Protein; Energenesis Biomedical Co., Ltd.) and the images
were captured using a ChemiDoc™ MP System (Bio-Rad Laboratories,
Inc.). LabTM Software (V6.0; Bio-Rad Laboratories, Inc.)
was used to semi-quantify the data from western blotting.
VEGF-A secretion quantification
ELISA was applied to assess VEGF-A secretion
(32). 3D culture cells were
treated with 25 µM doxazocin for 96 h. Then, the concentration of
VEGF-A in the supernatant was determined using a Human VEGF
Quantikine ELISA kit (cat. no. DEV00; R&D Systems, Inc.)
according to the manufacturer's instructions.
MMP-2 secretion and
quantification
3D culture cells were treated with 25 µM doxazocin
for 96 h. Then, the concentration of MMP-2 in the supernatant was
determined using a Human MMP-2 Quantikine ELISA kit (cat. no.
MMP200; R&D Systems, Inc.) according to the manufacturer's
instructions.
Statistical analysis
Data are presented as the mean ± SEM. Student's
unpaired t-test was performed for the statistical analysis of data
comparing two groups. One-way ANOVA followed by the Bonferroni's
post hoc test was used to perform the statistical analysis of
multiple groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
3D tubular structures form in A549
cells
A Matrigel-based VM formation assay was performed to
examine whether the NSCLC cell line, A549, generated channels in 3D
cultures. The results demonstrated that the cells formed
vessel-like structures when cultured on Matrigel (Fig. 1A). PAS staining was performed to
identify the glycoprotein-rich inner area of VM vessels, and
lumen-containing tubular structures were subsequently observed in
cultures using confocal microscopy and ZEN software (Fig. 1A and Video S1) (30). VM-related gene expression levels
were also determined. The results revealed that VEGF-A and
VE-cadherin expression levels were markedly upregulated in
VM-forming cells (Fig. 1B).
N-cadherin and vimentin (EMT markers) levels were
also increased in 3D cultures. In addition, the levels of
MMP-9, which is responsible for cell motility and ECM
remodeling (33), were raised.
MMP-2, which is involved in degrading basement membrane
components for cancer metastasis along with MMP-9 (33), expression levels showed an increased
trend, albeit this was not significant. FAK expression was
also increased, although not significantly These data demonstrated
that glycoprotein-rich lined tubular structures and VM-related
molecules were present in the constructed in vitro VM model.
Therefore, this model was utilized to evaluate the anti-VM effect
of doxazosin.
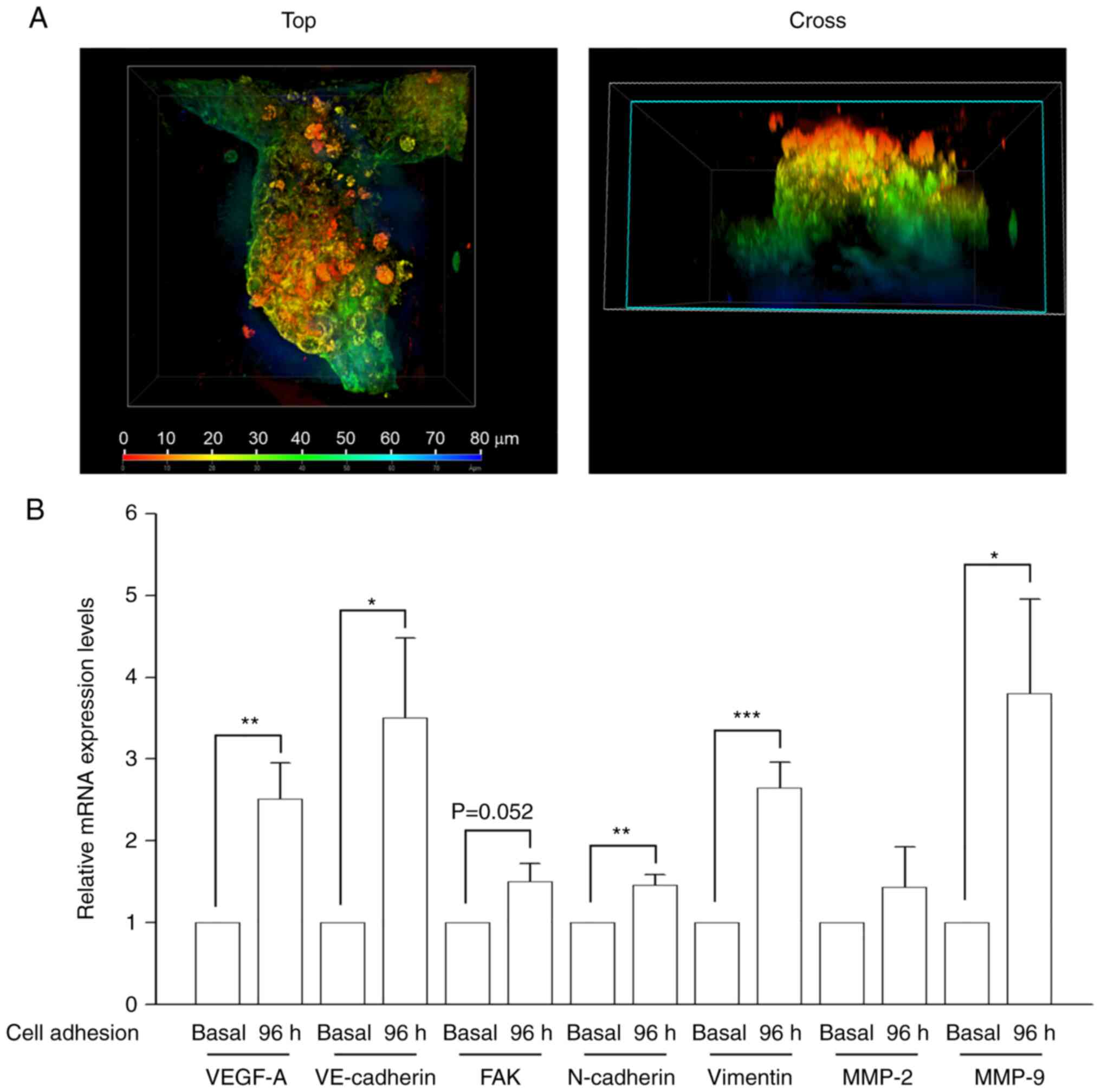 | Figure 1.Characterization of a VM in
vitro model of human non-small cell lung cancer. A549 cells
were seeded in serum-free RPMI medium onto Matrigel and cultured
for 96 h. Medium changes were performed every 2 days, then Corning
Cell Recovery Solution was used to recover cells from the 3D
Matrigel cultures. (A) Following Periodic Acid Schiff staining to
identify the glycoprotein-rich inner area of VM vessels,
3D-reconstruction of a 6-day-old 3D culture of A549 cells was
performed, with the resulting color map distinguishing between the
planes and lumen-containing tubular structures present in the
culture, using the confocal microscopy ZEN program. A view from the
top and cross sections are shown. The cross section identified
upper (red), middle (green) and lower sections (blue) of a tubular
structure. (B) VM-related gene expression after a 96-h cell
adhesion to Matrigel was determined by reverse
transcription-quantitative polymerase chain reaction. Data are
presented as the mean ± SEM of five experiments. *P<0.05,
**P<0.01, ***P<0.001 compared with basal condition (cell
adhesion to the Matrigel for 4 h). FAK, focal adhesion kinase; MMP,
matrix metalloproteinase; VE-cadherin, vascular
endothelial-cadherin; VEGF-A, vascular endothelial growth factor A;
VM, vasculogenic mimicry. |
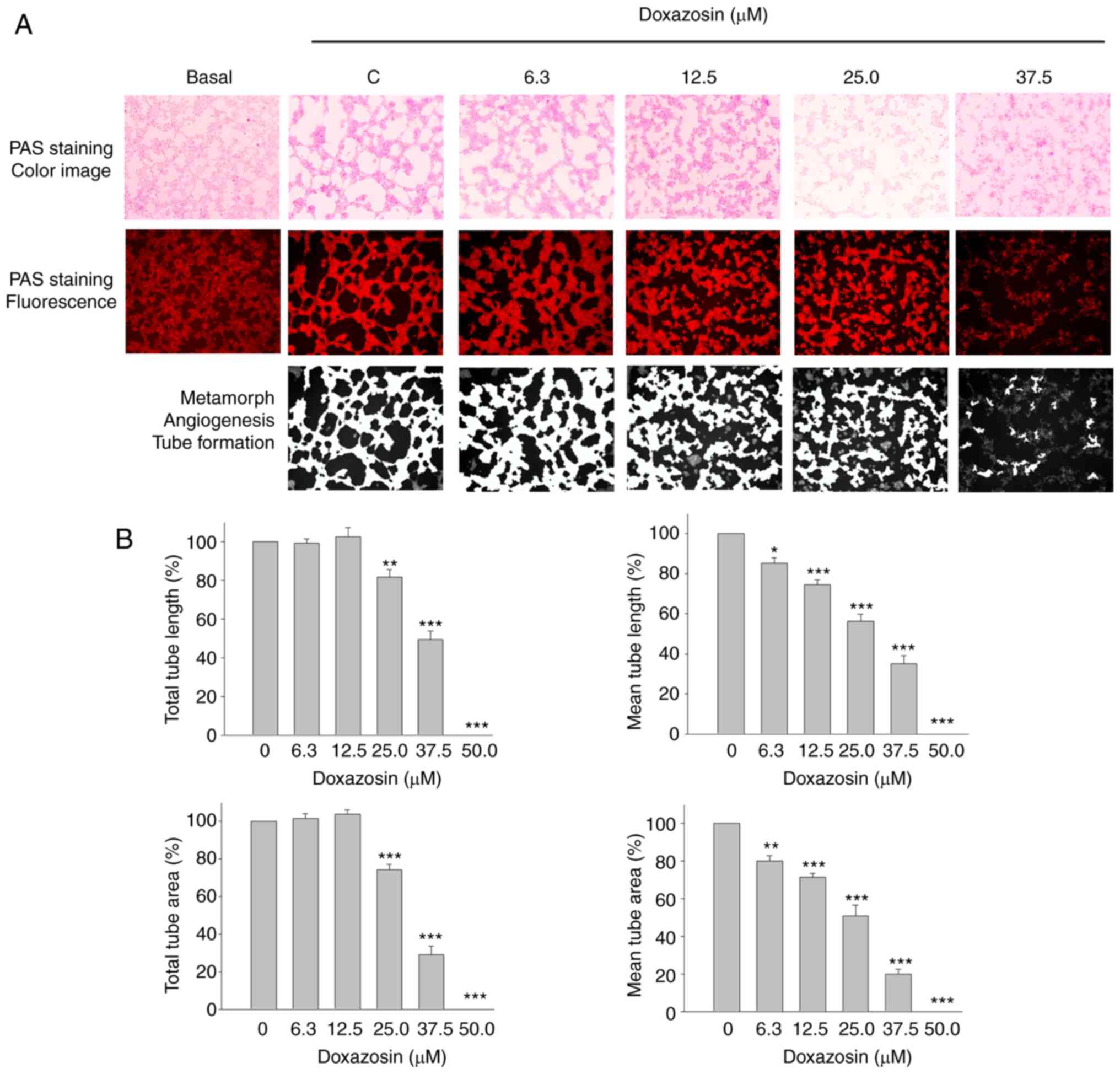
Effect of doxazosin on VM
formation
Quinazoline-based α1-adrenergic receptor
blockers are widely used therapeutic drugs for treating
hypertension (34), one of which
was examined in the present study using the constructed VM cell
model. Vasculogenic morphogenesis on a Matrigel surface was
visualized in the control group using fluorescence microscopy
following PAS staining (Figs. 1A
and 2A). Doxazosin inhibited
capillary-like tube formation and caused a dispersed morphology in
A549 cells (Fig. 2A). The total
tube length, mean tube length, total tube area and mean tube area
of mimetic vessels (quantified using MetaMorph software) were also
reduced by doxazosin, with IC50 values of 36.07±1.38,
28.38±2.10, 31.27±0.32 and 24.17±2.35 µM, respectively (Fig. 2B). The results therefore indicated
that doxazosin treatment of the 3D Matrigel cell culture model
significantly reduced VM channel formation.
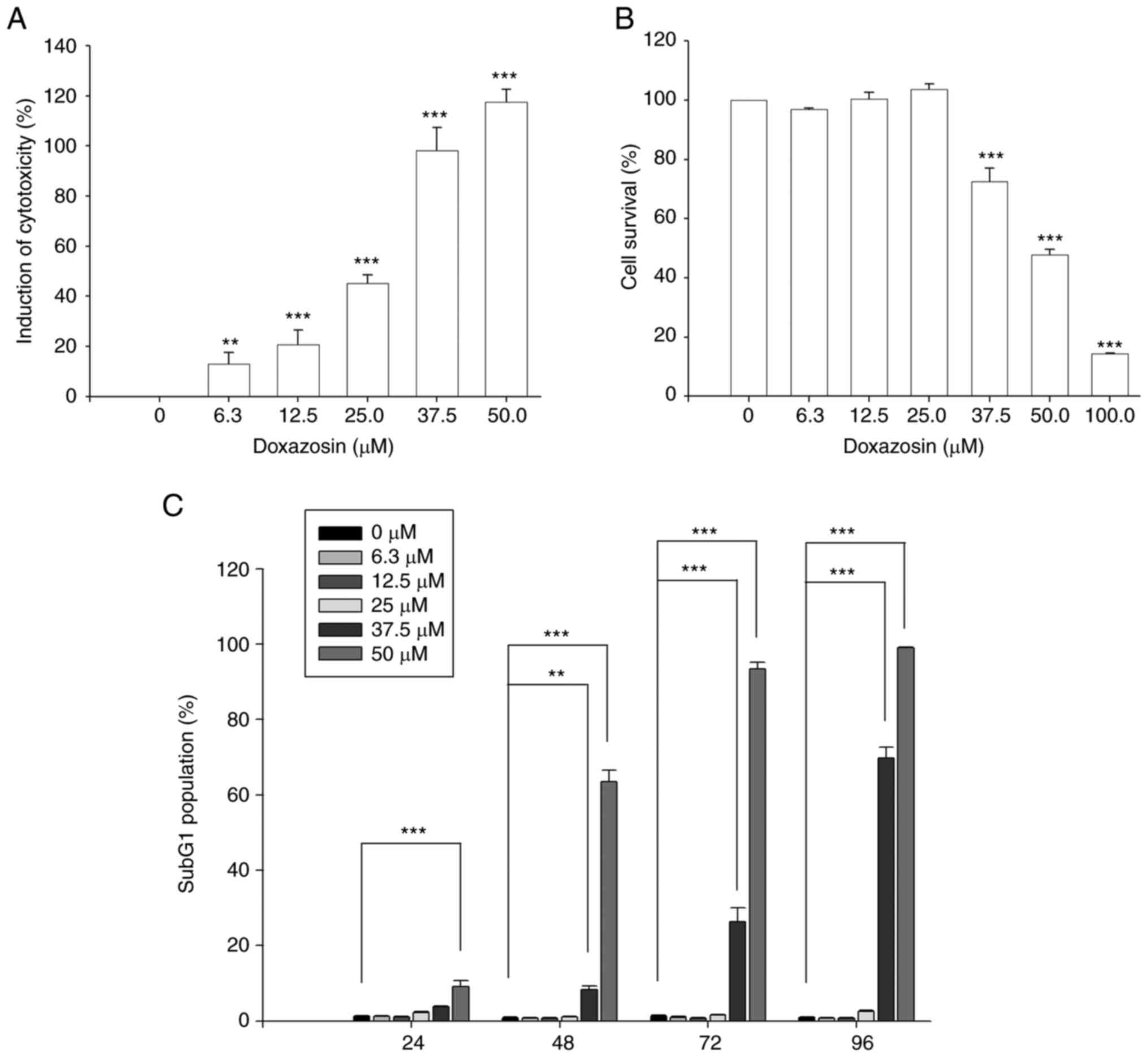
Effect of doxazosin on
cytotoxicity
The results of the SRB and MTT assays demonstrated
that doxazosin also induced cytotoxic effects in A549 cells
(Fig. 3). Doxazosin was more
effective in inducing toxicities (6.3 µM, 13.1±4.6%; 12.5 µM,
20.8±5.6%; 25.0 µM, 45.1±3.5%; 37.5 µM, 98.2±9.1%) when using the
SRB assay than when using MTT assay (cell survival: 37.5 µM,
72.6±4.4%; 50.0 µM, 47.8±1.8%; 100.0 µM, 14.5±0.1%) (Fig. 3A and B).
The effect of doxazosin on cell cycle progression
was also examined, which revealed an increase in the apoptotic
sub-G1 phase population with an initial apoptotic effect at 37.5
µM, compared with the 0 µM doxazosin control group (Figs. 3C and S1), which was similar to the
aforementioned cytotoxicity assessment (Fig. 3B). The results therefore indicated
that doxazosin was more effective in inducing anti-VM activities
than in inducing cytotoxic effects.
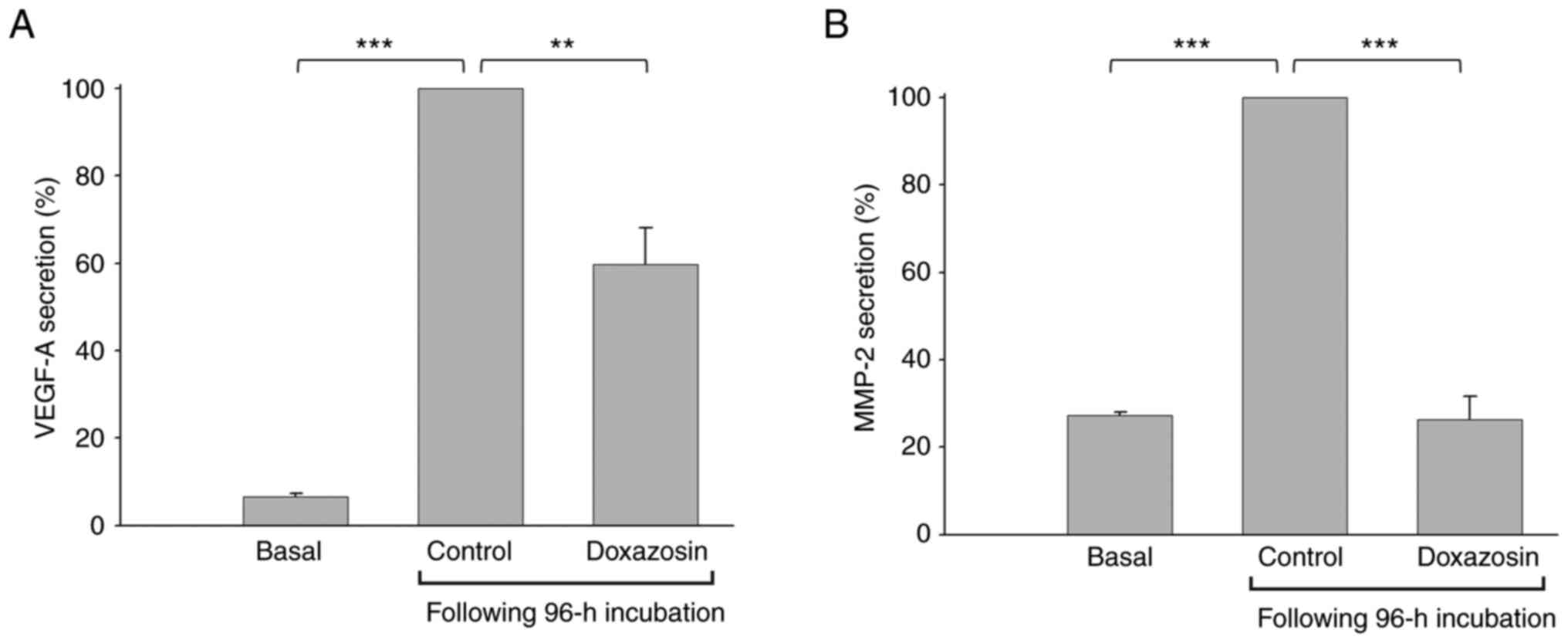
Effect of doxazosin on VEGF-A and
MMP-2 levels in 3D culture media
To further examine the doxazosin-mediated
VM-blocking effect on underlying signaling pathways, the levels of
two key mediators were determined in the medium during VM
formation. The results revealed that the levels of both VEGF-A and
MMP-2 in the control treatment group (0 µM doxazosin) were
significantly increased compared with basal condition group,
supporting VM formation (Fig. 4).
Treatment with doxazosin significantly decreased these secretion
levels.
Effect of doxazosin on the expression
of pro-VM formation regulators
VE-cadherin is required for proper vascular
development and is typically examined as an indicator of VM
formation (35–39). VE-cadherin expression was
significantly increased following a 96-h incubation of the 3D
Matrigel cultures (Fig. S2). The
VM-related protein expression levels were then examined following
treatment with doxazosin. For this, A549 cells were seeded in
serum-free RPMI medium onto Matrigel in the absence or presence of
25 µM doxazosin for 96 h. Then, Corning Cell Recovery Solution was
used to recover cells from the 3D Matrigel cultures. The protein
expression of several signaling pathways, including
VEGF-A/VE-cadherin/p-EphA2/p-PDK1/p-AKT/p-mTOR/p-p70S6k/p-ERK,
MMP-2/MMP-9/laminin 5γ2 and vimentin/fibronectin were determined by
western blotting. Doxazosin markedly decreased the cellular protein
expression of VEGF-A monomer and dimer (Fig. 5A). Furthermore, doxazosin
significantly decreased the protein expression of VE-cadherin and
EphA2, and significantly reduced the phosphorylation levels of
PDK1, AKT, mTOR, P70S6K and ERK in the 3D Matrigel cell culture
model (Fig. 5A). The aforementioned
results revealed the doxazosin-mediated suppression of VM
formation. MMP-2 and MMP-9 are reported to degrade collagen in the
basement membrane, supporting remodeling of the ECM and the
regulation of VM formation (40).
Although the laminin 5γ2 chain is mainly cleaved by activated MMP-2
(not MMP-9) to produce the 5γ2′ and 5γ2× cleaved fragments (which
subsequently trigger the migration and invasion of tumor cells),
both MMP-2 and MMP-9 colocalize with VM networks to assist VM
formation (41). In the present
study, doxazosin significantly reduced the protein expression
levels of MMP-2 and MMP-9 and downregulated the protein expression
levels of the cleaved forms of laminin, 5γ2′ and 5γ2× (Fig. 5B). EMT is reported to be implicated
in VM formation and is associated with the tumor
invasion-metastasis cascade (42).
Doxazosin also significantly downregulated the protein expression
levels of the two EMT markers, vimentin and fibronectin, in the 3D
Matrigel-cultured A549 model (Fig.
5C). Collectively, these results confirmed that doxazosin
displayed anti-VM activity in the NSCLC cell model.
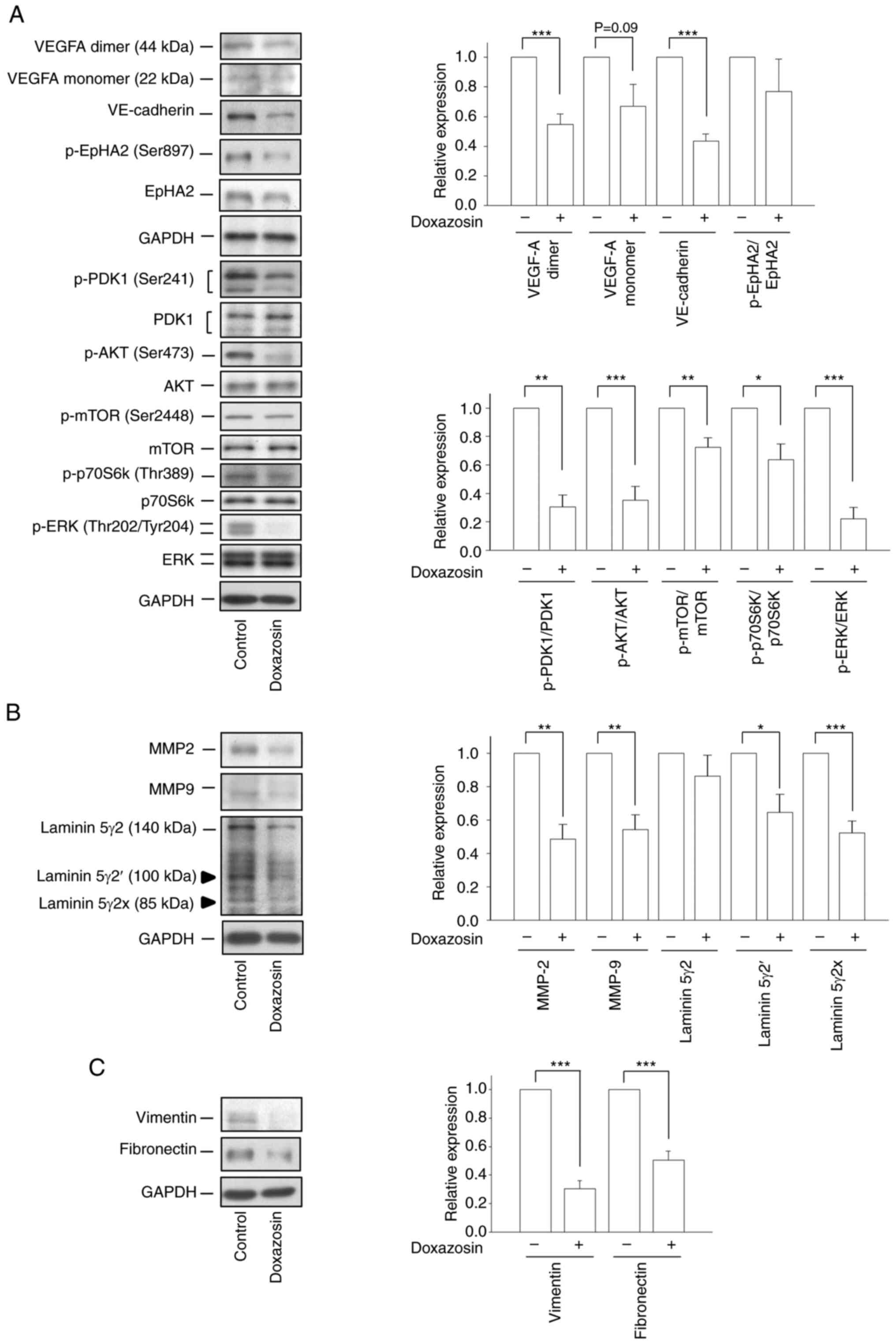 | Figure 5.Effect of doxazosin on the expression
of VM-related proteins. A549 cells were seeded in serum-free RPMI
medium onto Matrigel in the absence or presence of 25 µM doxazosin
for 96 h. Then, Corning Cell Recovery Solution was used to recover
cells from the 3D Matrigel cultures. The protein expression levels
of (A) VEGFA, VE-cadherin, p-EphA2, p-PDK1, p-AKT, p-mTOR,
p-p70S6k, p-ERK, (B) MMP2, MMP9, laminin 5γ2, and (C) vimentin and
fibronectin were determined by western blotting analysis. Data are
presented as the mean ± SEM of three to five experiments.
*P<0.05, **P<0.01, ***P<0.001, compared with the VM
control (0 µM doxazosin). EpHA2, Ephrin type-A receptor 2; MMP,
matrix metalloproteinase; p-, phosphorylated; PDK1,
3-phosphoinositide-dependent kinase 1; VE-cadherin, vascular
endothelial-cadherin; VEGF-A, vascular endothelial growth factor A;
VM, vasculogenic mimicry. |
Discussion
When tumors metastasize and grow in size,
neovasculature is required to achieve sufficient nutrition through
diffusion. VM serves as a mode of vascularization to mimic the
vasculogenesis of endothelial cells (11,12,43).
The vascular structure of VM has been reported to be built by
cancer cells on rich ECMs, including proteoglycans and
glycoproteins, which are positive for PAS staining. To identify VMs
in human tumor biopsies and experimental settings, positive PAS
staining in the absence of endothelial cell markers, such as CD31
or CD34, is generally used to assess vessel-like structures
(30,43). However, certain studies raise an
issue doubting the validity of concluding the presence of VM based
solely on positive PAS staining without a definitive proof of a
lumen in patterned vessel-like structures (44). In the present study, in accordance
with the definition of VM formation, confocal microscopy
examination was performed to detect structures with a lumen. The
images showed lumen-like tubular structures after a 6-day growth of
A549 cells in a Matrigel-cultured model. Furthermore, high
PAS+ staining of VM vessels was observed. Notably, the
gene expression levels of VEGF-A, VE-cadherin, N-cadherin,
vimentin and MMP-9, which are crucial molecules in
driving VM formation (9,44), were also markedly increased.
Collectively, these data indicated VM formation in the constructed
3D Matrigel-cultured A549 model.
Several anticancer mechanisms of doxazosin have been
reported, including activation of DNA damage (45,46),
the TGF-β pathway (47) and
autophagy (20), and the inhibition
of angiogenesis through regulating the VEGF/Akt/mTOR signaling
(23). In the present study, the
anti-VM effect of doxazosin was explored using the constructed
NSCLC A549 model. Doxazosin displayed inhibitory activity, as
demonstrated by the reduced PAS+ staining of VM vessels
and the quantified tube length and tube area. Notably, doxazosin
was more efficient in blocking VM formation than inducing
cytotoxicity by MTT assay and apoptosis. Although doxazosin induced
similar anti-VM efficacy and cytotoxicity by SRB assay, the
underlying mechanisms could be distinguished in which a broad panel
of markers were used for anti-VM identification, including the
protein expression of VEGF-A, MMP-2, MMP-9, VE-cadherin, EpHA2,
vimentin and fibronectin, and laminin 5γ2 cleavage.
VE-cadherin is responsible for homotypic cell-cell
interactions and vasculogenic events (48), and is exclusively expressed in
highly aggressive tumors (49).
Downregulation of VE-cadherin expression in aggressive melanoma
cells abolished the ability to generate vasculogenic networks
(49). Several studies have
examined VE-cadherin as the only indicator for VM formation due to
it having a prominent role in the acquisition of vascular-like
structures (36–38). Recent research and review articles
have also addressed the key role of VE-cadherin in VM formation
(38,39). Hepatocellular carcinoma cells,
cultured on plates coated with a fusion protein comprising a human
VE-cadherin extracellular domain and an immunoglobulin G Fc region
(hVE-cad-Fc), markedly formed patterned tubular structures and
exhibited increased levels or activity of EphA2, MMP-2, MMP-9 and
EMT makers (50). p-EphA2 has been
reported to trigger PI3K and increase membrane type 1 matrix
metalloproteinase/MMP/MMP-14 expression and MMP-2 activation
through the FAK and ERK1/2 pathways (8). Subsequently, the laminin 5γ2-chain is
cleaved into γ2′ and γ2× fragments that subsequently trigger
migration, invasion and VM formation in melanoma (8,41).
Apatinib and its combination with melatonin are reported to
decrease the expression of VE-cadherin and EphA2, and inhibit the
phosphorylation of PI3K and AKT, leading to the inhibition of VM
formation, survival and invasion of breast cancer stem cells
(51). In the present study,
similar inhibitory effects of doxazosin were observed on VM
formation in the A549 model through the downregulation of
VE-cadherin and the EphA2/PI3K/PDK-1/AKT/mTOR pathway. The
downregulation of both MMP-2 and MMP-9 also contributed to the
anti-VM mechanism. Furthermore, doxazosin significantly inhibited
the generation of γ2′ and γ2× fragments, further supporting its
anti-VM capability.
VEGF-A is another key mediator in VM formation.
Secreted as a dimer, VEGF-A binds to VEGFR-1, which is highly
expressed in malignant tumor cells with the capacity to induce VM
formation (52). VEGF-A exposure
triggers VM formation in several types of cancer cells, such as
ovarian carcinoma (53) and
melanoma (54), through increased
expression of VE-cadherin, EphA2, MMP-2 and MMP-9, indicating that
VEGF-A can stimulate tumor cell plasticity (53). VEGF gene silencing was
reported to reduce VM formation and impair the expression levels of
MMP-2 and MMP-9 via the PI3K/AKT-dependent pathway (55). Moreover, the blockade of EphA2
expression or activity inhibits VEGF expression and related
angiogenesis in animal models, suggesting a complex regulation of
these key regulators (56,57). In the present study, it was
demonstrated that the gene expression and medium content of VEGF-A
were upregulated in VM-forming cells, which was consistent with the
aforementioned previous studies. Following doxazosin treatment, the
reduction in VEGF-A expression might partly contribute to the
inhibition of downstream VM signaling. EMT-related regulatory
proteins are upregulated in VM-forming cells, suggesting a positive
association between EMT and VM (42). MMPs, secreted by tumor cells lining
VM networks, serve an important role in modifying cell-to-cell
junctions and cell-ECM interactions, in which the ECM facilitates
tumor invasion and metastasis (40). Due to the downregulation of MMP-2,
MMP-9, vimentin and fibronectin protein expression observed in the
present study following doxazosin treatment, doxazosin was
hypothesized to inhibit EMT pathways, although this requires
further validation.
It is noteworthy that several small molecules
including natural products, synthetic compounds and known
therapeutic drugs display anticancer potential through the
suppression of VM formation (58).
Furthermore, certain VM formation signaling pathways are suppressed
by these agents (59,60). For example, triptonide potently
inhibits VM by reducing the expression of VE-cadherin (59). Combretastatin A-4 and the synthetic
compound, DHPAC, inhibit AKT phosphorylation and decrease the
expression levels of VEGF, MMP2, MMP9 and Laminin 5 in NSCLC cell
models (60). Following validation
of the EphA2 receptor as a new target for treating tumors dependent
on angiogenesis and VM (61), a
novel synthetic compound, UniPR505, has been developed as an
antagonist of the EphA2 receptor (62). Similarly, the present study
demonstrated that doxazosin reduced the levels of VEGF-A, MMP-2,
MMP-9 and VE-cadherin and inhibited the EphA2/AKT/mTOR/Laminin-5γ2
signaling network. Collectively, these studies together with the
results of the present study support that the doxazosin-mediated
suppression of VM formation may be an addition to the anti-NSCLC
activities. However, the limitation of the present study is that it
is a mechanistic study using 3D cancer cell culture without the
consideration of interactions between other cell types and
environments, as with in real tissues.
In conclusion, the results of the present study
suggested that doxazosin displayed anti-VM activity in a 3D A549
model through the downregulation of VEGF-A and VE-cadherin levels,
and the suppression of signaling pathways in which the receptor
tyrosine kinase, EphA2, protein kinases, AKT and mTOR, and
proteases, MMP-2 and MMP-9, are involved. These data support the
add-on anti-VM effect of doxazosin as a potential agent against
NSCLC.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
The authors would like to thank Ms. Hwa-Man Hsu
(Imaging Core, First Core Labs, National Taiwan University College
of Medicine, Taipei, Taiwan), for technical assistance in image
acquisition and analysis.
Funding
This work was supported by grants from the National Science and
Technology Council (grant no. NSTC 112-2320-B-255-014) and the
Chang Gung University of Science and Technology (grant no.
ZURPF3N0091).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JLH, LCH, CHH and JHG contributed to the conception
and design of the experiments. JLH performed the experiments. JLH
and WJL analyzed the data. JLH, WJL and JHG confirm the
authenticity of all the raw data. JLH and JHG wrote the manuscript.
All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Wagle NS and Jemal
A: Cancer statistics, 2023. CA Cancer J Clin. 73:17–48. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li S, de Camargo Correia GS, Wang J,
Manochakian R, Zhao Y and Lou Y: Emerging targeted therapies in
advanced non-small-cell lung cancer. Cancers (Basel). 15:28992023.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tan AC and Tan DSW: Targeted therapies for
lung cancer patients with oncogenic driver molecular alterations. J
Clin Oncol. 40:611–625. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alexander M, Kim SY and Cheng H: Update
2020: Management of non-small cell lung cancer. Lung. 198:897–907.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jurišić V, Obradovic J, Pavlović S and
Djordjevic N: Epidermal growth factor receptor gene in
non-small-cell lung cancer: The importance of promoter polymorphism
investigation. Anal Cell Pathol (Amst). 2018:61921872018.PubMed/NCBI
|
|
6
|
Jurisic V, Obradovic J, Nikolic N, Javorac
J, Perin B and Milasin J: Analyses of P16INK4a gene
promoter methylation relative to molecular, demographic and
clinical parameters characteristics in non-small cell lung cancer
patients: A pilot study. Mol Biol Rep. 50:971–979. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun R, Hou Z, Zhang Y and Jiang B: Drug
resistance mechanisms and progress in the treatment of EGFR-mutated
lung adenocarcinoma. Oncol Lett. 24:4082022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kirschmann DA, Seftor EA, Hardy KM, Seftor
RE and Hendrix MJ: Molecular pathways: Vasculogenic mimicry in
tumor cells: Diagnostic and therapeutic implications. Clin Cancer
Res. 18:2726–2732. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cao Z, Bao M, Miele L, Sarkar FH, Wang Z
and Zhou Q: Tumour vasculogenic mimicry is associated with poor
prognosis of human cancer patients: A systemic review and
meta-analysis. Eur J Cancer. 49:3914–3923. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hendrix MJ, Seftor EA, Hess AR and Seftor
RE: Vasculogenic mimicry and tumour-cell plasticity: Lessons from
melanoma. Nat Rev Cancer. 3:411–421. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
van der Schaft DW, Seftor RE, Seftor EA,
Hess AR, Gruman LM, Kirschmann DA, Yokoyama Y, Griffioen AW and
Hendrix MJ: Effects of angiogenesis inhibitors on vascular network
formation by human endothelial and melanoma cells. J Natl Cancer
Inst. 96:1473–1477. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Angara K, Rashid MH, Shankar A, Ara R,
Iskander A, Borin TF, Jain M, Achyut BR and Arbab AS: Vascular
mimicry in glioblastoma following anti-angiogenic and anti-20-HETE
therapies. Histol Histopathol. 32:917–928. 2017.PubMed/NCBI
|
|
13
|
Song H, Ci H, Xu J, Xu Z, Zhang Y, Wang Y,
Wu S and Tao Y: Vasculogenic mimicry and expression of slug and
vimentin correlate with metastasis and prognosis in non-small cell
lung cancer. Int J Clin Exp Pathol. 11:2749–2758. 2018.PubMed/NCBI
|
|
14
|
Ci H, Xu Z, Xu J, Wang Y and Wu S:
Expressions of KAI1 and E-cadherin in nonsmall cell lung cancer and
their correlation with vasculogenic mimicry. Medicine (Baltimore).
97:e122932018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou X, Gu R, Han X, Wu G and Liu J:
Cyclin-dependent kinase 5 controls vasculogenic mimicry formation
in non-small cell lung cancer via the FAK-AKT signaling pathway.
Biochem Biophys Res Commun. 492:447–452. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Naeem A, Dakshanamurthy S, Walthieu H,
Parasido E, Avantaggiati M, Tricoli L, Kumar D, Lee RJ, Feldman A,
Noon MS, et al: Predicting new drug indications for prostate
cancer: The integration of an in silico proteochemometric network
pharmacology platform with patient-derived primary prostate cells.
Prostate. 80:1233–1243. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dalwadi SM, Hunt A, Bonnen MD and Ghebre
YT: Computational approaches for drug repurposing in oncology:
Untapped opportunity for high value innovation. Front Oncol.
13:11982842023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu H, Huang D, Zhou H, Sima X, Wu Z, Sun
Y, Wang L, Ruan Y, Wu Q, Wu F, et al: Metformin: A promising drug
for human cancers. Oncol Lett. 24:2042022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pantziarka P, Pirmohamed M and Mirza N:
New uses for old drugs. BMJ. 361:k27012018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Batty M, Pugh R, Rathinam I, Simmonds J,
Walker E, Forbes A, Anoopkumar-Dukie S, McDermott CM, Spencer B,
Christie D and Chess-Williams R: The role of α1-Adrenoceptor
antagonists in the treatment of prostate and other cancers. Int J
Mol Sci. 17:13392016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Forbes A, Anoopkumar-Dukie S,
Chess-Williams R and McDermott C: Relative cytotoxic potencies and
cell death mechanisms of α1-adrenoceptor antagonists in prostate
cancer cell lines. Prostate. 76:757–766. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bilbro J, Mart M and Kyprianou N:
Therapeutic value of quinazoline-based compounds in prostate
cancer. Anticancer Res. 33:4695–4700. 2013.PubMed/NCBI
|
|
23
|
Keledjian K, Garrison JB and Kyprianou N:
Doxazosin inhibits human vascular endothelial cell adhesion,
migration, and invasion. J Cell Biochem. 94:374–388. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Park MS, Kim BR, Dong SM, Lee SH, Kim DY
and Rho SB: The antihypertension drug doxazosin inhibits tumor
growth and angiogenesis by decreasing VEGFR-2/Akt/mTOR signaling
and VEGF and HIF-1α expression. Oncotarget. 5:4935–4944. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Scherbakov AM, Vorontsova SK, Khamidullina
AI, Mrdjanovic J, Andreeva OE, Bogdanov FB, Salnikova DI, Jurisic
V, Zavarzin IV and Shirinian VZ: Novel pentacyclic derivatives and
benzylidenes of the progesterone series cause anti-estrogenic and
antiproliferative effects and induce apoptosis in breast cancer
cells. Invest New Drugs. 41:142–152. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jurisic V, Bogdanovic G, Kojic V, Jakimov
D and Srdic T: Effect of TNF-alpha on Raji cells at different
cellular levels estimated by various methods. Ann Hematol.
85:86–94. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Skehan P, Storeng R, Scudiero D, Monks A,
McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S and Boyd MR:
New colorimetric cytotoxicity assay for anticancer-drug screening.
J Natl Cancer Inst. 82:1107–1112. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jurisic V, Srdic-Rajic T, Konjevic G,
Bogdanovic G and Colic M: TNF-α induced apoptosis is accompanied
with rapid CD30 and slower CD45 shedding from K-562 cells. J Membr
Biol. 239:115–122. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shaifer CA, Huang J and Lin PC:
Glioblastoma cells incorporate into tumor vasculature and
contribute to vascular radioresistance. Int J Cancer.
127:2063–2075. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Racordon D, Valdivia A, Mingo G, Erices R,
Aravena R, Santoro F, Bravo ML, Ramirez C, Gonzalez P, Sandoval A,
et al: Structural and functional identification of vasculogenic
mimicry in vitro. Sci Rep. 7:69852017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jurisic V: Multiomic analysis of cytokines
in immuno-oncology. Expert Rev Proteomics. 17:663–674. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cabral-Pacheco GA, Garza-Veloz I,
Castruita-De la Rosa C, Ramirez-Acuña JM, Perez-Romero BA,
Guerrero-Rodriguez JF, Martinez-Avila N and Martinez-Fierro ML: The
roles of matrix metalloproteinases and their inhibitors in human
diseases. Int J Mol Sci. 21:97392020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vincent J, Elliott HL, Meredith PA and
Reid JL: Doxazosin, an alpha 1-adrenoceptor antagonist:
Pharmacokinetics and concentration-effect relationships in man. Br
J Clin Pharmacol. 15:719–725. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Franco P, Camerino I, Merlino F, D'Angelo
M, Cimmino A, Carotenuto A, Colucci-D'Amato L and Stoppelli MP:
αV–Integrin-Dependent inhibition of glioblastoma cell migration,
invasion and vasculogenic mimicry by the uPAcyclin decapeptide.
Cancers (Basel). 15:47752023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Qin Y, Zhao W, Cai Z, Wang Q, Gao J, Ci H,
Feng Z and Ma L: The biomarker like the correlation between
vasculogenic mimicry, vascular endothelial cadherin,
sex-determiningregion on Y-Box transcription factor 17, and cyclin
D1 in oesophageal squamous cell carcinoma. J Oncol.
2022:89155032022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang Y, Tan Y, Liu S, Yin H, Duan J, Fan
L, Zhao X and Jiang B: Implications of Withaferin A for the
metastatic potential and drug resistance in hepatocellular
carcinoma cells via Nrf2-mediated EMT and ferroptosis. Toxicol Mech
Methods. 33:47–55. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Delgado-Bellido D, Garcia-Diaz A and
Oliver FJ: Co-immunoprecipitation of protein complexes from
different subcellular compartments in vasculogenic mimicry studies.
Methods Mol Biol. 2514:61–72. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Delgado-Bellido D, Oliver FJ, Vargas
Padilla MV, Lobo-Selma L, Chacón-Barrado A, Díaz-Martin J and de
Álava E: VE-Cadherin in cancer-associated angiogenesis: A deceptive
strategy of blood vessel formation. Int J Mol Sci. 24:93432023.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Winkler J, Abisoye-Ogunniyan A, Metcalf KJ
and Werb Z: Concepts of extracellular matrix remodelling in tumour
progression and metastasis. Nat Commun. 11:51202020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Seftor RE, Seftor EA, Koshikawa N, Meltzer
PS, Gardner LM, Bilban M, Stetler-Stevenson WG, Quaranta V and
Hendrix MJ: Cooperative interactions of laminin 5 gamma2 chain,
matrix metalloproteinase-2, and membrane
type-1-matrix/metalloproteinase are required for mimicry of
embryonic vasculogenesis by aggressive melanoma. Cancer Res.
61:6322–6327. 2001.PubMed/NCBI
|
|
42
|
Liu Q, Qiao L, Liang N, Xie J and Zhang J,
Deng G, Luo H and Zhang J: The relationship between vasculogenic
mimicry and epithelial-mesenchymal transitions. J Cell Mol Med.
20:1761–1769. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Maniotis AJ, Folberg R, Hess A, Seftor EA,
Gardner LM, Pe'er J, Trent JM, Meltzer PS and Hendrix MJ: Vascular
channel formation by human melanoma cells in vivo and in vitro:
Vasculogenic mimicry. Am J Pathol. 155:739–752. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Valdivia A, Mingo G, Aldana V, Pinto MP,
Ramirez M, Retamal C, Gonzalez A, Nualart F, Corvalan AH and Owen
GI: Fact or fiction, it is time for a verdict on vasculogenic
mimicry? Front Oncol. 9:6802019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lin SC, Chueh SC, Hsiao CJ, Li TK, Chen
TH, Liao CH, Lyu PC and Guh JH: Prazosin displays anticancer
activity against human prostate cancers: Targeting DNA and cell
cycle. Neoplasia. 9:830–839. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Arencibia JM, Del Rio M, Bonnin A, Lopes
R, Lemoine NR and López-Barahona M: Doxazosin induces apoptosis in
LNCaP prostate cancer cell line through DNA binding and
DNA-dependent protein kinase down-regulation. Int J Oncol.
27:1617–1623. 2005.PubMed/NCBI
|
|
47
|
Partin JV, Anglin IE and Kyprianou N:
Quinazoline-based alpha 1-adrenoceptor antagonists induce prostate
cancer cell apoptosis via TGF-beta signalling and I kappa B alpha
induction. Br J Cancer. 88:1615–1621. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Treps L, Le Guelte A and Gavard J:
Emerging roles of Semaphorins in the regulation of epithelial and
endothelial junctions. Tissue Barriers. 1:e232722013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hendrix MJ, Seftor EA, Meltzer PS, Gardner
LM, Hess AR, Kirschmann DA, Schatteman GC and Seftor RE: Expression
and functional significance of VE-cadherin in aggressive human
melanoma cells: Role in vasculogenic mimicry. Proc Natl Acad Sci
USA. 98:8018–8023. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Shuai Q, Cao L, Qin Z, Zhang Y, Gu Z and
Yang J: VE-cadherin fusion protein substrate enhanced the
vasculogenic mimicry capability of hepatocellular carcinoma cells.
J Mater Chem B. 8:1699–1712. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Maroufi NF, Rashidi M, Vahedian V,
Jahanbazi R, Mostafaei S, Akbarzadeh M, Kazemzadeh H, Nejabati HR,
Isazadeh A, Rashidi MR and Nouri M: Effect of Apatinib plus
melatonin on vasculogenic mimicry formation by cancer stem cells
from breast cancer cell line. Breast Cancer. 29:260–273. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Frank NY, Schatton T, Kim S, Zhan Q,
Wilson BJ, Ma J, Saab KR, Osherov V, Widlund HR, Gasser M, et al:
VEGFR-1 expressed by malignant melanoma-initiating cells is
required for tumor growth. Cancer Res. 71:1474–1485. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang JY, Sun T, Zhao XL, Zhang SW, Zhang
DF, Gu Q, Wang XH, Zhao N, Qie S and Sun BC: Functional
significance of VEGF-a in human ovarian carcinoma: Role in
vasculogenic mimicry. Cancer Biol Ther. 7:758–766. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Vartanian A, Stepanova E, Grigorieva I,
Solomko E, Baryshnikov A and Lichinitser M: VEGFR1 and PKCα
signaling control melanoma vasculogenic mimicry in a VEGFR2
kinase-independent manner. Melanoma Res. 21:91–98. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Xu X, Zong Y, Gao Y, Sun X, Zhao H, Luo W
and Jia S: VEGF induce vasculogenic mimicry of choroidal melanoma
through the PI3k signal pathway. Biomed Res Int. 2019:39091022019.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Cheng N, Brantley D, Fang WB, Liu H,
Fanslow W, Cerretti DP, Bussell KN, Reith A, Jackson D and Chen J:
Inhibition of VEGF-dependent multistage carcinogenesis by soluble
EphA receptors. Neoplasia. 5:445–456. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Brantley-Sieders DM, Fang WB, Hwang Y,
Hicks D and Chen J: Ephrin-A1 facilitates mammary tumor metastasis
through an angiogenesis-dependent mechanism mediated by EphA
receptor and vascular endothelial growth factor in mice. Cancer
Res. 66:10315–10324. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Guan YY, Luan X, Lu Q, Liu YR, Sun P, Zhao
M, Chen HZ and Fang C: Natural products with antiangiogenic and
antivasculogenic mimicry activity. Mini Rev Med Chem. 16:1290–1302.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Han H, Du L, Cao Z, Zhang B and Zhou Q:
Triptonide potently suppresses pancreatic cancer cell-mediated
vasculogenic mimicry by inhibiting expression of VE-cadherin and
chemokine ligand 2 genes. Eur J Pharmacol. 818:593–603. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Gong FL, Wang L, Yu LG, Dang YF, Jiang XN,
Zhao L and Guo XL: DHPAC, a novel microtubule depolymerizing agent,
suppresses angiogenesis and vasculogenic mimicry formation of human
non-small cell lung cancer. J Cell Biochem. 121:4756–4771. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Margaryan NV, Strizzi L, Abbott DE, Seftor
EA, Rao MS, Hendrix MJ and Hess AR: EphA2 as a promoter of melanoma
tumorigenicity. Cancer Biol Ther. 8:279–288. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Incerti M, Russo S, Corrado M, Giorgio C,
Ballabeni V, Chiodelli P, Rusnati M, Scalvini L, Callegari D,
Castelli R, et al: Optimization of EphA2 antagonists based on a
lithocholic acid core led to the identification of UniPR505, a new
3α-carbamoyloxy derivative with antiangiogenetic properties. Eur J
Med Chem. 189:1120832020. View Article : Google Scholar : PubMed/NCBI
|